Dear Editor,
Indole-3-carbinol (I3C; C9H9NO) is a phytochemical that is derived from the breakdown of the glucosinolate, glucobrassicin. I3C is present at relatively high levels in most cruciferous vegetables such as broccoli, cabbage, cauliflower, brussels sprouts, collard greens, and kale (Fujioka et al., 2016[14]; Licznerska and Baer-Dubowska, 2016[22]). The enzyme, myrosinase (β-thioglucosidase), catalyzes the hydrolysis of glucosinolates in intact plant cells (Zhao et al., 2015[37]). After chopping or chewing of raw cruciferous vegetables, the plant cells are damaged and glucobrassicin is exposed to myrosinase. This catalyzes the conversion of glucobrassicin to a glucose molecule and an unstable aglycone, which is hydrolyzed to thiohydroximate-O-sulfonate (de Vos et al., 2008[9]). If the sulfate ion is released spontaneously, this may form another unstable intermediate, 3-indolylmethylisothiocyanate. This released compound readily converts to a thiocyanate ion and I3C (Kim et al., 2008[20]).
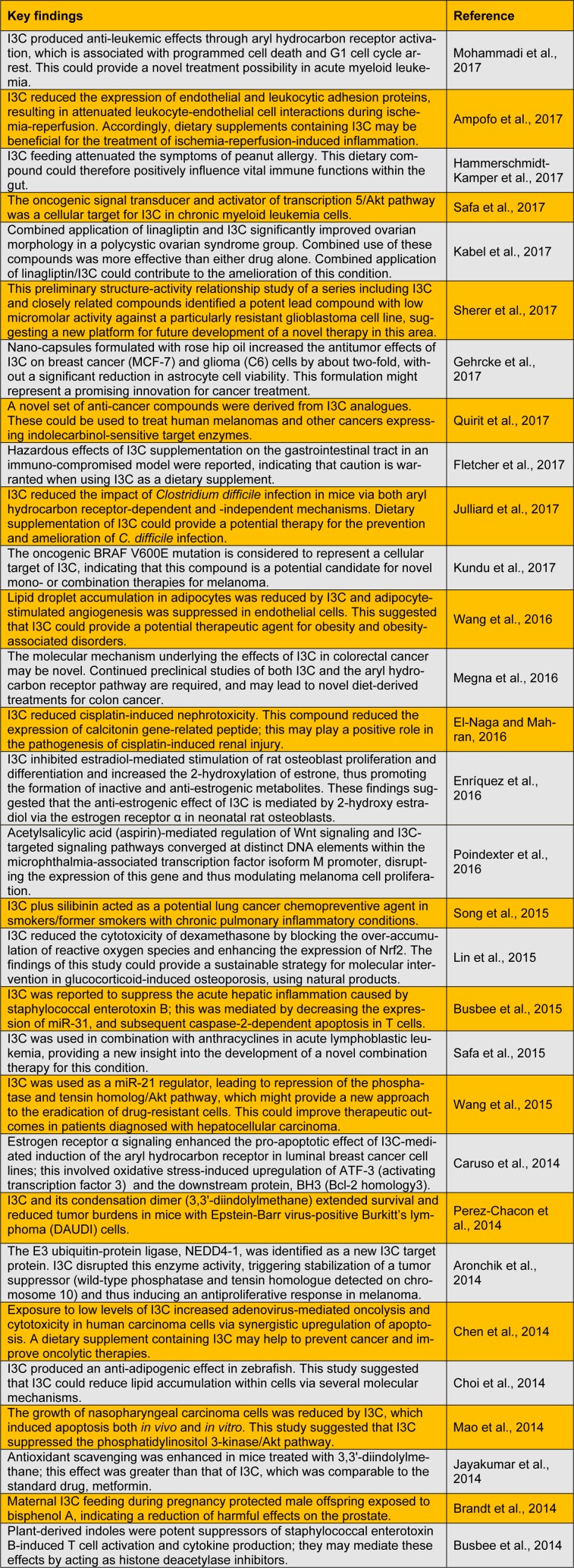
I3C has recently become available as a nutritional supplement and it provides an attractive natural product for drug development in the pharmaceutical industry. It has been reported to show diverse promising biological properties, with anti-atherogenic, antioxidant, anti-carcinogenic, and anti-inflammatory activities (Fuentes et al., 2015[13]; Maruthanila et al., 2014[25]). I3C has attracted considerable attention in recent years within the pharmaceutical and functional food industries. Here, we summarize recent studies performed to evaluate the biological and pharmacological activities of I3C (Table 1(Tab. 1); References in Table 1: Mohammadi et al., 2017[27]; Ampofo et al., 2017[1]; Hammerschmidt-Kamper et al., 2017[16]; Safa et al., 2017[31]; Kabel et al., 2017[19]; Sherer et al., 2017[33]; Gehrcke et al., 2017[15]; Quirit et al., 2017[30]; Fletcher et al., 2017[12]; Julliard et al., 2017[18]; Kundu et al., 2017[21]; Wang et al., 2016[35]; Megna et al., 2016[26]; El-Naga and Mahran, 2016[10]; Enríquez et al., 2016[11]; Poindexter et al., 2016[29]; Song et al., 2015[34]; Lin et al., 2015[23]; Busbee et al., 2015[4]; Safa et al., 2015[32]; Wang et al., 2015[36]; Caruso et al., 2014[6]; Perez-Chacon et al., 2014[28]; Aronchik et al., 2014[2]; Chen et al., 2014[7]; Choi et al., 2014[8]; Mao et al., 2014[24]; Jayakumar et al., 2014[17]; Brandt et al., 2014[3]; Busbee et al., 2014[5]).
Table 1. Recent studies on the biological and pharmacological activities of Indole-3-carbinol.
Acknowledgements
This research was supported by Golden Seed Project (213006051WTE11) funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS), Republic of Korea.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ampofo E, Lachnitt N, Rudzitis-Auth J, Schmitt BM, Menger MD, Laschke MW. Indole-3-carbinol is a potent inhibitor of ischemia-reperfusion-induced inflammation. J Surg Res. 2017;215:34–46. doi: 10.1016/j.jss.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Aronchik I, Kundu A, Quirit JG, Firestone GL. The antiproliferative response of indole-3-carbinol in human melanoma cells is triggered by an interaction with NEDD4-1 and disruption of wild-type PTEN degradation. Mol Cancer Res. 2014;12:1621–1634. doi: 10.1158/1541-7786.MCR-14-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt JZ, Silveira LT, Grassi TF, Anselmo-Franci JA, Fávaro WJ, Felisbino SL, et al. Indole-3-carbinol attenuates the deleterious gestational effects of bisphenol A exposure on the prostate gland of male F1 rats. Reprod Toxicol. 2014;43:56–66. doi: 10.1016/j.reprotox.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Busbee PB, Nagarkatti M, Nagarkatti PS. Natural indoles, indole-3-carbinol (I3C) and 3,3'-diindolylmethane (DIM), attenuate staphylococcal enterotoxin B-mediated liver injury by downregulating miR-31 expression and promoting caspase-2-mediated apoptosis. PLoS One. 2015;10(2):e0118506. doi: 10.1371/journal.pone.0118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busbee PB, Nagarkatti M, Nagarkatti PS. Natural indoles, indole-3-carbinol and 3,3'-diindolymethane, inhibit T cell activation by staphylococcal enterotoxin B through epigenetic regulation involving HDAC expression. Toxicol Appl Pharmacol. 2014;274:7–16. doi: 10.1016/j.taap.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso JA, Campana R, Wei C, Su CH, Hanks AM, Bornmann WG, et al. Indole-3-carbinol and its N-alkoxy derivatives preferentially target ERα-positive breast cancer cells. Cell Cycle. 2014;13:2587–2599. doi: 10.4161/15384101.2015.942210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Cheng PH, Rao XM, McMasters KM, Zhou HS. Indole-3-carbinol (I3C) increases apoptosis, represses growth of cancer cells, and enhances adenovirus-mediated oncolysis. Cancer Biol Ther. 2014;15:1256–1267. doi: 10.4161/cbt.29690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HS, Jeon HJ, Lee OH, Lee BY. Indole-3-carbinol, a vegetable phytochemical, inhibits adipogenesis by regulating cell cycle and AMPKα signaling. Biochimie. 2014;104:127–136. doi: 10.1016/j.biochi.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 9.de Vos M, Kriksunov KL, Jander G. Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol. 2008;146:916–926. doi: 10.1104/pp.107.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Naga RN, Mahran YF. Indole-3-carbinol protects against cisplatin-induced acute nephrotoxicity: role of calcitonin gene-related peptide and insulin-like growth factor-1. Sci Rep. 2016;6:29857. doi: 10.1038/srep29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enríquez J, Velázquez-Cruz R, Parra-Torres A, Gutiérrez-Sagal R, Larrea F. The anti-estrogenic activity of indole-3-carbinol in neonatal rat osteoblasts is associated with the estrogen receptor antagonist 2-hydroxyestradiol. J Endocrinol Invest. 2016;39:1149–1158. doi: 10.1007/s40618-016-0494-9. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher A, Huang H, Yu L, Pham Q, Yu L, Wang TT. Reversible toxic effects of the dietary supplement indole-3-carbinol in an immune compromised rodent model: intestine as the main target. J Diet Suppl. 2017;14:303–322. doi: 10.1080/19390211.2016.1215367. [DOI] [PubMed] [Google Scholar]
- 13.Fuentes F, Paredes-Gonzalez X, Kong AT. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3'-diindolylmethane: anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr Pharmacol Rep. 2015;1:179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujioka N, Fritz V, Upadhyaya P, Kassie F, Hecht SS. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol Nutr Food Res. 2016;60:1228–1238. doi: 10.1002/mnfr.201500889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gehrcke M, Giuliani LM, Ferreira LM, Barbieri AV, Sari MH, da Silveira EF, et al. Enhanced photostability, radical scavenging and antitumor activity of indole-3-carbinol-loaded rose hip oil nanocapsules. Mater Sci Eng C Mater Biol Appl. 2017;74:279–286. doi: 10.1016/j.msec.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt-Kamper C, Biljes D, Merches K, Steiner I, Daldrup T, Bol-Schoenmakers M, et al. Indole-3-carbinol, a plant nutrient and AhR-Ligand precursor, supports oral tolerance against OVA and improves peanut allergy symptoms in mice. PLoS One. 2017;12(6):e0180321. doi: 10.1371/journal.pone.0180321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayakumar P, Pugalendi KV, Sankaran M. Attenuation of hyperglycemia-mediated oxidative stress by indole-3-carbinol and its metabolite 3, 3'- diindolylmethane in C57BL/6J mice. J Physiol Biochem. 2014;70:525–534. doi: 10.1007/s13105-014-0332-5. [DOI] [PubMed] [Google Scholar]
- 18.Julliard W, De Wolfe TJ, Fechner JH, Safdar N, Agni R, Mezrich JD. Amelioration of Clostridium difficile infection in mice by dietary supplementation with indole-3-carbinol. Ann Surg. 2017;265:1183–1191. doi: 10.1097/SLA.0000000000001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabel AM, Al-Shehri AH, Al-Talhi RA, Abd Elmaaboud MA. The promising effect of linagliptin and/or indole-3-carbinol on experimentally-induced polycystic ovarian syndrome. Chem Biol Interact. 2017;273:190–199. doi: 10.1016/j.cbi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Lee BW, Schroeder FC, Jander G. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid) Plant J. 2008;54:1015–1026. doi: 10.1111/j.1365-313X.2008.03476.x. [DOI] [PubMed] [Google Scholar]
- 21.Kundu A, Quirit JG, Khouri MG, Firestone GL. Inhibition of oncogenic BRAF activity by indole-3-carbinol disrupts microphthalmia-associated transcription factor expression and arrests melanoma cell proliferation. Mol Carcinog. 2017;56:49–61. doi: 10.1002/mc.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Licznerska B, Baer-Dubowska W. Indole-3-carbinol and its role in chronic diseases. Adv Exp Med Biol. 2016;928:131–154. doi: 10.1007/978-3-319-41334-1_6. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Gao X, Chen G, Sun J, Chu J, Jing K, et al. Indole-3-carbinol as inhibitors of glucocorticoid-induced apoptosis in osteoblastic cells through blocking ROS-mediated Nrf2 pathway. Biochem Biophys Res Commun. 2015;460:422–427. doi: 10.1016/j.bbrc.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 24.Mao CG, Tao ZZ, Chen Z, Chen C, Chen SM, Wan LJ. Indole-3-carbinol inhibits nasopharyngeal carcinoma cell growth in vivo and in vitro through inhibition of the PI3K/Akt pathway. Exp Ther Med. 2014;8:207–212. doi: 10.3892/etm.2014.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruthanila VL, Poornima J, Mirunalini S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3'-diindolylmethane: a therapeutic marvel. Adv Pharmacol Sci. 2014;2014:832161. doi: 10.1155/2014/832161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megna BW, Carney PR, Nukaya M, Geiger P, Kennedy GD. Indole-3-carbinol induces tumor cell death: function follows form. J Surg Res. 2016;204:47–54. doi: 10.1016/j.jss.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi S, Seyedhosseini FS, Behnampour N, Yazdani Y. Indole-3-carbinol induces G1 cell cycle arrest and apoptosis through aryl hydrocarbon receptor in THP-1 monocytic cell line. J Recept Signal Transduct Res. 2017;37:506–514. doi: 10.1080/10799893.2017.1360351. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Chacon G, de Los Rios C, Zapata JM. Indole-3-carbinol induces cMYC and IAP-family downmodulation and promotes apoptosis of Epstein-Barr virus (EBV)-positive but not of EBV-negative Burkitt's lymphoma cell lines. Pharmacol Res. 2014;89:46–56. doi: 10.1016/j.phrs.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Poindexter KM, Matthew S, Aronchik I, Firestone GL. Cooperative antiproliferative signaling by aspirin and indole-3-carbinol targets microphthalmia-associated transcription factor gene expression and promoter activity in human melanoma cells. Cell Biol Toxicol. 2016;32:103–119. doi: 10.1007/s10565-016-9321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quirit JG, Lavrenov SN, Poindexter K, Xu J, Kyauk C, Durkin KA, et al. Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4-1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells. Biochem Pharmacol. 2017;127:13–27. doi: 10.1016/j.bcp.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Safa M, Jafari L, Alikarami F, Manafi Shabestari R, Kazemi A. Indole-3-carbinol induces apoptosis of chronic myelogenous leukemia cells through suppression of STAT5 and Akt signaling pathways. Tumour Biol. 2017;39(6):1010428317705768. doi: 10.1177/1010428317705768. [DOI] [PubMed] [Google Scholar]
- 32.Safa M, Tavasoli B, Manafi R, Kiani F, Kashiri M, Ebrahimi S, et al. Indole-3-carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells. Tumour Biol. 2015;36:3919–3930. doi: 10.1007/s13277-014-3035-1. [DOI] [PubMed] [Google Scholar]
- 33.Sherer C, Tolaymat I, Rowther F, Warr T, Snape TJ. Preliminary SAR on indole-3-carbinol and related fragments reveals a novel anticancer lead compound against resistant glioblastoma cells. Bioorg Med Chem Lett. 2017;27:1561–1565. doi: 10.1016/j.bmcl.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Song JM, Qian X, Molla K, Teferi F, Upadhyaya P, O’Sullivan G, et al. Combinations of indole-3-carbinol and silibinin suppress inflammation-driven mouse lung tumorigenesis by modulating critical cell cycle regulators. Carcinogenesis. 2015;36:666–675. doi: 10.1093/carcin/bgv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ML, Lin SH, Hou YY, Chen YH. Suppression of lipid accumulation by indole-3-carbinol is associated with increased expression of the aryl hydrocarbon receptor and CYP1B1 proteins in adipocytes and with decreased adipocyte-stimulated endothelial tube formation. Int J Mol Sci. 2016;17(8:pii):E1256. doi: 10.3390/ijms17081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, He H, Lu Y, Ren W, Teng KY, Chiang CL, et al. Indole-3-carbinol inhibits tumorigenicity of hepatocellular carcinoma cells via suppression of microRNA-21 and upregulation of phosphatase and tensin homolog. Biochim Biophys Acta. 2015;1853:244–253. doi: 10.1016/j.bbamcr.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Wang J, Liu Y, Miao H, Cai C, Shao Z, et al. Classic myrosinase-dependent degradation of indole glucosinolate attenuates fumonisin B1-induced programmed cell death in Arabidopsis. Plant J. 2015;81:920–933. doi: 10.1111/tpj.12778. [DOI] [PubMed] [Google Scholar]