Abstract
Many species exhibit prosocial behaviour, in which one individual's actions benefit another individual, often without an immediate benefit to itself. The neuropeptide oxytocin is an important hormonal mechanism influencing prosociality in mammals, but it is unclear whether the avian homologue mesotocin plays a similar functional role in birds. Here, we experimentally tested prosociality in pinyon jays (Gymnorhinus cyanocephalus), a highly social corvid species that spontaneously shares food with others. First, we measured prosocial preferences in a prosocial choice task with two different pay-off distributions: Prosocial trials delivered food to both the subject and either an empty cage or a partner bird, whereas Altruism trials delivered food only to an empty cage or a partner bird (none to subject). In a second experiment, we examined whether administering mesotocin influenced prosocial preferences. Compared to choices in a control condition, we show that subjects voluntarily delivered food rewards to partners, but only when also receiving food for themselves (Prosocial trials), and administration of high levels of mesotocin increased these behaviours. Thus, in birds, mesotocin seems to play a similar functional role in facilitating prosocial behaviours as oxytocin does in mammals, suggesting an evolutionarily conserved hormonal mechanism for prosociality.
Keywords: altruism, corvid, prosocial behaviour, prosocial choice task, mesotocin, oxytocin
1. Introduction
From helping injured nest-mates in ants to donating to charities in humans, many species exhibit prosocial behaviour, in which they behave in a way that benefits another individual [1]. In mammals, the neuropeptide oxytocin is a critical hormone regulating social behaviours, including prosociality. For example, administering oxytocin increases charitable donations in humans [2], social contact in marmosets [3], and levels of affiliation, social orientation and approach behaviours in dogs [4], though see [5] for summary of contrasting results. Among birds, administering an oxytocin antagonist impairs pair bond formation in zebra finches [6], while administering mesotocin—the avian homologue of oxytocin—increases the preference to associate with a larger social group [7]. Therefore, mesotocin also plays a key role in the social behaviours of birds. However, it remains unknown whether mesotocin's role in avian social behaviour carries over to prosociality.
Prosocial behaviour is often measured experimentally using the prosocial choice task [8]: subjects make a choice between two options that vary in their reward consequences to another individual. If subjects have prosocial preferences, then they will choose the option that delivers food to the other individual, sometimes even at a cost (altruism). Many corvids exhibit high rates of naturally occurring prosocial behaviours, such as voluntary food sharing [9–13]; however, only a handful of corvid species have been examined in experimental prosocial tasks [14–17]. Despite high rates of naturalistic food sharing, among these corvid species, only azure-winged magpies, Cyanopica cyana, have provided convincing evidence of prosociality in an experimental setting [18].
The current study aimed to test mesotocin as a hormonal mechanism of prosociality in pinyon jays, Gymnorhinus cyanocephalus, a highly social corvid species that voluntarily shares food [9]. Like magpies, pinyon jays exhibit facultative cooperative breeding [19], which may facilitate the expression of prosocial behaviour [20]. Given their highly social nature and voluntary food sharing, our first experiment examined whether pinyon jays choose to provide benefits to same-sex partners in a prosocial choice task. Our second experiment then investigated whether administering mesotocin influenced the proportion of subjects' prosocial choices. We hypothesized that (i) pinyon jays would preferentially choose to provide benefits to another individual and (ii) mesotocin administration would increase these prosocial choices.
2. Methods
(a). Subjects
In Experiment 1, we tested three female and six male captive adult pinyon jays. In Experiment 2, we tested the same individuals, except for two males. In Experiment 1, subjects rotated through three same-sex partners, whereas, in Experiment 2, they had a single same-sex partner (electronic supplementary material, table S1).
(b). Experimental apparatus
We placed three adjacent cages in front of a choice apparatus with two trays resting on a shelf (figure 1). Each tray contained two dishes in which food (a mealworm) could be placed. To begin a trial, both trays remained out of the birds’ reach. Subjects chose by pecking one of two wires extending from the apparatus, which resulted in an experimenter pushing forward the corresponding tray, giving access to food dishes on that tray. Subjects chose from the centre cage, with a partner in either the left or right cage (side counterbalanced across sessions).
Figure 1.
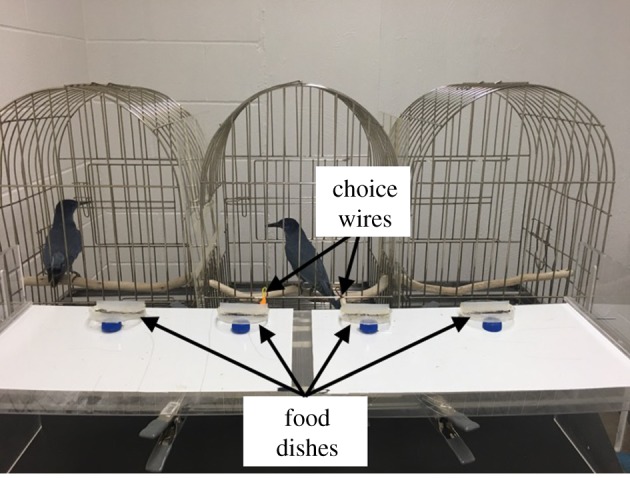
Experimental apparatus. Subjects in centre cage pecked one of two possible choice wires. An experimenter pushed forward the chosen side thereby giving the subject access to one of the innermost food dishes and the partner access to an outermost food dish (if the tray on the partner's side was chosen). The trial type (Attention, Bias, Altruism, Prosocial) determined the distribution of food across food dishes.
(c). Experimental sessions
Subjects experienced training to ensure that they understood the consequences of their choices (see the electronic supplementary material). All experimental sessions consisted of 16 trials: four Attention trials, followed by four Bias trials, and then four each, in pseudorandomized order, of Prosocial and Altruism trials (table 1).
Table 1.
Experimental trial types.
| trial type | reward distribution (food dishes left to right: 1, food present; 0, absent) | explanation | purpose |
|---|---|---|---|
| Attention | 0010 or 0100 | one mealworm was placed on either the L- or R- centre dishes | these trials ensured that subjects started each session attending to where food rewards were distributed |
| Bias | 0110 | one mealworm was placed on each of the centre dishes, thus either an L- or R- choice resulted in a food reward | since the outcome to subjects is equivalent, these trials reflect (i) the overall preference for choosing left or right (side bias) and (ii) the potential role of social facilitation, where the presence of a partner could influence which side the subject chooses |
| Altruism | 1001 | one mealworm was placed on each of the outermost dishes. Though neither an L- nor R- choice would give the subject a reward, an L-choice would deliver one mealworm to the left cage and R-choice to right cage | subjects do not get food regardless of side chosen, but if they prefer to be altruistic, they will choose the same side as the partner. That is, an altruistic choice would deliver no food to the subject, thus benefiting the partner at a low cost to subject |
| Prosocial | 1111 | one mealworm was placed on all dishes. Any choice resulted in a food reward for subject; an L-choice would deliver one mealworm to the left cage and R-choice to right cage | subjects will get food regardless of side chosen, but if they prefer to be prosocial, they will choose the same side as the partner. That is, a prosocial choice would deliver food to both the subject and partner |
(d). Measurement of choice and analyses
To account for potential biases in the subjects' prosocial and altruistic choices, such as social facilitation, we corrected the amount of matching (i.e. choosing the tray on the same side as the partner) observed in Prosocial and Altruism trials by subtracting the amount of bias matching. For each comparison, we first calculated the absolute change in partner-side matching from Bias to Prosocial/Altruism trials (absolute tendency, see Pt in [21]). We also calculated a relative, weighted tendency (see Pt’ in [21]); however, results from both measures agreed for all analyses, so we present only absolute tendency here (see electronic supplementary material). The greater the amount of prosocial/altruistic choices relative to their bias, the more positive a subject's tendencies will be (see electronic supplementary material, table S2 for definition of each term). To test whether the amount of matching differed from that observed in Bias trials, we compared the absolute and weighted tendencies against 0. We used Bayes factors (BF) to measure the strength of evidence for hypotheses of group differences over null hypotheses of no difference [22].
(e). Hormonal manipulation
For Experiment 2, an experimenter intranasally administered one of three possible solutions (high-mesotocin: 30 mg (15 IU) dose; low-mesotocin: 15 mg (7.5 IU) and a saline control) 30 min prior to each session. For each administration, an experimenter dripped the corresponding solution into the subject's nares using a needleless 1-ml syringe. We based administration time frames and dosages on mammalian oxytocin studies [3].
3. Results
(a). Experiment 1: do pinyon jays preferentially deliver food to others?
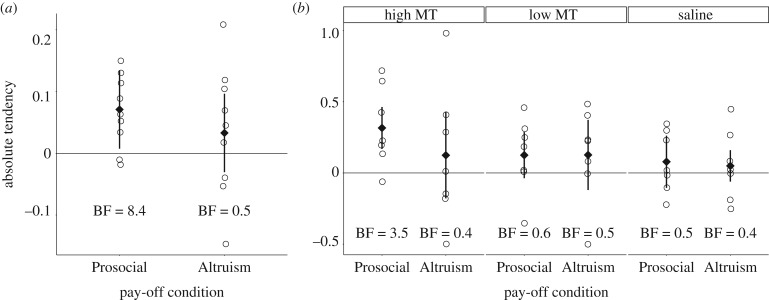
Compared to Bias trials, pinyon jays increased their delivery of food to a partner by 7.1% in Prosocial trials (figure 2a) and by 3.3% in Altruism trials. Therefore, there is evidence for pinyon jays choosing prosocially (prosocial absolute tendency; one sample t-test: t8 = 3.6, BF = 8.4) but not altruistically (altruistic absolute tendency; t8 = 0.9, BF = 0.5).
Figure 2.
Absolute tendency for both experiments. (a) In Experiment 1, compared to Bias trials, subjects preferentially delivered food to partners in Prosocial but not Altruism trials. (b) In Experiment 2, subjects who were administered high levels of mesotocin preferentially delivered food to partners in Prosocial trials but not in any other condition. BF, Bayes factor; MT, mesotocin. Circles represent individual subjects' mean absolute tendency, diamonds represent the overall means and bars represent within-subjects 95% CIs.
(b). Experiment 2: does administration of mesotocin increase prosocial and altruistic choices?
Compared to Bias trials, pinyon jays increased prosocial matching by 31.6% in the high-mesotocin condition (prosocial absolute tendency; t6 = 3.0, BF = 3.5; figure 2b), by 12.5% in the low-mesotocin condition (t6 = 1.3, BF = 0.6), and by 7.9% in the saline condition (t6 = 1.0, BF = 0.5). Therefore, there is evidence for pinyon jays choosing prosocially only in the high-mesotocin condition. There is no evidence for altruism in any condition (altruistic absolute tendency; high-mesotocin: mean = 12.4%, t6 = 0.7, BF = 0.4; low-mesotocin: 12.6%, t6 = 1.0, BF = 0.5; saline: 5.0%, t6 = 0.5, BF = 0.4).
4. Discussion
In Experiment 1, pinyon jays preferentially chose to deliver food rewards to a partner but only in trials when also receiving benefits for themselves (i.e. in Prosocial but not Altruism trials). In Experiment 2, when given a high dose of mesotocin, subjects preferentially chose to deliver food during Prosocial trials. However, there was no evidence of preferentially delivering food when given a low dose of mesotocin or a saline control. Lastly, pinyon jays did not preferentially deliver food in Altruism trials regardless of hormone condition. Thus, pinyon jays are prosocial, but not altruistic, in a prosocial choice task, and mesotocin can enhance prosocial behaviour.
These data are important in at least two ways. First, our measures of prosocial and altruistic tendency account for individual biases, such as local enhancement and social facilitation, and our results do not change whether we account for the initial degree of bias or not. Thus, pinyon jays join magpies [18] in corvids that show evidence of prosocial behaviour not due to social facilitation in an experimental setting, which is consistent with the notion that cooperatively breeding species tend to exhibit unsolicited prosociality [20]. Second, this study is the first to show that mesotocin, the avian homologue of mammalian oxytocin, influences prosocial behaviour in birds. Thus, whereas others have shown that mesotocin and oxytocin play a similar functional role in other social behaviours across birds and mammals [7], we provide the first evidence that the similarity extends to prosociality. This suggests that oxytocin and mesotocin may serve as an evolutionarily conserved hormonal mechanism for prosociality across mammals and birds.
Despite evidence for choosing prosocially in Experiment 1, the pinyon jays did not show this in the saline condition of Experiment 2, which most closely resembled Experiment 1. Characteristics of the subject, partner and their interaction, such as degree of affiliation, could mediate decisions in the prosocial choice task, as well as the behavioural effects of mesotocin administration. Indeed, individuals showed considerable variation in their preferences in both experiments (electronic supplementary material, tables S4 and S5), and partner identity influenced their decisions (electronic supplementary material, table S3), replicating the variability in food sharing that donors exhibit across recipients [9]. In Experiment 2, we reduced the number of partners to decrease variation in the data. However, the partners chosen for Experiment 2 happened to receive fewer prosocial choices than other partners in Experiment 1 (electronic supplementary material, table S3). Thus, we may have biased subjects' decisions towards fewer prosocial choices, leading to this discrepancy.
Another possible cause of this discrepancy is that handling the subjects when administering the hormones may have elevated stress, which could have disrupted prosocial behaviour. In mammals, oxytocin buffers stress responsiveness [23], which could explain why our high dose of mesotocin resulted in prosocial preferences. Thus, both handling stress and partner preferences may have contributed to a reduction in overall prosocial preferences in Experiment 2.
In mammals, contextual factors and individual differences (e.g. familiarity of partners and genetic variation) moderate how oxytocin influences behaviour [24]. Here, though mesotocin administration influenced prosociality, subjects differed in how they responded to this hormone (electronic supplementary material, table S5). Future studies exploring how contextual and individual characteristics influence prosocial preferences, as well as how different individuals respond to hormonal administration, may reveal the factors that underlie variation in avian prosociality.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Adaptive Decision Making Lab for their thoughtful feedback on manuscript drafts. Particular thanks go to the undergraduate research assistants (Chandler Dulin, Krysten Fries, Marisa Howell, Haley Kizer, Athena Manning, Anna Rodriguez and Emily Stockwell) who collected the data, laboratory technician Jesse Baumann for maintaining the bird colony, and Jeff French for advice on mesotocin administration.
Ethics
The University of Nebraska-Lincoln Institutional Animal Care and Use Committee approved this project (protocol #1354).
Data accessibility
Summary tables for subjects and partners, data and R code are available in the electronic supplementary material in the Dryad Data Repository (http://dx.doi.org/10.5061/dryad.g38qb00) [25] and the Open Science Framework (https://osf.io/358hs) [26].
Authors' contributions
J.F.D. and J.R.S. developed study design, compiled and analysed data, and drafted manuscript; H.A. and W.L. assisted with study design, collected data and commented on the manuscript. All authors agree to be held accountable for the content herein and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This research was supported, in part, by a Nebraska EPSCoR FIRST Award and a University of Nebraska-Lincoln Layman Award to J.R.S. and a National Science Foundation Graduate Research Fellowship Program award (DGE-10410000) to J.F.D.
References
- 1.Marshall-Pescini S, Dale R, Quervel-Chaumette M, Range F. 2016. Critical issues in experimental studies of prosociality in non-human species. Anim. Cogn. 19, 679–705. ( 10.1007/s10071-016-0973-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barraza JA, McCullough ME, Ahmadi S, Zak PJ. 2011. Oxytocin infusion increases charitable donations regardless of monetary resources. Horm. Behav. 60, 148–151. ( 10.1016/j.yhbeh.2011.04.008) [DOI] [PubMed] [Google Scholar]
- 3.Smith AS, Ågmo A, Birnie AK, French JA. 2010. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 57, 255–262. ( 10.1016/j.yhbeh.2009.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero T, Nagasawa M, Mogi K, Hasegawa T, Kikusui T. 2014. Oxytocin promotes social bonding in dogs. Proc. Natl Acad. Sci. USA 111, 9085–9090. ( 10.1073/pnas.1322868111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartz JA, Zaki J, Bolger N, Ochsner KN. 2011. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15, 301–309. ( 10.1016/j.tics.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 6.Pedersen A, Tomaszycki ML. 2012. Oxytocin antagonist treatments alter the formation of pair relationships in zebra finches of both sexes. Horm. Behav. 62, 113–119. ( 10.1016/j.yhbeh.2012.05.009) [DOI] [PubMed] [Google Scholar]
- 7.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. 2009. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science 325, 862–866. ( 10.1126/science.1174929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silk JB, Brosnan SF, Vonk J, Henrich J, Povinelli DJ, Richardson AS, Lambeth SP, Mascaro J, Schapiro SJ. 2005. Chimpanzees are indifferent to the welfare of unrelated group members. Nature 437, 1357–1359. ( 10.1038/nature04243) [DOI] [PubMed] [Google Scholar]
- 9.Duque JF, Stevens JR. 2016. Voluntary food sharing in pinyon jays: the role of reciprocity and dominance. Anim. Behav. 122, 135–144. ( 10.1016/j.anbehav.2016.09.020) [DOI] [Google Scholar]
- 10.von Bayern AMP, de Kort SR, Clayton NS, Emery NJ. 2007. The role of food- and object-sharing in the development of social bonds in juvenile jackdaws (Corvus monedula). Behaviour 144, 711–733. ( 10.1163/156853907781347826) [DOI] [Google Scholar]
- 11.de Kort SR, Emery NJ, Clayton NS. 2006. Food sharing in jackdaws, Corvus monedula: what, why and with whom? Anim. Behav. 72, 297–304. ( 10.1016/j.anbehav.2005.10.016) [DOI] [Google Scholar]
- 12.Ostojić L, Shaw RC, Cheke LG, Clayton NS. 2013. Evidence suggesting that desire-state attribution may govern food sharing in Eurasian jays. Proc. Natl Acad. Sci. USA 110, 4123–4128. ( 10.1073/pnas.1209926110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheid C, Schmidt J, Noë R. 2008. Distinct patterns of food offering and co-feeding in rooks. Anim. Behav. 76, 1701–1707. ( 10.1016/j.anbehav.2008.07.023) [DOI] [Google Scholar]
- 14.Schwab C, Swoboda R, Kotrschal K, Bugnyar T. 2012. Recipients affect prosocial and altruistic choices in jackdaws, Corvus monedula. PLoS ONE 7, e34922 ( 10.1371/journal.pone.0034922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Lascio F, Nyffeler F, Bshary R, Bugnyar T. 2013. Ravens (Corvus corax) are indifferent to the gains of conspecific recipients or human partners in experimental tasks. Anim. Cogn. 16, 35–43. ( 10.1007/s10071-012-0548-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert ML, Massen JJM, Seed AM, Bugnyar T, Slocombe KE. 2017. An ‘unkindness’ of ravens? Measuring prosocial preferences in Corvus corax. Anim. Behav. 123, 383–393. ( 10.1016/j.anbehav.2016.11.018) [DOI] [Google Scholar]
- 17.Massen JJM, Lambert M, Schiestl M, Bugnyar T. 2015. Subadult ravens generally don't transfer valuable tokens to conspecifics when there is nothing to gain for themselves. Front. Psychol. 6, 885 ( 10.3389/fpsyg.2015.00885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn L, Scheer C, Bugnyar T, Massen JJM. 2016. Proactive prosociality in a cooperatively breeding corvid, the azure-winged magpie (Cyanopica cyana). Biol. Lett. 12, 20160649 ( 10.1098/rsbl.2016.0649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzluff JM, Balda RP. 1992. The pinyon jay: behavioral ecology of a colonial and cooperative corvid. London, UK: T&AD Poyser. [Google Scholar]
- 20.Burkart JM, Hrdy SB, Van Schaik CP. 2009. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186. ( 10.1002/evan.20222) [DOI] [Google Scholar]
- 21.Massen JJM, Luyten I, Spruijt B, Sterck E. 2011. Benefiting friends or dominants: prosocial choices mainly depend on rank position in long-tailed macaques. Primates 52, 237–247. ( 10.1007/s10329-011-0244-8) [DOI] [PubMed] [Google Scholar]
- 22.Wagenmakers E-J. 2007. A practical solution to the pervasive problems of p values. Psychon. Bull. Rev. 14, 779–804. ( 10.3758/BF03194105) [DOI] [PubMed] [Google Scholar]
- 23.Smith AS, Wang Z. 2014. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76, 281–288. ( 10.1016/j.biopsych.2013.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. 2013. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 38, 1883–1894. ( 10.1016/j.psyneuen.2013.06.019) [DOI] [PubMed] [Google Scholar]
- 25.Duque JF, Leichner W, Ahmann H, Stevens JR.2018. Data from: Mesotocin influences pinyon jay prosociality. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
- 26.Duque JF, Leichner W, Ahmann H, Stevens JR. 2018. Data from: Mesotocin influences pinyon jay prosociality Open Science Framework. (https://osf.io/358hs/) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Duque JF, Leichner W, Ahmann H, Stevens JR.2018. Data from: Mesotocin influences pinyon jay prosociality. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
- Duque JF, Leichner W, Ahmann H, Stevens JR. 2018. Data from: Mesotocin influences pinyon jay prosociality Open Science Framework. (https://osf.io/358hs/) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Summary tables for subjects and partners, data and R code are available in the electronic supplementary material in the Dryad Data Repository (http://dx.doi.org/10.5061/dryad.g38qb00) [25] and the Open Science Framework (https://osf.io/358hs) [26].