Abstract
Rituximab is a highly effective agent that is used in the treatment of B-cell lymphoma. Rituximab-induced acute thrombocytopenia is a rare side effect that has previously been reported in a small number of patients with malignant lymphoma; its mechanism is still unknown. We herein report the case of a 74-year old man who was diagnosed with follicular lymphoma and who developed severe acute thrombocytopenia the day after the administration of rituximab. Coagulation abnormality, which mimicked disseminated intravascular coagulation, also appeared. When physicians use rituximab to treat high-risk patients, the platelet count should be closely monitored to avoid possible adverse events.
Keywords: rituximab, acute thrombocytopenia, follicular lymphoma
Introduction
Rituximab, a chimeric monoclonal antibody directed against the CD20 antigen, is commonly used to treat B-cell lymphoma, chronic lymphocytic leukemia and some autoimmune disorders (1). Infusion reactions, which manifest with symptoms such as flushing, itching, dyspnea, heartache, fever, and nausea, are the most common adverse effect of rituximab (2). Late-onset pancytopenia, which usually occurs several weeks to months after the administration of rituximab, is also common, but usually self-limited. On the other hand, rituximab-induced acute thrombocytopenia (RIAT), which usually occurs within a few days after the administration of rituximab, is very unusual and the pathogenesis remains unclear (3-18). RIAT has been almost exclusively reported in patients with mantle cell lymphoma, which is a minor sub-group of B-cell lymphoma (4,6,8-12,14,15). Recently, Ureshino et al. reported the first case of RIAT in a patient with follicular lymphoma (16). We herein report the 2nd case of RIAT in a patient with follicular lymphoma; in the present case, the condition developed on the 3rd and 4th cycles of rituximab therapy.
Case Report
A 74-year-old man with inguinal lymphadenopathy was referred to our hospital. His medical history revealed chronic atrial fibrillation, which was treated with warfarin. A physiological examination showed bilateral cervical, subclavian, axillary and left inguinal lymphadenopathy. Laboratory tests revealed pancytopenia: white blood cell (WBC) count, 2,900 /μL, with 2% atypical lymphocytes; hemoglobin (Hb), 12.0 g/dL; and platelet (PLT) count, 61,000 /μL. Computed tomography revealed systemic lymphadenopathy and massive splenomegaly. The histological examination of a left axillary lymph node biopsy specimen revealed grade 2 follicular lymphoma. Immunohistochemistry revealed that the lymphoma cells were positive for CD20, CD10, Bcl-2, and negative for CD3 and CD5. A chromosome analysis revealed 46, XY, add(1)(p36,3), t(14;18)(q32;q21. 3) in the tumorous lymph nodes. A polymerase chain reaction detected immunoglobulin heavy chain gene rearrangement. Bone marrow biopsy revealed the infiltration of the bone marrow by lymphoma cells (abnormal lymphoid cells that were positive for CD20 and Bcl-2).
The patient was scheduled to receive rituximab, cyclophosphamide, vincristine, predonisolone (R-CVP) chemotherapy. Doxorubicin was eliminated due to the patient's low cardiac function. The patient was pretreated with prednisolone to avoid the risk of tumor lysis syndrome; cyclophosphamide and vincristine were then administered. On day 14 of CVP therapy, he underwent rituximab infusion. He developed fever and shivering during the infusion. The infusion was stopped for a few hours and then restarted at a slow rate without any complications. On day 2 of the 2nd cycle of R-CVP, he underwent a 3-hour infusion of rituximab without any reactions to the infusion. The next day, a blood test showed a drop in the PLT count from 75,000 /μL to 36,000 /μL with a slight elevation in the prothrombin time-international normalized ratio (PT-INR) from 2.31 to 2.62. He did not experience any major adverse events until the end of the 2nd cycle of R-CVP therapy.
On day 2 of the 3rd cycle of the R-CVP therapy, he received a 3-hour rituximab infusion. A blood test before the administration of rituximab revealed the following findings: WBC count, 3,400 /μL; Hb, 10.5 g/dL; PLT count, 63,000 /μL; D-dimer, 6.3 μg/mL; and PT-INR, 1.90. The next day, a blood test showed a drastic drop in the PLT count to 14,000 /μL, the elevation of the D-dimer level to 33.3 μg/mL and the elevation of the PT-INR to 2.97. Transfusions were performed two days in a row with 20 units of platelets, 8 units of fresh frozen plasma, and 19,200 units of recombinant thrombomodulin alpha. The post-transfusion PLT count was 63,000 /μL. The serum levels of creatinine, uric acid, lactate dehydrogenase and electrolytes were not affected by the administration of rituximab, which ruled out an association with tumor lysis syndrome. Contrast-enhanced computed tomography detected no intravenous thrombosis and the patient's enlarged spleen was found to have shrunk from the 1st cycle of R-CVP therapy. His platelet count spontaneously recovered to 110,000 /μL within one week. The fourth administration of rituximab also caused acute thrombocytopenia. The following day, the PLT count decreased from 61,000 /μL to 9,000 /μL, the D-dimer level increased from 4.6 μg/mL to 64.2 μg/mL, and the PT-INR increased from 2.56 to 3.10 without a decrease in either the WBC count or the Hb level. The thrombin-antithrombin complex (TAT) level was 4.9 ng/mL and the plasmin-α2 plasmin inhibitor complex (PIC) level was 6.1 μg/mL, which indicated coagulopathy. The extent of thrombocytopenia in the 4th cycle of rituximab therapy was much greater than that observed during the 3rd cycle. The serum biochemistry and electrolytes values showed almost no change after the administration of rituximab. His platelet counts spontaneously recovered again within one week. Figure shows the platelet count in the 4th course of rituximab infusion. Platelet associated immunoglobulin G (PA-IgG) was not detected by an enzyme-linked immunosorbent assay (ELISA) of blood samples obtained on day 1 after the administration of rituximab. Furthermore, human anti-chimeric antibodies (HACA) to the murine fragment of rituximab were not detected by an ELISA (HACA to rituximab was measured by Falco Biosystems, Kyoto, Japan). Rituximab was omitted from the subsequent cycles of treatment to avoid the risk of life-threatening thrombocytopenia.
Figure.
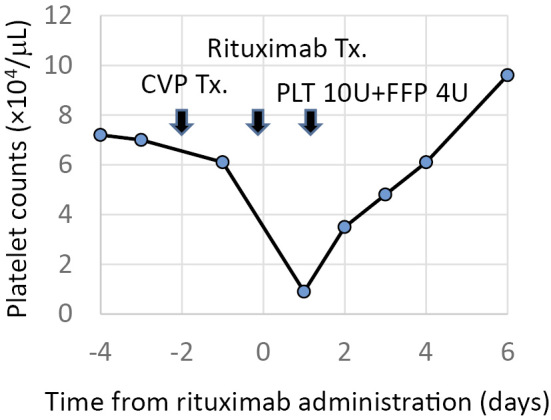
The time course of treatment and the platelet counts in the 4th course of rituximab infusion.
Discussion
Rituximab is commonly used in the treatment of B-cell lymphoma and its use is generally well-tolerated. RIAT has been reported as a rare side effect and its mechanism remains unclear. We searched the PubMed database using the terms “rituximab” and “acute thrombocytopenia”. The search yielded a total of 21 cases, the diagnoses of the cases included mantle cell lymphoma (n=13) (4,6,8-12,14,15), hairy cell leukemia (n=3) (6,7,13), prolymphocytic leukemia (n=1) (5), lymphoplasmacytic lymphoma (n=1) (3), follicular lymphoma (n=1) (16), pre-B acute lymphoblastic leukemia (n=1) (17), and autoimmune hemolytic anemia (n=1) (18). Table shows the reports on RIAT. As described above, in most cases, RIAT occurred in patients with mantle cell lymphoma. The incidence of RIAT in this lymphoma subtype differs from the incidence of the histological lymphoma subtype (the frequency of mantle cell lymphoma is low in Japan and throughout the rest of the world). This means that development of RIAT is strongly associated with the lymphoma subtype. We herein report the 2nd case of a patient with follicular lymphoma who developed severe acute thrombocytopenia as a result of the administration of rituximab. It is important that RIAT also occurs in follicular lymphoma, which is a more frequent subtype of lymphoma.
Table.
Patients Characteristic and Clinical Status of RIAT Cases.
| Age, Sex | Disease | BM invasion | Splenomegaly | Coagulopathy | Previous R infusion without RIAT |
Re-infusion/recurrence | Reference | |
|---|---|---|---|---|---|---|---|---|
| 60 | F | LPL | + | + | - | - | +/- | [3] |
| 57 | M | MCL | + | + | unknown | - | +/+ | [4] |
| 75 | M | PLL | + | + | unknown | - | - | [5] |
| 41 | M | HCL | + | + | unknown | - | - | [6] |
| 64 | M | MCL | + | unknown | unknown | - | unknown | [6] |
| 44 | M | HCL | + | unknown | + | - | unknown | [7] |
| 63 | M | MCL | unknown | unknown | - | + | +/+ | [8] |
| 84 | M | MCL | unknown | unknown | unknown | unknown | unknown | [9] |
| 71 | F | MCL | unknown | unknown | - | + | unknown | [10] |
| 58 | M | MCL | + | + | - | - | +/+ | [11] |
| 73 | F | MCL | + | + | unknown | - | +/+ | [12] |
| 74 | M | HCL | + | + | + | - | unknown | [13] |
| 63 | F | MCL | + | + | unknown | - | +/- | [14] |
| 72 | M | MCL | + | + | unknown | - | +/- | [14] |
| 60 | M | MCL | + | + | unknown | + | +/- | [14] |
| 64 | F | MCL | + | + | unknown | - | +/- | [14] |
| 76 | M | MCL | + | + | C | unknown | unknown | [14] |
| 66 | F | MCL | + | + | unknown | - | +/- | [15] |
| 65 | M | FL | unknown | + | + | - | - | [16] |
| 59 | M | ALL | + | unknown | + | - | +/+ | [17] |
| 3 | M | AIHA | - | + | unknown | + | unknown | [18] |
| 74 | M | FL | + | + | + | + | +/+ | our case |
LPL: lymphoplasmacytic lymphoma, MCL: mantle cell lymphoma, PLL: prolymphocytic leukemia, HCL: hairy cell leukemia, FL: follicular lymphoma, ALL: acute lymphoblastic leukemia, AIHA: autoimmune hemolytic anemia
Giezen et al. reported on the incidence of rituximab-induced thrombocytopenia (platelet count, <100,000 /μL within 30 days after the administration of rituximab) in a cohort study (19). Among 90 rituximab-treated patients, 27 patients developed thrombocytopenia (30%); the diagnoses of these patients included Burkitt lymphoma (n=3), diffuse large B-cell lymphoma (n=6), NHL (n=11), CLL (n=2) and other (n=5).
Our case shared some common clinical features with the cases of previous reports, specifically, massive splenomegaly, and bone marrow infiltration (Table). On the other hand, our case was distinguished from previous cases in that the patient developed RIAT from the 3rd cycle of rituximab onwards and the lymphoma subtype was follicular lymphoma. Although the tumor burden and the degree of splenomegaly were still severe when RIAT first developed, the tumor volume was lower and the size of the spleen was smaller in comparison to the 1st chemotherapy cycle. This means that the tumor burden itself may not independently explain the pathogenesis of RIAT. Furthermore, our case had coagulopathy. Some of the other RIAT cases showed coagulopathy while others did not (Table). Although our case developed an infusion reaction on the first administration of rituximab, he did not develop an infusion reaction during the subsequent courses of rituximab therapy. However, in many of the RIAT cases, the infusion reaction occurred simultaneously; thus, this may be a risk factor.
Our hypothesized mechanisms of RIAT are as follows. First, in some RIAT cases, including ours, the patient simultaneously developed coagulopathy (7,13,16,17). Thus, infused rituximab interacts with lymphoma cells, which might activate immune components and cause consumption coagulopathy, which expends platelets (17). Second, platelets have an Fc receptor (20); thus, rituximab antibodies, which bind to lymphoma cells, also bind the platelets through their Fc receptors, leading to platelet degradation. Previous reports have suggested that the presence of soluble CD20 antigen in the circulation may cause an antigen-antibody reaction and immune-mediated cell lysis (21).
There have been several reports on the re-infusion of rituximab in patients with RIAT (Table). In some cases (including our own), RIAT recurred (4,8,11,12,17). In other cases, there was no recurrence of RIAT after rituximab re-infusion (3,14,15). Furthermore, in several cases (including our own), RIAT occurred first after several rituximab infusions (8,10,14). At present, the reason for these differences is unknown.
The incidence of RIAT may be underestimated because platelet counts are not always monitored in the first days after rituximab therapy, especially when patients are treated in the outpatient setting. Moreover, it is possible that a patient's thrombocytopenia could be so mild that RIAT goes unnoticed. In our case, mild thrombocytopenia occurred after the second infusion of rituximab and might have also been RIAT.
We herein reported the case of a patient with follicular lymphoma who developed RIAT in the 3rd and 4th cycles of rituximab chemotherapy. Routine blood count monitoring should be considered for a few days after the administration of rituximab, especially for high-risk lymphoma patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Engelhard M. Anti-CD20 antibody treatment of non-Hodgkin lymphomas. Clin Immunol 172: 101-104, 2016. [DOI] [PubMed] [Google Scholar]
- 2. McLaughlin P, Grillo-López AJ, Link BK, et al. . Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16: 2825-2833, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Rigamonti C, Volta C, Colombi S, et al. . Severe thrombocytopenia and clinical bleeding associated with rituximab infusion in a lymphoma patient with massive splenomegaly without leukemic invasion. Leukemia 15: 186-187, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Shah C, Grethlein SJ. Case report of rituximab-induced thrombocytopenia. Am J Hematol. 75: 263, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Pamuk GE, Donmez S, Turgut B, Demir M, Vural O. Rituximab-induced acute thrombocytopenia in a patient with prolymphocytic leukemia. Am J Hematol 78: 81, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Otrock ZK, Mahfouz RA, Oghlakian GO, Salem ZM, Bazarbachi A. Rituximab-induced acute thrombocytopenia: a report of two cases. Haematologica 90 (Suppl): ECR23, 2005. [PubMed] [Google Scholar]
- 7. Thachil J, Mukherje K, Woodcock B. Rituximab-induced haemorrhagic thrombocytopenia in a patient with hairy cell leukaemia. Br J Haematol 135: 273-274, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Rosado M, Chao H, Rose M. Severe acute thrombocytopenia following rituximab therapy. Leuk Lymphoma 48: 2239-2240, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Dhand S, Bahrain H. Rituximab-induced severe acute thrombocytopenia: a case report and review of literature. Cancer Invest 26: 913-915, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Ram R, Bonstein L, Gafter-Gvili A, Ben-Bassat I, Shpilberg O, Raanani P. Rituximab-associated acute thrombocytopenia: an under-diagnosed phenomenon. Am J Hematol 84: 247-250, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Yi JH, Kim SJ, Ahn HK, Lee SJ, Chang MH, Kim WS. Rituximab-induced acute thrombocytopenia: a case report and review of the literature. Med Oncol 26: 45-48, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Parajuli R, Hire E, Shah BK. Rituximab-induced acute severe thrombocytopenia. Br J Haematol 149: 804, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Kotsianidis I, Goutzouvelidis A, Anastasiades A, et al. . Severe thrombocytopenia and fibrinolysis mimicking disseminated intravascular coagulation after rituximab infusion. Am J Hematol 85: 146, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Sadashiv SK, Rao R, Fazal S, Lister J. Rituximab-induced acute severe thrombocytopenia: a case series in patients with mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 13: 602-605, 2013. [DOI] [PubMed] [Google Scholar]
- 15. El-Osta H, Nair B. Rituximab-induced acute thrombocytopenia: an underappreciated entity. Leuk Lymphoma 54: 2736-2737, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Ureshino H, Nishioka A, Kojima K, et al. . Rituximab-induced acute thrombocytopenia in high tumor burden follicular lymphoma. Intern Med 55: 2061-2064, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Rafei H, Nassereddine S, Garcia IF. Disseminated intravascular coagulation-like reaction following rituximab infusion. BMJ Case Rep 8: 1-6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larrar S, Guitton C, Willems M, Bader-Meunier B. Severe hematological side effects following Rituximab therapy in children. Haematologica 91 (8 Suppl): ECR36, 2006. [PubMed] [Google Scholar]
- 19. Giezen TJ, Mantel-Teeuwisse AK, ten Berg MJ, et al. . Rituximab-induced thrombocytopenia: a cohort study. Eur J Haematol 89: 256-266, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Qiao J, Al-Tamimi M, Baker RI, Andrews RK, Gardiner EE. The platelet Fc receptor, FcγRIIa. Immunol Rev 268: 241-252, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Manshouri T, Do KA, Wang X, et al. . Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood 101: 2507-2513, 2003. [DOI] [PubMed] [Google Scholar]


