Abstract
Risk perception plays an important role in testing behaviour for sexually transmitted infections, but is rarely included in mathematical models exploring the impact of testing. We explored the impact of incorporating sexual behaviour (SB), risk perception (RP) and differential testing uptake in SB–RP groups on prevalence, using chlamydia as an example. We developed a pair model with a susceptible–infected–susceptible structure representing heterosexuals aged 16–26 years. The effect of testing on chlamydia prevalence was compared between a model with only SB (SB model) and a model with SB and RP (SB–RP model). In the SB–RP model, a scenario without differential testing uptake in SB–RP groups was compared to scenarios with differential testing uptake in SB–RP groups. Introducing testing into the SB–RP model resulted in a slightly smaller reduction in chlamydia prevalence (−38.0%) as compared to the SB model (−40.4%). In the SB–RP model, the scenario without differential testing uptake in SB–RP groups overestimated the reduction in chlamydia prevalence (with 4.8%), especially in the group with high SB and low RP (19.8%). We conclude that mathematical models incorporating RP and differential testing uptake in SB–RP groups improve the impact assessment of testing and treatment on chlamydia prevalence.
Keywords: Chlamydia trachomatis, mathematical model, sexual behaviour, risk perception, testing, sexually transmitted diseases
1. Background
Risk perception plays an important role in behaviour related to transmission of sexually transmitted infections (STIs), such as condom use or testing for STIs [1–3]. For example, individuals who do not consider themselves to be at risk for STIs might be less inclined to use condoms or to seek testing [4,5]. Previous research has suggested that, concerning risk perception in the context of sexual behaviour, a distinction can be made between individuals who realistically perceive their risk (realists), overestimate their risk (pessimists) or underestimate their risk (optimists) [6,7].
Mathematical models are widely used to study transmission of STIs and to predict the impact of intervention measures to guide public health policy decisions [8–11]. It has previously been suggested that models including greater heterogeneity in STI risk might provide more accurate estimates of the impact of interventions [12,13]. However, risk perception is hardly ever included in these models. To our knowledge, there are only two mathematical modelling studies exploring the impact of risk perception on transmission dynamics, but they focused on HIV only and did not include the influence of risk perception on testing behaviour [14,15]. A mathematical model exploring the influence of risk perception on the transmission of curable STIs and testing behaviour has not yet been developed [16,17].
Chlamydia trachomatis (chlamydia) is the most commonly reported curable STI in young heterosexual men and women in many Western countries [18–20]. Infection is associated with increased risk of sequelae in women, such as pelvic inflammatory disease (PID), ectopic pregnancy and tubal subfertility [21–23]. To date, control of chlamydia has proved to be challenging. It is unclear why the prevalence of chlamydia has not declined even in countries with chlamydia screening guidelines or programmes, such as England, Australia, Canada and the United States [24–27]. One of the possible reasons for the unchanged chlamydia prevalence in young adults could be that many do not perceive themselves to be at risk [1,28] and are, therefore, less likely to seek chlamydia testing [4,5]. This could have serious consequences for the transmission of chlamydia, because for many individuals this perception is incorrect [4,5] and because chlamydia is usually asymptomatic, most people are unaware of their infection [9,29]. As a result, testing rates might not be high enough to decrease the estimated population prevalence of chlamydia [27] and this makes incorporating risk perception in models evaluating the influence of testing and treatment on chlamydia prevalence essential.
In this study, the first aim was to explore the influence of incorporating risk perception in a mathematical model that estimates the effect of testing and treatment on the prevalence of chlamydia. The second aim was to estimate the impact of incorporating differential testing uptake based on risk perception on chlamydia prevalence.
2. Methods
2.1. Data
To parametrize the model, we used data from a Dutch online survey on sexual behaviour and STI risk perception conducted among heterosexual men and women aged 16–26 years in May 2016. Participants were recruited via social media, by promoting the survey on Facebook and Twitter. To increase the number of lower educated individuals, participants were also recruited at a vocational school in Amersfoort, the Netherlands. The survey was approved by the Medical Ethics Committee Noord-Holland, Alkmaar, the Netherlands (M016-024). All participants in the survey provided informed consent. Of the 296 participants who agreed to participate in the survey, 123 (42%) did not complete the questionnaire, leaving 173 (58%) fully completed questionnaires available for analyses.
Sexual behaviour was assessed by questions about the number of sex partners in the last year, condom use at the most recent sex act, number of sex acts in the last four weeks and whether the participants were planning to have sex with their current partner in the future (to determine the percentage of people in a partnership). The sexual behaviour questions were based on several validated questionnaires [25,27,30–32]. Risk perception was assessed with two items, asking respondents to estimate their own risk of chlamydia in the coming year and in their lifetime on a scale from 0 to 100 [7,30,32–34]. To obtain a more robust estimate of a complex construct, such as risk perception, it is common to combine multiple items that represent one psychological construct [35]. For example, perceived risk of chlamydia for the coming year is strongly dependent on the context in the present, whereas lifetime risk might give an indication of behaviour independent of context. Therefore, individuals' personal risk estimates for chlamydia were defined as the mean of both answers providing a more reliable measure of perceived risk of chlamydia, in general.
2.2. Definition of sexual behaviour and risk perception groups
We defined sexual behaviour (SB) and risk perception (RP) groups based on the survey data. First, the participants were divided into two sexual behaviour groups (SB groups) based on the reported number of partners: low- and high-risk sexual behaviour groups, similar to previous research [36,37]. Individuals with three or more partners were considered the high-risk group, because the majority of heterosexual STI clinic visitors in the Netherlands report three or more partners per year [20,38], and STI clinic visitors tend to be at higher risk for STIs than the general population [25]. Second, individuals were divided into two risk perception groups based on the personal risk estimates for chlamydia (RP groups): low and high risk perception. It is unclear what cut-off value for risk perception would result in clinically relevant behavioural differences. Therefore, the risk perception groups were constructed using a median split. The resulting four groups (SB–RP groups) were referred to as follows based on previous work [6,7]. Individuals with few partners, in other words low-risk sexual behaviour, who considered themselves to be high risk were referred to as ‘pessimists’. Individuals who viewed themselves correctly to be low risk were referred to as ‘low-risk realists’. Similarly, people engaged in high-risk sexual behaviour who considered themselves correctly to be high risk were referred to as ‘high-risk realists’. If they viewed themselves to be low risk, they were referred to as ‘optimists’.
2.3. Description of the model
A deterministic pair compartmental model was developed with a susceptible–infected–susceptible (SIS) structure representing heterosexuals aged 16–26 years, based on existing pair models describing chlamydia transmission [9,38,39]. A detailed description of the model is presented in electronic supplementary material, text S1. In short, people can either be susceptible (S) or infected (I) with chlamydia. Individuals can become infected with a transmission probability per condomless sex act in a pair of a susceptible and an infected individual. Infected individuals can become susceptible again after natural clearance or after testing and treatment, assuming everyone who is diagnosed accepts treatment and treatment is 100% effective. For simplicity, no period of immunity after natural clearance or after testing and treatment was assumed. In the model, the pair formation and separation processes are independent of the infection status, meaning that anyone can form a pair with anyone of the opposite sex at any time. An advantage of the use of pair models is that we could explicitly incorporate partnership duration in our model, which can improve the estimated impact of interventions on chlamydia prevalence [39,40].
To explore the influence of incorporating risk perception in mathematical models, we developed two variants of the model. In the first variant, we incorporated the two sexual behaviour groups (SB groups) defined above without further dividing these groups into risk perception groups (SB model). In the second variant, we incorporated the four groups (SB–RP groups) defined above (pessimists, low-risk realists, high-risk realists and optimists, see electronic supplementary material, figure S2), including both sexual behaviour and risk perception in the model (SB–RP model). We assumed that all individuals remained in the same group for the period considered in the simulations. Mixing between individuals in the different groups was incorporated using a mixing parameter, which could be varied from fully assortative (only like-with-like mixing) to fully proportionate mixing (random mixing). A detailed description of mixing between males and females in the different groups is provided in electronic supplementary material, text S1.4.
2.4. Model parametrization
For both models, the baseline infection parameters, the transmission probability and the duration of infection were informed by the literature (table 1) and were assumed to be the same for each group. The transmission probability was calibrated to a steady-state prevalence of 4% [27,39] using the uniroot function [43], a widely used minimization procedure to determine a zero of a function, with fixed baseline values for all other parameters. The distribution of the model population over the groups and the behavioural parameters including the number of partners per year, the number of sex acts, the percentage of the population in a relationship and condom use were informed by the survey (table 1). Differences in these parameters between the two SB groups (SB model) and the four SB–RP groups (SB–RP model) were explored. Parameters were assumed to be the same between males and females, because the low number of male participants (n = 34) in the survey hampered a gender-stratified analysis. The number of partners per year and the corresponding percentage of the population in a sexual partnership at any time were used to calculate the pair formation and separation rates (see electronic supplementary material, text S1.3.1). The number of sex acts per week was calculated by dividing the number of reported sex acts in the past four weeks by the number of weeks they had sex in the past month (based on reported most recent sex act) (see electronic supplementary material, text S1.3.2). In partnerships of individuals from different SB–RP groups, the mean of the parameter values from each group was used. For example, when a pessimist forms a partnership with an optimist, the corresponding parameter value for the number of sex acts was determined by calculating the mean number of sex acts in the pessimist group and in the optimist group.
Table 1.
Baseline parameter values informed by the literature and the survey among heterosexual males and females aged 16–26 years. SB, sexual behaviour; RP, risk perception.
| parameters | baseline values | |||
|---|---|---|---|---|
| infection parameters (informed by literature) | value | source | ||
| chlamydia prevalence (%) | 4 | [27,39] | ||
| transmission probability (per sex act)a | 0.07 | |||
| duration of infection (years) | 1 | [41,42] | ||
| behavioural parameters (informed by the survey)b | ||||
| SB model and SB–RP model | low-risk sexual behaviour (n = 120) | high-risk sexual behaviour (n = 53) | ||
| number of partners, per year (median) | 1 | 4 | ||
| percentage of population in a partnership (%) | 73.7 | 69.2 | ||
| SB model only | ||||
| percentage of group in total population (%) | 70 | 30 | ||
| number of sex acts, past four weeks (median) | 6 | 4 | ||
| condom use most recent sex act (%) | 27.2 | 39.6 | ||
| SB–RP model only | pessimist (n = 32) | low-risk realist (n = 88) | high-risk realist (n = 35) | optimist (n = 18) |
| percentage of group in total population (%) | 18.9 | 51.1 | 19.8 | 10.2 |
| number of sex acts, past four weeks (median) | 6 | 7 | 3 | 5.3 |
| condom use most recent sex act (%) | 24.1 | 28.2 | 37.1 | 44.4 |
aCalibrated to the steady-state prevalence.
bCalculated using behavioural data from the survey (see electronic supplementary material, S1.3).
2.5. Model analyses
We explored the impact of an overall testing uptake of 10% per year [44–46] for five consecutive years in the model. We considered two testing scenarios: one in which we assumed differential uptake in the SB groups (the baseline scenario), and another one where we assumed differential testing uptake in the RP groups in addition to differential uptake in the SB groups (scenario with differential uptake in SB–RP groups). In both scenarios, the overall testing uptake remained 10% per year. In the baseline scenario, we assumed that 60% of all the testers were high-risk realists and optimists (high-risk sexual behaviour) and 40% of all the testers were pessimists and low-risk realists (low-risk sexual behaviour), because individuals with high-risk sexual behaviour test more frequently for STIs than people at lower risk [5,25,47]. In the scenario with differential testing uptake in SB–RP groups, we assumed that 90% of all the testers in each sexual behaviour group was pessimist or high-risk realist (high risk perception), and 10% was low-risk realist or optimist (low risk perception), because individuals with high risk perception might be more inclined to seek testing than individuals with low risk perception [4,5]. A detailed description of the calculations of the corresponding testing rates in each group can be found in electronic supplementary material, text S2.1 and figure S2.
To explore the influence of incorporating risk perception in mathematical models, we compared the effect of the baseline testing scenario (without differential uptake in SB–RP groups) on chlamydia prevalence in a model without risk perception (SB model) and a model incorporating risk perception (SB–RP model). To explore the impact of incorporating differential testing uptake in SB–RP groups on chlamydia prevalence, we used the SB–RP model and compared the reduction in the prevalence of chlamydia between the baseline scenario and the testing scenario with differential uptake in SB–RP groups (electronic supplementary material, figure S2). The impact of incorporating differential testing uptake in SB–RP groups was defined as the relative difference in chlamydia prevalence between the baseline testing scenario and the testing scenario with differential uptake in SB–RP groups for each consecutive year after the introduction of testing uptake.
2.6. Uncertainty analyses
First, we tested the uncertainty of our results for the assumed overall testing uptake by conducting the analyses in the SB–RP model with an overall testing uptake of 20% and 30%. Second, we examined the effects of changing the mixing parameter value to zero (fully assortative mixing) or one (fully proportionate mixing) on the relative difference in chlamydia prevalence in the four SB–RP groups. The other parameter values remained fixed at the baseline values. For each value of the mixing parameter, the transmission probability was recalibrated to result in an overall prevalence of 4%. Last, we explored different distributions of testing uptake in the SB–RP groups, from 0% in the low-risk realists and optimists, and 100% in the pessimists and high-risk realists, to 100% in the low-risk realists and optimists, and 0% in the pessimists and high-risk realists. The overall testing uptake in these analyses remained 10% per year. The impact of changing the baseline assumptions of the overall testing uptake, mixing parameter and distributions of testing uptake in the SB–RP groups was defined in a similar way as described above: the relative difference in chlamydia prevalence between the baseline testing scenario and the testing scenario with differential uptake in SB–RP groups. All analyses were done using R v. 3.4.0 [48]. Data and R codes are available upon request.
3. Results
3.1. Survey data
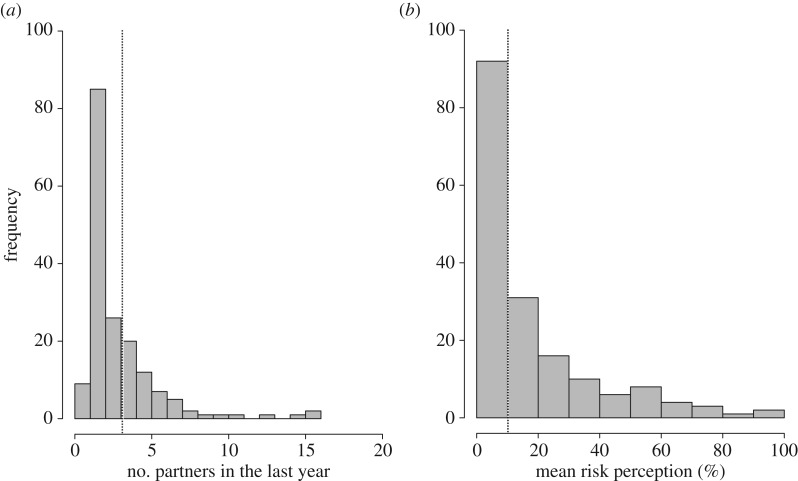
In total, 34 heterosexual males and 139 heterosexual females (n = 173) aged 16–26 years provided a complete questionnaire. Many individuals reported only few partners in the last year (figure 1a). Individuals reporting less than three partners were allocated to the low-risk sexual behaviour group (70%) and individuals reporting three partners or more in the last year were allocated to the high-risk sexual behaviour group (30%). Individuals were further divided into a low or high risk perception group based on the median risk estimate for chlamydia (figure 1b). Individuals reporting personal risk estimates for chlamydia higher than 10% were allocated to the high risk perception group (27% of the low-risk sexual behaviour group and 66% of the high-risk sexual behaviour group). Individuals with risk estimates lower than or equal to 10% were allocated to the low risk perception group (73% of the low-risk sexual behaviour group and 34% of the high-risk sexual behaviour group). As a result, pessimists made up 19%, low-risk realists 51%, high-risk realists 20% and optimists 10% of the survey population.
Figure 1.
Number of reported partners in the last year (a) and perceived personal risk of acquiring chlamydia ranging from low (0%) to high risk perception (100%) (b) among heterosexuals aged 16–26 years. The dotted line indicates the cut-off point used to define the SB–RP groups in the model population (i.e. 4th quartile of the number of reported partners and the median personal risk estimate for chlamydia of the total population).
The number of partners per year and condom use was lower, and the percentage of the population in a partnership and the number of sex acts in the past four weeks was higher in the low-risk compared with the high-risk sexual behaviour group in the survey. Furthermore, individuals in the low-risk sexual behaviour group had more condomless sex acts (number of sex acts multiplied by fraction non-condom use) than individuals in the high-risk sexual behaviour group. These results were used to inform the baseline values of the behavioural parameters in the SB model (table 1; electronic supplementary material, table S1). To inform the behavioural parameters in the SB–RP model (table 1, SB–RP model), the survey data were also analysed for the pessimists, low-risk realists, high-risk realists and optimists (the SB–RP groups). Individuals with low risk perception (low-risk realists and optimists) had more condomless sex acts than individuals with high risk perception (pessimists and high-risk realists). The number of sex acts and condom use differed between the SB–RP groups, whereas the number of partners per year and the percentage of the population in a partnership did not differ between the four groups (table 1; electronic supplementary material, table S1). Hence, no further discrimination in the SB–RP groups for the latter two parameters was made to inform the SB–RP model (electronic supplementary material, text S1.3).
3.2. Influence of incorporating RP in models
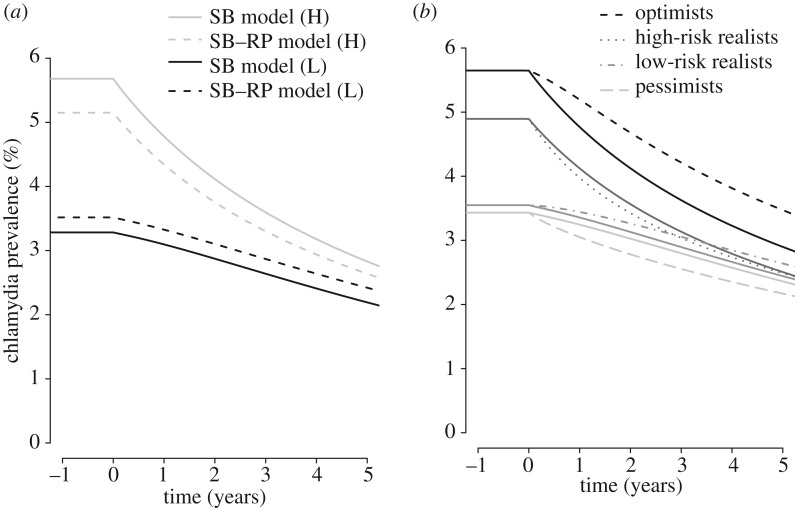
The overall steady-state population prevalence of chlamydia before introducing the baseline testing scenario was 4% in the model without risk perception (SB model) and the model with risk perception (SB–RP model), but the prevalences in the SB groups were different between the two models. The prevalence of chlamydia in the low-risk sexual behaviour group was 3.3% in the SB model and 3.5% in the SB–RP model, and in the high-risk sexual behaviour group 5.7% in the SB model and 5.1% in the SB–RP model. We compared the reduction in chlamydia prevalence 5 years after the introduction of the baseline testing scenario between the SB model and the SB–RP model (electronic supplementary material, text S2.2 and figure S3). In the SB–RP model, the estimated reduction in the prevalence of chlamydia was slightly smaller in the total population (38.0% versus 40.4%) and both SB groups (31.2% versus 33.2% in low-risk and 48.8% versus 50.2% in the high-risk sexual behaviour group) compared with the SB model (figure 2a).
Figure 2.
Estimated chlamydia prevalence after the introduction of the baseline testing uptake scenario for five consecutive years. (a) The estimated chlamydia prevalence after the introduction of the baseline testing scenario; solid lines denote the model without incorporating risk perception (SB model) and dashed lines denote the model with risk perception (SB–RP model). (b) The estimated chlamydia prevalence in each group after the introduction of the baseline testing scenario (solid lines) and the testing scenario with differential uptake in SB–RP groups (dashed, dotted and dash-dot) in the SB–RP model.
3.3. Impact of incorporating differential testing uptake in SB–RP groups
The SB–RP model was used to compare the reduction in chlamydia prevalence in the baseline testing scenario (without differential uptake in SB–RP groups) to the reduction in chlamydia prevalence in the testing scenario with differential uptake in SB–RP groups. The estimated steady-state prevalence before introducing the testing uptake scenarios was 3.4% in the pessimists, 3.5% in the low-risk realists, 4.9% in the high-risk realists and 5.6% in the optimists in both scenarios. Five years after the introduction of testing, the reduction in chlamydia prevalence in the testing scenario with differential uptake in SB–RP groups was smaller compared with the reduction in the baseline scenario in the total population (35.0% versus 38.0%), especially in the optimists (38.5% versus 48.7%). In the optimists, the prevalence of chlamydia was 19.8% higher in the testing scenario with differential uptake in SB–RP groups compared with baseline scenario (electronic supplementary material, text S2.3 and figure S4). In the low-risk realists, the prevalence was 8.0% higher in the model with differential uptake in SB–RP groups, and 8.1% lower in the pessimists and 0.7% lower in the high-risk realists (figure 2b) compared with the baseline testing scenario (4.8% higher in the total population).
3.4. Uncertainty analyses differential testing uptake in SB–RP groups in the SB–RP model
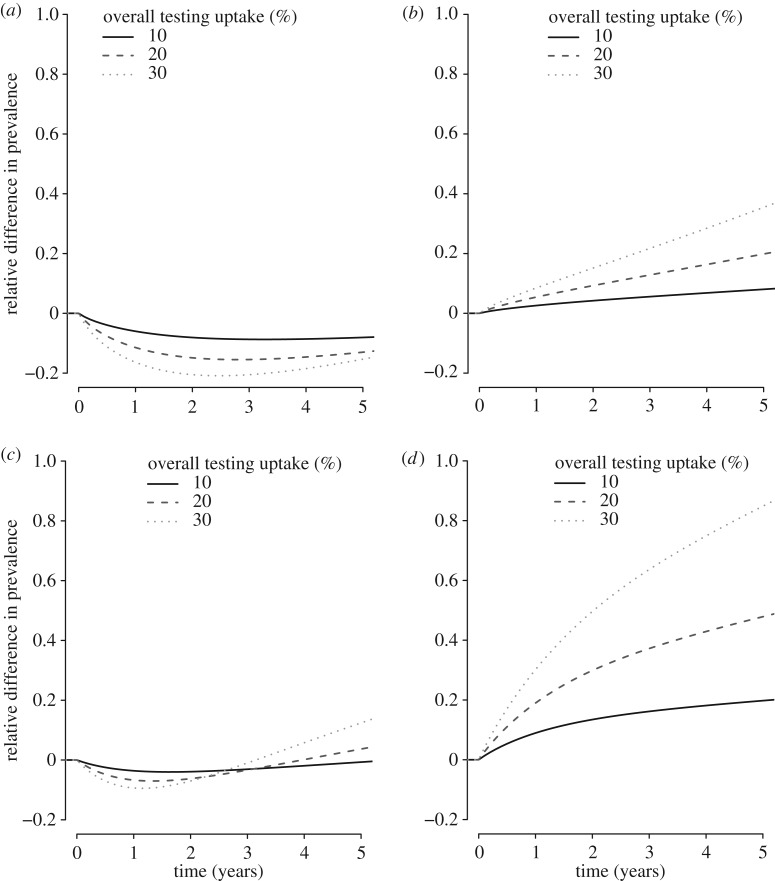
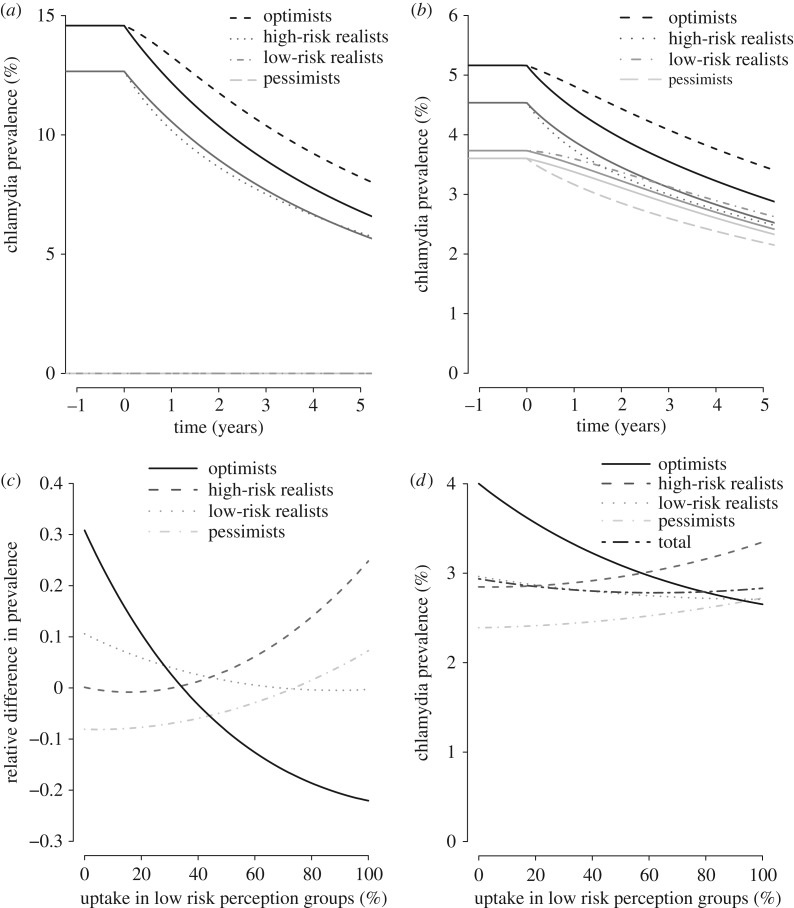
We explored how changing the overall testing uptake, changing the mixing parameter to 0 (fully assortative) or 1 (fully proportionate), and changing the distribution of testing uptake over the SB–RP groups in the SB–RP model affected the relative difference between the baseline testing scenario and the testing scenario with differential uptake in SB–RP groups. In all uncertainty analyses, we found that the optimist group was most sensitive to changes in the assumptions (greatest relative differences). First, we found that when the overall testing uptake increased to 20% or 30%, the relative difference increased in all SB–RP groups (figure 3). Second, although steady-state prevalences in the SB–RP groups differed for different values of the mixing parameter (figure 4a,b), the relative difference in chlamydia prevalence between the baseline testing scenario and the testing scenario with differential uptake in SB–RP groups, was hardly affected by the assumed value of the mixing parameter. Last, we showed that the impact of the testing scenario with differential testing uptake in SB–RP groups is equal to the impact of the baseline testing scenario (relative difference = 0) when the distribution of testing uptake is 34% in the optimists and 66% in the high-risk realists, and when the distribution of testing uptake is 73% in the low-risk realists and 27% in the pessimists (figure 4c). Furthermore, there is an optimal distribution of testing uptake in the SB–RP groups (44% in pessimists and high-risk realists, and 56% in low-risk realists and optimists), where the overall prevalence of chlamydia is lowest (figure 4d).
Figure 3.
Relative difference in chlamydia prevalence in the SB–RP model between the baseline testing scenario and the testing scenario with differential uptake in SB–RP groups for five consecutive years for different values of the overall testing uptake for pessimists (a), low-risk realists (b), high-risk realists (c) and optimists (d).
Figure 4.
Uncertainty analyses with the mixing parameter and different distributions of the testing uptakes in the SB–RP groups (SB–RP model). (a,b) The estimated chlamydia prevalence in each group for five consecutive years after the introduction of the baseline testing scenario (solid lines) and the testing scenario with differential uptake in SB–RP groups (dashed lines) for a value of the mixing parameter of zero (fully assortative mixing, a) or one (fully proportionate mixing, b). Note that the prevalence in the pessimists and low-risk realists is zero when the value of the mixing parameter is set to zero. (c) The relative difference in chlamydia prevalence between the baseline testing scenario and the testing scenario with differential uptake in SB–RP groups after 5 years is shown for different distributions of the testing uptake in the SB–RP groups, keeping an overall testing uptake of 10%. The testing uptake distribution over the SB–RP groups was explored continuously by steps of 1%, starting at uptake in low risk perception groups = 0 (0% uptake in the low-risk realists and optimists, and 100% uptake in the pessimists and high-risk realists) to uptake in low risk perception groups = 100 (100% uptake in the low-risk realists and optimists, and 0% uptake in the pessimists and high-risk realists). (d) The prevalence of chlamydia in each group and the total population, for these different distributions of testing uptake in SB–RP groups.
4. Discussion
This study showed that the effect of testing on chlamydia prevalence in the total population and different sexual behaviour groups is slightly smaller in a mathematical model incorporating risk perception as compared to a model without risk perception. In the model with risk perception, introducing differential testing uptake in risk perception groups resulted in an even smaller reduction in the overall chlamydia prevalence and the low risk perception groups, compared to a baseline testing scenario without differential uptake in risk perception groups. The impact of incorporating differential testing uptake in risk perception groups was largest in the optimist group (high-risk sexual behaviour in combination with low risk perception).
A strength of this study is that, to our knowledge, this is the first mathematical modelling study incorporating risk perception in the field of curable STIs. Two mathematical modelling studies have considered the effects of risk perception in the context of HIV [14,15]. In these models, risk perception was included as a factor influencing sexual behaviour, whereas in our model risk perception was incorporated as a factor influencing healthcare seeking behaviour. Another strength of this study is that the model was informed by real life data.
There are also limitations. First, the sample size of the survey to inform the model was small and the low response rate might have resulted in selection bias. However, parameter values obtained for this model (i.e. number of partners per year and percentage of people in a partnership) are comparable with parameter values used in other chlamydia models that were informed by large general population surveys [9,39]. Second, in the model, we assumed serial monogamy, but it is likely that some individuals have concurrent partnerships. Nevertheless, a previous modelling study exploring the impact of testing interventions and partner notification in a general population showed that including concurrency hardly affected the results [49]. Third, similar to other chlamydia modelling studies [36,38], we did not include a period of immunity after natural clearance of chlamydia in our model, because it is not clear if and for how long a period of immunity exists [50]. Fourth, we performed univariate uncertainty analyses only, changing a single parameter and keeping the other parameters fixed at their baseline values. Nevertheless, the univariate uncertainty analyses provided good insight into which parameters were most sensitive to changes in the assumptions. Furthermore, we did not perform uncertainty analyses using different cut-off values for defining the risk groups in the model. However, we used a median split to define the cut-off value for the risk perception groups and propose that increasing this cut-off value would result in larger low risk perception groups, and hence a larger overestimation of the effect of testing on the prevalence of chlamydia, especially in the optimists. Last, we assumed that testing uptake was the same for males and females. Previous research has shown that females are more likely to perceive themselves to be at high risk for STIs than males [51]. In line with this finding, estimates of the National Chlamydia Screening Programme (NSCP) in England and the Chlamydia Screening Implementation (CSI) in the Netherlands suggest that testing uptake in males is only half of that in females [45,52]. We speculate that adding these complexities to the model might change the distribution of males and females over the risk perception groups, and the impact of incorporating differential testing uptake might become larger in males than females.
Our results from the survey suggest that the number of sex acts and condom use is not only different between individuals with low- or high-risk sexual behaviour, but also within these groups between individuals with low or high risk perception. For example, in the high-risk sexual behaviour group, optimists considered themselves to be at lower risk for chlamydia than the high-risk realists, even though the number of condomless sex acts was higher in that group. The results from our model analyses suggest that models that do not incorporate these differences in sexual behaviour between risk perception groups (models without risk perception) slightly overestimate the effect of testing on the prevalence of chlamydia. However, the impact of incorporating differential testing uptake in risk perception groups is larger, which implies that the focus on models incorporating risk perception should be on differential testing uptake.
Models without differential testing uptake in risk perception groups overestimate the reduction in chlamydia prevalence, especially in the optimists. This means that interventions promoting STI testing targeted at the general population and/or high-risk sexual behaviour groups might not be enough to limit the transmission of chlamydia. Optimists are less likely to seek chlamydia testing, which means that the prevalence of chlamydia in this group remains higher than estimated in a model without risk perception. On the other hand, low-risk sexual behaviour does not mean that there is no risk. Testing uptake in the low-risk realists, who make up more than 50% of the model population, is low, and therefore many chlamydia infections in this group might remain undetected. Since optimists and low-risk realists also have partnerships with individuals in the other groups, the transmission of chlamydia in the total population will continue at a higher level than expected. Moreover, optimists have higher partner change rates than the other groups, which might make them possible drivers of chlamydia transmission, and this might contribute to the unchanged population chlamydia prevalence in young adults observed in many countries [24–27].
We found that increasing testing uptake in the low-risk realists and optimists (individuals with low risk perception), resulted in a slightly larger reduction in the overall chlamydia prevalence compared to increasing testing uptake in the pessimists and high-risk realists (individuals with high risk perception). This suggests that individuals with low risk perception, and especially individuals with low risk perception and high-risk sexual behaviour, might be a core group to be targeted in future. It has previously been suggested that health education interventions and health risk messages aimed at changing risk perception can contribute to increasing an individual's healthcare seeking behaviour, such as STI testing or vaccination [5,53,54]. Future modelling efforts could focus on exploring the impact of these types of interventions on individual risk perception, healthcare-seeking behaviour and the prevalence of chlamydia. This might improve our understandings and estimates of the impact of testing interventions on reducing chlamydia prevalence.
In conclusion, mathematical models incorporating risk perception and differential testing uptake in risk perception groups improve the impact assessment of testing and treatment on chlamydia prevalence. In interventions aimed at reducing chlamydia prevalence, more attention should be given to how individuals perceive their own risk of acquiring chlamydia and these interventions should be targeted more towards optimists.
Supplementary Material
Acknowledgement
We thank the survey participants for their time and contributions.
Ethics
The online survey was approved by the Medical Ethics Committee Noord-Holland, Alkmaar, the Netherlands (M016-024). All participants provide informed consent for participation in the survey.
Data accessibility
Data and R codes are available upon request.
Authors' contributions
C.d.D., J.C.M.H. and D.A.v.W. designed the survey. J.C.M.H. and D.A.v.W. developed the mathematical model. All authors contributed to the interpretation of the results, reviewed the manuscript and approved the final version for publication.
Competing interests
We declare we have no competing interests.
Funding
This project was funded by the Strategic Programme (SPR) of the National Institute for Public Health and the Environment (RIVM) (project number S/113004/01/IP). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript
References
- 1.Abel G, Brunton C. 2005. Young people's use of condoms and their perceived vulnerability to sexually transmitted infections. Aust. N Z J. Public Health 29, 254–260. ( 10.1111/j.1467-842X.2005.tb00764.x) [DOI] [PubMed] [Google Scholar]
- 2.Clifton S, Nardone A, Field N, Mercer CH, Tanton C, Macdowall W, Johnson AM, Sonnenberg P. 2016. HIV testing, risk perception, and behaviour in the British population. AIDS 30, 943–952. ( 10.1097/QAD.0000000000001006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravningen K, Braaten T, Schirmer H. 2015. Self-perceived risk and prevalent chlamydia infection among adolescents in Norway: a population-based cross-sectional study. Sex. Transm. Infect. 92, 91–96. ( 10.1136/sextrans-2014-051927) [DOI] [PubMed] [Google Scholar]
- 4.Ten Hoor GA, Ruiter RAC, van Bergen JEAM, Hoebe CJPA, Dukers-Muijrers NHTM, Kok G. 2016. Predictors of Chlamydia Trachomatis testing: perceived norms, susceptibility, changes in partner status, and underestimation of own risk. BMC Public Health 16, 55 ( 10.1186/s12889-016-2689-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfers ME, de Zwart O, Kok G. 2011. Adolescents in the Netherlands underestimate risk for sexually transmitted infections and deny the need for sexually transmitted infection testing. AIDS Patient Care STDS 25, 311–319. ( 10.1089/apc.2010.0186) [DOI] [PubMed] [Google Scholar]
- 6.van der Pligt J, Richard R. 1994. Changing adolescents’ sexual behaviour: perceived risk, self-efficacy and anticipated regret. Patient Educ. Couns. 23, 187–196. ( 10.1016/0738-3991(94)90034-5) [DOI] [PubMed] [Google Scholar]
- 7.Van der Velde FW, Hooykaas C, van der Pligt J. 1992. Risk perception and behavior: pessimism, realism, and optimism about AIDS-related health behavior. Psychol. Health 6, 23–38. ( 10.1080/08870449208402018) [DOI] [Google Scholar]
- 8.Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. 2011. Mathematical models in the evaluation of health programmes. Lancet 378, 515–525. ( 10.1016/S0140-6736(10)61505-X) [DOI] [PubMed] [Google Scholar]
- 9.Heijne JCM, Herzog SA, Althaus CL, Tao G, Kent CK, Low N. 2013. Insights into the timing of repeated testing after treatment for Chlamydia trachomatis: data and modelling study. Sex. Transm. Infect. 89, 57–62. ( 10.1136/sextrans-2011-050468) [DOI] [PubMed] [Google Scholar]
- 10.Kretzschmar MEE, Turner KM, Barton PM, Edmunds WJ, Low N. 2009. Predicting the population impact of chlamydia screening programmes: comparative mathematical modelling study. Sex. Transm. Infect. 85, 359–366. ( 10.1136/sti.2009.036251) [DOI] [PubMed] [Google Scholar]
- 11.Kretzschmar MEE, van Duynhoven YT, Severijnen AJ. 1996. Modeling prevention strategies for gonorrhea and Chlamydia using stochastic network simulations. Am. J. Epidemiol. 144, 306–317. ( 10.1093/oxfordjournals.aje.a008926) [DOI] [PubMed] [Google Scholar]
- 12.Althaus CL, Turner KM, Schmid BV, Heijne JCM, Kretzschmar MEE, Low N. 2012. Transmission of Chlamydia trachomatis through sexual partnerships: a comparison between three individual-based models and empirical data. J. R Soc. Interface 2, 136–146. ( 10.1098/rsif.2011.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MI, Ghani AC, Edmunds WJ. 2009. A metapopulation modelling framework for gonorrhoea and other sexually transmitted infections in heterosexual populations. J. R Soc. Interface 6, 775–791. ( 10.1098/rsif.2008.0394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tully S, Cojocaru M, Bauch CT. 2013. Coevolution of risk perception, sexual behaviour, and HIV transmission in an agent-based model. J. Theor. Biol. 337, 125–132. ( 10.1016/j.jtbi.2013.08.014) [DOI] [PubMed] [Google Scholar]
- 15.Tully S, Cojocaru M, Bauch CT. 2015. Sexual behavior, risk perception, and HIV transmission can respond to HIV antiviral drugs and vaccines through multiple pathways. Sci. Rep. 5, 15411 ( 10.1038/srep15411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronn MM, Wolf EE, Chesson H, Menzies NA, Galer K, Gorwitz R, Gift T, Hsu K, Salomon JA. 2017. The use of mathematical models of chlamydia transmission to address public health policy questions: a systematic review. Sex. Transm. Dis. 44, 278–283. ( 10.1097/OLQ.0000000000000598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verelst F, Willem L, Beutels P. 2016. Behavioural change models for infectious disease transmission: a systematic review (2010–2015). J. R Soc. Interface 13, 20160820 ( 10.1098/rsif.2016.0820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control E. 2016. Chlamydia—Annual Epidemiological Report 2016 [2014 data]. Stockholm: ECDC. See https://ecdc.europa.eu/en/publications-data/chlamydia-annual-epidemiological-report-2016-2014-data.
- 19.Van den Broek IVF, et al. 2016. Sexually Transmitted Infections in the Netherlands in 2015. Bilthoven: Centre for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM) See http://www.rivm.nl/Documenten_en_publicaties/Wetenschappelijk/ Rapporten/2016/juni/Sexually_transmitted_infections_in_the_Netherlands_in_2015. [Google Scholar]
- 20.Visser M, et al. 2017. Sexually transmitted infections including HIV, in the Netherlands in 2016. Bilthoven: Centre for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM) See http://www.rivm.nl/Documenten_en_publicaties/Wetenschappelijk/ Rapporten/2017/Juni/Sexually_transmitted_infections_including_HIV_in_the_Netherlands_in_2016. [Google Scholar]
- 21.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. 2010. Risk of sequelae after Chlamydia trachomatis genital infection in women. J. Infect. Dis. 201(Suppl. 2), S134–S155. ( 10.1086/652395) [DOI] [PubMed] [Google Scholar]
- 22.Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L et al. . 2008. Sexually transmitted diseases, 4th edn New York, NY: McGraw-Hill. [Google Scholar]
- 23.Low N, Heijne JC, Herzog SA, Althaus CL. 2014. Reinfection by untreated partners of people treated for Chlamydia trachomatis and Neisseria gonorrhoeae: mathematical modelling study. Sex. Transm. Infect. 90, 254–256. ( 10.1136/sextrans-2013-051279) [DOI] [PubMed] [Google Scholar]
- 24.Datta SD, Torrone E, Kruszon-Moran D, Berman S, Johnson R, Satterwhite CL, Papp J, Weinstock H. 2012. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sex. Transm. Dis. 39, 92–96. ( 10.1097/OLQ.0b013e31823e2ff7) [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg P, et al. 2013. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the national surveys of sexual attitudes and lifestyles (Natsal). Lancet 382, 1795–1806. ( 10.1016/S0140-6736(13)61947-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unemo M, et al. 2017. Sexually transmitted infections: challenges ahead. Lancet Infect. Dis. 17, e235–e279. ( 10.1016/S1473-3099(17)30310-9) [DOI] [PubMed] [Google Scholar]
- 27.van den Broek IVF, et al. 2012. Effectiveness of yearly register-based screening for chlamydia in the Netherlands: controlled trial with randomised stepped wedge implementation. BMJ 345, e4316 ( 10.1136/bmj.e4316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samkange-Zeeb F, Pottgen S, Zeeb H. 2013. Higher risk perception of HIV than of chlamydia and HPV among secondary school students in two German cities. PLoS ONE 8, e61636 ( 10.1371/journal.pone.0061636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller WC, Ford CA, Morris MK, Handcock MS, Schmitz JL, Hobbs MM et al. . 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291, 2229–2236. ( 10.1001/jama.291.18.2229) [DOI] [PubMed] [Google Scholar]
- 30.de Graaf H, Meijer S, Poelman J, Vanwesenbeeck I. 2005. Seks onder je 25e: seksuele gezondheid van jongeren in nederland anno 2005 [In Dutch]. Delft, The Netherlands: Uitgeverij Eburon. [Google Scholar]
- 31.Mollema L, De Melker H, Hahné S, Van Weert J, Berbers G, Van Der Klis F.2010. PIENTER 2-project: second research project on the protection against infectious diseases offered by the national immunization programme in the Netherlands. See http://www.rivm.nl/Documenten_en_publicaties/Wetenschappelijk/ Rapporten/2010/maart/PIENTER_2_project_second_research_project_on_the_protection_against_ infectious_diseases_offered_by_the_national_immunization_programme_in_the_Netherlands .
- 32.Wolfers ME, Kok G, Mackenbach JP, de Zwart O. 2010. Correlates of STI testing among vocational school students in the Netherlands. BMC Public Health 10, 725 ( 10.1186/1471-2458-10-725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan AD, Aiken LS, West SG. 1997. Young women's condom use: the influence of acceptance of sexuality, control over the sexual encounter, and perceived susceptibility to common STDs. Health Psychol. 16, 468 ( 10.1037/0278-6133.16.5.468) [DOI] [PubMed] [Google Scholar]
- 34.Lauby JL, Bond L, Eroğlu D, Batson H. 2006. Decisional balance, perceived risk and HIV testing practices. AIDS Behav. 10, 83–92. ( 10.1007/s10461-005-9029-7) [DOI] [PubMed] [Google Scholar]
- 35.Strauss ME, Smith GT. 2009. Construct validity: advances in theory and methodology. Annu. Rev. Clin. Psychol. 5, 1–25. ( 10.1146/annurev.clinpsy.032408.153639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzog SA, Heijne JC, Scott P, Althaus CL, Low N. 2013. Direct and indirect effects of screening for Chlamydia trachomatis on the prevention of pelvic inflammatory disease: a mathematical modeling study. Epidemiology 24, 854–862. ( 10.1097/EDE.0b013e31829e110e) [DOI] [PubMed] [Google Scholar]
- 37.Johnson AM, et al. 2001. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet 358, 1835–1842. ( 10.1016/S0140-6736(01)06883-0) [DOI] [PubMed] [Google Scholar]
- 38.Heijne JCM, van Liere GA, Hoebe CJ, Bogaards JA, van Benthem BH, Dukers-Muijrers NH. 2016. What explains anorectal chlamydia infection in women? Implications of a mathematical model for test and treatment strategies. Sex. Transm. Infect. 93, 270–275. ( 10.1136/sextrans-2016-052786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heijne JCM, Althaus CL, Herzog SA, Kretzschmar M, Low N. 2010. The role of reinfection and partner notification in the efficacy of chlamydia screening programs. J. Infect. Dis. 203, 372–377. ( 10.1093/infdis/jiq050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kretzschmar MEE, Dietz K. 1998. The effect of pair formation and variable infectivity on the spread of an infection without recovery. Math. Biosci. 148, 83–113. ( 10.1016/S0025-5564(97)10008-6) [DOI] [PubMed] [Google Scholar]
- 41.Althaus CL, Heijne JCM, Roellin A, Low N. 2010. Transmission dynamics of Chlamydia trachomatis affect the impact of screening programmes. Epidemics 2, 123–131. ( 10.1016/j.epidem.2010.04.002) [DOI] [PubMed] [Google Scholar]
- 42.Price MJ, Ades AE, Angelis DD, Welton NJ, Macleod J, Soldan K, Turner K, Simms I, Horner PJ. 2013. Mixture-of-exponentials models to explain heterogeneity in studies of the duration of Chlamydia trachomatis infection. Stat. Med. 32, 1547–1560. ( 10.1002/sim.5603) [DOI] [PubMed] [Google Scholar]
- 43.Brent RP. 2013. Algorithms for minimization without derivatives. Courier Corporation.
- 44.Rutgers. 2016. Kerncijfers leefstijlmonitor seksuele gezondheid 2016 [In Dutch]. See https://www.rutgers.nl/sites/rutgersnl/files/PDF/Leefstijlmonitor%20SG%202016_0.pdf
- 45.Schmid BV, et al. 2013. Effects of population based screening for Chlamydia infections in the Netherlands limited by declining participation rates. PLoS ONE 8, e58674 ( 10.1371/journal.pone.0058674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Broek IVF, et al. 2010. Evaluation design of a systematic, selective, internet-based, Chlamydia screening implementation in the Netherlands, 2008–2010: implications of first results for the analysis. BMC Infect. Dis. 10, 89 ( 10.1186/1471-2334-10-89) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velicko I, Ploner A, Sparen P, Marions L, Herrmann B, Kuhlmann-Berenzon S. 2016. Sexual and testing behaviour associated with Chlamydia trachomatis infection: a cohort study in an STI clinic in Sweden. BMJ Open 6, e011312 ( 10.1136/bmjopen-2016-011312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Development Core Team. 2017. R: A language and environment for statistical computing. V:3.4.2. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 49.Althaus CL, Heijne JCM, Herzog SA, Roellin A, Low N. 2012. Individual and population level effects of partner notification for Chlamydia trachomatis. PLoS ONE 7, e51438 ( 10.1371/journal.pone.0051438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlieb SL, Martin DH, Xu F, Byrne GI, Brunham RC. 2010. Summary: The natural history and immunobiology of Chlamydia trachomatis genital infection and implications for Chlamydia control. J. Infect. Dis. 201(Suppl. 2), S190–S204. ( 10.1086/652401) [DOI] [PubMed] [Google Scholar]
- 51.Leval A, Sundstrom K, Ploner A, Dahlstrom LA, Widmark C, Sparen P. 2011. Assessing perceived risk and STI prevention behavior: a national population-based study with special reference to HPV. PLoS ONE 6, e20624 ( 10.1371/journal.pone.0020624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Public Health England N.C.S.P. 2016. Sexually transmitted infections and chlamydia screening in England, 2015. Public Health England. See https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables.
- 53.De Wit JBF, Das E, Vet R. 2008. What works best: objective statistics or a personal testimonial? An assessment of the persuasive effects of different types of message evidence on risk perception. Health Psychol. 27, 110 ( 10.1037/0278-6133.27.1.110) [DOI] [PubMed] [Google Scholar]
- 54.de Wit JBF, Vet R, Schutten M, van Steenbergen J. 2005. Social-cognitive determinants of vaccination behavior against hepatitis B: an assessment among men who have sex with men. Prev. Med. 40, 795–802. ( 10.1016/j.ypmed.2004.09.026) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R codes are available upon request.