Abstract
Background
Lower-limb endoprosthetic operations and spinal operations are among the more common types of orthopedic procedures. Postoperative wound-healing disturbances and infections can lead to longer periods of hospital stay and recovery as well as to higher morbidity and mortality.
Methods
209 patients who had been judged to have an indication for a primary knee or hip endoprosthesis or for a primary spinal operation were included in this randomized trial (ClinicalTrials.gov: NCT01988818) over the period June 2014–February 2015. After randomization, patients in the intervention group were given a trial dressing (Mepilex-Border Post-Op) and those in the control group were given a conventional adhesive dressing (Cosmopor). The primary endpoint was blister formation.
Results
In the overall study population, only a single case of blister formation was seen. The affected patient belonged to the intervention group but was mistakenly given a control dressing and developed blisters on the 6th day after surgery. Dressings were changed less frequently in the intervention group, and this difference was statistically significant (p<0.001). The patients, nurses, and physicians all expressed greater satisfaction with the trial dressings than with the control dressings (p<0.001).
Conclusion
The intervention group did not differ from the control group with respect to the primary endpoint, postoperative blister formation. The patients, nurses, and physicians all judged the dressing used in the intervention group more favorably than the conventional dressing.
Total hip and knee replacement surgery (arthroplasty) as well as spinal surgery are among the most commonly performed orthopedic surgical procedures (1). Typically, a suitable wound dressing is used to protect the wound closure by providing external cover and creating a physical barrier against contamination. In addition, an ideal wound dressing should absorb wound exudate without causing excessive dryness of the wound, allowing a microclimate to form that promotes wound healing (2). A wide range of dressings designed for surgical wounds are available. However, at present the existing data do not allow to make evidence-based recommendations as to which wound care product applied after surgical procedures is superior in terms of infection rate, scarring, pain, dressing change, and, most importantly, patient acceptance (2).
Despite impeccable surgical technique and highest hygienic standards, complications of wound healing may occur after orthopedic procedures (3– 6). Relevant wound complications do not only develop after major surgery, but also in day-to-day clinical practice after minor surgical procedures or injuries, both in an out- and in-patient setting (3– 5). In patients with risk factors, such as diabetes mellitus, advanced age, ischemia, and bacterial infection, seemingly small and harmless wounds can lead to complications and chronic wounds (7). The incidence of complications of wound healing after primary orthopedic knee and hip procedures is reported to be 0 to 1.25% (5, 8– 12) and even 0 to 16% after spinal surgery (1, 13– 15). After elective orthopedic surgery, sepsis occurred in 0 to 0.34% of patients (16, 17). Here, harmless wounds can result in acute life-threatening complications (18).
Besides impaired wound healing, blistering is a potential post-operative complication with incidence rates of 6 to 24% reported in the literature (12, 13, 19, 20). Lack of elasticity and insufficient flexibility of adhesive dressings are among the factors promoting the formation of blisters (21, 22). Here, shear forces between skin and wound dressing/plaster play a key role (22). These occur when the epidermis separates from the dermis as the result of continuous abrasion (23). Other factors include age-related skin changes, postoperative soft-tissue edema, the type of wound dressing and the way a wound dressing is applied (22, 24). Due to the irritation of dermal nerve endings resulting from injury to the epidermis, patients often experience discomfort and pain during dressing changes (24). Following the breakdown of the skin barrier, superficial wound infection may occur which, in the worst case, can progress to deep wound infection (24). Moist wounds are associated with faster healing, less infections, and less wound pain compared to dry wounds (25). However, dressings with low adsorption capacity may result in the exposure of the wound to excessive wound exudate. This wet environment favors moisture-associated skin damage (maceration) and bacterial contaminations of the wound, especially if the dressing has a mechanical leak (26). Postoperative wound complications or infections may result in increased rehabilitation time (27, 28), hospital length of stay as well as morbidity and mortality rates (18, 29).
Here, primary prevention is the most effect way to avoid blistering, complications of wound healing and secondary infection. Selection of the best suited wound dressing is key (30). According to Ousey et al., criteria for the fitting wound dressing include adhesion level, thickness of wound dressing, shape of the direct wound contact area of the dressing, absorbability of the dressing, as well as friction (4, 20). Similar criteria have been compiled by the National Institute for Health and Clinical Excellence (NICE) (31).
In everyday clinical practice, conventional dressings are typically used. The regular wound dressing changes required with conventional dressings are associated with pain and a slight risk of infection (32). Currently published data from prospective randomized trials (9, 12) and reviews (10, 21) suggest that absorbent wound dressings already established for the treatment of chronic wounds may also offer benefits in standard postoperative wound management.
In view of these facts, we designed a randomized study comparing a conventional adhesive dressing (Cosmopor E, Hartmann) with an absorbent wound dressing in an orthopedic surgery setting. From the many absorbent wound dressings available in the market, we selected Mepilex Border Post-Op (Mölnlycke, Gothenburg, Sweden)—a flexible, self-adhering and absorbent dressing coated with a soft silicone layer. The integrated Mepilex wound dressing combined with the Safetac adhesive technology used had already shown good results in terms of adhesibility, usability and patient satisfaction in previous studies (12, 21).
The aim of this study was to evaluate the performance of this absorbent wound dressing and to compare it with that of a conventional dressing.
Material and methods
This randomized study conducted at the University Hospital Cologne between June 2014 and February 2015 included a total of 209 adult patients with an indication for primary total knee or hip replacement or primary spinal surgery and an expected duration of the post-operative hospital stay of at least 6 days. Patients were allocated to either of the two treatments at a ratio of 1:1. Allocation was performed in a stratified manner (hip, knee, spine), using permuted blocks of variable lengths. This study was blinded with the help of computer-generated, pseudo-random treatment allocations in sequentially numbered, opaque, sealed envelopes.
The primary endpoint of this study was the incidence of blistering in patients after total hip or knee replacement or after primary spinal surgery. The sample size was calculated on this basis (13). Secondary endpoints were dressing performance, wearing comfort, conformability, and patient acceptance of the dressing. Fulfilling the Consolidated Statement of Reporting Trials (CONSORT) criteria, we highlight here within the framework of selective reporting of endpoints that contrary to the requirement set in the study protocol both wound care provision and wound outcome assessment were performed by the same persons.
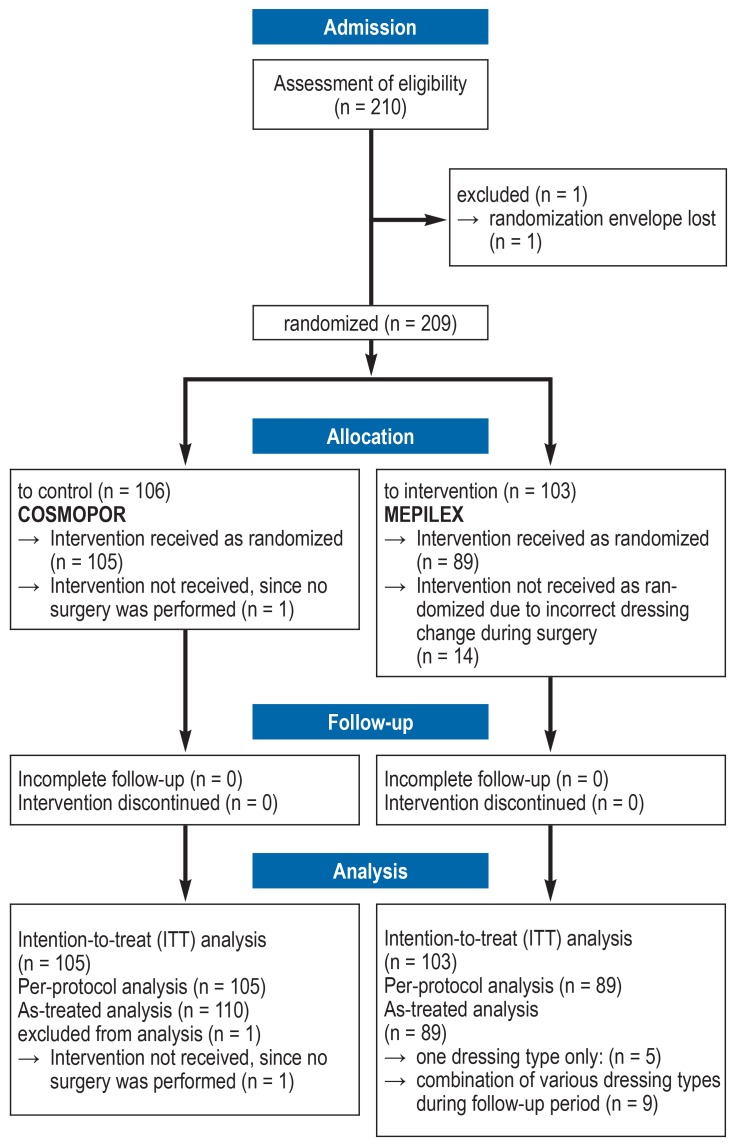
The detailed study protocol of this study was published in advance (13). The randomization method used is depicted in the CONSORT flowchart (Figure). The approval of this study by the relevant ethics committee of the University Hospital Cologne is available (reference number 13–348). This study was registered in advance (ClinicalTrials.gov number: NCT01988818). A comprehensive description of the methods used is provided in the eMethods section.
Figure 1.
Flowchart of the study according to CONSORT guidelines
CONSORT, Consolidated Statement of Reporting Trials
Results
Of the 209 patients enrolled in this study, 208 could be included in the analysis. Of these, 105 were allocated to the control group and 103 to the intervention group. The mean age was 66.2 years (33–90 years) in the control group and 66.8 years (37–92 years) in the intervention group. In the control group, 55.2% of patients were male and 44.8% female. In the intervention group, 47.6% of patients were male and 52.4% female. There were no significant differences in demographic characteristics between the 3 surgery groups (total knee replacement, total hip replacement and spinal surgery) (table 1).
Table 1. Comparison of baseline data of the two groups.
| Control group | Intervention group | p value | |
| Age (years) | 66.2 | 66.8 | 0.696 |
| Men (%) | 55.2 | 47.6 | 0.269 |
| Women (%) | 44.8 | 52.4 | |
| Hip surgery (%) | 34.3 | 33.0 | 0.981 |
| Knee surgery (%) | 34.3 | 33.0 | 0.981 |
| Spinal surgery (%) | 33.3 | 34.0 | 0.981 |
In the entire study population, blistering was observed in one patient on postoperative day 6 (primary endpoint). This patient was in the intervention group, but had accidentally received a control wound dressing. Table 2 shows overall patient satisfactions, broken down by type of surgery performed. In all 3 groups (knee, hip, spine), patients in the intervention group reported higher satisfaction levels (p<0.001).
Table 2. Overall patient rating (postoperative day 6; p<0.001).
|
Standard group [%] |
Intervention group [%] |
|
| excellent | 1.9 | 50.5 |
| very good | 20 | 30.1 |
| good | 39 | 17.5 |
| poor | 39 | 1.9 |
In the intervention group, patients rated the wearing comfort of the dressing significantly better compared to the control group (table 3). The ratings in terms of usability, assessment of the wound situation through the dressing and removal of the adhesive dressing were significantly more favorable in the intervention group (p<0.001) (Tables 4– 6). In the overall evaluation, nursing staff/physicians rated the usability of the dressing in the intervention group as being better (p<0.001) (table 4).
Table 3. Patient-rated comfort (postoperative day 6; p<0.001).
|
Standard group [%] |
Intervention group [%] |
|
| excellent | 1.9 | 53.4 |
| very good | 19 | 27.2 |
| good | 40 | 17.5 |
| poor | 39 | 1.9 |
Table 4. Rating of usability by nursing staff/physicians (postoperative day 6; p<0.001).
|
Standard group [%] |
Intervention group [%] |
|
| excellent | 1 | 30.9 |
| very good | 44 | 50 |
| good | 23 | 17 |
| poor | 32 | 2.1 |
Taking into consideration pain after dressing change—measured using a visual analog scale [VAS]—, the intervention group experienced significantly less discomfort compared to the control group (postoperative day 6; p = 0.006), regardless of the type of surgery (hip, knee or spine) performed (etable).
eTable. Comprehensive overview of the results.
| Variables | Rating | Postoperative day 2 | Postoperative day 3 | Postoperative day 6 | ||||
| Percentage | p value | Percentage | p value | Percentage | p value | |||
| Blistering | Intervention group | 0 | 0 | 0 | ||||
| Control group | 0 | 0 | 0 | |||||
|
Dressing changes performed |
Intervention group | 15.7 | <0.0001 | 18.4 | <0.0001 | 94.2 | 0.497 | |
| Control group | 59 | 59 | 96.2 | |||||
|
Visual analog scale (VAS) before dressing change |
Intervention group | 0 = 100 | 0.632 | 0 = 100 | 0.513 | 0 = 99 | 0.085 | |
| 2 = 1 | ||||||||
| Control group | 0 = 98.4 | 0 = 92.2 | 0 = 96 | |||||
| 1 = 1.6 | 1 = 3.1 | 1 = 4 | ||||||
| 2 = 4.7 | ||||||||
|
VAS during dressing change |
Intervention group | 0 = 78.6 | 0.001 | 0 = 50 | 0.16 | 0 = 85.6 | <0.0001 | |
| 1 = 14.3 | 1 = 6.3 | 1 = 10.3 | ||||||
| 2 = 7.1 | 2 = 31.3 | 2 = 2.1 | ||||||
| 4 = 12.4 | 3 = 1 | |||||||
| 5 = 1 | ||||||||
| Control group | 0 = 21.6 | 0 = 23.3 | 0 = 29.7 | |||||
| 1 = 26.3 | 1 = 31.4 | 1 = 23.8 | ||||||
| 2 = 21.1 | 2 = 20.3 | 2 = 27.7 | ||||||
| 3 = 9.2 | 3 = 12.5 | 3 = 5.9 | ||||||
| 4 = 9.2 | 4 = 6.3 | 4 = 8.9 | ||||||
| 5 = 2.6 | 5 = 1.6 | 5 = 3 | ||||||
| 6 = 3.1 | 6 = 1 | |||||||
| 7 = 1.6 | ||||||||
|
VAS after dressing change |
Intervention group | 0 = 92.9 | 0.073 | 0 = 75 | 0.245 | 0 = 96.9 | 0.006 | |
| 2 = 7.1 | 1 = 12.5 | 1 = 2.1 | ||||||
| 2 = 12.5 | 2 = 1 | |||||||
| Control group | 0 = 77.6 | 0 = 75 | 0 = 82.2 | |||||
| 1 = 19.2 | 1 = 21.9 | 1 = 15.8 | ||||||
| 2 = 2.6 | 2 = 3.1 | 2 = 1 | ||||||
| 9 = 1 | ||||||||
|
Dressing change time (mean) |
Intervention group | 2.2 minutes | 2.5 minutes | 2.3 minutes | ||||
| Control group | 2.2 minutes | 2.6 minutes | 2.5 minutes | |||||
|
Patient satisfaction, overall |
Intervention group | poor | 3.9 | <0.0001 | 2.9 | <0.0001 | 1.9 | <0.0001 |
| good | 21.6 | 15.7 | 17.5 | |||||
| very good | 57.8 | 50 | 30.1 | |||||
| excellent | 16.7 | 31.4 | 50.5 | |||||
| Control group | poor | 15.2 | 26.7 | 39 | ||||
| good | 59 | 53.3 | 39 | |||||
| very good | 25.7 | 17.1 | 20 | |||||
| excellent | 0 | 2.9 | 1.9 | |||||
|
Patient satisfaction, comfort |
Intervention group | poor | 3.9 | <0.0001 | 2.9 | <0.0001 | 1.9 | <0.0001 |
| good | 21.6 | 14.7 | 17.5 | |||||
| very good | 57.8 | 51 | 27.2 | |||||
| excellent | 16.7 | 31.4 | 53.4 | |||||
| Control group | poor | 14.3 | 28.6 | 39 | ||||
| good | 60 | 50.5 | 40 | |||||
| very good | 24.8 | 19 | 19 | |||||
| excellent | 1 | 1.9 | 1.9 | |||||
|
Assessor rating, overall |
Intervention group | poor | 3 | <0.0001 | 4 | <0.0001 | 1 | <0.0001 |
| good | 26.7 | 14.9 | 17.5 | |||||
| very good | 56.4 | 49.5 | 33 | |||||
| excellent | 13.9 | 31.7 | 48.5 | |||||
| Control group | poor | 14.4 | 19.9 | 35.2 | ||||
| good | 61.5 | 56.7 | 37.1 | |||||
| very good | 24 | 22.1 | 26.7 | |||||
| excellent | 0 | 1.9 | 1 | |||||
|
Assessor rating, size of wound dressing |
Intervention group | poor | 2.9 | <0.0001 | 2.9 | <0.0001 | 1.9 | <0.0001 |
| good | 20.6 | 15.7 | 17.5 | |||||
| very good | 63.7 | 56.9 | 42.7 | |||||
| excellent | 12.7 | 24.5 | 37.9 | |||||
| Control group | poor | 6.7 | 14.4 | 19 | ||||
| good | 62.9 | 53.8 | 46.7 | |||||
| very good | 29.5 | 31.7 | 34.3 | |||||
| excellent | 1 | 0 | 0 | |||||
|
Assessor rating, shape of wound dressing |
Intervention group | poor | 2.9 | <0.0001 | 2.9 | <0.0001 | 1 | <0.0001 |
| good | 19.6 | 15.7 | 18.4 | |||||
| very good | 65.7 | 57.8 | 42.7 | |||||
| excellent | 11.8 | 23.5 | 37.9 | |||||
| Control group | poor | 8.6 | 16.3 | 21.9 | ||||
| good | 62.9 | 53.8 | 45.7 | |||||
| very good | 28.6 | 29.8 | 32.4 | |||||
| excellent | 0 | 0 | 0 | |||||
|
Assessor rating, transparency of wound dressing |
Intervention group | poor | 8.8 | <0.0001 | 13.9 | <0.0001 | 12.6 | <0.0001 |
| good | 17.6 | 13.9 | 12.6 | |||||
| very good | 16.7 | 11.9 | 6.8 | |||||
| excellent | 56.9 | 60.4 | 68 | |||||
| Control group | poor | 76.2 | 72.4 | 81.9 | ||||
| good | 22.9 | 24.8 | 15.2 | |||||
| very good | 0 | 1 | 1.9 | |||||
| excellent | 1 | 1.9 | 1 | |||||
|
Assessor rating, removal of wound dressing |
Intervention group | poor | 13.3 | <0.0001 | 25 | <0.0001 | 4.3 | <0.0001 |
| good | 20 | 35 | 12.8 | |||||
| very good | 46.7 | 25 | 18.1 | |||||
| excellent | 20 | 15 | 64.9 | |||||
| Control group | poor | 57.9 | 29.9 | 60 | ||||
| good | 33.3 | 22.4 | 28 | |||||
| very good | 8.8 | 44.8 | 11 | |||||
| excellent | 0 | 3 | 1 | |||||
|
Assessor rating, application of wound dressing |
Intervention group | poor | 6.7 | 0.083 | 17.6 | 0.573 | 2.1 | <0.0001 |
| good | 33.3 | 35.3 | 17 | |||||
| very good | 53.3 | 41.2 | 50 | |||||
| excellent | 6.7 | 5.9 | 30.9 | |||||
| Control group | poor | 21.4 | 29.9 | 32 | ||||
| good | 44.6 | 22.4 | 23 | |||||
| very good | 33.9 | 44.8 | 44 | |||||
| excellent | 0 | 3 | 1 | |||||
| Mobilization | Intervention group | confined to bed | 53.4 | 0.459 | 18.4 | 0.7 | 11.7 | 0.459 |
| confined to chair | 17.5 | 28.2 | 12.6 | |||||
| mobile | 29.1 | 52.4 | 75.7 | |||||
| Control group | confined to bed | 58.1 | 18.1 | 6.7 | ||||
| confined to chair | 11.4 | 25.7 | 13.3 | |||||
| mobile | 30.5 | 55.2 | 80 | |||||
| Tissue consistency | Intervention group | normal | 96.1 | 0.362 | 98.1 | 0.575 | 99 | 0.227 |
| firm | 1.9 | 1 | 1 | |||||
| boggy | 1.9 | 1 | 0 | |||||
| other | 0 | 0 | 0 | |||||
| Control group | normal | 96.2 | 95.2 | 98.1 | ||||
| firm | 0 | 1 | 0 | |||||
| boggy | 2.9 | 2.9 | 1.9 | |||||
| other | 0 | 1 | 0 | |||||
| Skin temperature | Intervention group | normal | 86.4 | 0.524 | 95.1 | 0.139 | 99 | 0.166 |
| warm | 0 | 4.9 | 1 | |||||
| cold | 13.6 | 0 | 0 | |||||
| Control group | normal | 82.9 | 86.7 | 94.2 | ||||
| warm | 1 | 10.5 | 4.8 | |||||
| cold | 16.2 | 1.9 | 1 | |||||
| Skin status | Intervention group | normal | 99 | 0.225 | 97.1 | 0.261 | 98 | 0.056 |
| dry | 0 | 1.9 | 1 | |||||
| scaly | 0 | 0 | 0 | |||||
| oily | 0 | 1 | 0 | |||||
| moist | 0 | 0 | 1 | |||||
| other | 1 | 0 | 0 | |||||
| Control group | normal | 98.1 | 93.3 | 88.5 | ||||
| dry | 1.9 | 5.7 | 7.7 | |||||
| scaly | 0 | 1 | 1 | |||||
| moist | 0 | 0 | 2.9 | |||||
| other | 0 | 0 | 0 | |||||
|
Patient-reported skin status |
Intervention group | normal | 97.1 | 0.05 | 94.2 | <0.0001 | 96.1 | <0.0001 |
| itchy | 2.9 | 3.9 | 1.9 | |||||
| burning | 0 | 0 | 0 | |||||
| numb | 0 | 0 | 0 | |||||
| combined | 0 | 2 | 2 | |||||
| Control group | normal | 86.7 | 66.7 | 61 | ||||
| itchy | 11.4 | 28.6 | 21 | |||||
| burning | 1 | 0 | 0 | |||||
| numb | 1 | 0 | 0 | |||||
| combined | 0 | 4.8 | 18,2 | |||||
| Sweating | Intervention group | none | 95.1 | 0.497 | 95.1 | 0.711 | 96 | 0.515 |
| mild | 3.9 | 4.9 | 4 | |||||
| moderate | 1 | 0 | 0 | |||||
| severe | 0 | 0 | 0 | |||||
| Control group | none | 93.3 | 96.2 | 93.3 | ||||
| mild | 5.7 | 3.8 | 5.8 | |||||
| moderate | 0 | 0 | 0 | |||||
| severe | 1 | 0 | 1 | |||||
| Edema | Intervention group | none | 82.5 | 0.626 | 84.5 | 0.319 | 87 | 0.762 |
| mild | 4.9 | 7.8 | 11 | |||||
| moderate | 9.7 | 5.8 | 1 | |||||
| severe | 2.9 | 1.9 | 1 | |||||
| Control group | none | 82.9 | 80 | 91.3 | ||||
| mild | 7.6 | 12.4 | 6.7 | |||||
| moderate | 8.6 | 7.6 | 1 | |||||
| severe | 1 | 0 | 1 | |||||
| Amount of exudate | Intervention group | none | 70.9 | 0.366 | 73.8 | 0.932 | 81 | 0.057 |
| little | 22.3 | 17.5 | 12 | |||||
| moderate | 1 | 3.9 | 1 | |||||
| copious | 5.8 | 4.9 | 6 | |||||
| Control group | none | 80 | 71.4 | 92.3 | ||||
| little | 14.3 | 20 | 3.8 | |||||
| moderate | 1.9 | 4.8 | 1.9 | |||||
| copious | 3.8 | 3.8 | 1.9 | |||||
| Quality of exudate | Intervention group | serous | 0 | 0.12 | 0.557 | 11.1 | 0.296 | |
| serous/ hemorrhagic | 20 | 14.8 | 16.7 | |||||
| purulent | 85.2 | |||||||
| hemorrhagic | 80 | 72.2 | ||||||
| Control group | serous | 0 | 33.3 | |||||
| serous/ hemorrhagic | 4.8 | 10 | 22.2 | |||||
| purulent | 86.7 | |||||||
| hemorrhagic | 95.2 | 3.3 | 44.4 | |||||
| Absorbency | Intervention group | not stated | 70.9 | 0.016 | 73.8 | 0.004 | 83 | 0.015 |
| little | 2.9 | 1 | ||||||
| good | 6.8 | 7.8 | 3 | |||||
| very good | 12.6 | 10.7 | 4 | |||||
| excellent | 6.8 | 6.8 | 10 | |||||
| Control group | not stated | 80 | 72.4 | 92.3 | ||||
| little | 7.6 | 7.6 | 1.9 | |||||
| good | 7.9 | 15.2 | 3.8 | |||||
| very good | 2.9 | 2.9 | ||||||
| excellent | 1.9 | 1.9 | 1.9 | |||||
| Wound odor | Intervention group | none | 99 | 0.311 | 100 | 100 | 0.368 | |
| mild | ||||||||
| moderate | 1 | |||||||
| Control group | none | 100 | 100 | 99 | ||||
| mild | 1 | |||||||
| Drainage | Intervention group | external | 9.7 | 0,079 | 1 | 0,111 | ||
| none | 1,9 | 87,4 | 100 | |||||
| removed today | 88.3 | 11.7 | ||||||
| Control group | external | 3.8 | ||||||
| none | 95.2 | 100 | ||||||
| removed today | 96.2 | 4.8 | ||||||
The number of dressing changes during the study period was significantly lower when the silicone dressing (approved for 7 days) was applied (p<0.001) (table 7). In 70.9% of patients, no change of dressing was required during 6 postoperative days. In the control group, by contrast, all patients had a dressing change during this period, as this was required by the hospital’s general hygiene rules. On average, patients in the intervention group needed 0.3 dressings after the intraoperatively applied dressing, compared to 1.2 dressings per patient in the control group (table 2). No difference between the dressings was found with regard to the time required for dressing change. During the study period, no wound complications were observed. The eTable provides an overview of all results.
Table 7. Number of dressing changes (p<0.001).
| Number of dressing changes | Control group | Intervention group |
| 0 | 6 (5.7 %) | 73 (70.9 %) |
| 1 | 74 (70.5 %) | 25 (24.3 %) |
| 2 | 25 (23.8 %) | 5 (4.9 %) |
Discussion
The primary study hypothesis that the use of absorbent wound dressing will reduce blistering was not confirmed. For conventional adhesive dressings, blistering rates of 6 to 24% have been reported in the literature (13, 23). However, these findings were not confirmed in our patient population. This may be due to the fact that the incidence of blistering can generally be reduced if the dressing is applied without tension, daily dressing changes are avoided, and the dressing is gently separated from the skin and cautiously removed.
However, our study showed that patients in the intervention group reported less postoperative pain, required less dressing changes overall, and were more satisfied with the dressing. Likewise, user satisfaction findings were in favor of the intervention group. In the analyses of the data collected for all primary and secondary parameters (etable), no differences between the two studied groups were found for numerous parameters.
The wound dressing used in the intervention group of this study meets all requirements set in the 2008 National Health and Clinical Excellence (NICE) guideline.
This dressing’s permeability had already been shown in previous studies (12) and been rated as satisfactory. This rating is supported by the absence of blistering and the low frequency of dressing changes in the intervention group of our study.
Our results show that the wound dressing in the intervention group can be removed without causing relevant pain—a key criterion of patients when rating a wound dressing. Consequently, the absorbent adhesive dressing is significantly superior to today’s standard dressing.
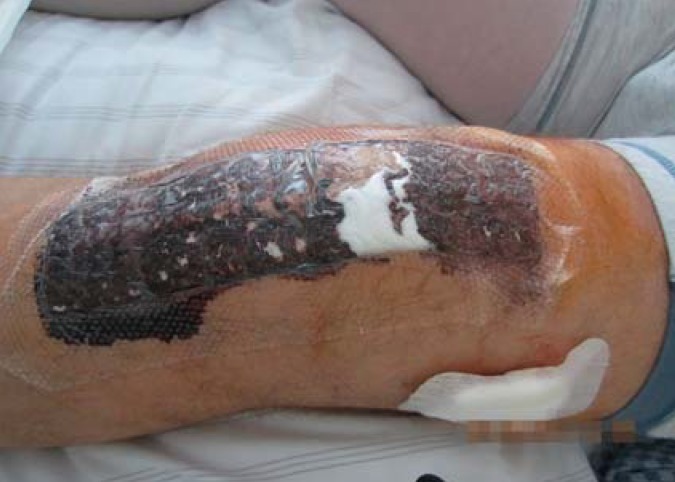
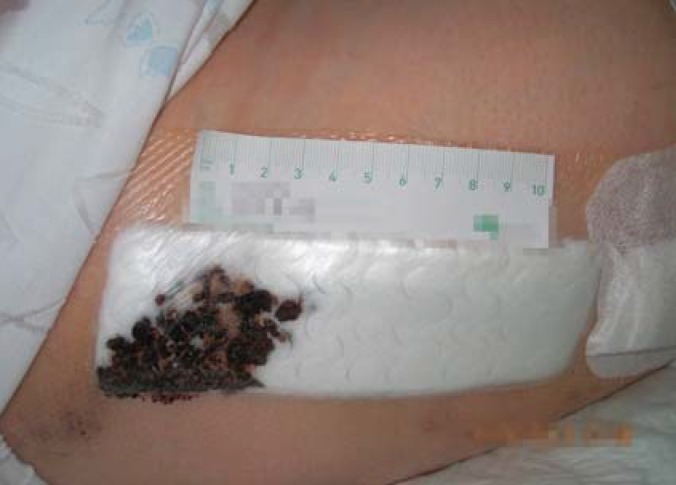
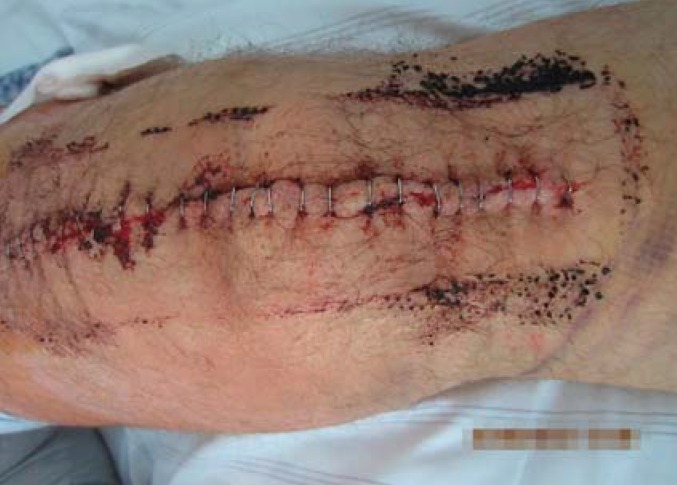
Here, it is worth mentioning that nursing staff/physicians starting to use absorbent dressing are confronted with a significant change in wound care procedures. Usually, the surgeon who applied the dressing assesses the patient’s surgical wound postoperatively every 2 days to make sure that no wound complications have occurred. However, the silicone dressing used in this study limited the visibility of the area surrounding the wound (Figures 2 and 3). While a highly exuding section of the wound can be localized through the dressing, proper assessment requires a dressing change, as in the group with conventional dressing (figure 4). Dressing changes were performed at the surgeon’s discretion. For both nursing staff/physicians and patients, it was unfamiliar to have to wait for 7 days after surgery to see the skin incision wound for the first time. With the wound dressing in place, only the peripheral skin areas were visible, but not the surgical incision. However, this did not result in any medical disadvantages for the patients, as no impairment of wound healing or wound infection was overlooked. Complete transparency of the dressing could resolve this issue.
Figure 2.
Wound before dressing change with silicone dressing (intervention group)
Figure 3.
Wound before dressing change with silicone dressing (intervention group)
Figure 4.
Wound after removal of silicone dressing (intervention group)
To avoid unnecessary dressing changes appears to make sense, as each dressing change is associated with the risk of contamination of the wound (32). In addition, cellular wound healing is disrupted for 3 to 5 hours due to a fall in skin temperature, resulting in delayed healing (33). It is likely that the selection of the right dressing helps to prevent infection of surgical wounds. Intraoperatively, it was made sure that the exit site of the surgical drain, if any, was located outside of the area to be covered by the wound dressing. Only by keeping drain exit site and drain dressing separate, it was possible to leave the dressing in place on the surgical incision and remove the drain in time (figure 5). Staples were used for skin closure after primary knee and hip surgery. According to a recently published meta-analysis, using a stapler for skin closure results in the lowest rate of wound complications after total knee replacement (34). After spinal surgery, the skin was closed with a running subcuticular suture.
Figure 5.
Wound after application of the silicone dressing (intervention group) and external pedunculated exit of surgical drain
In our study, both patients and nursing staff/physicians reported greater satisfaction in the intervention group compared to the control group. These results have been supported by recent randomized trials with absorbent wound dressings after knee or hip surgery (6, 24, 35– 37).
In addition, Sharma et al. conclude in their meta-analysis of currently published randomized trials that despite these hints no evidence is available to confirm that absorbent wound dressings can reduce joint infection (36).
For the first time, our study also included and assessed patients with spinal disorders. Especially in this region of the body, wound dressings are exposed to stronger forces as these patients spend more time lying on the wound dressing. Consequently, the risk that dressings are displaced by shearing forces is potentially greater in the spinal region compared to the extremities. However, in spinal surgery patients the new dressing applied in the intervention group was significantly better than that in the control group and met all NICE criteria.
The initially applied wound dressing should ideally be left in place as long as possible to reduce the risk of infection and increase patient comfort. Requirements for the use of the new dressings are that no signs of infection or exudate production beyond the capacity of the dressing are detected. These new wound dressings are already commercially available and offered by various manufacturers. In other countries, it is common practice that patients take a shower on the second postoperative day—without increased rates of wound infections (38– 40). Thus, the advantage of leaving absorbent wound dressings in place longer should be critically evaluated and put into perspective.
One limitation of this study was its short study period of only 6 days. Nevertheless, this period was sufficiently long to evaluate the primary study hypothesis. However, this study was not designed to determine the infection rate during the subsequent wound healing period. In addition, it cannot entirely be ruled out that the assessments by the nursing staff/physicians and the patients were biased as the endpoints were determined by the same persons who had earlier changed the dressings. Furthermore, after surgery the wrong dressing was used in 14 patients (control group dressing instead of intervention group dressing). It is desirable to perform an independent follow-up study to confirm these results.
Further studies should take into account that no data on revision surgery or treatment of patients with preexisting conditions have yet been reported in the literature. In addition, the aspect of the study of Rasmussen et al. from 1993 (28) could be revisited and the indication for absorbent adhesive dressings (occlusive wound dressing) could be extended to pediatric patients. Especially in the pediatric patient population, treatment could be significantly facilitated or improved by greater patient satisfaction as the result of almost painless dressing changes. Based on these considerations, the evident dogma of performing dressing changes every 2 to 3 days should be reconsidered in favor of longer wearing periods, provided wound and dressing status allow for it. An important point would be to ensure that the conventional adhesive dressings used in the control group provide reliable adhesion over a comparably long period of time.
Conclusion
No difference between the control and the intervention group was found for the primary endpoint, postoperative blistering. The dressing used in the intervention group was better tolerated by patients and nursing staff/physicians reported greater satisfaction with its application. Based on the results of this study, the postoperative use of absorbent wound dressings in patients with primary total hip or knee replacements or spinal surgery can be recommended as it offers increased patient comfort.
Supplementary Material
eMETHODS
Exclusion criteria comprised known allergies or hypersensitivities to components of the wound dressing as well as known bone fractures, patients after polytrauma, cancer surgery and preexisting wounds in the surgical field. Additional exclusion criteria were neurological deficits at the surgical site and skin diseases.
After inclusion of the patient, the allocated wound dressing was applied in the operating room. In line with the standards of the hospital, patients were followed up on postoperative days 2, 3 and 6. At each visit, the following parameters were assessed:
In case of a dressing change, the time from getting the material until completion of the dressing change was measured and documented. Dressing changes were performed as specified in the study protocol and in the general hygiene rules of the hospital. In the control group, dressing changes were performed not later than on the second postoperative day and subsequently as required, but not later than every second day. In the intervention group, the dressing was only changed before the sixth postoperative day if medically indicated, as the manufacturer recommends to leave the dressing in situ for this period of time to achieve optimum outcomes. Indications for a dressing change included apparent bleeding into the dressing, exudate penetrating the dressing, patient discomfort, and signs of infection.
Patients rated their pain before, during and after removal of the dressing and on application of the new wound dressing, using a visual analog scale (VAS). In addition, patient satisfaction was measured based on wearing comfort and overall rating of the wound dressing. In addition, outcome assessors documented their ratings for wound dressing size, shape, transparency and overall impression. The visit on postoperative day 6 was the final visit; subsequently, the conventional dressing was used for further wound care.
Patients marked their answers on the VAS and rated their satisfaction using Likert scales (from 0–10 or from “poor“ to “good“ and “very good“ to “excellent“). Furthermore, at the study visits patients were asked to rate their own skin adjacent to the surgical site and the wound dressing.
Patients had the opportunity to report whether sensitivity had changed or whether they had experienced unusual sensations potentially related to the wound dressing.
The choice of answers included “normal“, “itchy“, “burning“, “biting“, “pricking”, “formication“, “numb“, “other—with more precise description“.
Validated quality-of-life questionnaires were not used in this study.
Statistical analysis
The primary analysis was performed according to the “intention-to-treat” principal (full analysis set). A patient was eligible to be included in this analysis if he underwent surgery and received a study treatment. Incomplete participation in the study was categorized as a treatment failure. A secondary analysis included all patients essentially treated and followed up according to the study protocol (per-protocol set).
Primary outcome analysis
The proportion of patients with blistering in each of the two groups were compared using the Mantel-Haenszel test. A two-sided type 1 error of 5% was used and data were stratified by the type of surgical procedure. The null hypothesis H0 (the odds ratio for blistering is 1) was tested against the alternative hypothesis HA(the odds ratio for blistering is ? 1).
Secondary outcome analysis
Pain, number of dressing changes/blistering and performance/assessor and patient ratings were analyzed using rang-based tests (Wilcoxon signed-rank test or Wilcoxon rank-sum test). Other wound complications were compared using the chi-square test or Fisher‘s exact test.
skin status, including edema and temperature assessments
status with regard to blistering
sensation of the patient underneath the dressing
wound exudate quantity and quality
absorbency of the dressing.
Table 5. Rating of wound dressing removal by nursing staff/ physicians (postoperative day 6; p<0.001).
|
Standard group [%] |
Intervention group [%] |
|
| excellent | 1 | 64.9 |
| very good | 11 | 18.1 |
| good | 28 | 12.8 |
| poor | 60 | 4.5 |
Table 6. Ability to assess wound/wound complications with dressing in situ (postoperative day 6; p<0.001).
|
Standard group [%] |
Intervention group [%] |
|
| excellent | 1 | 68 |
| very good | 1.9 | 6.8 |
| good | 15.2 | 12.6 |
| poor | 81.9 | 12.6 |
Key Messages.
No difference between control and intervention group was found for the primary endpoint, postoperative blistering.
Patient ratings for wearing comfort, overall satisfaction, usability, and removal of the adhesive dressing were significantly in favor of the intervention group compared to the control group.
In the overall evaluation, the users (nursing staff/physicians) rated the wound dressing in the intervention group as better.
Limitations of the study include the short 6-day follow-up period which was not long enough to allow a final evaluation of the incidence of blistering and postoperative infection.
In addition, assessment was biased because the endpoints were assessed by the same persons who had before changed the dressings.
Acknowledgments
Translated from the original German by Ralf Thoene, M.D.
Footnotes
Conflict of interest
Dr. Bredow received reimbursement of congress fees and travel expenses as well as study support from Mölnlycke (free supply of Mepilex Border Post-Op for the intervention group).
Dr. Oppermann received study support (third-party funding) from Mölnlycke (supply of adhesive dressing for the study).
Dr. Zarghooni received reimbursement of congress fees and travel expenses, lecture fees, and study support of Mölnlycke.
Professor Eysel received study support (third-party funding) from Mölnlycke (supply of adhesive dressing for the study)
The remaining authors declare no conflict of interest.
References
- 1.Niethard F, Malzahn J, Schafer T. Endoprothetik und Wirbelsäuleneingriffe: Uneinheitliches Versorgungsgeschehen. Dtsch Arztebl. 2013;110:A1362–A1365. [Google Scholar]
- 2.Dumville JC, Gray TA, Walter CJ, Sharp CA, Page T. Dressings for the prevention of surgical site infection. Cochrane Database of Syst Rev. 2014 doi: 10.1002/14651858.CD003091.pub3. CD003091. [DOI] [PubMed] [Google Scholar]
- 3.Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59:1713–1720. doi: 10.1002/art.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ousey K, Gillibrand W, Stephenson J. Achieving international consensus for the prevention of orthopaedic wound blistering: results of a Delphi survey. Int Wound J. 2013;10:177–184. doi: 10.1111/j.1742-481X.2012.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6 489 total knee replacements. Clin Orthop Relat Res. 2001:15–23. [PubMed] [Google Scholar]
- 6.Ravnskog FA, Espehaug B, Indrekvam K. Randomised clinical trial comparing hydrofiber and alginate dressings post-hip replacement. J Wound Care. 2011;20:136–142. doi: 10.12968/jowc.2011.20.3.136. [DOI] [PubMed] [Google Scholar]
- 7.Scalise A, Bianchi A, Tartaglione C, et al. Microenvironment and microbiology of skin wounds: the role of bacterial biofilms and related factors. Semin Vasc Surg. 2015;28:151–159. doi: 10.1053/j.semvascsurg.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620x.86b5.14887. [DOI] [PubMed] [Google Scholar]
- 9.Burke NG, Green C, McHugh G, McGolderick N, Kilcoyne C, Kenny P. A prospective randomised study comparing the jubilee dressing method to a standard adhesive dressing for total hip and knee replacements. J Tissue Viability. 2012;21:84–87. doi: 10.1016/j.jtv.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Grimer RJ, Abudu A. Infection after total hip arthroplasty. J Bone Joint Surg Br. 2005;87 doi: 10.1302/0301-620X.87B4.16232. [DOI] [PubMed] [Google Scholar]
- 11.Villar RN. Failed hip replacements. BMJ. 1992;304:3–4. doi: 10.1136/bmj.304.6818.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarghooni K, Bredow J, Siewe J, Deutloff N, Meyer HS, Lohmann C. Is the use of modern versus conventional wound dressings warranted after primary knee and hip arthroplasty? Results of a prospective comparative study. Acta Orthop Belg. 2015;81:768–775. [PubMed] [Google Scholar]
- 13.Bredow J, Oppermann J, Hoffmann K, et al. Clinical trial to evaluate the performance of a flexible self-adherent absorbent dressing coated with a soft silicone layer compared to a standard wound dressing after orthopedic or spinal surgery: study protocol for a randomized controlled trial. Trials. 2015;16 doi: 10.1186/s13063-015-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90:62–69. doi: 10.2106/JBJS.F.01515. [DOI] [PubMed] [Google Scholar]
- 15.Pawar AY, Biswas SK. Postoperative spine infections. Asian Spine J. 2016;10:176–183. doi: 10.4184/asj.2016.10.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohl DD, Sershon RA, Fillingham YA, Della Valle CJ. Incidence, risk factors, and sources of sepsis following total joint arthroplasty. J Arthroplasty. 2016;31:2875–2879e2. doi: 10.1016/j.arth.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Nota SP, Braun Y, Ring D, Schwab JH. Incidence of surgical site infection after spine surgery: what is the impact of the definition of infection? Clin Orthop Relat Res. 2015;473:1612–1619. doi: 10.1007/s11999-014-3933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vince K, Chivas D, Droll KP. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22:39–44. doi: 10.1016/j.arth.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Clarke MT, Longstaff L, Edwards D, Rushton N. Tourniquet-induced wound hypoxia after total knee replacement. J Bone Joint Surg Br. 2001;83:40–44. doi: 10.1302/0301-620x.83b1.10795. [DOI] [PubMed] [Google Scholar]
- 20.Ousey K, Edward KL, Lui S. Identifying and exploring physical and psychological morbidity and patient and family caregiver resilience following acute wound development and/or wound blistering post orthopaedic surgery: a systematic review. Int Wound J. 2015;12:63–69. doi: 10.1111/iwj.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies P, Rippon M. Evidence review: the clinical benefits of safetac technology in wound care. J Wound Care. 2008;(3-31) [PubMed] [Google Scholar]
- 22.Gupta SK, Lee S, Moseley LG. Postoperative wound blistering: is there a link with dressing usage? J Wound Care. 2002;11:271–273. doi: 10.12968/jowc.2002.11.7.26413. [DOI] [PubMed] [Google Scholar]
- 23.Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care. 2005;14:27–29. doi: 10.12968/jowc.2005.14.1.26722. [DOI] [PubMed] [Google Scholar]
- 24.Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: aquacel and tegaderm versus cutiplast. Ann R Coll Surg Engl. 2006;88:18–22. doi: 10.1308/003588406X82989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field FK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S–6S. doi: 10.1016/0002-9610(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 26.Bowler PG, Jones SA, Davies BJ, Coyle E. Infection control properties of some wound dressings. J Wound Care. 1999;8:499–502. doi: 10.12968/jowc.1999.8.10.26356. [DOI] [PubMed] [Google Scholar]
- 27.Eastburn S, Ousey K, Rippon MG. A review of blisters caused by wound dressing components: can they impede post-operative rehabilitation and discharge? Int J Orthop Trauma Nurs. 2016;21:3–10. doi: 10.1016/j.ijotn.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen H, Larsen MJ, Skeie E. Surgical wound dressing in outpatient paediatric surgery A randomised study. Dan Med Bull. 1993;40:252–254. [PubMed] [Google Scholar]
- 29.Tustanowski J. Effect of dressing choice on outcomes after hip and knee arthroplasty: a literature review. J Wound Care. 2009;18:449–450. doi: 10.12968/jowc.2009.18.11.44985. 52, 54, passim. [DOI] [PubMed] [Google Scholar]
- 30.Downie F, Egdell S, Bielby A, Searle R. Barrier dressings in surgical site infection prevention strategies. Br J Nurs. 2010;19:42–46. doi: 10.12968/bjon.2010.19.Sup10.79693. [DOI] [PubMed] [Google Scholar]
- 31.Leaper D, Burman-Roy S, Palanca A, et al. Prevention and treatment of surgical site infection: summary of NICE guidance. BMJ. 2008;337 doi: 10.1136/bmj.a1924. [DOI] [PubMed] [Google Scholar]
- 32.Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty. 2010;25:103–107. doi: 10.1016/j.arth.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 33.McGuiness W, Vella E, Harrison D. Influence of dressing changes on wound temperature. J Wound Care. 2004;13:383–385. doi: 10.12968/jowc.2004.13.9.26702. [DOI] [PubMed] [Google Scholar]
- 34.Kim KY, Anoushiravani AA, Long WJ, Vigdorchik JM, Fernandez-Madrid I, Schwarzkopf R. A meta-analysis and systematic review evaluating skin closure after total knee arthroplasty-what is the best method? J Arthroplasty. 2017;32:2920–2927. doi: 10.1016/j.arth.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Dobbelaere A, Schuermans N, Smet S, van der Straeten C, Victor J. Comparative study of innovative postoperative wound dressings after total knee arthroplasty. Acta Orthop Belg. 2015;81:454–461. [PubMed] [Google Scholar]
- 36.Sharma G, Lee SW, Atanacio O, Parvizi J, Kim TK. In search of the optimal wound dressing material following total hip and knee arthroplasty: a systematic review and meta-analysis. Int Orthop. 2017;41:1295–1305. doi: 10.1007/s00264-017-3484-4. [DOI] [PubMed] [Google Scholar]
- 37.Springer BD, Beaver WB, Griffin WL, Mason JB, Odum SM. Role of surgical dressings in total joint arthroplasty: a randomized controlled trial. Am J Orthop (Belle Mead NJ) 2015;44:415–420. [PubMed] [Google Scholar]
- 38.Dayton P, Feilmeier M, Sedberry S. Does postoperative showering or bathing of a surgical site increase the incidence of infection? A systematic review of the literature. J Foot Ankle Surg. 2013;52:612–614. doi: 10.1053/j.jfas.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Harrison C, Wade C, Gore S. Postoperative washing of sutured wounds. Ann Med Surg (Lond) 2016;11:36–38. doi: 10.1016/j.amsu.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neues C, Haas E. Modification of postoperative wound healing by showering. Chirurg. 2000;71:234–236. doi: 10.1007/s001040050040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMETHODS
Exclusion criteria comprised known allergies or hypersensitivities to components of the wound dressing as well as known bone fractures, patients after polytrauma, cancer surgery and preexisting wounds in the surgical field. Additional exclusion criteria were neurological deficits at the surgical site and skin diseases.
After inclusion of the patient, the allocated wound dressing was applied in the operating room. In line with the standards of the hospital, patients were followed up on postoperative days 2, 3 and 6. At each visit, the following parameters were assessed:
In case of a dressing change, the time from getting the material until completion of the dressing change was measured and documented. Dressing changes were performed as specified in the study protocol and in the general hygiene rules of the hospital. In the control group, dressing changes were performed not later than on the second postoperative day and subsequently as required, but not later than every second day. In the intervention group, the dressing was only changed before the sixth postoperative day if medically indicated, as the manufacturer recommends to leave the dressing in situ for this period of time to achieve optimum outcomes. Indications for a dressing change included apparent bleeding into the dressing, exudate penetrating the dressing, patient discomfort, and signs of infection.
Patients rated their pain before, during and after removal of the dressing and on application of the new wound dressing, using a visual analog scale (VAS). In addition, patient satisfaction was measured based on wearing comfort and overall rating of the wound dressing. In addition, outcome assessors documented their ratings for wound dressing size, shape, transparency and overall impression. The visit on postoperative day 6 was the final visit; subsequently, the conventional dressing was used for further wound care.
Patients marked their answers on the VAS and rated their satisfaction using Likert scales (from 0–10 or from “poor“ to “good“ and “very good“ to “excellent“). Furthermore, at the study visits patients were asked to rate their own skin adjacent to the surgical site and the wound dressing.
Patients had the opportunity to report whether sensitivity had changed or whether they had experienced unusual sensations potentially related to the wound dressing.
The choice of answers included “normal“, “itchy“, “burning“, “biting“, “pricking”, “formication“, “numb“, “other—with more precise description“.
Validated quality-of-life questionnaires were not used in this study.
Statistical analysis
The primary analysis was performed according to the “intention-to-treat” principal (full analysis set). A patient was eligible to be included in this analysis if he underwent surgery and received a study treatment. Incomplete participation in the study was categorized as a treatment failure. A secondary analysis included all patients essentially treated and followed up according to the study protocol (per-protocol set).
Primary outcome analysis
The proportion of patients with blistering in each of the two groups were compared using the Mantel-Haenszel test. A two-sided type 1 error of 5% was used and data were stratified by the type of surgical procedure. The null hypothesis H0 (the odds ratio for blistering is 1) was tested against the alternative hypothesis HA(the odds ratio for blistering is ? 1).
Secondary outcome analysis
Pain, number of dressing changes/blistering and performance/assessor and patient ratings were analyzed using rang-based tests (Wilcoxon signed-rank test or Wilcoxon rank-sum test). Other wound complications were compared using the chi-square test or Fisher‘s exact test.
skin status, including edema and temperature assessments
status with regard to blistering
sensation of the patient underneath the dressing
wound exudate quantity and quality
absorbency of the dressing.