Abstract
We herein report a case of dural arteriovenous fistula (DAVF) at the cavernous sinus that was diagnosed by arterial spin-labeled imaging (ASL). A 67-year-old woman was referred to our hospital due to double vision and bilateral conjunctival injection. Conventional magnetic resonance imaging findings were normal. However, abnormal hyperintense signals on ASL were detected. Furthermore, the abnormality disappeared after successful endovascular embolization. Although conventional digital subtraction angiography is the standard tool for diagnosing DAVF, we speculated that ASL might be useful to this end as well.
Keywords: MRI, dural arteriovenous fistula, arterial spin-labeling, cavernous sinus
Introduction
Since patients with dural arteriovenous fistula (DAVF) present with a wide range of symptoms, such as tinnitus, ophthalmoplegia, convulsion, consciousness disturbance, and stroke-like symptoms, DAVF tends to be misdiagnosed. Conventional digital subtraction angiography (DSA) has been a useful tool for the diagnosis of DAVF. However, DSA is invasive and carries a number of risks. Recently, magnetic resonance imaging (MRI), especially susceptibility-weighted imaging (1,2), has been reported to be useful for diagnosing DAVF with cortical venous reflux. However, in most patients with DAVF, the blood flow through the arteriovenous shunt is pooled in diseased veins or sinuses. Stagnant flow may be detected as hyperintense signals on arterial spin-labeled imaging (ASL) in patients with large vessel occlusion (3). We herein report a patient who presented with ophthalmoplegia and conjunctival injection due to DAVF at the cavernous sinus and was able to be diagnosed using ASL.
Case Report
A 67-year-old woman with ophthalmoplegia and conjunctival injection was admitted to our hospital. Her medical history included type 2 diabetes mellitus (5 years) and hypertension (10 years). She did not have a history of drinking alcohol or smoking. Her family history was unremarkable. Furthermore, she did not have a history of headache or head trauma that might have caused carotid cavernous fistula. She had only ophthalmoplegia one month before admission. She had been suspected of having Tolosa-Hunt syndrome and was treated with corticosteroids (0.5 mg/kg) at her previous hospital. However, her symptoms did not improve. Furthermore, conjunctival injection appeared additionally. Her primary care physician at her previous hospital consulted our department, and she was admitted to our hospital.
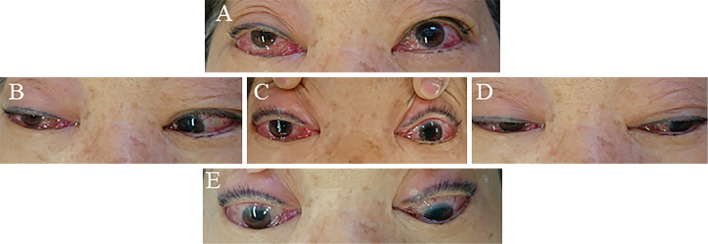
On admission, the patient presented with bilateral conjunctival injection (Fig. 1). A physical examination showed no remarkable findings except for a neurological deficit. A neurological examination revealed no muscle weakness or any sensory disturbance but did show hyporeflexia due to diabetes mellitus. She also presented with incomplete right oculomotor and abducens nerve palsy, indicating incomplete ophthalmoplegia, right ptosis, and no abnormality of pupils. She did not present with trochlear nerve palsy (Fig. 1). Chest radiography and 12 channel electrocardiography showed no remarkable abnormalities. There were no remarkable abnormalities on laboratory findings except for elevated hemoglobin A1c based on National Glycohemoglobin Standardization Program (NGSP HbA1c). There was clinical suspicion of DAVF at the cavernous sinus indicating indirect arteriovenous shunt through meningeal branches to the cavernous sinus and direct carotid cavernous fistula (CCF) indicating direct arteriovenous shunt from the internal carotid artery to the cavernous sinus due to head injury or rupture of aneurysm at the ICA. However, it was difficult to distinguish between DAVF and CCF, clinically. Therefore, MRI was performed, including ASL. Conventional MRI revealed no abnormality. However, we did detect an abnormal hyperintense signal on ASL.
Figure 1.
Manifestation of the eyes at the time of admission. A: Upward gaze, B: Rightward gaze, C: Front vision, D: Leftward gaze, E: Downward gaze. We noted limitation in the gazes in all directions in the right eye along with bilateral conjunctival injection. Furthermore, right ptosis can be seen (permission to use these pictures obtained from the patient).
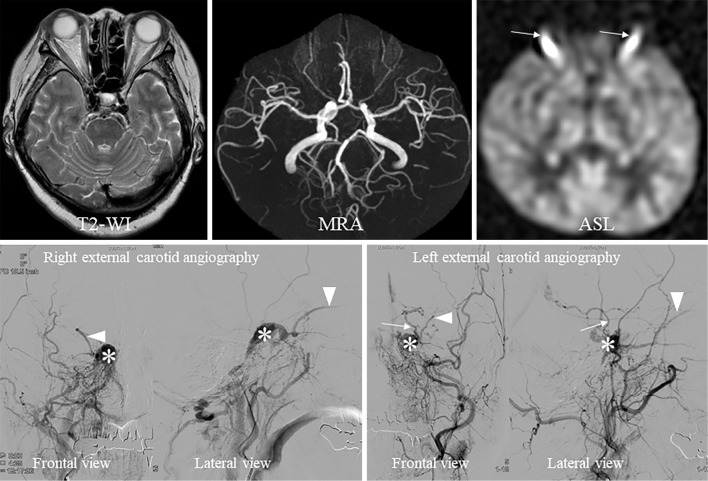
We also performed conventional DSA to confirm whether or not the patient had arteriovenous shunt, such as DAVF and CCF. We detected many meningeal arteries that were concentrated at the cavernous sinus (right ascending pharyngeal artery, artery of foramen rotundum, middle meningeal artery), thus indicating indirect arteriovenous shunt. Blood was regurgitated to the bilateral superior ophthalmic veins (SOVs) and left uncal vein. The bilateral inferior petrosal sinuses became constricted, which may have caused the high blood pressure observed at the cavernous sinus and reflux of blood into the bilateral SOVs (Fig. 2).
Figure 2.
Radiological findings. The upper row is magnetic resonance imaging, and the lower row is digital subtraction angiography (DSA). T2 and MRA show no abnormalities. However, we detected abnormal hyperintense signals (arrows) at the bilateral orbital cavities on ASL. DSA shows arteriovenous shunt at the cavernous sinus. The feeding arteries from the right external carotid artery concentrate in the cavernous sinus (*) and bilateral superior ophthalmic veins (arrow heads) and left uncal vein (white arrow) at the arterial phase on DSA. T2-WI: T2-weighted imaging, MRA: magnetic resonance angiography, ASL: arterial spin-labeling imaging
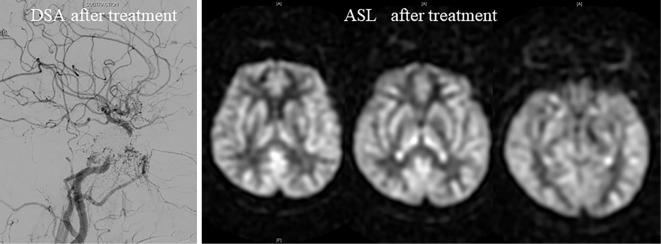
We performed transvenous embolization to obliterate arteriovenous shunt. Under general anesthesia, we placed 6-Fr sheaths into the right femoral vein and artery and advanced a 5-Fr guiding catheter to the right external carotid artery and a 6-Fr guiding catheter to the right internal juglar vein. We also advanced a microcatheter using a microguide wire to the cavernous sinus. First, we performed coil embolization via the SOVs; we then performed sinus packing at the cavernous sinus using platinum coils to treat the cerebral aneurysm. We were able to obliterate the aneurysm completely in this manner. We also performed MRI five days after treatment and confirmed the disappearance of the abnormal hyperintense signals on ASL (Fig. 3).
Figure 3.
Digital subtraction angiography and arterial spin-labeling imaging after treatment. We confirmed the disappearance of arteriovenous shunt on DSA after treatment. Abnormal signals detected on ASL disappeared after embolization. DSA: digital subtraction angiography, ASL: arterial spin-labeling imaging
Discussion
ASL might be useful for evaluating DAVF, especially in patients with negative findings on conventional MRI. In patients with DAVF, meningeal arterial blood magnetically labeled on ASL might not distribute properly, instead being concentrated in the diseased cavernous sinus. This concentrated blood might be detected as emphasized ASL signals. Furthermore, we were able to confirm the disappearance of hyperintense signals on ASL after successful endovascular embolization in our case. This suggests that the hyperintense signals appeared due to arteriovenous shunt. We speculate that identifying abnormal hyperintense signals on ASL might improve the detection of DAVF. The ASL findings, including our results, may improve the rate of misdiagnosis in patients with suspected DAVF.
ASL is a useful tool with MRI that enables the measurement of tissue perfusion without the use of exogenous contrast agents by magnetically tagging the water in inflowing blood (4). ASL has mainly been used to detect hypoperfusion territories in patients with acute ischemic stroke. However, we use ASL to detect occluded sites of cerebral major arteries (3) and to exclude stroke mimics (5). Furthermore, our present findings suggested that ASL might be useful for detecting DAVF.
However, although the patient had venous drainage to the uncal vein from the cavernous sinus, we failed to detect abnormal hyperintense signals at the sylvian fissure. We speculated that these false negative findings, indicating negative findings on ASL despite regurgitation to the uncal vein, might have been caused by the limited spatial resolution and the determined post-labeling delay time. Because ASL imaging was determined by the post-labeled delay from labeling at the proximal potion of carotid artery, the presentation of abnormal hyperintense signals on ASL might depend on how long it takes for blood to pass through the fistula's point. In the present case, blood regurgitated to the SOVs was stagnant due to stenosis of the facial veins. However, blood regurgitated to the uncal vein was not stagnant because the blood was able to pass freely. This may have been why we detected no abnormal hyperintense signals at the sylvian fissure.
When evaluating DAVF using ASL, a multi-delay sequence may need to be considered in order to improve the detection rate of abnormal hyperintense signals on ASL due to venous reflux and/or stagnant flow in patients with DAVF and different hemodynamic statuses (6).
The authors state that they have no Conflict of Interest (COI).
References
- 1. Gasparetto EL, Pires CE, Domingues RC. Susceptibility-weighted MR phase imaging can demonstrate retrograde leptomeningeal venous drainage in patients with dural arteriovenous fistula. Am J Neuroradiol 32: E54, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saini J, Thomas B, Bodhey NK, Periakaruppan A, Babulal JM. Susceptibility-weighted imaging in cranial dural arteriovenous fistulas. Am J Neuroradiol 30: E6, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tada Y, Satomi J, Abe T, Kuwayama K, Sogabe S, Fujita K, Yamamoto N, Kaji R, Harada M, Nagahiro S. Intra-arterial signal on arterial spin labeling perfusion MRI to identify the presence of acute middle cerebral artery occlusion. Cerebrovasc Dis 38: 191-196, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 89: 212-216, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo X, Xu S, Wang G, Zhang Y, Guo L, Zhao B. Asymmetry of cerebral blood flow measured with three-dimensional pseudocontinuous arterial spin-labeling mr imaging in temporal lobe epilepsy with and without mesial temporal sclerosis. J Magn Reson Imaging 42: 1386-1397, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Jung Y, Wong EC, Liu TT. Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magn Reson Med 64: 799-810, 2010. [DOI] [PubMed] [Google Scholar]