Abstract
Octopus and cephalopods are able to regenerate injured tissues. Recent advancements in the study of regeneration in cephalopods appear promising encompassing different approaches helping to decipher cellular and molecular machinery involved in the process. However, lack of specific markers to investigate degenerative/regenerative phenomena and inflammatory events occurring after damage is limiting these studies. Label-free multiphoton microscopy is applied for the first time to the transected pallial nerve of Octopus vulgaris. Various optical contrast methods including coherent anti-Stokes Raman scattering (CARS), endogenous two-photon excited fluorescence (TPEF) and second harmonic generation (SHG) have been used. We detected cells and structures often not revealed with classical staining methods. CARS highlighted the involvement of haemocytes in building up scar tissue; CARS and TPEF facilitated the identification of degenerating fibres; SHG allowed visualization of fibrillary collagen, revealing the formation of a connective tissue bridge between the nerve stumps, likely involved in axon guidance. Using label-free multiphoton microscopy, we studied the regenerative events in octopus without using any other labelling techniques. These imaging methods provided extremely helpful morpho-chemical information to describe regeneration events. The techniques applied here are species-specific independent and should facilitate the comparison among various animal species.
Keywords: Octopus vulgaris, label-free multiphoton microscopy, CARS, TPEF, SHG, nerve regeneration
1. Introduction
Efficient regeneration, which is represented by restoration of lost structures and functions, can occur at several levels, i.e. tissues, structures, organs, including the striking feature of whole body regeneration [1]. Among all the tissues that can regenerate, the ability of nervous structures to re-grow after damage, even with some limitations depending on the species, appears to be widespread across invertebrate and vertebrate species [2–5], including mammals. Cephalopods are invertebrates that possess the capacity of healing and full regeneration of appendages (i.e. arms, tentacles, fins) [6–8], internal organs (e.g. brachial gland, brachial heart) [9] and tissues (i.e. skin, nerves, muscles) [10–12].
Nerve regeneration in these species appears of particular interest, as complete transection of a nerve is followed by full recovery of the structure and re-innervation of the end targets [13]. Pallial nerves, a pair of nerves connecting the central brain to the periphery, proved to be a valuable model for the investigation of nervous tissue regeneration in this species. The structural changes occurring within the nerve after injury were described at the beginning of the twentieth century by Sereni & Young [11], extended by Sanders & Young [13] and recently re-examined in detail [14]. Unilateral complete transection of this nerve in O. vulgaris determines in the peripheral stump a process similar to Wallerian degeneration, characterized by intense axon fragmentation. Fibres in the central stump regenerate quickly, followed two weeks later by regeneration of axons in the peripheral stump. Haemocytes (i.e. octopus' blood cells) appear to have a leading role in the process: they enrich at the lesion site, contribute to scar formation between the two stumps and become highly proliferative especially between regenerating fibres. Connective tissue appears to be involved in driving the two stumps towards each other.
Although direct imaging of the injured tissues represents an extremely advantageous approach for the evaluation of the degenerative and regenerative phenomena, there are only a limited number of specific markers commercially available for researchers working with cephalopods or other invertebrate species.
In recent years, new microscopy methods were made available for the study of regeneration in vertebrate models. Label-free multiphoton microscopy including coherent anti-Stokes Raman scattering (CARS) and two-photon microscopy proved indeed to be extremely helpful for the imaging and study of axonal injury after spinal cord or sciatic nerve contusion or lesioning ex vivo and in vivo [15–18]. In the mammalian nervous system, CARS microscopy has been mainly used to detect tissue lipid content (particularly myelin); using two-photon excited fluorescence (TPEF), endogenous fluorescent molecules can be detected while second harmonic generation (SHG) highlights myosin and fibrillar collagen [19–21]. The combination of these techniques provided a great deal of information on axons and myelin degeneration and regeneration, extracellular matrix composition and microglia/macrophages distribution without the need for any marker or antibody. This imaging technology visualizes preserved chemical tissue properties and is, therefore, not depending on species-related epitopes.
Here, we applied label-free multiphoton microscopy on lesioned pallial nerves of O. vulgaris for the first time to characterize the regenerative response of cells and tissue involved without the need for antibodies or other labelling approaches.
2. Material and methods
2.1. Animals
Octopus vulgaris (N = 9; body weight: 250–350 g) of both sexes (males = 4, females = 5) were caught in the Bay of Naples (Italy) and kept in laboratory conditions (seawater temperature range: 18–20°C) where they were daily fed with live crabs, according to standardized procedures [22]. Animals were selected for the absence of any kind of injury or regenerative phenomena [14].
Cephalopod molluscs are included in Directive 2010/63/EU and, therefore, the sole invertebrate taxon ‘regulated’ at EU level for scientific research purposes [23,24]. Experiments with live octopuses included in this study were carried out before transposition of Directive 2010/63/EU in Italy. Although no authorization was required, all procedures were performed in order to minimize the suffering and distress of the animals involved [23–25].
2.2. Surgery and samples collection
Surgery was performed on anaesthetized animals (3.5% MgCl2 in seawater for 15 min), as described in Imperadore et al. [14]. Briefly, animals were turned on their ventral side and a scalpel was used to incise muscles and connective tissue enveloping the pallial nerve. The two nerves (from left and right side) were exposed with a hooklet. The left one was then positioned back intact and served as sham control, while the right one was completely transected with fine scissors under a stereo microscope. Following surgery, all animals were allowed to recover and positioned back in their tank until they were sacrificed at 3 (N = 3), 7 (N = 3) or 14 (N = 3) days post lesion. Tissue at harvesting was fixed in paraformaldehyde 4% in seawater (90 min), washed in PBS and embedded in freezing and blocking medium (OCT; Leica Biosystems). Samples were stored at −80°C until use. Subsequently, sections (30 µm) were obtained with a cryostat and processed.
2.3. Multiphoton microscopy
After thawing, cryostat sections were rehydrated in PBS, covered with a glass coverslip and subjected to label-free multiphoton microscopy. Imaging was obtained with an optical microscope Axio Examiner Z.1 coupled to a laser scanning module LSM 7 (Carl Zeiss AG, Jena, Germany) equipped with non-descanned detectors. An erbium fibre laser (Femto Fiber pro NIR from Toptica Photonics AG, Munich, Germany) provides excitation for TPEF and SHG by emitting at 781 nm with a pulse length of 1.2 ps and a maximum nominal power of 100 mW.
The TPEF signal was acquired in the spectral range 500–550 nm, while the SHG signal was retrieved using a bandpass filter centred at 390 nm. CARS excitation needed a second laser source, i.e. the Femto Fiber pro TNIR from Toptica Photonics AG which is tunable in the range 850–1100 nm and has a pulse length of 0.8 ps. In all CARS experiments, the wavelength was set to 1005 nm (emitted power 1.5 mW), in order to resonantly excite the symmetric stretching vibration of methylene groups at 2850 cm−1. CARS, TPEF and SHG were simultaneously excited and acquired with a W Plan-Apochromat 20×/1.0 (Carl Zeiss AG) to build multimodal RGB images (red channel: CARS; green channel: TPEF; blue channel: SHG).
2.4. Light microscopy
Following imaging, the coverslip was removed from the slides using a PBS bath. Slides were then either used for immunohistochemistry or stained with haematoxylin and eosin (H&E) or Nauta–Gygax. H&E staining was performed as follows: 2 min in Meyer haematoxylin followed by 5 min in tap water and 20 s in eosin.
Nauta–Gygax staining protocol adapted for cephalopod tissues was performed following Lund [26]. In brief, sections were washed in distilled water and placed in 50% ethyl alcohol (100 parts) and ammonia solution (one part) for 6 h. After three changes in distilled water, slides were immersed in a bath of 10% silver nitrate (in water) for 16 h. The following day, they were washed again in distilled water and transferred to an ammoniacal silver bath: 4.5% silver nitrate (40 ml), ethyl alcohol 100% (20 ml), ammonia solution (4 ml), sodium hydroxide 2.5% (2.8 ml) for 1 min. Afterwards, slides were transferred to the standard Nauta reducer for 1 min: distilled water (50 ml), ethyl alcohol 100% (5.6 ml), formalin 4% (1.7 ml), citric acid 1% (1.7 ml), washed briefly in distilled water and fixed in 5% sodium thiosulfate for 1 min.
Immunohistochemical staining was performed as previously described [14]. In brief, after blocking in normal goat serum (NGS) 5% in PBT (PBS + Tween 0.1%) 1 h at room temperature, sections were incubated with primary antibody (anti-acetylated tubulin, SIGMA T6793; 1:1000) in PBT and NGS 1%, overnight at 4°C. Following washing in PBT, sections were incubated with secondary antibodies (1:250, Alexa Fluor goat anti-rat IgG (H + L) 488) for 1 h at room temperature. DAPI (diluted 1:1000 in PBS) was then used to counterstain nuclei.
Sections were mounted in PBS and imaged again with multiphoton microscopy; stained sections (H&E or Nauta–Gygax) were instead dehydrated in an ethanol series, cleared in xylene, coverslipped using DePex and imaged using either Axio Examiner Z.1 (Carl Zeiss AG) equipped with camera AxioCam or Axio Scope.A1 (Carl Zeiss AG) equipped with camera Canon DS126231.
3. Results
After unilateral pallial nerve transection, the animals were sacrificed at 3-, 7- or 14-days post lesion. Both pallial nerves (sham and cut) connected to the stellate ganglion were harvested and imaged using label-free multiphoton microscopy. To facilitate image interpretation, a schematic drawing depicting the connections between the brain and stellate ganglion through the pallial nerves is presented in each figure.
3.1. Sham nerve
Two examples of sham control nerves are displayed in figures 1 and 2. CARS imaging (displayed in red in merged images) enabled assessment of the entire structure of the pallial nerve and the stellate ganglion, as it was possible to detect signal from axons in the nerve (figures 1b–d and 2b) and from the neuropil and neurons in the stellate ganglion (figure 1b,e). In cephalopods, axons are not myelinated. However, axonal structures and cells are visualized by CARS based on the signals of CH2 groups in membrane lipids and cytoplasmic proteins and fatty acids.
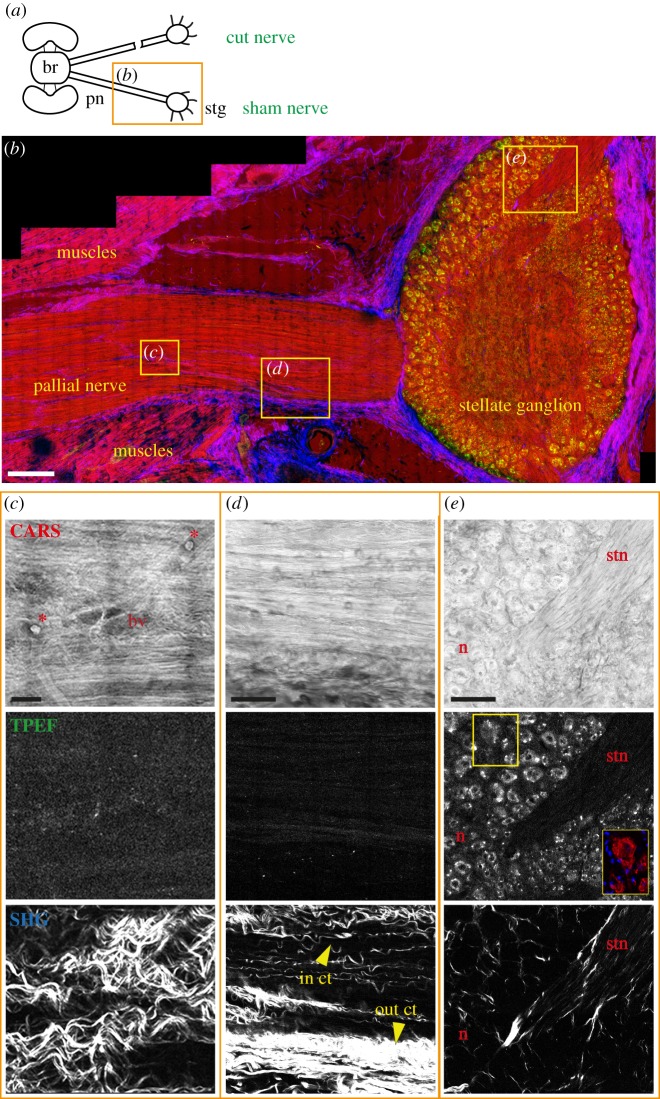
Figure 1.
Sham control nerve. (a) Schematic drawing showing the connections between the brain (br) and stellate ganglion (stg) through the right and left pallial nerves (pn). The orange rectangle delimits the area of the nerve shown in the figure. The sham nerve is shown in (b) after multiphoton imaging. CARS signals (red in b) highlighted the structure of axons in the pallial nerve (b–d) and the neuropil, neurons (n) and stellar nerves (stn) inside the stellate ganglion (b,e). CARS signal also showed blood vessels (bv) and haemocytes (red asterisks) in the nerve (c). In (b) also muscles appear to give a strong signal in CARS. SHG (blue in b) allowed visualization of the outer connective tissue (out ct) enwrapping the pallial nerve (b,d) and the stellate ganglion (b), and the inner connective tissue (in ct) surrounding bundles of fibres inside the pallial nerve (c,d). TPEF signal (in green) was strongly detected only in the neurons of the stellate ganglion (e). Neurons were identified via acetylated tubulin labelling, visible in red in the yellow box in (e). Glial cells around neurons are not highlighted using multiphoton microscopy, but their nuclei are detected via DAPI counterstain (blue in the yellow box in e). Scale bars: (b) 250 µm, (c) 20 µm, (d,e) 50 µm.
Figure 2.
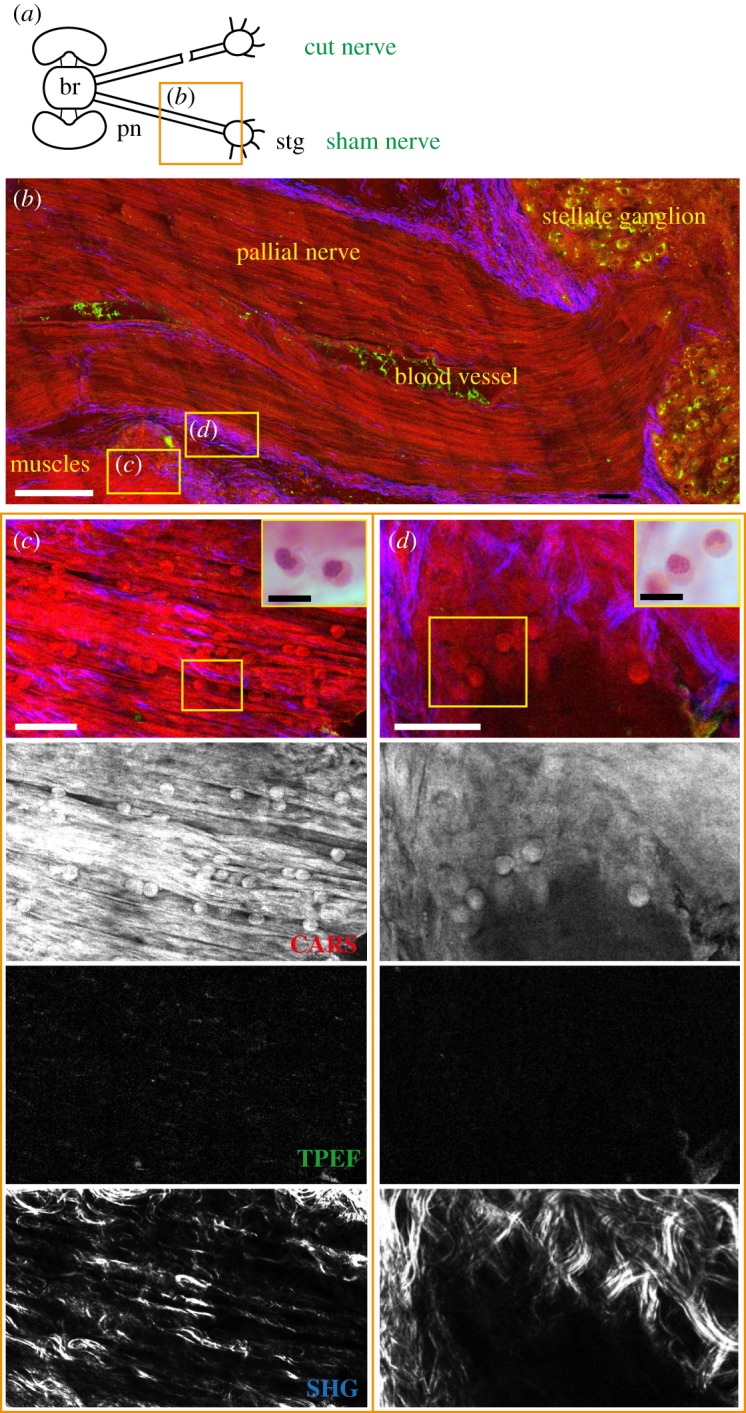
Haemocytes infiltrating sham tissues. (a) Schematic drawing showing the connections between the brain (br) and stellate ganglion (stg) through the right and left pallial nerves (pn). The orange rectangle delimits the area of the nerve shown in the figure panel. (b) A blood vessel is detected inside the sham nerve via CARS. Haemocytes were visualized in this vessel and infiltrating the damaged tissues, like muscles (c) and connective tissue (d) injured to expose the nerve, as round spheres with a diameter of 7–12 µm. This is confirmed by H&E staining, which highlighted the classical U-shaped nuclei of haemocytes (yellow box enlargements in c and d). TPEF did not give detectable signal in muscles or connective tissue as shown in single channel images (TPEF in c and d). Connective tissue is detected in the pallial nerve forming axon bundles, enveloping the whole nerve (b). It also gives a strong signal inside the muscle (c) surrounding the nerve. Scale bars: (b) 250 µm, (c) 20 µm, (d) 25 µm, yellow box in (c,d) 10 µm.
Tissue and cellular structures including muscles, blood vessels and haemocytes were clearly visualized using CARS (figures 1 and 2). It was possible to identify small vessels (with a diameter of around 15–20 µm) including their branches (figure 1c), as well as the larger vessel running within the pallial nerve (figure 2b). Haemocytes were highlighted inside the nerve as round structures with a diameter ranging between 7 and 12 µm. They were identified either within the nerve-associated blood vessels (figure 1c), or within the muscles (figure 2c) and connective tissue (figure 2d) lesioned to expose the nerve. Reference staining of the same section, indeed, allowed identification of these blood cells recognized by their characteristic U-shaped nuclei (see yellow box magnifications in figure 2c,d).
Using TPEF (shown in green in merged images), it was also possible to clearly reveal the cytoplasm of the neurons inside the stellate ganglion (figure 1b,e). Conversely, glial cells around these neurons did not give any signal in TPEF, thus allowing a distinction between the two (figure 1e). This was also confirmed by antibody labelling, as neurons were marked with anti-acetylated tubulin (showed in red in the yellow box in figure 1e), while glia and other cells were counterstained with DAPI.
A weak TPEF signal was detected in the intact axons both of the pallial nerve and of the stellar nerves or into the neuropil of the stellate ganglion (figure 1c–e).
SHG highlighted the connective tissue in the investigated structures (displayed in blue in merged images; figures 1 and 2). It was possible to distinguish the outer connective tissue (out ct; figure 1d) enwrapping the whole nerve (figures 1b and 2b) and ganglion (figure 1b) and also the inner connective tissue (in ct; figure 1d), which divides axon fibres into bundles (figure 1d,e). Connective tissue also appears to be involved in sealing the lesioned tissues around the sham lesioned nerves.
Muscular tissue around the nerve was perfectly distinguishable from the nervous structures using CARS and SHG contrast (figures 1b and 2b,c), whereas no TPEF signal is detected (figure 2c).
3.2. Injured nerve
3.2.1. Three days post lesion
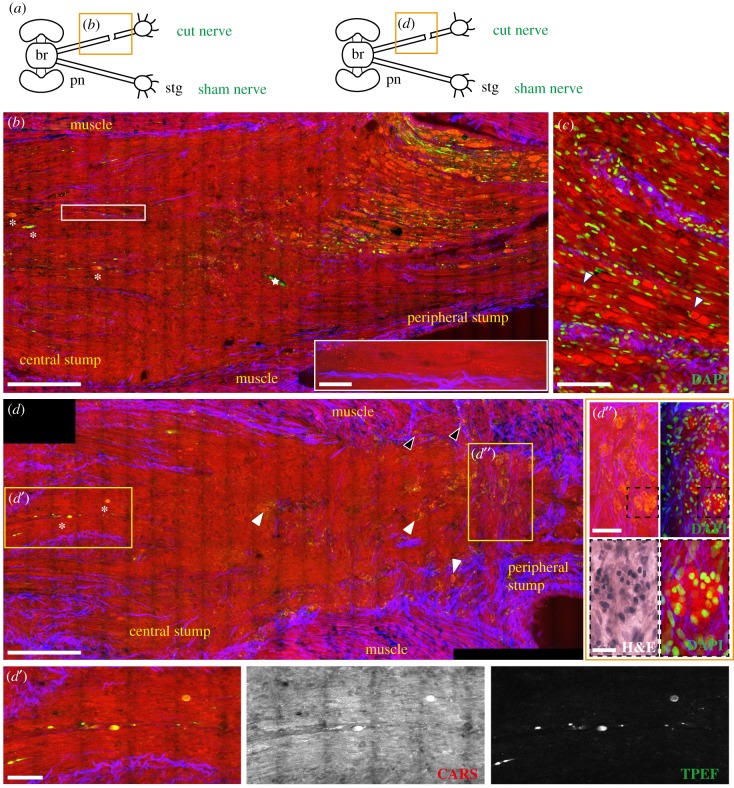
As for the sham control nerves, CARS allowed imaging of the entire structure of the nerve and ganglion and, additionally, also of the injured site. In this case, it was indeed possible to visualize the two stumps induced by the lesion (namely the central and the peripheral stumps) (figure 3b) and the scar separating the two (figure 3b–d).
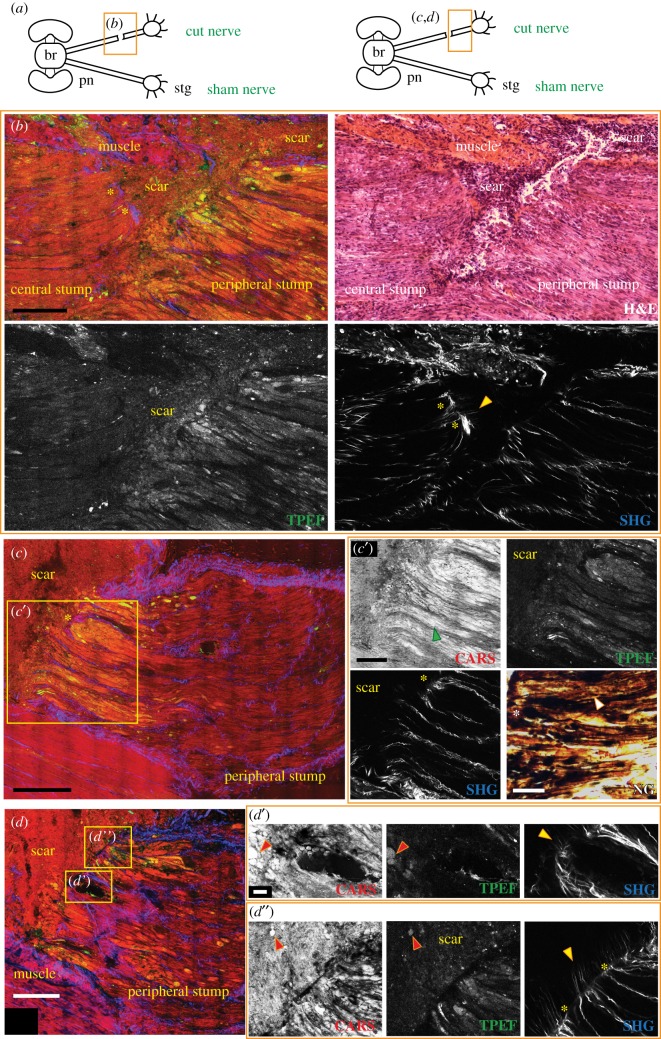
Figure 3.
Injured nerve 3 days post lesion. (a) Schematic drawing showing the connections between the brain (br) and stellate ganglion (stg) through the right and left pallial nerves (pn). The orange rectangle delimits the area of the nerve shown in the figure panel. (b) The lesion site of a nerve 3 days post lesion imaged by multiphoton microscopy and later stained using H&E. In the merged image, the two stumps of the nerve appear to be separated by a scar which also forms in the muscular tissue. The scar is highlighted by CARS and TPEF. Axons in both stumps are seen to degenerate, especially in the peripheral stump characterized by fibres swelling and fragmenting (b,c,c′, CARS and TPEF; see green arrow in c′ CARS) and debris were also detected as round structures inside the scar that forms between the two stumps (highlighted by red arrows in d′ and d″, CARS and TPEF). Degeneration is further confirmed by Nauta–Gygax (NG) staining (c′) where axon tip swelling is marked by white asterisks and small granules of fragmenting axons are highlighted by white arrows. Connective tissue appears to seal around cut axons of both stumps (b,c′, SHG marked with yellow asterisks) but also to stick out straight from them invading the scar (b,d′,d″, SHG, marked with yellow arrows). A blood vessel is also visible in the peripheral stump. It contains haemocytes that are released inside the scar (d′, CARS) and contribute to its formation. Scale bars: (b–d) 250 µm, (c′) 125 µm, (c′, NG) 50 µm, (d′,d″) 20 µm.
The structure of the axons, especially in the peripheral stump, appeared remarkably altered already 3 days post injury. In particular, degenerating fibres belonging to this stump appeared fragmented and swollen (figure 3b,c,c′, green arrow), especially if compared with the uninjured ones of the sham nerves (figures 1 and 2) and with those far from the lesion, which were not affected yet by degenerative phenomena at 3 days post lesion. Confirmation of the degenerative nature of these fibres was obtained by Nauta–Gygax staining which allowed discrimination between degenerating and intact axons [26], the former having the appearance of small granules and presenting tip swelling (figure 3c′ NG, white arrow and asterisk, respectively), while the latter appearing as continuous fibres.
Increased CARS signal intensity was observed in the degenerating axons than in the intact ones. Some of these fragmented axons also showed an intense TPEF signal (figure 3b,c′), which is again hardly visible in intact fibres (figure 1c,d). This massive increase in TPEF and CARS signal intensity in degenerating fibres 3 days after transection was found in all three animals investigated and made it possible to measure the area affected by degenerative phenomena in the peripheral stump which was around 500–700 µm from the scar.
The central stump was also characterized by degenerative phenomena, visible in CARS and TPEF, although to a lesser extent than the peripheral stump (figure 3b).
The scar structures forming between the stumps and inside the surrounding tissues were visualized by CARS and TPEF (figure 3b). They allowed detection of small round spheres with a diameter around 10 µm localized at the edge of the peripheral stump and within the scar (red arrows, figure 3d′,d″). Using H&E staining, it was possible to identify the composition of the scar which appeared mainly to consist of cells and degeneration debris (i.e. axon fragments) (figure 3b, H&E) that co-localize with the spheres.
It is known that haemocytes contribute to the formation of the cicatricial tissue. Indeed, injured vessels inside both nerve stumps were captured releasing blood cells into the scar (figure 3d,d′).
The inner connective tissue (imaged by SHG) was observed to seal around transected axons of both stumps (yellow asterisks in figure 3b,c,c′,d″), but it also appeared to stick out straight in thin filaments penetrating the scar (yellow arrows in figure 3b,d′,d″, SHG).
3.2.2. Seven days post lesion
At 7 days after injury, degenerating fibres in the peripheral stump were likewise characterized by intense TPEF and CARS signal intensity. The area affected by degeneration increased with time: the majority of the axon fragments were observed in an area of around 1100–1300 µm from the scar for the three animals (based on increased CARS signal and TPEF) (figure 4b,d,d′) although some degenerating fibres had reached the ganglion (white arrows in figure 4d, CARS and TPEF). At this time point, axon fragments displayed an ovoidal shape, detected again by CARS and TPEF (magnification in white box in figure 4b).
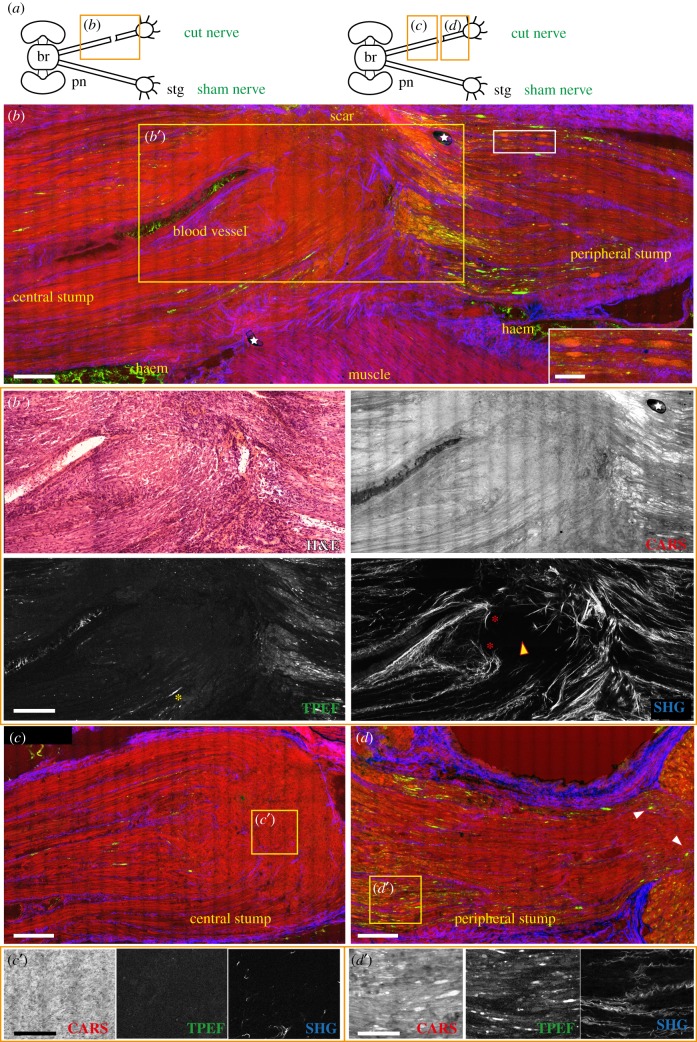
Figure 4.
Injured nerve 7 days post lesion. (a) Schematic drawing showing the connections between the brain (br) and stellate ganglion (stg) through the right and left pallial nerves (pn). The orange rectangle delimits the area of the nerve shown in the figure panel. The lesioned nerve 7 days post lesion is shown in (b). The scar has now a limited size, mainly confined to the side. Blood vessels running in the central stump and haemorrhagic areas (haem) around the nerve are visible, especially due to blood giving a strong signal in TPEF (green in b). Degenerating fibres occupy a large area of the peripheral stump, observable via CARS and TPEF. Some of them assume an ovoidal shape (highlighted in the white box enlargement in b). Some degenerating fibres are also visible in the central stump (b′) with TPEF (highlighted by yellow asterisk) well organized in rows. The inner connective tissue grows backwards to seal the cut axons (red asterisks in b′, SHG). Beyond this point, regenerating axons from the stump grow as a bulk of disorganized fibres (c′, CARS) where connective tissue is not present (also marked with yellow arrow in b′, SHG). (d) Degeneration in the peripheral stump involves a large number of fibres (d′, CARS and TPEF) which also get closer to the ganglion (see white arrows in d). White stars in figure (b) indicate artefacts. Scale bars: (b,b′,c,d) 250 µm, (white insert in b) 100 µm, (c′,d′) 50 µm.
The TPEF signal appeared even more evident in the few degenerating axons of the central stump, where they were extremely limited in number (figure 4b,b′). Degenerating fibres here appeared to be well organized in rows (yellow asterisk in figure 4b′, TPEF).
At 7 days post lesion, the nerve was also characterized by intense regeneration of the fibres in the central stump. Even though growing fibres are not highlighted by multiphoton imaging, it is still possible to recognize them. The original site of injury could be indeed identified by the peculiar behaviour of the internal connective tissue, appearing to grow backward around severed axons (red asterisk in figure 4b′, SHG). Beyond this point, regenerating fibres grew towards the opposite stump in an area delimited by the outer connective tissue. In the area of new growing axons, connective tissue is mainly absent (yellow arrow in figure 4b′, and c,c′; figure 5a,a′, SHG) confirming our previous data [14]. These axons differed from uninjured or degenerating ones as they appeared in CARS as a disorganized bulk of fibres (figure 4b,c).
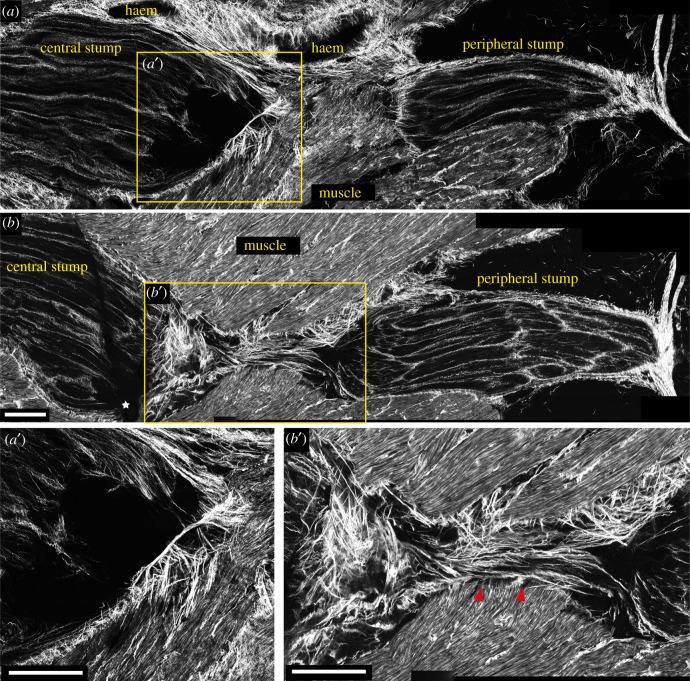
Figure 5.
Connective tissue (SHG signal) in injured nerve 7 days post lesion. (a,b) The outer connective tissue sealed around the cut stumps of the nerve 7 days post lesion, shaping the central stump in a spike-like structure. (a′) Regenerating fibres in the central stump lack inner connective tissue. (b,b′) Shows how close to the muscular tissue underneath the lesioned nerve, connective tissue forms a bridge that fills the gap between the two stumps (marked by red arrows in b′). White stars in figure (b) indicate artefacts. Scale bar (a,a′,b,b′): 250 µm.
The analysis of the SHG signal revealed that the outer connective tissue narrowed around the two stumps, starting to shape the central stump to a spike-like structure (figure 5a,b). Proceeding deeper in the tissue, closer to the muscular tissue underneath the nerve, it was possible to observe the formation of a connective tissue bridge connecting the two stumps (figure 5b, red arrows in figure 5b′).
The area occupied by the scar was highly reduced compared to 3 days after injury and was located laterally to the nerve (figure 4b).
Haemorrhagic areas in the muscles and connective tissues around the nerve were also observable, filled with blood highlighted by TPEF (figure 4b). Those areas were caused by the procedure of nerve exposure as they were visible at all time points and also in sham control nerves.
3.2.3. Two weeks post lesion
Swollen axons and ovoidal fragments (figure 6b,c, CARS and TPEF) increased in number two weeks post injury and mostly occupied the entire length of the peripheral stump. Although some of them appeared to have a circular structure inside resembling a nucleus, DAPI counterstain allowed us to exclude the presence of cells within the axonal fragments, (arrows in figure 6c, CARS and DAPI). Again, a limited number of degenerating axons were visible in the central stump (white asterisks in figure 6b,d, and figure 6d′, CARS and TPEF). Two weeks after lesion was, however, characterized by the presence of small granules with intense CARS signal (white rectangle in figure 6b and figure 6d′), apparently aligned to axonal orientation.
Figure 6.
Injured nerve 14 days post lesion. (a) Schematic drawing showing the connections between the brain (br) and stellate ganglion (stg) through the right and left pallial nerves (pn). The orange rectangle delimits the area of the nerve shown in the figure panel. (b,d) The central and peripheral stumps of a nerve 14 days post lesion. The majority of the fibres in the peripheral stump appear swollen and fragmented, while the few degenerating axons in the central stump are marked with white asterisks (also visible in d′, CARS and TPEF). Small granules, arranged in rows are visible in the central stump (enlargement in the white rectangle in b). In (c) the peripheral stump is counterstained with DAPI, showing that cell nuclei are outside the ovoidal fragments and not infiltrating them. Some of these fragments contain a structure resembling a nucleus (marked with white arrows) which, however, does not stain with DAPI. Drop shaped structures were identified in the lesioned nerve (white arrows in d; enlargement in d″) and muscles (black arrows in d), highlighted by CARS and TPEF (red and green in d). Counterstain with DAPI allowed identification of those structures as cell clusters (d″) surrounded by connective tissue (d″ SHG). H&E staining (black dotted box in d″) slightly stained these structures. White stars in figure (b) indicate artefacts. Scale bars: (b,d) 250 µm, (c) 100 µm, (d′,d″) 50 µm, (e) 20 µm, white rectangle in (b) 25 µm.
Spread between the central and the peripheral stumps, proximal to the original site of lesion, we identified peculiar structures never observed before. These drop-shaped structures, visible in CARS and TPEF, appeared to be filled with small round granules that gave a strong signal in CARS (white arrows in figure 6d and figure 6d″). They were slightly highlighted via H&E staining (see black dotted box in figure 6d″, H&E). Those structures were only found 14 days after injury and in all three animals investigated at this time point. Counterstaining with DAPI allowed the identification of these structures as cluster of cells (figure 6d″, DAPI). Some of these clusters were also observed in the muscles that were injured to expose the nerve (black arrows in figure 6d).
Connective tissue appeared to tightly wrap around these structures, growing in an unusual direction, from one side of the nerve to the other (figure 6d,d″, SHG).
4. Discussion
Despite the recent increasing interest of the scientific community towards cephalopods, one of the main issues in their use as research animals remains the lack of specific antibodies, markers and advanced techniques for imaging [27], and tracking the biological machinery involved, for example, in regenerative phenomena [14]. Direct imaging of lesioned structures is, indeed, one of the most advantageous and informative approaches used in regenerative studies. Synergically with the development of custom-made markers for this taxon, the establishment of methods alternative to classical approaches is a priority in our view.
It is for this aim that we tested multiphoton microscopy on O. vulgaris samples of intact and lesioned pallial nerve. The combination of CARS, TPEF and SHG not only allowed confirmation of the results obtained with classical imaging methods, but also provided additional information that could not be obtained via immunohistochemistry and histological staining.
Old and recent studies [11,14] on the unilateral complete transection of the pallial nerve revealed the occurrence of an inflammatory response involving haemocytes which infiltrate the two stumps and form a scar in between the two. Additionally, while the peripheral stump appears mainly characterized by degeneration, fibres in the central stump intensely regenerate towards the stellate ganglion during the first two weeks.
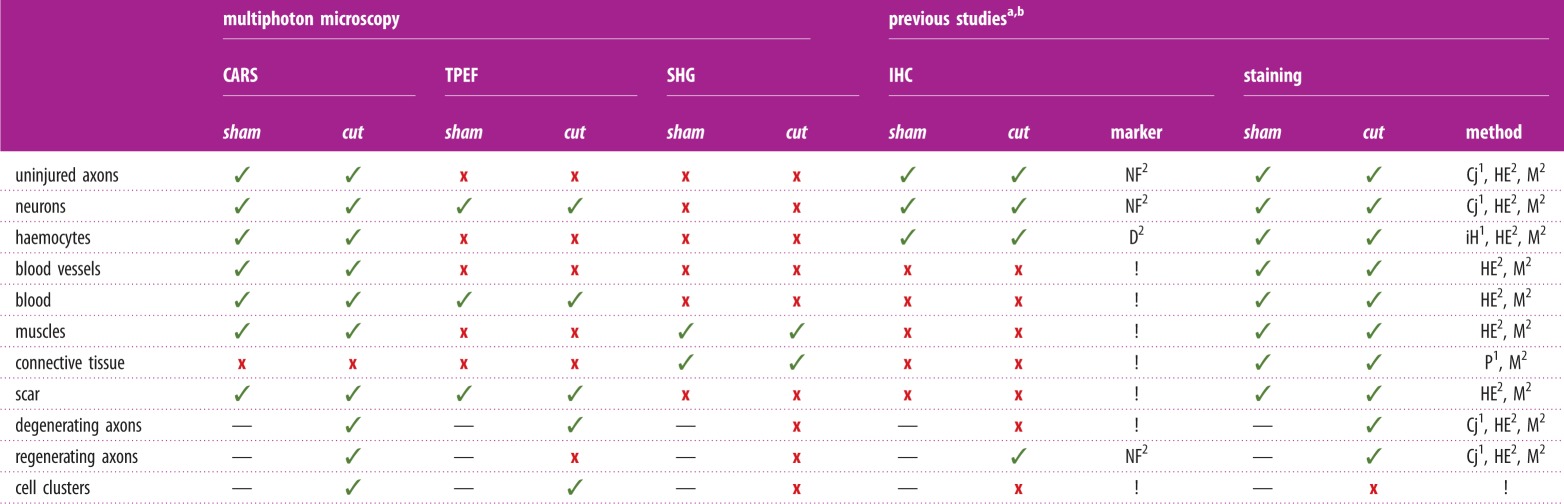
Imaging using label-free multiphoton microscopy for the first time in octopus proved to be extremely valuable as it was possible to assess tissue morphology in total and to highlight structures that are usually hardly or not detectable with classical methods, which remain hidden in the tissue (table 1).
Table 1.
Structure detection via multiphoton microscopy and classical approaches. An overview of the structures highlighted in sham and transected (cut) pallial nerves using multiphoton microscopy. CARS, TPEF and SHG signals, using one single scanning, allow detection of all the structures of interest. Similar outcomes are obtained with classical approaches, as performed in Sereni & Young [11] or Imperadore et al. [14], and different markers and histological staining have to be used. For structures detected (✓), not detected (x), not present (—), marker not available or not used for this study (!). NF, NF200 antibody; D, DAPI; HE, haematoxylin and eosin; M, Masson's trichrome; Cj, Cajal's stain; iH, iron haematoxylin; P, picronigrosin. (Online version in colour.)
 |
First of all, the CARS signal of muscles, axons and neurons, revealed the structure of the pallial nerve and stellate ganglion, while TPEF mainly highlighted neurons in the ganglia, likely due to fluorescence emitted by the metabolic waste product lipofuscin, which is known to accumulate in cephalopod nervous tissues [28,29].
Transection of the nerve, however, determined axon degeneration. This was highlighted by a strong TPEF signal and also by an increase of CARS signal intensity at all time points investigated. The two signals were extremely helpful for the detection of the few degrading fibres of the central stump, which are usually difficult to observe with other methodologies. Underlying causes might include accumulation of lipin and waste material. Sereni & Young [11], indeed, observed that lesioned axons (in the pallial or stellar nerves) accumulate lipins, while phagocytic haemocytes appear filled with lipid droplets.
Haemocytes were also visualized by CARS, confirming unambiguously their contribution in the inflammatory response triggered by the insult. Indeed, these cells are usually only recognized by nuclei shape and topographic cues when using IHC, as no specific marker has ever been identified.
Clusters of cells, probably involved in debris removal, were also highlighted; they were shaped in drop-like structures which gave an intense and dotted signal in CARS.
Connective tissue was instead detected by SHG, which allowed us to image outer connective tissue enwrapping the whole nerve and ganglion and inner connective tissue enveloping bundle of axons in the nerve. The sheets of connective tissue, enwrapping individual axons in the nerve are instead only slightly visible in SHG, possibly due to the different nature of these structures. Only fibrillar collagens are SHG-active (mainly type I and less type III). We postulate that this connective tissue is a non-fibrillar collagen (like collage IV), but no evidence is available at the moment on any cephalopod collagen tissue, to the best of our knowledge.
Seven days post lesion, connective tissue was observed to bridge between the two stumps of the nerve, which might provide guidance for axons and re-innervation of their target tissue.
These results are obtained by multiphoton microscopy with one single scanning sweep allowing detection of all structures of interest. Furthermore, the integrity of the molecules and structures is not altered by this process, allowing additional processing and staining of these same samples, e.g. antibody-based staining and imaging.
Compared to conventional methodologies, similar results can be achieved. However, different approaches (IHC and histological staining) have to be used to highlight the different structures, increasing the number of samples to be processed (table 1).
Nonlinear optical microscopy (NLOM) provides excellent optical contrast but is not molecule specific. Therefore, NLOM is not specific for the structure observed, as in the case of haemocytes. The latter are indeed also detected using DAPI, HE or Masson's trichrome; however; they highlight all nuclei present in the tissues and not only blood cells. Detection of blood cells with CARS is instead univocal and unmistakable.
Although in this study, multiphoton imaging was applied on tissue sections, this technology holds the potential for in vivo investigations as it is non-invasive and can be applied without any induction of tissue photodamage [30,31]. The combination of microscopy and endoscope-like graded-index lenses has already opened the possibility of performing imaging of nervous structures in rodents in vivo [32–34] and multiphoton endoscopic imaging systems were developed [35–37]. In the near future, these might enable deep tissues to be accessed in vivo by the use of fibre-based devices.
The promising outcomes obtained in this study open new strategies to study regeneration in cephalopods. Lack of specific markers for this taxon appears to be at least partially overcome by label-free multiphoton imaging. These methods might be extended to other lines of cephalopod research and different non-mammalian animal species lacking specific nerve markers.
For instance, it remains to be seen whether some regeneration patterns, including age-related decline in nerve regeneration ability [38–40], may also be conserved in cephalopods and explore the possibility of using multiphoton approach for the investigation.
Acknowledgements
This work has been initiated through a Short Term Scientific Mission of the COST Action FA1301 granted to P.I. The authors acknowledge COST and the COST Action FA1301 ‘A network for improvement of cephalopod welfare and husbandry in research, aquaculture and fisheries (CephsInAction)’ for support and for increasing networking capabilities. The authors are grateful to Dr G. Ponte for the continuous advice.
Data accessibility
This article has no additional data.
Authors' contributions
P.I. carried out all experiments, analysed the data and drafted the manuscript; O.U. and R.G. contributed to two-photon imaging applications to cephalopod tissue and drafted the manuscript; M.K. and G.S. contributed to the experimental design; G.F. designed the experiments and revised the final manuscript. All authors contributed to the final writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was also supported by the Stazione Zoologica Anton Dohrn (Napoli, Italy) and the RITMARE Flagship Project (SP2-WP4-A3; MIUR & CNR, Italy) to G.F. P.I. is supported by the Association for Cephalopod Research-CephRes.
References
- 1.Bely AE, Nyberg KG. 2010. Evolution of animal regeneration: re-emergence of a field. Trends Ecol. Evol. 25, 161–170. ( 10.1016/j.tree.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 2.Bejjani RE, Hammarlund M. 2012. Neural regeneration in Caenorhabditis elegans. Annu. Rev. Genet. 46, 499–513. ( 10.1146/annurev-genet-110711-155550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockes JP, Kumar A. 2008. Comparative aspects of animal regeneration. Annu. Rev. Cell Dev. Biol. 24, 525–549. ( 10.1146/annurev.cellbio.24.110707.175336) [DOI] [PubMed] [Google Scholar]
- 4.Chen Z-L, Yu W-M, Strickland S. 2007. Peripheral regeneration. Annu. Rev. Neurosci. 30, 209–233. ( 10.1146/annurev.neuro.30.051606.094337) [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Brockes JP. 2012. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci 35, 691–699. ( 10.1016/j.tins.2012.08.003) [DOI] [PubMed] [Google Scholar]
- 6.Fossati SM, Candiani S, Nodl MT, Maragliano L, Pennuto M, Domingues P, Benfenati F, Pestarino M, Zullo L. 2015. Identification and expression of acetylcholinesterase in Octopus vulgaris arm development and regeneration: a conserved role for ACHE? Mol. Neurobiol. 52, 45–56. ( 10.1007/s12035-014-8842-2) [DOI] [PubMed] [Google Scholar]
- 7.Lange MM. 1920. On the regeneration and finer structure of the arms of the cephalopods. J. Exp. Zool. 31, 1–57. ( 10.1002/jez.1400310102) [DOI] [Google Scholar]
- 8.Tressler J, Maddox F, Goodwin E, Zhang Z, Tublitz N. 2014. Arm regeneration in two species of cuttlefish Sepia officinalis and Sepia pharaonis. Invert. Neurosci. 14, 37–49. ( 10.1007/s10158-013-0159-8) [DOI] [PubMed] [Google Scholar]
- 9.Taki I. 1964. On the morphology and physiology of branchial gland in Cephalopoda. J. Fac. Fish. Anim. Husbandry Hiroshima Univ. 5, 345–417. [Google Scholar]
- 10.Polglase JL, Bullock AM, Roberts RJ. 1983. Wound healing and the hemocyte response in the skin of the lesser octopus Eledone cirrhosa (Mollusca, Cephalopoda). J. Zool. 201, 185–204. ( 10.1111/j.1469-7998.1983.tb04269.x) [DOI] [Google Scholar]
- 11.Sereni E, Young JZ. 1932. Nervous degeneration and regeneration in cephalopods. Pubbl. Staz. Zool. Napoli. 12, 173–208. [Google Scholar]
- 12.Zullo L, Fossati SM, Imperadore P, Nödl MT. 2017. Molecular determinants of cephalopod muscles and their implication in muscle regeneration. Front. Cell Dev. Biol. 5, 53 ( 10.3389/fcell.2017.00053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders GD, Young JZ. 1974. Reappearance of specific colour patterns after nerve regeneration in Octopus. Proc. R. Soc. Lond. B 186, 1–11. ( 10.1098/rspb.1974.0031) [DOI] [PubMed] [Google Scholar]
- 14.Imperadore P, Shah SB, Makarenkova HP, Fiorito G. 2017. Nerve degeneration and regeneration in the cephalopod mollusc Octopus vulgaris: the case of the pallial nerve. Sci. Rep. 7, 46564 ( 10.1038/srep46564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uckermann O, et al. 2015. Endogenous two-photon excited fluorescence provides label-free visualization of the inflammatory response in the rodent spinal cord. BioMed Res. Int. 2015, 1–9. ( 10.1155/2015/859084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenzana AO, Lee JK, Mui M, Chang A, Zheng B. 2015. A surviving intact branch stabilizes remaining axon architecture after injury as revealed by in vivo imaging in the mouse spinal cord. Neuron 86, 947–954. ( 10.1016/j.neuron.2015.03.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli R, et al. 2012. Vibrational spectroscopic imaging and multiphoton microscopy of spinal cord injury. Anal. Chem. 84, 8707–8714. ( 10.1021/ac301938m) [DOI] [PubMed] [Google Scholar]
- 18.Bélanger E, Henry FP, Vallée R, Randolph MA, Kochevar IE, Winograd JM, Lin CP, Côté D. 2011. In vivo evaluation of demyelination and remyelination in a nerve crush injury model. Biomed. Opt. Express 29, 2698–2708. ( 10.1364/BOE.2.002698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. 2003. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl Acad. Sci. USA 100, 7075–7080. ( 10.1073/pnas.0832308100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schain AJ, Hill RA, Grutzendler J. 2014. Label-free in vivo imaging of myelinated axons in health and disease with spectral confocal reflectance microscopy. Nat. Med. 20, 443–449. ( 10.1038/nm.3495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imitola J, Côté D, Rasmussen S, Xie XS, Liu Y, Chitnis T, Sidman RL, Lin CP, Khoury SJ. 2011. Multimodal coherent anti-Stokes Raman scattering microscopy reveals microglia-associated myelin and axonal dysfunction in multiple sclerosis-like lesions in mice. J. Biomed. Opt. 16, 021109 ( 10.1117/1.3533312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amodio P, Andrews PLR, Salemme M, Ponte G, Fiorito G. 2014. The use of artificial crabs for testing predatory behavior and health in the octopus. Altex 31, 1–12. ( 10.14573/altex.1401282) [DOI] [PubMed] [Google Scholar]
- 23.Fiorito G, et al. 2014. Cephalopods in neuroscience: regulations, research and the 3Rs. Invert. Neurosci. 14, 13–36. ( 10.1007/s10158-013-0165-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorito G, et al. 2015. Guidelines for the care and welfare of cephalopods in research—a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 49, 1–90. ( 10.1177/0023677215580006) [DOI] [PubMed] [Google Scholar]
- 25.Andrews PLR, Darmaillacq AS, Dennison N, Gleadall IG, Hawkins P, Messenger JB, Osorio D, Smith VJ, Smith JA. 2013. The identification and management of pain, suffering and distress in cephalopods, including anesthesia, analgesia and humane killing. J. Exp. Mar. Biol. Ecol. 447, 46–64. ( 10.1016/j.jembe.2013.02.010) [DOI] [Google Scholar]
- 26.Lund RD. 1965. The staining of degeneration in the nervous system of the octopus by modified silver methods. J. Cell Sci. 3, 115–117. [Google Scholar]
- 27.Wollesen T, Loesel R, Wanninger A. 2009. Pygmy squids and giant brains: mapping the complex cephalopod CNS by phalloidin staining of vibratome sections and whole-mount preparations. J. Neurosci. Methods 179, 63–67. ( 10.1016/j.jneumeth.2009.01.021) [DOI] [PubMed] [Google Scholar]
- 28.Semmens J, Pecl G, Villanueva R, Jouffre D, Sobrino I, Wood J, Rigby P. 2004. Understanding octopus growth: patterns, variability and physiology. Mar. Freshw. Res. 55, 367–377. ( 10.1071/MF03155) [DOI] [Google Scholar]
- 29.Doubleday ZA, Semmens JM. 2011. Quantification of the age-pigment lipofuscin in known-age octopus (Octopus pallidus): a potential tool for age determination. J. Exp. Mar. Biol. Ecol. 397, 8–12. ( 10.1016/j.jembe.2010.11.010) [DOI] [Google Scholar]
- 30.Fu Y, Wang H, Shi R, Cheng J-X. 2006. Characterization of photodamage in coherent anti-Stokes Raman scattering microscopy. Opt. Express. 14, 3942–3951. ( 10.1364/OE.14.003942) [DOI] [PubMed] [Google Scholar]
- 31.Galli R, Uckermann O, Andresen EF, Geiger KD, Koch E, Schackert G, Steiner G, Kirsch M. 2014. Intrinsic indicator of photodamage during label-free multiphoton microscopy of cells and tissues. PLoS ONE 9, e110295 ( 10.1371/journal.pone.0110295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belanger E, Crépeau J, Laffray S, Vallée R, De Koninck Y, Côté D. 2012. Live animal myelin histomorphometry of the spinal cord with video-rate multimodal nonlinear microendoscopy. J. Biomed. Opt. 17, 021107 ( 10.1117/1.JBO.17.2.021107) [DOI] [PubMed] [Google Scholar]
- 33.Prouty AM, Wu J, Lin D-T, Camacho P, Lechleiter JD. 2006. Multiphoton laser scanning microscopy as a tool for Xenopus oocyte research. In Xenopus protocols. Methods in molecular biology (ed. Liu XJ.), pp. 87–101. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- 34.Kim P, Pouris’ haag M, Côté D, Lin CP, Yun S-H. 2008. In vivo confocal and multiphoton microendoscopy. J. Biomed. Opt. 13, 010501 ( 10.1117/1.2839043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Légaré F, Evans CL, Ganikhanov F, Xie XS. 2006. Towards CARS endoscopy. Opt. Express 14, 4427–4432. ( 10.1364/OE.14.004427) [DOI] [PubMed] [Google Scholar]
- 36.Deladurantaye P, et al. 2014. Advances in engineering of high contrast CARS imaging endoscopes. Opt. Express 22, 25 053–25 064. ( 10.1364/OE.22.025053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukić A, Dochow S, Chernavskaia O, Latka I, Matthäus C, Schwuchow A, Schmitt M, Popp J. 2016. Fiber probe for nonlinear imaging applications. J. Biophotonics 9, 138–143. ( 10.1002/jbio.201500010) [DOI] [PubMed] [Google Scholar]
- 38.Zou Y, Chiu H, Zinovyeva A, Ambros V, Chuang C-F, Chang C. 2013. Developmental decline in neuronal regeneration by the progressive change of two intrinsic timers. Science 340, 372–376. ( 10.1126/science.1231321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Painter MW, et al. 2014. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 83, 331–343. ( 10.1016/j.neuron.2014.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivakhnitskaia E, Lin RW, Hamada K, Chang C. 2018. Timing of neuronal plasticity in development and aging. Wiley Interdiscip. Rev. Dev. Biol. 7, e305 ( 10.1002/wdev.305) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.