Abstract
The relationship between body size and temperature of mammals is poorly resolved, especially for large keystone species such as bison (Bison bison). Bison are well represented in the fossil record across North America, which provides an opportunity to relate body size to climate within a species. We measured the length of a leg bone (calcaneal tuber, DstL) in 849 specimens from 60 localities that were dated by stratigraphy and 14C decay. We estimated body mass (M) as M = (DstL/11.49)3. Average annual temperature was estimated from δ18O values in the ice cores from Greenland. Calcaneal tuber length of Bison declined over the last 40,000 years, that is, average body mass was 37% larger (910 ± 50 kg) than today (665 ± 21 kg). Average annual temperature has warmed by 6°C since the Last Glacial Maximum (~24–18 kya) and is predicted to further increase by 4°C by the end of the 21st century. If body size continues to linearly respond to global temperature, Bison body mass will likely decline by an additional 46%, to 357 ± 54 kg, with an increase of 4°C globally. The rate of mass loss is 41 ± 10 kg per°C increase in global temperature. Changes in body size of Bison may be a result of migration, disease, or human harvest but those effects are likely to be local and short‐term and not likely to persist over the long time scale of the fossil record. The strong correspondence between body size of bison and air temperature is more likely the result of persistent effects on the ability to grow and the consequences of sustaining a large body mass in a warming environment. Continuing rises in global temperature will likely depress body sizes of bison, and perhaps other large grazers, without human intervention.
Keywords: Bergmann’s rule, body size change, climate change, fossil, North America, ungulate
1. INTRODUCTION
Variation in body size of American bison (Artiodactyla, Bovidae) has been a contentious topic for more than 7 decades (Dary, 1974; Hill, Hill, & Widga, 2008; McDonald, 1981; Skinner & Kaisen, 1947). In North America, Skinner and Kaisen (1947) synthesized and synonymized 52 species of bison down to eight species using primarily skulls and horn cores which respond plastically to sexual selection. McDonald (1981) and Pinsof (1991) synthesized and synonymized those eight species of bison to five, again based on cranial morphology. Bison priscus and B. latifrons, which denote sister taxa groups to the extant bison clade and represent the larger, more giant end of the body size spectrum, appear to go extinct circa 30kya. The extant bison clade in North America traditionally includes Bison bison, B. occidentalis, and B. antiquus, which represent a smaller body size in comparison with the larger, giant bison (B. priscus and B. latifrons). Yet, the skulls of these smaller species still represent plastic variation, likely due to sexual selection, not representative of overall body size. To avoid the issues surrounding problems with cranial morphology, our study here focuses on the postcranial body size reconstruction, particularly of a mechanistic element to the structure of the skeleton. Our assessment is that the extant bison clade species may represent a linear chronospecies and is supported by recent ancient DNA assessments (Froese et al., 2017; Shapiro et al., 2004). Likely, B. antiquus and B. occidentalis did not go extinct, but through phenotypic and morphologic adaptation to changing climatic conditions, evolved into what is traditionally referred to as B. bison that we have throughout the Holocene and this is what we present below.
Extant Bison are one of eight ungulate genera to survive the most recent deglaciation in North America (Koch & Barnosky, 2006; Kurtén & Anderson, 1980; McDonald, 1981). Bison bison (the extant species in North America) has also survived a more recent near‐extinction event by market hunters in the late 19th century (Dary, 1974; McDonald, 1981). Modern bison of the early 20th‐century bottleneck have rebounded in population to approximately 400,000 bison today because of conservation efforts from public and private sectors (Gates, Freese, Gogan, & Kotzman, 2010; United States Department of Agriculture, 2016). During the Holocene in North America, Bison had the largest distribution of any contemporary ungulate; from Pacific to Atlantic coasts and from arctic to the tropical ecoregions (Feranec, Hadly, & Paytan, 2009; McDonald, 1981; Skinner & Kaisen, 1947). Although it is often assumed that Bison are obligate grazers (occasionally referred to as hyper‐grazers (MacFadden & Cerling, 1996; Leng, 2006)), Bison have shown to be adaptable and variable in diet selection (Bergman, Fryxell, Gates, & Fortin, 2001; Feranec & MacFadden, 2000; Miquelle, 1985; Widga, 2006). Bison have inhabited North America (south of 55°N latitude) for approximately 200,000 years (Barnosky et al., 2014; Bell et al., 2004; Pinsof, 1991) and have occupied Beringia for nearly 300,000 years (Froese et al., 2017; McDonald, 1981; Shapiro et al., 2004).
Despite conservation efforts, modern bison face increasing temperatures and increasing variability in climate (IPCC Working Group 1, 2014). Global temperature in the 21st century is expected to rise between 1 and 4°C above the 20th‐century average (IPCC Working Group 1, 2014). Past global and regional climates can be reconstructed using isotopic markers from ice cores and marine sediments and using limnological data such as species of pollen and diatoms, and charcoal in geological context. Currently, the longest and highest resolution records for reconstructing past atmospheric conditions are stable isotopes of oxygen (18O) from continental ice sheets in Greenland (<120,000 years (Alley et al., 1993)) and Antarctica (<800,000 years (Jouzel et al., 2007)). Values for δ18O from the Greenland Ice Sheet Project (GISP2) index decadal temperatures that would have been experienced by Bison in the Northern Hemisphere.
Species that are affected by climate change may alter their distribution and adapt through changes in morphology, physiology, behavior, and life history (Smith, Murray, Harding, Lease, & Martin, 2014; Smith et al., 2010). Small mammals appear to be able to adapt morphology and life history to environmental shifts within one to three generations (Crews & Gore, 2012; Mifsud et al., 2011). However, the adaptive responses of large mammals to climate change are poorly understood. In comparison with small mammals, large species can better avoid harsh environments by moving long distances, tolerate austere conditions with large bodies, and recover over multiple seasons to reproduce over long lifespans (Barboza, Parker, & Hume, 2009). Impacts of climate change on animals are twofold: direct effects of temperature on the animal (i.e., energetic load as heat) and indirect effects of temperature on the animal's food supply (Figure 1). Warm temperatures advance the seasonal growth of grasses to reduce the availability of nitrogen for growth of cattle and bison (Craine, 2013; Craine, Elmore, Olson, & Tolleson, 2010; Craine, Towne, Joern, & Hamilton, 2009; Craine et al., 2012). Ambient air temperature directly affects the costs of thermoregulation of the animal in cold winters and the ability to lose excess heat in warm summers (Long et al., 2014; Speakman & Król, 2010). Seasonal patterns of air temperature affect the onset, duration, and intensity of plant production that sets the quantity and quality of food for growth and reproduction of herbivores from spring through autumn (Albon et al., 2017; Huston & Wolverton, 2011).
Figure 1.
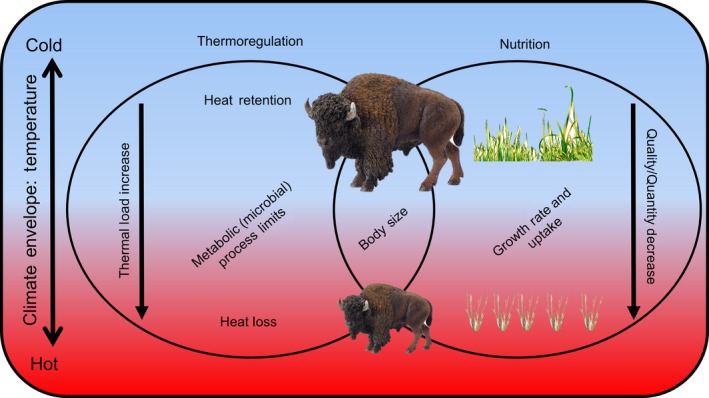
Conceptual model of the direct and indirect effects of elevated ambient temperature on body size of Bison
At least four biological concepts attempt to explain the phenomenon of changing body size. Cope's rule recognizes the tendency of vertebrate animals to increase body size over geological time scales (Stanley, 1973). Bergmann's rule emphasizes the positive relationship between body size and latitude, which suggests that the ability to retain body heat favors larger bodies at cooler temperatures as long as net primary production is adequate for animals of large size (Bergmann, 1847; Huston & Wolverton, 2011; Watt, Mitchell, & Salewski, 2010). The Metabolic Theory of Ecology emphasizes the allometric scaling of body size and the underlying relationships between the volume of animals and the surfaces that are exposed to the environment (Brown & Sibly, 2006). The Heat Dissipation Limit Theory emphasizes heat load as a driver of body size because metabolism can produce excess energy (heat), which may be more difficult to dissipate as body size and metabolic work increase (Speakman & Król, 2010). However, these relationships alone are not sufficient to accurately project the effect of climate change on the body size of large species. Although the fossil record provides abundant evidence of changes in the body size of vertebrate animals (i.e., dinosaurs, proboscideans) that have been linked to global shifts in climate (Sander et al., 2011), taxa differ in the direction, rate, and extent of response to warming and cooling (Lovegrove & Mowoe, 2013). Among large mammals, changes in body size at a continental scale declined quickly with rising temperature but rose more slowly with cooling over the past 100 million years (Evans et al., 2012).
The relationship between body size and temperature of mammals is poorly resolved especially for ecological keystone species of large mammals, such as bison (Knapp et al., 1999). Bison modify ecosystems through selective grazing (Coppedge & Shaw, 1998; Fahnestock & Knapp, 1994), wallowing (Coppedge, Fuhlendorf, Engle, Carter, & Shaw, 1999; Polley & Collins, 1984), transporting nutrients (Plumb & Dodd, 1994; Towne, 2000), herd movements (Bergman et al., 2001; Van Vuren, 2001), and physical disturbance of soil and vegetation (Allred, Fuhlendorf, & Hamilton, 2011; Coppedge & Shaw, 2000). Fossilized skeletal elements can be used to study body size over long‐time frames. Our study focuses on the calcaneum (the heel bone; Figure 3), an anatomically functional element, that is, conserved evolutionarily. We used the calcaneum to estimate body mass, whereas previous authors have focused on skull metrics (McDonald, 1981; Skinner & Kaisen, 1947) that are more susceptible to sexual selection and vary widely among species. In contrast, sexual dimorphism in bison, while noticeable in modern contexts, is lost in the fossil record without adequate comparison of other representatives of the correct species at that time. Moreover, using osteometrics and ratios on postcranial elements are unable to determine the intermediate‐sized individuals within a fossil population, stated another way, mature females and immature bulls overlap in size and all immature individuals overlap in size (Lewis, Johnson, Buchanan, & Churchill, 2007). Bison are well represented in the fossil record across North America, which provides an opportunity to relate body size to climate within a taxon over the last 40,000 years. In this study, we used the historical and prehistorical records of Bison to test the hypothesis that large‐scale changes in climate drive changes in body size.
Figure 3.
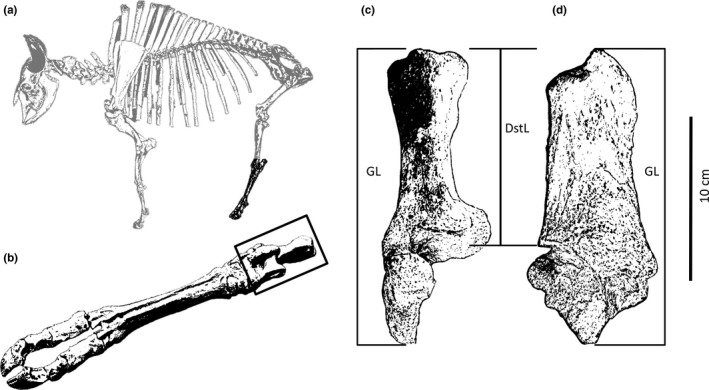
Standard metrics on a typical fossil calcaneum from a Bison (a) hock (b) shown in dorsal view (c) and medial view (d). Two measures for assessing body size of bison are illustrated: GL, greatest length; DstL, distal tuber length. Additional measures of the calcaneum are described by Von Den Driesch (1976) and Hill (1996)
2. MATERIALS AND METHODS
We used curated specimens from modern and fossil Bison. Data S1 lists specimen numbers and sponsoring collections. Physiographic and chronological information about localities is summarized in Data S2, and osteometric information about specimens at each locality is summarized in Data S3. J.I. Mead and J.M. Martin accrued a database of 2,400 Bison calcanea representing 60 localities (geological strata at geographic locations) in North America (Figure 2, Data S2). We used determinations of radiocarbon age only after 1990 for consistent accuracy of radiometric estimates (Data S2) that were calibrated using OxCal Online Tool (https://c14.arch.ox.ac.uk/) by employing the IntCal13 curve (Reimer, Bard, & Bayliss, 2013). Calibrated ages and errors are reported in Data S1; ages and errors in analyses are assumed accurate but not precise due to variability of the radiocarbon curve (Reimer et al., 2013). Specimens lacking adequate chronologies or osteometrics (<3 measures) were omitted from subsequent analyses, thus providing 1,169 samples.
Figure 2.
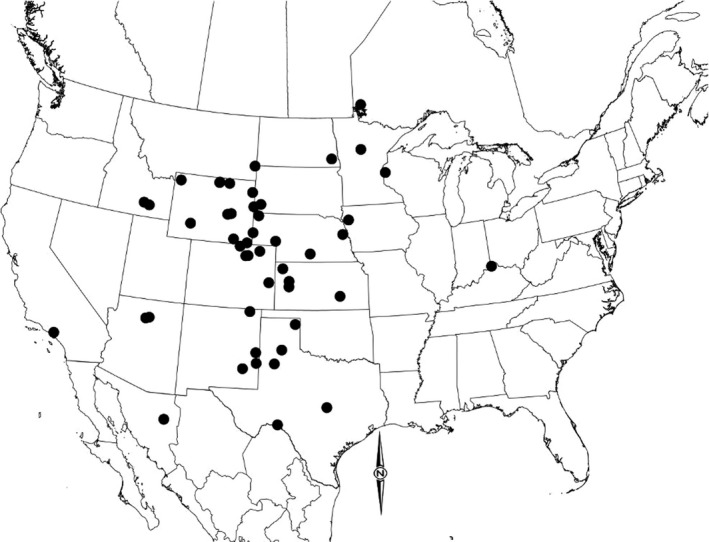
Localities (n = 60) of fossil specimens in North America that correspond with body mass estimates of bison with calibrated age. Sites are further described in Data S2
Fossil calcanea were reported as belonging to one of three species of Bison (e.g., B. bison, B. antiquus, and B. occidentalis) in collection databases based on associated diagnostic elements with specific shape and morphological landmarks (e.g., horn cores, (Skinner & Kaisen, 1947; Balkwill & Cumbaa, 1992)). Some of the specimens were originally identified as Bison bison antiquus, (nomen dubium), which has been synonymized with B. antiquus (McDonald, 1981). Six standard linear measurements were taken on the calcaneum (Hill, 1996; McDonald, 1981; Miller & Brotherson, 1979; Olsen, 1960; Von Den Driesch, 1976): distal breadth of calcaneal tuber (DstBr), greatest breadth of calcaneum at the sustentaculum (GBr), distal depth of calcaneal tuber (DstDp), distal length of calcaneal tuber (DstL), greatest length of calcaneum (GL), and greatest depth of calcaneum at the sustentaculum (GDp, Figure 3). We used DstL to estimate live body mass (M) by the relationship of Christiansen (2002, p. 688).
| (1) |
We assume that global temperature is relative to the Greenland Ice Sheet Project (GISP2) ice core paleotemperature proxy data (Grootes, Stuiver, White, Johnsen, & Jouzel, 1993). Proxy data from reconstructing global paleoclimatic temperature in °C were derived from GISP2 δ18O values (‰; Grootes et al., 1993; Alley, 2000; Alley & Ágústsdóttir, 2005) and were related to average age of the locality. The global temperature anomaly was derived by scaling the GISP2 data to the estimated Last Glacial Maximum temperature, which was on average 6°C colder than the 20th‐century average global temperature.
We used mixed model regressions for each metric of the calcaneum to compare species as a fixed effect with B. bison as the base for the comparison (Stata v14.2, 2015, StataCorp, College Station, TX, USA). Similarly, mixed models were used to compare DstL with other calcaneal metrics with species as a fixed effect. The fixed effects of species, temperature, and latitude were included in the model to analyze DstL and estimates of body mass from measures of DstL (Christiansen, 2002). We used two estimates of temperature in the models: GISP2 temperatures and the relative global temperature anomaly. All mixed models included site as a random effect to account for repeated measures within each location. We used the robust “sandwich estimator” to relax assumptions of normal distribution and homogeneity of variance for the regression (Bolker et al., 2009; Rabe‐Hesketh & Skrondal, 2012). Pairwise group comparisons among predicted margins from each model were made with Bonferroni's correction (α = 0.05).
3. RESULTS
Species significantly affected all metrics of calcaneal size (Table 1), that is, specimens from B. antiquus were larger than those of B. bison. Similarly, the intercept of the positive relationship between the depth or breadth of the calcaneum and its tuber length (DstL) was greater for B. antiquus than for B. bison (Table 2). Estimated body mass decreased over time from B. antiquus (802 ± 183 kg) to B. occidentalis (678 ± 105 kg) to modern B. bison (479 ± 177 kg; (Figure 4).
Table 1.
Summary statistics [ ± SD (n)] calcaneal osteometrics (mm) of Bison
| Parameter | Bison bison | B. occidentalis a | B. antiquus a |
|---|---|---|---|
| GL | 142.1 ± 12.2 (428) A | 155.9 ± 8.7 (35) B | 161.8 ± 11.3 (568) C |
| DstL | 88.4 ± 12.0 (273) A | 100.7 ± 5.3 (36) B | 106.2 ± 8.0 (540) C |
| DstBr | 36.17 ± 3.8 (164) A | 38.3 ± 4.1 (35) B | 41.5 ± 4.7 (569) B |
| DstDp | 39.3 ± 3.5 (164) A | 42.2 ± 3.5 (38) B | 44.3 ± 4.5 (589) C |
| GBr | 48.0 ± 4.5 (433) A | 50.3 ± 4.2 (33) B | 55.2 ± 5.2 (545) B |
| GDp | 55.5 ± 4.5 (400) A | 58.3 ± 4.6 (34) B | 63.7 ± 5.0 (563) B |
DstBr, distal tuber breadth; DstDp, distal tuber depth; DstL, distal tuber length, GBr, greatest breadth; GDp, greatest depth; GL, greatest length.
Uppercase letters indicate significant pairwise differences (p < .05) between species within each measure (row).
Extinct.
Table 2.
Regression relationships for estimating distal tuber length in Bison
| Parameter | Obs. | Sites | Intercept (± SE) | Slope (± SE) |
|---|---|---|---|---|
| GLa | 743 | 53 | −6.22 ± 2.48 | 0.68 ± 0.01 |
| DstBr | 645 | 48 | 46.69 ± 2.06 + 3.14 ± 1.04 (B.a.) + 2.37 ± 1.18 (B.o.) | 1.34 ± 0.05 |
| DstDp | 662 | 47 | 40.38 ± 2.78 + 4.53 ± 1.52 (B.a.) + 2.45 ± 1.45 (B.o.) | 1.36 ± 0.07 |
| GBr | 714 | 51 | 40.13 ± 4.57 + 6.00 ± 1.93 (B.a.) + 5.99 ± 2.00 (B.o.) | 1.08 ± 0.07 |
| GDp | 723 | 52 | 29.49 ± 4.88 + 5.77 ± 2.29 (B.a.) + 6.30 ± 2.35 (B.o.) | 1.11 ± 0.07 |
B.a., Bison antiquus; B.o., B. occidentalis.
(DstL) from other measures of the calcaneum (GL, DstBr, DstDp, GBr, GDp) in Bison using mixed models with site as a random effect and B. bison as the comparison base for species.
No linear effect of species on GL (p < .05).
Figure 4.
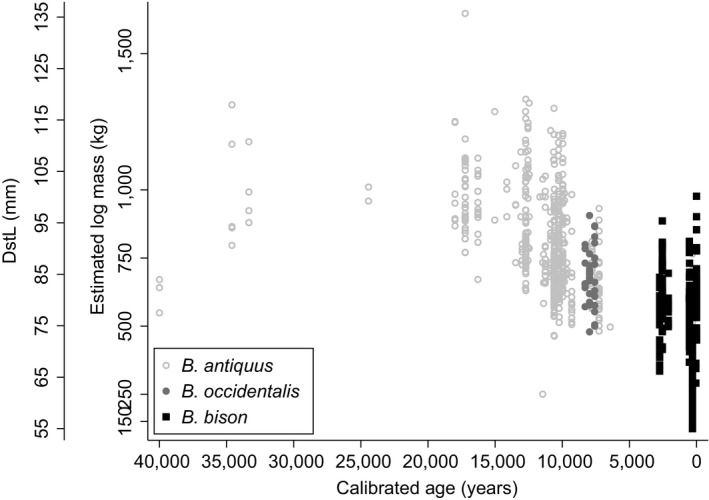
Average body size of fossil bison measured as calcaneal lengths (DstL) and body mass at 60 localities in North America from 40,000 years ago (left) to today (right)
The greatest proportion of specimens (50%) were those of B. antiquus and B. occidentalis that were dated between 7,000 and 13,000 years ago, whereas 38% of the specimens were those of B. bison from 3,000 years ago to present. Average annual temperatures varied over 25°C on the scale of Greenland temperature over the last 40,000 years, which was equivalent to a span of 6°C on the relative global scale (Figure 5).
Figure 5.
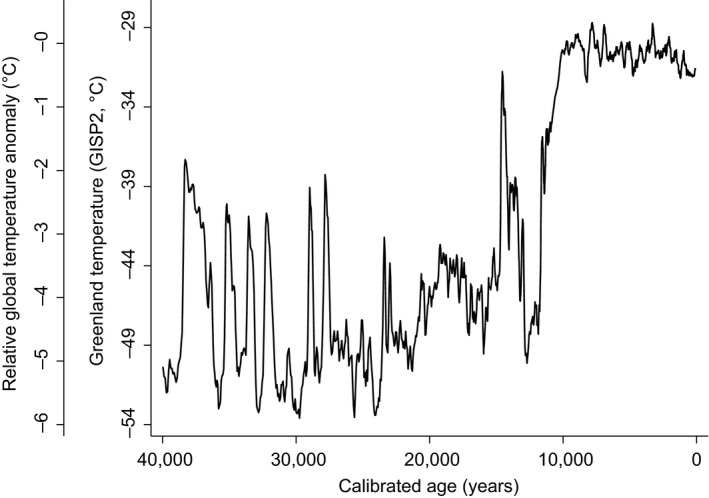
Sequence of Greenland mean annual temperature (°C derived from GISP2 δ18O values (Alley & Ágústsdóttir, 2005)) and relative global temperature anomaly derived from modern Greenland temperatures (μ29.9°C mean annual temperature) from 40,000 years ago (left) to today (right)
The largest proportion of Bison specimens were associated with two large fluctuations from 15,000 years ago to present that included warming in the Bølling–Allerød period (15,000 years to 13,000 years ago), cooling in the Younger Dryas (13,000 to 12,000 years ago), and warming through the Holocene period to present with small undulations in temperature, such as the Medieval Climatic Anomaly (approximately 1,000 to 700 years ago) and Little Ice Age (approximately 700 to 150 years ago; Figure 5).
Calcaneal distal tuber length (DstL) was negatively related to Greenland temperature (slope: −0.45 mm/°C ± 0.11; z = −3.95 p < .001) with intercepts at 78 ± 4 mm for B. bison, 90 ± 3 mm for B. antiquus, and 87 ± 2 mm for B. occidentalis. The relationship between calcaneal distal tuber length (DstL) and relative global temperature was −1.77 mm/°C ± 0.45 (z = −3.95, p < .001) with intercepts at 92 ± 2 mm for B. bison, 103 ± 3 mm for B. antiquus, and 101 ± 2 mm for B. occidentalis. Consequently, the slope of estimated body mass with global temperature was also negative at −41 kg/°C (± 10; z = −4.10, p < .001) with intercepts at 521 ± 36 kg for B. bison, 737 ± 45 kg for B. antiquus, and 676 ± 36 kg for B. occidentalis (Figure 6). This relationship predicts that B. bison will decrease by 164 ± 40 kg to 357 ± 54 kg if global temperature rises from 0°C to +4°C (Figure 6).
Figure 6.
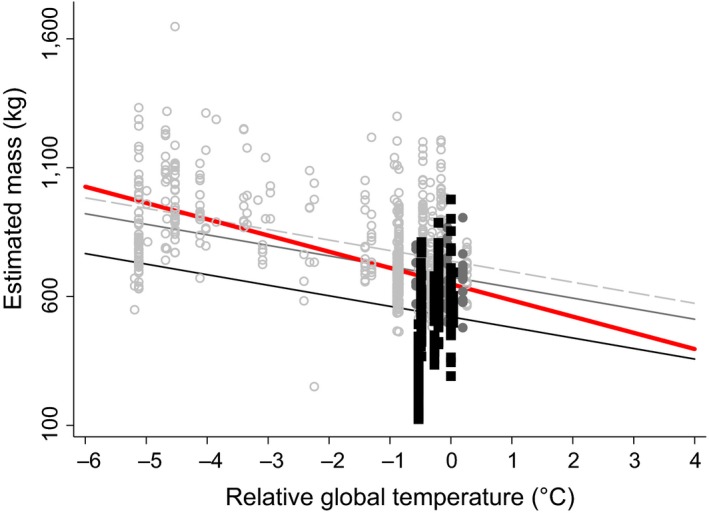
Relationship between estimated body mass (kg; ± SE) and the linear effect of relative global temperature (°C derived from GISP2 δ18O values) from the mixed model regression with fixed effects of temperature and the random effect of site. Regression line (y = −40.9 kg/°C ± 10) with lines for specific regressions (intercepts for B. bison (black): 520.9 ± 36.1; B. occidentalis (dark gray): 675.6 ± 36.2; B. antiquus (light gray): 737.3 ± 44.7; p < .001, n = 849, N = 53). Regression line for the small‐sized Bison clade (red line) is −63 kg/°C (± 10; z = −6.11 p < .001) with an intercept at 648 ± 26 kg
4. DISCUSSION
Our data supported our hypothesis that global climate change drives body size of Bison spp., that is, as temperatures warmed, Bison became smaller. Generally, described as Bergmann’s Rule (Bergmann, 1847), endotherms increase in body size with increasing latitude (Huston & Wolverton, 2011). It is likely that negative correlation between temperature and latitude is driving Bergmann's rule (i.e., body size) because even though we found that bison are larger at cooler temperatures, we were unable to correlate a significant effect of latitude over the geologic record (p > .94). The negative relationship between body mass and global temperature may reflect underlying relationships between body size and net primary production as well as heat loads (Speakman & Król, 2010; Huston & Wolverton, 2011; Figure 1).
Paleontologists have long used skeletal elements from extant animals to reconstruct body mass and body shape of fossils (Christiansen, 2002; Damuth & MacFadden, 1990; Gingerich, 1990). Data from some bones indicate body size more accurately than others. Indices of body size in mammals, including Bison, are best indicated by bones of the hind foot (elements of the ankle, calcaneum, and astragalus), and front foot (elements of the wrist, scaphoid, and magnum), along with the toes (podial digits and distal and proximal phalanges; (Damuth & MacFadden, 1990)). The bulk of the foot bones precisely reflects body mass because they bear the weight of the animal, whereas the shape of the bones reflects the functional anatomy for locomotion through the attachment of tendons and muscle (Scott, 1990). Longer bones of limbs (femora and humeri) are also good proxies for reconstructing body size. Unfortunately, long bones in the fossil record are typically broken, whereas the calcanea, astragali, and phalanges are commonly well preserved, likely because these dense elements resist degradation. Consequently, podial elements are well studied within Bovinae, which includes cattle (Bos taurus, (Lawrence, 1951; Olsen, 1960; Balkwill & Cumbaa, 1992)), and Antilopinae, mountain goats (Oreamnos sp., (Carpenter, 2003)), bighorn sheep (Ovis sp., (Todd & Rapson, 1988; Rothschild & Martin, 2003), among others). However, it is difficult to distinguish taxa using podial elements. Bison and Bos can be resolved from traits of podial elements by the methods of Balkwill and Cumbaa (1992) but we cannot resolve Bison species based upon podial elements alone. Species designations in our dataset originated from whole collections of associated podial and cranial material that may not distinguish mixes of species at each location. For example, American Falls Reservoir in Idaho contains at least four co‐existing species of Bison (Pinsof, 1991). If we ignore species designations and analyze our data at the clade level, the slope of podial size with increasing temperatures becomes steeper; −63 kg/°C (±10; z = −6.11 p < .001) with an intercept at 648 ± 26 kg for Bison spp., as compared to the −41 kg/°C for Bison bison (Figure 6). This slope may change regionally with latitudinal differences in body size of extant Bison.
Bison crania exhibit plastic morphology, likely due to a combination of environmental and sexual selection, whereas postcranial elements—podial elements specifically—exhibit a more conservative and accurate reflection of body size due to functional anatomy of the appendicular skeleton (Clifford, 2009, 2010). Historically, it has been difficult to identify Bison fossil species (Bison bison, B. occidentalis, and B. antiquus) based on skeletal remains without skulls, especially those without horn cores (McDonald, 1981; Skinner & Kaisen, 1947). This issue continues today (Grayson, 2006; Lyman, 2004; McDonald & Lammers, 2002), with the exceptions of B. latifrons (Giant bison (Hopkins, 1951; Schultz & Hillerud, 1977; Pinsof, 1991)) and B. priscus (Steppe bison; (Gee, 1993; Zazula, MacKay, & Andrews, 2009; Boeskorov et al., 2014)), which are distinct because of their massive size. Many of the above authors rely on cranial elements alone to specifically classify Bison, but recent studies suggest that the diagnostic Bison cranial characters vary widely (Krasinska, 1988) and do not reflect conservative morphological variability in the skeleton. Cranial elements of Bison are now thought too variable to rely on for taxonomic classification (Prothero & Foss, 2007). Widga (2013) attempted to synthesize a large dataset of bison horn‐core metrics and illustrates the noise inherent in these samples (Hill, Hawley, Widga, Monahan, & Wanamaker, 2014; Wilson, 1974).
Some researchers suggest that the past several millennia of anthropogenic selection by Paleoindians, conservationists, and producers may have directly and indirectly selected traits that scale to body size (i.e., large heart girths, large heads, straight vertebral column; (Todd, 1983; Grayson, 2000, 2001)). Undoubtedly, early arrivals of modern humans were having impacts on the available bison through hunting some 14,000 years ago (Barnosky et al., 2014; Grayson, 2000); however, these effects were limited by small human populations dispersed over a large continent and were therefore local impacts (Hawley, Hill, & Widga, 2013; Hill et al., 2008, 2014). Others have acknowledged that any selection has not made significant changes in morphology (Hawley et al., 2013; Hill et al., 2008, 2014). Climate is the most parsimonious explanation for shaping Bison morphology (Hill et al., 2008; Lewis et al., 2007; Shapiro et al., 2004). Changes in body size of Bison could be a result of migration or disease but those effects are geographically local and not likely to persist over the long time scale of the fossil record (Hamel et al., 2016). Wilson, Hills, and Shapiro (2008) postulate the decrease in body size of Bison is a consequence of dispersal theory, that is, expansion of range, over the last 80,000 years (Wilson, 1996). A more cogent argument explaining decrease in body size is the rapidly warming global climate, characterizing the termination of the Younger Dryas period.
This study demonstrates a strong inverse correlation between increasing global temperatures and body size of bison over the last 40,000 years. We hypothesize that increasing temperature alters both metabolic demands and available resources (Figure 1).
The IPCC Working Group 1 (2014) predicts 4°C rise in global temperatures by year 2100. While the absolute increase in 4°C is not unprecedented in the evolutionary history of Bison, the rate of temperature change is 30 times faster than the Bølling–Allerød period, the transition from the Last Glacial Maximum to Holocene climate conditions. The Last Glacial Maximum corresponds with a global temperature 6°C cooler than the 20th century, when Bison mass was 910 kg. If global temperature warms to +4°C as predicted for the 21st century, Bison body mass will likely decline from 665 kg to 357 kg (Figure 6), if body size declines at the long‐term average. The greatest decline in body size of Bison apparently occurred between 12,500 and 9,250 years ago, when mass declined by 26% (906 kg to 670 kg) in approximately 3,000 years. If generation time of Bison is 3–10 years (Evans et al., 2012; Gingerich, 1993), the change in body size occurred in 325–1,080 generations producing an average rate of change of 0.2–0.7 kg per generation. It is unclear whether Bison can adapt body size to a 4°C warming within 10 generations by year 2100.
Bison today express a 30% body mass gradient from north to south, that is, Bison in Saskatchewan (52°N) are at least 30% larger than those in Texas (30°N (Craine, 2013, p. 3)). This body size gradient is likely associated with latitudinal variation in timing of reproduction and parturition as well as windows for growth (Barboza et al., 2009). Quantifying and comparing physiological thresholds and mechanisms driving body size change are imperative for managing Bison and other large herbivores (Figure 1). Conservation goals among latitudinally disparate Bison herds in North America should consider that resident Bison will likely grow smaller and more slowly in the south than in the north, which will impact management strategies at both regional and continental scales.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
JMM conceived and developed the study. JMM and JIM acquired the data. JMM, JIM, and PSB interpreted and analyzed the data. JMM, JIM, and PSB drafted, revised, and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank R.A. Short‐Martin, C. Widga, R. Shively, K. Hollingsworth, K. Oster, S. C. Wallace, S.L. Swift, T. Martin, and K. Martin for facilitating discussions improving this study. We are in hock to the contributions provided by the anonymous reviewers improving the manuscript. We thank M.E. Hill, M.G. Hill, E. Scott, H.G. McDonald, J.N. McDonald, and C. Widga for supplying additional calcaneal metrics. We thank R.A. Fowler, C.I. Rodda, C. Frost, G. Lima, and T. Lessard from the Climate Change Institute at the University of Maine for contributing to previous interpretations of the data and earlier versions of the paper. Funding for this research was supported in part by the Western Bison Association research grant, the Throlson American Bison Foundation Scholarship at the National Bison Association, the Larry D. Agenbroad Legacy Fund at The Mammoth Site of Hot Springs, South Dakota; and the US National Science Foundation Adaptation to Abrupt Climate Change IGERT program at the Climate Change Institute of the University of Maine, grant #DGE‐1144423. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. We are grateful to the many institutions listed in S2 that provided over 2,400 specimens and more than 7,300 measurements for this study.
Martin JM, Mead JI, Barboza PS. Bison body size and climate change. Ecol Evol. 2018;8:4564–4574. https://doi.org/10.1002/ece3.4019
REFERENCES
- Albon, S. D. , Irvine, R. J. , Halvorsen, O. , Langvatn, R. , Loe, L. E. , Ropstad, E. , … Stien, A. (2017). Contrasting effects of summer and winter warming on body mass explain population dynamics in a food‐limited Arctic herbivore. Global Change Biology, 23, 1374–1389. https://doi.org/10.1111/gcb.13435 [DOI] [PubMed] [Google Scholar]
- Alley, R. B. (2000). The Younger Dryas cold interval as viewed from central Greenland. Quaternary Science Reviews, 19, 213–226. https://doi.org/10.1016/S0277-3791(99)00062-1 [Google Scholar]
- Alley, R. B. , & Ágústsdóttir, A. M. (2005). The 8k event: Cause and consequences of a major Holocene abrupt climate change. Quaternary Science Reviews, 24, 1123–1149. https://doi.org/10.1016/j.quascirev.2004.12.004 [Google Scholar]
- Alley, R. B. , Meese, D. A. , Shuman, C. A. , Gow, A. J. , Taylor, K. C. , Grootes, P. M. , … Zielinski, G. A. (1993). Abrupt increase in Greenland snow accumulation at the end of the Younger Dryas event. Nature, 362, 527–529. https://doi.org/10.1038/362527a0 [Google Scholar]
- Allred, B. W. , Fuhlendorf, S. D. , & Hamilton, R. G. (2011) The role of herbivores in Great Plains conservation: Comparative ecology of bison and cattle. Ecosphere, 2, 1–17. https://doi.org/10.1890/ES10-00152.1art26. [Google Scholar]
- Balkwill, D. M. , & Cumbaa, S. L. (1992). A guide to the identification of postcranial bones of Bos taurus and Bison bison(pp. 277). Ottawa: Syllogeus, 71. [Google Scholar]
- Barboza, P. S. , Parker, K. L. , & Hume, I. D. (2009). Integrative Wildlife Nutrition (p. 350). Berlin, Heidelberg: Verlag, Springer; https://doi.org/10.1007/978-3-540-87885-8 [Google Scholar]
- Barnosky, A. D. , Holmes, M. , Kirchholtes, R. , Lindsey, E. , Maguire, K. C. , Poust, A. W. , … Wogan, G. O. U. (2014). Prelude to the Anthropocene: Two new North American Land Mammal Ages (NALMAs). The Anthropocene Review, 1, 225–242. https://doi.org/10.1177/2053019614547433 [Google Scholar]
- Bell, C. J. , Lundelius, E. L. Jr , Barnosky, A. D. , Graham, R. W. , Lindsay, E. H. , Ruez, D. R. Jr , … Zakrzewski, R. J. (2004). The Blancan, Irvingtonian, and Rancholabrean Mammal Ages In Woodburne M. O. (Ed.), Late Cretaceous and Cenozoic mammals of North America: Biostratigraphy and Geochronology (pp. 232–314). New York City, NY: Columbia University Press. [Google Scholar]
- Bergman, C. M. , Fryxell, J. M. , Gates, C. C. , & Fortin, D. (2001). Ungulate foraging strategies: Energy maximizing or time minimizing? Journal of Animal Ecology, 70, 289–300. https://doi.org/10.1046/j.1365-2656.2001.00496.x [Google Scholar]
- Bergmann, C. (1847). Über die verhaltnisse der warmeokonomie der thiere zu ihrer grosse (translation: On the Conditions of the Thermal Economy of Animals to Their Size) (p. 116). Germany: Göttingen. [Google Scholar]
- Boeskorov, G. G. , Potapova, O. R. , Mashchenko, E. N. , Protopopov, A. V. , Kuznetsova, T. V. , Agenbroad, L. , & Tikhonov, A. N. (2014) Preliminary analyses of the frozen mummies of mammoth (Mammuthus primigenius), bison (Bison priscus) and horse (Equus sp.) from the Yana‐Indigirka Lowland, Yakutia, Russia. Integrative Zoology, 9(4), 471–480. [DOI] [PubMed] [Google Scholar]
- Bolker, B. M. , Brooks, M. E. , Clark, C. J. , Geange, S. W. , Poulsen, J. R. , Stevens, M. H. H. , & White, J. S. S. (2009). Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology and Evolution, 24, 127–135. https://doi.org/10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Brown, J. H. , & Sibly, R. M. (2006). Life‐history evolution under a production constraint. Proceedings of the National Academy of Sciences of the United States of America, 103, 17595–17599. https://doi.org/10.1073/pnas.0608522103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, M. C. (2003). Late Pleistocene Aves, Chiroptera, Perissodactyla, and Artiodactyla from Rampart Cave, Grand Canyon (p. 333). Arizona: Northern Arizona University. [Google Scholar]
- Christiansen, P. (2002). Locomotion in terrestrial mammals: The influence of body mass, limb length and bone proportions on speed. Zoological Journal of the Linnean Society, 136, 685–714. https://doi.org/10.1046/j.1096-3642.2002.00041.x [Google Scholar]
- Clifford, A. B. (2009) Evolution and Mechanics of Unguligrady in Artiodactyls. Brown University, 1‐145 pp.
- Clifford, A. B. (2010). The evolution of the unguligrade manus in artiodactyls. Journal of Vertebrate Paleontology, 30, 1827–1839. https://doi.org/10.1080/02724634.2010.521216 [Google Scholar]
- Coppedge, B. R. , Fuhlendorf, S. D. , Engle, D. M. , Carter, B. J. , & Shaw, J. H. (1999). Grassland soil depressions: Relict bison wallows or inherent landscape heterogeneity? American Midland Naturalist, 142, 382–392. https://doi.org/10.1674/0003-0031(1999)142[0382:GSDRBW]2.0.CO;2 [Google Scholar]
- Coppedge, B. R. , & Shaw, J. H. (1998). Bison grazing patterns on seasonally burned tallgrass prairie. Journal of Range Management, 51, 258–264. https://doi.org/10.2307/4003408 [Google Scholar]
- Coppedge, B. R. , & Shaw, J. H. (2000). American bison Bison bison wallowing behavior and wallow formation on tallgrass prairie. Acta Theriologica, 45, 103–110. https://doi.org/10.4098/0001-7051 [Google Scholar]
- Craine, J. M. (2013). Long‐term climate sensitivity of grazer performance: A cross‐site study. PLoS One, 8, e67065 https://doi.org/10.1371/journal.pone.0067065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine, J. M. , Elmore, A. J. , Olson, K. C. , & Tolleson, D. (2010). Climate change and cattle nutritional stress. Global Change Biology, 16, 2901–2911. https://doi.org/10.1111/j.1365-2486.2009.02060.x [Google Scholar]
- Craine, J. M. , Nippert, J. B. , Elmore, A. J. , Skibbe, A. M. , Hutchinson, S. L. , & Brunsell, N. A. (2012). Timing of climate variability and grassland productivity. Proceedings of the National Academy of Sciences, 109, 3401–3405. https://doi.org/10.1073/pnas.1118438109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine, J. M. , Towne, E. G. , Joern, A. , & Hamilton, R. G. (2009). Consequences of climate variability for the performance of bison in tallgrass prairie. Global Change Biology, 15, 772–779. https://doi.org/10.1111/j.1365-2486.2008.01769.x [Google Scholar]
- Crews, D. , & Gore, A. C. (2012). Epigenetic synthesis: A need for a new paradigm for evolution in a contaminated world. Biology Reports, 43410, 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuth, J. , & MacFadden, B. J. (1990). Body Size in Mammalian Paleobiology: Estimation and Biological Implications (p. 401). Cambridge, Massachusetts: Cambridge University Press. [Google Scholar]
- Dary, D. A. (1974). The Buffalo Book: The Full Saga of the American animal. Chicago, Illinois: The Swallow Press. [Google Scholar]
- Evans, A. R. , Jones, D. , Boyer, A. G. , Brown, J. H. , Costa, D. P. , Ernest, S. K. , … Uhen, M. D. (2012). The maximum rate of mammal evolution. Proceedings of the National Academy of Sciences, 109, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock, J. T. , & Knapp, A. K. (1994). Plant responses to selective grazing by bison: Interactions between light, herbivory and water stress. Vegetatio, 115, 123–131. [Google Scholar]
- Feranec, R. S. , Hadly, E. A. , & Paytan, A. (2009). Stable isotopes reveal seasonal competition for resources between late Pleistocene bison (Bison) and horse (Equus) from Rancho La Brea, southern California. Palaeogeography, Palaeoclimatology, Palaeoecology, 271, 153–160. https://doi.org/10.1016/j.palaeo.2008.10.005 [Google Scholar]
- Feranec, R. S. , & MacFadden, B. J. (2000). Evolution of the grazing niche in Pleistocene mammals from Florida: Evidence from stable isotopes. Palaeogeography, Palaeoclimatology, Palaeoecology, 162, 155–169. https://doi.org/10.1016/S0031-0182(00)00110-3 [Google Scholar]
- Froese, D. , Stiller, M. , Heintzman, P. D. , Reyes, A. V. , Zazula, G. D. , Soares, A. E. , … Shapiro, B. (2017). Fossil and genomic evidence constrains the timing of bison arrival in North America. Proceedings of the National Academy of Sciences of the United States of America, 114(13), 3457–3462. https://doi.org/10.1073/pnas.1620754114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates C. C., Freese C. H., Gogan P. J. P. & Kotzman M., (eds). (2010). American bison: Status survey and conservation guidelines(pp. 134). Gland, Switzerland: IUCN. [Google Scholar]
- Gee, H. (1993). Distinction between postcranial bones of Bos primigenius Bojanus, 1827 and Bison priscus Bojanus, 1827 from the British Pleistocene and the taxonomic status of Bos. Journal of Quaternary Science, 8, 79–92. https://doi.org/10.1002/(ISSN)1099-1417 [Google Scholar]
- Gingerich, P. D. (1990). Prediction of Body Mass in Mammalian Species From Long Bone Lengths and Diameters pp. (79–92). Ann Arbor: Contributions From theMuseum of Paleontology, University of Michigan, 28. [Google Scholar]
- Gingerich, P. D. (1993). Rates of evolution in Plio‐Pleistocene mammals: Six case studies In Martin R. A., & Barnosky A. D. (Eds.), Morphological change in Quaternary mammals of North America (pp. 84–106). Cambridge, Massachusetts: Cambridge University Press; https://doi.org/10.1017/CBO9780511565052 [Google Scholar]
- Grayson, D. K. (2000). Mammalian responses to Middle Holocene climatic change in the Great Basin of the western United States. Journal of Biogeography, 27, 181–192. https://doi.org/10.1046/j.1365-2699.2000.00383.x [Google Scholar]
- Grayson, D. (2001). The archaeological record of human impacts on animal populations. Journal of World Prehistory, 15, 1–68. https://doi.org/10.1023/A:1011165119141 [Google Scholar]
- Grayson, D. K. (2006). Holocene bison in the Great Basin, western USA. Holocene, 16, 913–925. https://doi.org/10.1191/0959683606hol982fa [Google Scholar]
- Grootes, P. , Stuiver, M. , White, J. , Johnsen, S. , & Jouzel, J. (1993). Comparison of oxygen isotope records from the GISP2 and GRIP Greenland ice cores. Nature, 366, 552–554. https://doi.org/10.1038/366552a0 [Google Scholar]
- Hamel, S. , Gaillard, J. , Yoccoz, N. G. , Albon, S. , Côté, S. D. , Craine, J. M. , … Tveraa, T. (2016). Cohort variation in individual body mass dissipates with age in large herbivores. Ecological Monographs, 86, 517–543. https://doi.org/10.1002/ecm.1232 [Google Scholar]
- Hawley, M. F. , Hill, M. G. , & Widga, C. C. (2013). New Deal Era discovery and investigation of middle Holocene bonebeds in the Upper Midwest (pp. 29–35). Washington, DC: The SAA Archaeological Record. [Google Scholar]
- Hill, M. G. (1996). Size comparison of the Mill Iron Site bison calcanea In Frison G. C. (Ed.), The Mill Iron Site (pp. 231–237). Albuquerque, New Mexico: University of New Mexico Press. [Google Scholar]
- Hill, M. G. , Hawley, M. F. , Widga, C. C. , Monahan, L. A. H. , & Wanamaker, A. D. Jr (2014). The Nye site, Wisconsin: The search for early man in the Upper Midwest, investigative incursions, and paleozoology. The Wisconsin Archeologist, 95, 200–238. [Google Scholar]
- Hill, M. E. , Hill, M. G. , & Widga, C. C. (2008). Late Quaternary Bison diminution on the Great Plains of North America: Evaluating the role of human hunting versus climate change. Quaternary Science Reviews, 27, 1752–1771. https://doi.org/10.1016/j.quascirev.2008.07.002 [Google Scholar]
- Hopkins, M. (1951). Bison (Gigantobison) latifrons and Bison (Simobison) alleni in southeastern Idaho. Journal of Mammalogy, 32, 192–197. https://doi.org/10.2307/1375374 [Google Scholar]
- Huston, M. A. , & Wolverton, S. (2011). Regulation of animal size by eNPP, Bergmann's rule, and related phenomena. Ecological Monographs, 81, 349–405. https://doi.org/10.1890/10-1523.1 [Google Scholar]
- IPCC Working Group 1 . (2014) IPCC Fifth Assessment Report (AR5) ‐ The physical science basis. IPCC.
- Jouzel, J. , Masson‐Delmotte, V. , Cattani, O. , Dreyfus, G. , Falourd, S. , Hoffmann, G. , … Wolff, E. W. (2007). Orbital and millennial Antarctic climate variability over the past 800,000 years. Science, 317, 793–796. https://doi.org/10.1126/science.1141038 [DOI] [PubMed] [Google Scholar]
- Knapp, A. K. , Blair, J. M. , Briggs, J. M. , Collins, S. L. , Hartnett, D. C. , Johnson, L. C. , & Towne, E. G. (1999). The Keystone Role of Bison in North American Tallgrass Prairie. BioScience, 49, 39–50. https://doi.org/10.2307/1313492 [Google Scholar]
- Koch, P. L. , & Barnosky, A. D. (2006). Late Quaternary extinctions: State of the debate. Annual Review of Ecology, Evolution, and Systematics, 37, 215–250. https://doi.org/10.1146/annurev.ecolsys.34.011802.132415 [Google Scholar]
- Krasinska, M. (1988). Variability of the Skull Shape in Hybrids between European bison and Domestic Cattle. Acta Theriologica, 33, 147–186. https://doi.org/10.4098/0001-7051 [Google Scholar]
- Kurtén, B. , & Anderson, E. (1980). Pleistocene mammals of North America (p. 442). New York, NY: Columbia University Press. [Google Scholar]
- Lawrence, B. (1951). Mammals found at the Awatovi Site: Post‐cranial skeletal characters of deer, pronghorn, and sheep‐goat with notes on Bos and Bison, Vol. 3 (p. 43). Cambridge: Massachusetts. [Google Scholar]
- Leng, M. (2006). Isotopes in palaeoenvironmental research (pp. 322). Berlin: Springer Science & Business Media; https://doi.org/10.1007/1-4020-2504-1 [Google Scholar]
- Lewis, P. J. , Johnson, E. , Buchanan, B. , & Churchill, S. E. (2007). The Evolution of Bison bison: A View from the Southern Plains. Bulletin of the Texas Archeological Society, 78, 197–204. [Google Scholar]
- Long, R. A. , Bowyer, R. T. , Porter, W. P. , Mathewson, P. , Monteith, K. L. , & Kie, J. G. (2014). Behavior and nutritional condition buffer a large‐bodied endotherm against direct and indirect effects of climate. Ecological Monographs, 84(3), 513–532. https://doi.org/10.1890/13-1273.1 [Google Scholar]
- Lovegrove, B. G. , & Mowoe, M. O. (2013). The evolution of mammal body sizes: Responses to Cenozoic climate change in North American mammals. Journal of Evolutionary Biology, 26, 1317–1329. https://doi.org/10.1111/jeb.12138 [DOI] [PubMed] [Google Scholar]
- Lyman, R. L. (2004). Late‐Quaternary diminution and abundance of prehistoric bison (Bison sp.) in eastern Washington state, U.S.A. Quaternary Research, 62, 76–85. https://doi.org/10.1016/j.yqres.2004.04.001 [Google Scholar]
- MacFadden, B. J. , & Cerling, T. E. (1996). Mammalian herbivore communities, ancient feeding ecology, and carbon isotopes: A 10 million‐year sequence from the Neogene of Florida. Journal of Vertebrate Paleontology, 16, 103–115. https://doi.org/10.1080/02724634.1996.10011288 [Google Scholar]
- McDonald, J. N. (1981). North American bison: Their classification and evolution (p. 316). Los Angeles, California: University of California Press. [Google Scholar]
- McDonald, J. N. , & Lammers, G. (2002). Bison antiquus from Kenora, Ontario, and notes on the evolution of North American Holocene Bison . Smithsonian Contributions to Paleobiology, 93, 83–97. [Google Scholar]
- Mifsud, K. R. , Gutièrrez‐Mecinas, M. , Trollope, A. F. , Collins, A. , Saunderson, E. A. , & Reul, J. M. H. M. (2011). Epigenetic mechanisms in stress and adaptation. Brain, Behavior, and Immunity, 25, 1305–1315. https://doi.org/10.1016/j.bbi.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Miller, W. E. , & Brotherson, J. D. (1979). Size variation in foot elements of Bison from Rancho La Brea(pp. 1–19). Los Angeles: Natural History Museum, Contributions in Science, 323. [Google Scholar]
- Miquelle, D. (1985) Food habits and range conditions of bison and sympatric ungulates on the Upper Chita River, Wrangell‐Saint Elias National Park and Preserve. Alaska Region Research Resources Management Report AR‐8.
- Olsen, S. J. (1960). Post‐cranial skeletal characters of Bison and Bos . Papers of the Peabody Museum of Archaeology and Ethnology, Harvard University, 35, 1–15. [Google Scholar]
- Pinsof, J. D. (1991). A cranium of Bison alaskensis (Mammalia: Artiodactyla: Bovidae) and comments on fossil Bison diversity in the American Falls area, southeastern Idaho. Journal of Vertebrate Paleontology, 11, 509–514. https://doi.org/10.1080/02724634.1991.10011418 [Google Scholar]
- Plumb, G. E. , & Dodd, J. L. (1994). Foraging ecology of bison and cattle. Rangelands Archieves, 16, 107–109. [Google Scholar]
- Polley, H. W. , & Collins, S. L. (1984). Relationships of vegetation and environment in buffalo wallows. American Midland Naturalist, 112, 178–186. https://doi.org/10.2307/2425471 [Google Scholar]
- Prothero, D. R. , & Foss, S. E. (2007). The evolution of artiodactyls (p. 384). Baltimore, Maryland: Johns Hopkins University Press. [Google Scholar]
- Rabe‐Hesketh, S. , & Skrondal, A. (2012). Multilevel and longitudinal modeling using Stata, 3rd edn College Station, Texas: Stata Press. [Google Scholar]
- Reimer, P. , Bard, E. , & Bayliss, A. (2013). IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon, 55, 1869–1887. https://doi.org/10.2458/azu_js_rc.55.16947 [Google Scholar]
- Rothschild, B. M. , & Martin, L. D. (2003). Frequency of pathology in a large natural sample from natural trap cave with special remarks on erosive disease in the Pleistocene. Reumatismo, 55, 58–65. [DOI] [PubMed] [Google Scholar]
- Sander, P. M. , Christian, A. , Clauss, M. , Fechner, R. , Gee, C. T. , Griebeler, E. M. , … Witzel, U. (2011). Biology of the sauropod dinosaurs: The evolution of gigantism. Biological Reviews, 86, 117–155. https://doi.org/10.1111/j.1469-185X.2010.00137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, C. B. , & Hillerud, J. M. (1977). The antiquity of Bison latifrons (Harlan) in the Great Plains of North America. Transactions of the Nebraska Academy of Sciences, 4, 103–116. [Google Scholar]
- Scott, K. M. (1990). Post‐cranial predictors of ungulates as predictors of body size In Damuth J., & Macfadden B. J. (Eds.), Body size in mammalian paleobiology (pp. 301–336). Cambridge, Massachusetts: Cambridge University Press. [Google Scholar]
- Shapiro, B. , Drummond, A. J. , Rambaut, A. , Wilson, M. C. , Matheus, P. E. , Sher, A. V. , … Cooper, A. (2004). Rise and fall of the Beringian Steppe Bison . Science, 306, 1561–1565. https://doi.org/10.1126/science.1101074 [DOI] [PubMed] [Google Scholar]
- Skinner, M. F. , & Kaisen, O. C. (1947). The fossil Bison of Alaska and preliminary revision of the genus. Bulletin of the American Museum of Natural History, 89, 123–256. [Google Scholar]
- Smith, F. A. , Boyer, A. G. , Brown, J. H. , Costa, D. P. , Dayan, T. , Ernest, S. K. , … Uhen, M. D. (2010). The evolution of maximum body size of terrestrial mammals. Science, 330, 1216–1219. https://doi.org/10.1126/science.1194830 [DOI] [PubMed] [Google Scholar]
- Smith, F. A. , Murray, I. W. , Harding, L. E. , Lease, H. M. , & Martin, J. (2014). Life in an extreme environment: a historical perspective on the influence of temperature on the ecology and evolution of woodrats. Journal of Mammalogy, 95, 1128–1143. https://doi.org/10.1644/13-MAMM-S-070 [Google Scholar]
- Speakman, J. R. , & Król, E. (2010). Maximal heat dissipation capacity and hyperthermia risk: Neglected key factors in the ecology of endotherms. Journal of Animal Ecology, 79, 726–746. [DOI] [PubMed] [Google Scholar]
- Stanley, S. (1973). An explanation for Cope's Rule. Evolution, 27, 1–26. https://doi.org/10.1111/j.1558-5646.1973.tb05912.x [DOI] [PubMed] [Google Scholar]
- Todd, L. (1983) The Horner Site: Taphonomy of an Early Holocene Bison bonebed. University of New Mexico, 340 pp.
- Todd, L. C. , & Rapson, D. J. (1988). Long bone fragmentation and interpretation of faunal assemblages: Approaches to comparative analysis. Journal of Archaeological Science, 15, 307–325. https://doi.org/10.1016/0305-4403(88)90067-2 [Google Scholar]
- Towne, E. G. (2000). Prairie vegetation and soil nutrient responses to ungulate carcasses. Oecologia, 122, 232–239. https://doi.org/10.1007/PL00008851 [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture (2016). Health and management practices on U.S. ranched‐bison operations, 2014 (p. 184). Colorado: Fort Collins. [Google Scholar]
- Van Vuren, D. H. (2001). Spatial relations of American bison (Bison bison) and domestic cattle in a montane environment. Animal Biodiversity and Conservation, 24, 117–124. [Google Scholar]
- Von Den Driesch, A. (1976). A guide to the measurement of animal bones from archeological sites, 1st edn. Peabody Museum: Harvard University, Harvard, Massachusetts. [Google Scholar]
- Watt, C. , Mitchell, S. , & Salewski, V. (2010). Bergmann's rule; A concept cluster? Oikos, 119, 89–100. https://doi.org/10.1111/j.1600-0706.2009.17959.x [Google Scholar]
- Widga, C. C. (2006). Niche variability in late Holocene bison: A perspective from Big Bone Lick, KY. Journal of archaeological science, 33, 1237–1255. https://doi.org/10.1016/j.jas.2005.12.011 [Google Scholar]
- Widga, C. C. (2013). Evolution of the High Plains Paleoindian landscape: The paleoecology of Great Plains faunal assemblages In Knell E. J., & Muniz M. P. (Eds.), Paleoindian lifeways of the Cody Complex (pp. 69–92). Salt Lake City, Utah: The University of Utah Press. [Google Scholar]
- Wilson, M. C. (1974). The Casper local fauna and its fossil bison In Frison G. C. (Ed.), The Casper Site, a Hell Gap bison kill on the High Plains (pp. 124–171). New York, NY: Academic Press. [Google Scholar]
- Wilson, M. C. (1996). Late quaternary vertebrates and the opening of the ice‐free corridor, with special reference to the genus Bison . Quaternary International, 32, 97–105. [Google Scholar]
- Wilson, M. C. , Hills, L. V. , & Shapiro, B. (2008). Late Pleistocene northward‐dispersing Bison antiquus from the Bighill Creek Formation, Gallelli gravel pit, Alberta, Canada, and the fate of Bison occidentalis . Canadian Journal of Earth Sciences, 45, 827–859. https://doi.org/10.1139/E08-027 [Google Scholar]
- Zazula, G. D. , MacKay, G. , & Andrews, T. D. (2009). A late Pleistocene steppe bison (Bison priscus) partial carcass from Tsiigehtchic, Northwest Territories, Canada. Quaternary Science Reviews, 28, 2734–2742. https://doi.org/10.1016/j.quascirev.2009.06.012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


