Abstract
Salmonella spp. are food-borne pathogens that usually cause gastroenteritis, although bacteremia and subsequent focal metastatic infection can also occasionally occur. Of the known Salmonella spp., Salmonella houtenae is a rare subspecies, comprising less than 1% of all Salmonella strains. We herein report the first case of S. houtenae-induced empyema complicated with chronic tuberculous empyema, which was successfully treated by antibacterial agents alone. We wish to highlight the importance of being aware that Salmonella spp. can cause empyema in cases suffering from chronic tuberculous empyema; moreover, despite the successful completion of treatment with antibacterial agents, periodical follow-up is mandatory in such cases.
Keywords: Salmonella, Salmonella houtenae, chronic tuberculous empyema, empyema
Introduction
Salmonella spp. are food-borne pathogens that usually cause gastroenteritis, although bacteremia and subsequent focal metastatic infection may also occasionally occur (1). More than 2,500 Salmonella serotypes have been identified to date, all of which belong to either of 2 main species: Salmonella bongori and Salmonella enterica. The latter serotype comprises 6 subspecies, including S. enterica (I), S. salamae (II), S. arizonae (IIIa), S. diarizonae (IIIb), S. houtenae (IV), and S. indica (VI). Although S. enterica subsp. enterica strains represent the vast majority of Salmonella strains isolated from warm-blooded animals including humans, the other subspecies and S. bongori are typically isolated from the intestinal tracts of cold-blooded animals (2).
S. houtenae (IV) is a rare subspecies, comprising less than 1% of all Salmonella strains (3). Although several reports on empyema due to Salmonella species have been documented (1,4-6), few have so far been reported in association with Salmonella species-induced empyema, especially that of S. houtenae, resulting as a complication of chronic tuberculous empyema. Moreover, no clear treatments for empyema due to Salmonella species have yet been established.
We report the first known case of S. houtenae-induced empyema complicated with chronic tuberculous empyema, which was successfully treated by antibacterial agents alone.
Case Report
A 76-year-old man with a history of left chronic tuberculous empyema was admitted to our hospital with symptoms of bloody sputum and fever. Four days prior to admission, he experienced a productive cough and was treated with oral cefditoren pivoxil, a third-generation cephalosporin, by a general practitioner. Of note, he had been diagnosed with pulmonary tuberculosis 23 years prior to this presentation and suffered from left chronic tuberculous empyema with a reduction of his total lung volume after chemotherapy. His other underlying conditions included chronic heart failure, atrial fibrillation, and mitral valve regurgitation. He was a retired mechanic and did not smoke or consume alcohol. He had no recent history of gastroenteritis or any contact with animals.
On physical examination, the patient was alert, but had a mild fever of 37.9℃. His blood pressure and heart rate were normal, but arrhythmic. His respiratory rate was normal. The respiratory sounds on his left side were diminished. A chest radiograph showed a reduced left lung volume and decreased permeability in the left upper lung field, with a tracheal shift to the left; there was no permeability in the left lower lung field (Fig. 1). Routine laboratory investigations revealed the following: leukocytosis, 10,100 cells/mm3 and C-reactive protein, 20.5 mg/dL. Other laboratory data are shown in Table 1. Chest computed tomography (CT) revealed a volume reduction secondary to fibrous change in the left upper lobe, and encysted empyema in the left lower thoracic cavity. Consolidation in the left lower lobe indicated pneumonia (Fig. 2A and B).
Figure 1.
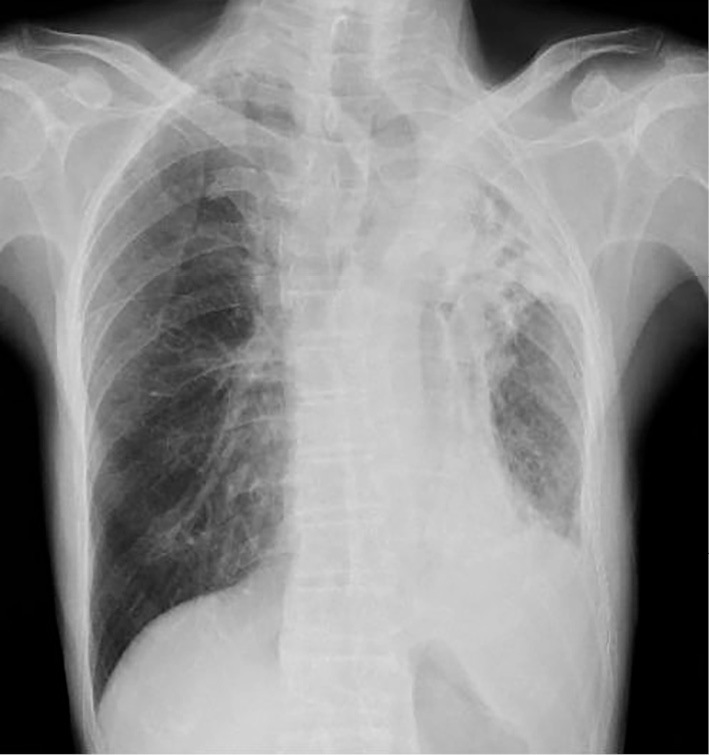
Chest radiograph on day of admission. Chest radiograph showing volume reduction of the left lung and decreased permeability in the left upper lung field with a tracheal shift to the left; there is no permeability in the left lower lung field.
Table 1.
Laboratory Data on Day of Admission.
| TP | 7.4 | g/dL | WBC | 10,100 | /mL |
| Alb | 3.4 | g/dL | Neutrophil | 88 | % |
| T-bil | 1.9 | mg/dL | Lymphocyte | 4 | % |
| AST | 21 | IU/L | Eosinophil | 0 | % |
| ALT | 8 | IU/L | Basophil | 0 | % |
| LDH | 205 | IU/L | Monocyte | 8 | % |
| ALP | 575 | IU/L | RBC | 435 | ×106/mL |
| CK | 95 | IU/L | Hb | 13 | g/dL |
| BUN | 17.8 | mg/dL | Ht | 40 | % |
| Cre | 1.0 | mg/dL | Plt | 14.7 | ×104/mL |
| Na | 129 | mEq/L | |||
| K | 3.5 | mEq/L | NT-proBNP | 3,767 | pg/mL |
| Cl | 94 | mEq/L | |||
| CRP | 20.5 | mg/dL |
Figure 2.
Chest computed tomography (CT) findings. A, B: Day of admission. A CT image showing volume reduction due to fibrous change in the left upper lobe and encysted empyema in the left lower thoracic cavity. Consolidation (arrowhead) in the left lower lobe indicates pneumonia. C: Day 5 of hospitalization. A contrast-enhanced CT image showing encysted empyema penetrating into the parenchyma of the left lower lobe (arrow). D: Four months after discharge. A CT image showing remission of pneumonia and a slight decrease in the size of the empyema.
Based on a diagnosis of left lower lobe bacterial pneumonia, intravenous ampicillin/sulbactam 3.0 g every 12 hours was initiated. After 24 hours of antibiotic treatment, his fever increased to 39.0℃, following which, intravenous ciprofloxacin 400 mg daily was added to the treatment. His fever subsided temporarily, but rose again at night, suggestive of periodic fever. On day 5 of hospitalization, contrast-enhanced CT revealed encysted empyema penetrating into the parenchyma of the left lower lobe, thus indicating a possible bronchopleural fistula (Fig. 2C). All three consecutive sputum cultures obtained on the day of admission grew Salmonella species, which were sensitive to ampicillin and levofloxacin (Table 2). A sensitivity test for cefditoren pivoxil and ciprofloxacin was not available. Acid-fast bacillus was not detected on Ziehl-Neelsen staining and a polymerase chain reaction analysis. Bronchoscopy and thoracocentesis were performed to evaluate the bronchial pathways; in the latter procedure, aspiration of the left pleural fluid was difficult, and only 1 mL of blood clot-like pleural fluid was obtained. Both bronchial lavage fluid (taken from the left lower bronchus) and pleural fluid also grew Salmonella species. Based on the antibacterial sensitivity, the antibacterial regime was changed to intravenous levofloxacin 500 mg daily. A blood culture yielded no growth after 7 days of incubation at 37℃. The organisms cultured from the sputum, bronchial lavage fluid, and pleural fluid specimens were subsequently identified as S. enterica subspecies houtenae (Salmonella IV O40 : z4, z23 : -).
Table 2.
Antibacterial Minimum Inhibitory Concentration Results for S. Houtenae. (Susceptibility Testing and Isolate Identification Performed by Walk Away 40 Plus Automated Microbiology System; Beckman Coulter, Inc, Brea CA, USA).
| Antibacterial agent | Minimum inhibitory concentration (mg/mL) |
|---|---|
| Ampicillin | ≤8 Sensitive |
| Ampicillin/Sulbactam | ≤8/4 Sensitive |
| Cefazolin | ≤4 Sensitive |
| Cefditren pivoxil | NA |
| Cefepime | ≤2 Sensitive |
| Ceftriaxon | ≤1 Sensitive |
| Ciprofloxacin | NA |
| Gentamicin | ≤2 Sensitive |
| Levofloxacin | ≤0.5 Sensitive |
| Meropenem | ≤ 1 Sensitive |
| Piperacillin/Tazobactam | ≤16 Sensitive |
| Tetracycline | ≤2 Sensitive |
| Trimetprim/Sulfamethoxazole | ≤2/38 Sensitive |
NA: Not available
Although surgical treatments including extrapleural pneumonectomy were considered, we gave priority to conservative treatment with antibacterial agents. The estimated risk of perioperative complications was high considering the patient's history of cardiovascular disease. His fever subsided on day 10 of hospitalization, and subsequent sputum culture samples were negative for Salmonella species. Levofloxacin 500 mg was continued orally. He was discharged on day 35 of hospitalization, after completing 6 weeks of levofloxacin therapy. Four months post-discharge, the patient remained afebrile and healthy. Repeat chest CT showed a remission of pneumonia and a slight reduction in the size of the empyema (Fig. 2D).
Discussion
We identified two interesting clinical issues in relation to the present case.
First, S. houtenae, a rare Salmonella subspecies, can cause empyema in a patient with underlying chronic tuberculous empyema. A review of pleuropulmonary complications due to Salmonella species by Crum revealed only 39 such cases reported until 2005, with Salmonella typhimurium as the most common non-typhi species associated with empyema (4). Our search of the pertinent English literature based on relevant keywords through the PubMed database yielded no reports on pleuropulmonary manifestations due to S. houtenae. Moreover, no reports of S. houtenae-induced empyema as a complication of chronic tuberculous empyema were found. Therefore, to the best of our knowledge, this is the first case of its kind.
Second, conservative treatment with antibacterial agents alone was effective in this case. Our patient had a long history of chronic tuberculous empyema, leading to chronic inflammation that had progressed through the phases of exudative effusion, fibrinopurulent empyema thoracis, and organizing fibrothorax. Pleural drainage is ineffective in such cases due to the presence of thick pus and fibrous septa (7). Although extrapleural pneumonectomy may be considered, it is associated with a high incidence of postoperative complications, such as bronchopleural fistula and postoperative empyema (8,9). Extrapleural pneumonectomy is performed only when absolutely necessary, and in carefully-selected patients. Moreover, due to underlying cardiovascular disorders, there was an increased risk for complications in this case if conservative treatment proved to be ineffective.
The optimal treatment of empyema due to Salmonella species has not yet been fully established. In Cohen et al.'s review of 36 cases of Salmonella empyema, which included 5 patients with prior structural abnormalities of the lungs or pleura, over 85% of the patients who were treated with chest tube or open surgical drainage survived; in contrast, only 60% (3 out of 5 patients) who received antibacterial agents alone survived (1). Regarding empirical treatment, third-generation cephalosporins or fluoroquinolones are initially recommended before antibiotic susceptibility testing is finalized (10). Although resistance towards antibacterial agents is increasing these days (11), the isolate in this case was sensitive to most of the antibiotics tested, including levofloxacin, which has the lowest minimum inhibitory concentration and wide bioavailability. The patient experienced no relapse following the completion of treatment. Previous case reports of S. houtenae infections involving other sites have also shown a good response to antibacterial agents (12-15). We thus believe that conservative treatment with antibacterial agents alone is sufficient for the effective treatment of S. houtenae infections.
The route of transmission in the present case was considered to be hematogenous dissemination via the intestinal mucosa and settlement in chronic tuberculosis empyema. In addition, S. houtenae was also identified in sputum, bronchial lavage, and pleural fluid specimens. Pleural penetration may lead to pneumonia-like symptoms, while local structural abnormalities may cause turbulence of the blood flow, obstruction, and flow stasis, favoring bacterial localization and an impairment of host defense mechanisms (1,16). With regard to the sterile blood culture, we believe that the administration of oral cefditoren pivoxil prior to admission may have suppressed bacteremia.
In conclusion, S. houtenae-induced empyema may occur in patients with underlying chronic tuberculous empyema. In this case, conservative treatment with antibacterial agents alone proved to be effective. Moreover, despite the successful completion of treatment, periodical multidisciplinary follow-up is mandatory in such cases.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank the Infectious Disease Surveillance Center in the National Institute of Infectious Diseases, Japan for identifying our specimens. We are also grateful to Yano Masatoshi's helpful contributions regarding bacterial knowledge.
References
- 1. Cohen JI, Bartlett JA, Corey GR. Extra-intestinal manifestations of Salmonella infections. Medicine (Baltimore) 66: 349-388, 1987. [DOI] [PubMed] [Google Scholar]
- 2. Desai PT, Porwollik S, Long F, et al. Evolutionary genomics of Salmonella enterica subspecies. MBio 4: e00579-e12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tavechio AT, Ghilardi AC, Peresi JT, et al. Salmonella serotypes isolated from nonhuman sources in São Paulo, Brazil, from 1996 through 2000. J Food Prot 65: 1041-1044, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Crum NF. Non-typhi Salmonella empyema: case report and review of the literature. Scand J Infect Dis 37: 852-857, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Afridi FI, Farooqi BJ, Hussain A. Pleural empyema due to Salmonella typhi. J Coll Physicians Surg Pak 22: 803-805, 2012. [PubMed] [Google Scholar]
- 6. Saeed NK. Salmonella pneumonia complicated with encysted empyema in an immunocompromised youth: case report and literature review. J Infect Dev Ctries 10: 437-444, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Sihoe AD, Shiraishi Y, Yew WW. The current role of thoracic surgery in tuberculosis management. Respirology 14: 954-968, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Shiraishi Y, Nakajima Y, Koyama A, et al. Morbidity and mortality after 94 extra pleural pneumonectomies for empyema. Ann Thorac Surg 70: 1202-1207, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Zellos L, Jaklitsch MT, Al-Mourgi MA, et al. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg 19: 355-359, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis 32: 263-269, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Medalla F, Hoekstra RM, Whichard JM, et al. Increase in resistance to ceftriaxone and nonsusceptibility to ciprofloxacin and decrease in multidrug resistance among Salmonella strains, United States, 1996-2009. Foodborne Pathog Dis 10: 302-309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wybo I, Potters D, Plaskie K, et al. Salmonella enterica subspecies houtenae serotype 44:z4, z23:- as a rare cause of meningitis. Acta Clin Belg 59: 232-234, 2004. [PubMed] [Google Scholar]
- 13. Ma JS, Chen PY, Lau YJ, et al. Brain abscess caused by Salmonella enterica subspecies houtenae in a patient with chronic granulomatous disease. J Microbiol Immunol Infect 36: 282-284, 2003. [PubMed] [Google Scholar]
- 14. Nimir AR, Ibrahim R, Ibrahim IA. Salmonella meningitis in a pediatric patient caused by Salmonella enterica serotype houtenae. BMJ Case Rep 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lourenço MC, dos Reis EF, Valls R, et al. Salmonella enterica subsp houtenae serogroup O:16 in a HIV positive patient: case report. Rev Inst Med Trop Sao Paulo 46: 169-170, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Bobra ST. Chronic empyema due to Salmonella oranienburg; complication of an old chest wound. Can Med Assoc J 78: 599-602, 1958. [PMC free article] [PubMed] [Google Scholar]



