Abstract
Sclerostin, a product of the Sost gene, is a Wnt-inhibitor and thus negatively regulates bone accrual. Canonical Wnt/β-catenin signalling is also known to be activated in mechanotransduction. Sclerostin neutralizing antibodies are being tested in ongoing clinical trials to target osteoporosis and osteogenesis imperfecta but their interaction with mechanical stimuli on bone formation remains unclear. Sost knockout (KO) mice were examined to gain insight into how long-term Sost deficiency alters the local mechanical environment within the bone. This knowledge is crucial as the strain environment regulates bone adaptation. We characterized the bone geometry at the tibial midshaft of young and adult Sost KO and age-matched littermate control (LC) mice using microcomputed tomography imaging. The cortical area and the minimal and maximal moment of inertia were higher in Sost KO than in LC mice, whereas no difference was detected in either the anterior–posterior or medio-lateral bone curvature. Differences observed between age-matched genotypes were greater in adult mice. We analysed the local mechanical environment in the bone using finite-element models (FEMs), which showed that strains in the tibiae of Sost KO mice are lower than in age-matched LC mice at the diaphyseal midshaft, a region commonly used to assess cortical bone formation and resorption. Our FEMs also suggested that tissue mineral density is only a minor contributor to the strain distribution in tibial cortical bone from Sost KO mice compared to bone geometry. Furthermore, they indicated that although strain gauging experiments matched strains at the gauge site, strains along the tibial length were not comparable between age-matched Sost KO and LC mice or between young and adult animals within the same genotype.
Keywords: Sost, sclerostin, maturation, mechanical strain, finite-element analysis
1. Introduction
Sclerostin, a product of the Sost gene, negatively regulates bone accrual through Wnt inhibition [1–4]. A deficiency in sclerostin leads to a high bone mass phenotype that is associated with increased bone strength in humans and mice [1]. Mutations of the Sost gene and a non-coding deletion 35 kb downstream of this gene leads, respectively, to sclerosteosis and van Buchem's disease in humans [5,6]. Both conditions have similar phenotypes characterized by osteosclerosis and hyperostosis. Apart from syndactyly and minor digital deformities, prenatal skeletal development is normal in humans with sclerosteosis. Postnatal development is marked by increased longitudinal growth, although closure of the growth plates occurs normally [7]. Also an increased bone mineral density (BMD) with increasing age has been reported in sclerosteosis patients, starting from early childhood [7–9]. Studies on bone material properties performed by Lierop and colleagues [7] revealed lower mineralization and higher heterogeneity of mineralization in young and adult sclerosteosis patients. Also the mineral maturity to crystallinity ratio was lower in these patients compared to healthy controls. These findings are likely to be explained, at least in part, by the higher bone formation observed in sclerosteosis patients, since newer tissue has a lower mineralization.
The Sost knockout (KO) mouse model also presents a high bone mass phenotype [10,11], although limited characterization of the bone composition [12,13] and morphology [10,14] has been published. Two studies found the mineral-to-matrix ratio of newly formed cortical and trabecular bone to be significantly lower in Sost KO mice than in age-matched wild-type (WT) mice [12,13], while no differences in global bone mineralization were found between the Sost KO and WT mice when looking at the whole bone cross section and not just at the newly formed tissue [13]. Contrarily, Li et al. and Kramer et al. have shown significant higher tissue mineral density (TMD) in trabecular bone of Sost KO mice compared to WT [10,11].
As most studies have concentrated on young mice, little is known as to how Sost deficiency alters whole bone geometry and TMD after skeletal maturation. Whole bone geometry and TMD affect the local strain environment within the bone, and thus regulate bone adaptation processes [15,16]. Main et al. [17] showed that the bones of 26 week old C57Bl/6 mice are more curved than those of 10 week old C57Bl/6 mice, which increases their bending stresses under axial compression loading. The increase in the curvature counteracts effects that age-related increases in mineral and geometric properties of the cross-section have on the bending stresses. No clinical or preclinical studies have yet examined how changes in bone mass or whole bone morphology due to Sost deficiency affect the local mechanical strain environment within the bone. This knowledge is particularly relevant since the local strain environment regulates the anabolic response to loading.
Since the anabolic response to loading did not occur with high Sost levels [18], it appears that lower levels of Sost are essential for the anabolic response to loading to occur. We recently showed an increased bone formation surface (MS/BS) after in vivo mechanical loading in 10 week and 26 week old Sost KO mice compared to age-matched littermate control (LC) mice [19]. We also observed a greater load-induced (interlimb difference) total bone gain (Ct.Ar/T.Ar, Ct.Th) in both young and adult Sost KO mice compared to LC mice, the interlimb difference being the difference between the loaded and non-loaded limb of the same animal. Histomorphometric parameters revealed that this gain was achieved through a higher load-induced bone formation at both the periosteal and endocortical surface in the adult Sost KO, whereas just the periosteal surface showed a higher load-induced response to loading in young Sost KO compared to young LC mice. In contrast, Robling et al. [20], showed that load-induced ulnar periosteal bone formation occurred normally in Sost KO mice. Morse et al. [14] observed a greater response to loading in 10 week old Sost KO mice compared to WT at the metaphyseal region (Ct.Th: +23% in Sost KO, +8% in WT), but a similar response at the diaphyseal region located at 37% of the total tibial length (Ct.Th: +15% in Sost KO, +15% in WT). We recently showed in WT mice that the tibial metaphyseal and mid-diaphyseal regions have a different strain-threshold above which an anabolic response to loading in cortical bone occurs [21], which may explain the region-specific differences. At the 50% mid-diaphyseal region, the same region reported in our study, Morse et al. observed a slightly lower response to loading in Sost KO compared to WT mice (Ct.Th: 11% in Sost KO, 14% in WT).
It is known that the bone anabolic response to loading is diminished in adult compared to young individuals, both in humans and mice [1,22]. Our data show that there is also a reduction of bone mechanoresponsiveness in Sost KO mice occurring with maturation [19]. Clinical trials are currently underway to examine the effect of monoclonal antibodies inhibiting sclerostin on bone mass of individuals with osteoporosis [23,24], an age-associated disease, and osteogenesis imperfecta [25], a lifelong disease associated with low bone mass and compromised bone quality. As we are interested in the potential additive effect of loading and sclerostin antibodies on bone gain during growth and skeletal maturation, it is important that we understand how the strain environment changes with skeletal maturation under long-term Sost deficiency. Therefore, the aim of this study is to investigate how long-term Sost deficiency alters whole bone geometry and TMD and subsequently affects the local strain environment within the bone, since this regulates bone adaptation. Furthermore, we aim to comprehend how the strain environment changes with skeletal maturation under long-term Sost deficiency. We hypothesized that strains will be lower in Sost KO than in LC mice in regions different from the strain gauge site despite being identical in that particular position (anterior–medial surface of the cortical mid-diaphysis). We tested this hypothesis by investigating the local strain distribution in the bone by in vivo strain gauge experiments and finite-element models (FEMs) of young and adult female Sost KO and LC mice. These data are critical to our understanding of how long-term Sost deficiency is affected by skeletal maturation and whether mechanical loading could enhance bone mass concurrently with sclerostin-neutralizing antibody treatment.
2. Material and methods
2.1. Animals and strain gauging experiments
In this study, mouse tibiae were used to investigate alterations in the bone mechanical behaviour due to Sost deficiency and maturation. These mouse tibiae were obtained from 10 and 26 week old Sost KO and LC mice (n = 5 for 10 week old LC, n = 7 for 26 week old LC and 10 week and 26 week old Sost KO), which had been used in a previous strain gauging experiment [19]. The limbs were excised after in vivo, experimental strain measurement and euthanization.
2.2. Ex vivo microCT
Ex vivo microCT imaging of the Sost KO and LC bone tibiae was performed. For scan acquisition (Skyscan 1172, Bruker, Kontich, Belgium), the following settings were used: isotropic voxel resolution of 9.91 µm, 100 kVp, 100 µA, 360°, using 0.3° rotation steps and 3 frames averaging. We segmented background and soft tissue voxels from bone voxels by applying a global threshold of 0.650 g cm−3 in both ages and genotypes. This threshold, which kept both cancellous and cortical bone voxels, was calculated using the linear attenuation coefficient (μ) distribution of the whole tibia and calculating the minimum between the bone and the soft tissue peaks. Two phantoms with densities of 0.25 and 0.75 g HA cm−3 were used to calculate the tissue mineral density (TMD) based on the μ of the scans. Frequency plots of the microCT images after densitometric calibration can be found in the electronic supplementary material, data S2.
2.3. Bone curvature and morphometric parameters of the cortical bone
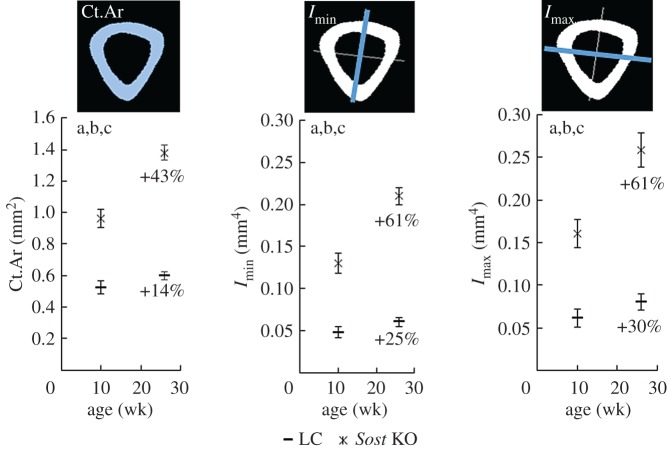
We analysed bone curvature and bone morphometric parameters using microCT images (n = 5, for 10 week old LC; n = 7, 26 week old LC and 10 week and 26 week old Sost KO). The binarized scans were manually aligned using commercial software (DataViewer, Bruker, Kontich, Belgium). The minimal and maximal moments of inertia (Imin, Imax) were calculated for each microCT slice located in the tibial midshaft (5% of the total tibial length centred at 50% of the total tibial length) and averaged. Also the cortical area (Ct.Ar) was determined and averaged in this region. All parameters were calculated using open-source image processing software (BoneJ, ImageJ, NIH, Bethesda, USA) [26]. Since small rotations of the bone cross-section can have a considerable effect on Ct.Ar and moments of inertia, we used not just a three-dimensional view of the bone when aligning it but also projections engendered when cutting it in several planes. After bone realignment, slices at the metaphysis, the tibial central section, the distal tibiofibular junction and the ankle were checked. If one bone was not well aligned, we realigned it again.
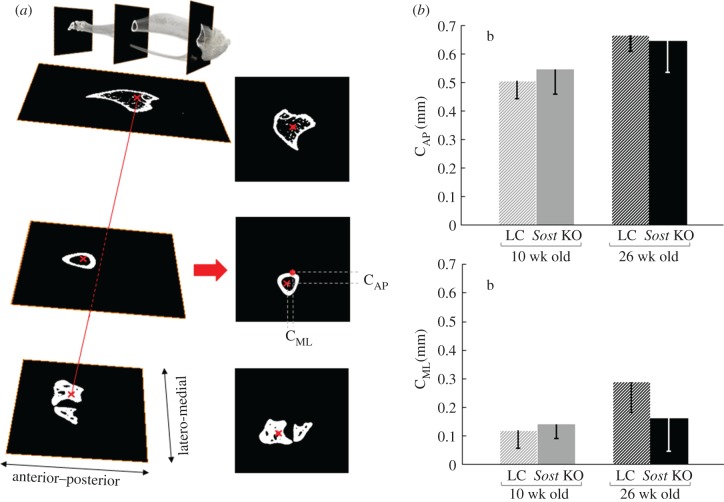
We characterized the bone curvature at the central section (CS) of the midshaft by calculating the distance of the centroid of each section to a reference line passing through the centroid of the widest cross-section at the ankle (malleoli) and the centroid of a proximal cross-section located at 10% of the total tibial length (TL) from the tibial condyles (figure 2a). This distance was calculated in anterior–posterior (CAP) and in medio-lateral (CML) directions. The fibula was manually removed from the images before the centroids were calculated. We also measured the TL from the microCT images.
Figure 2.
(a) Graphical description of the method used to quantify bone curvature from microCT images. (b) Bone curvature in mediolateral (CML) and anteroposterior directions (CAP). ANOVA. Main effects: (a) genotype, (b) age; Interactions: (c) genotype × age. p < 0.05.
After determining with a Shapiro–Wilks test that the data had a normal distribution, we performed parametric statistics. The between-subject effects of genotype (Sost KO mice and LCs) and age (10 week old, 26 week old) as well as their interactions were assessed using a two-way ANOVA (SAS 9.3, Cary, USA). Subanalyses were performed using independent Student's t-test for some datasets. A p-value ≤0.05 was considered significant.
The per cent difference between genotypes is presented as (Sost KO − LC)/LC) × 100%. Age related differences for each of the genotypes are calculated as (26 weeks − 10 weeks)/10 weeks) × 100%.
2.4. Finite-element modelling
FEMs of 10 and 26 week old Sost KO and LC tibiae (n = 1/age/genotype) were built based on the ex vivo microCT data of strain gauged mice tibiae described in §2.2. After image segmentation, a triangular approximation of the bone surface was calculated using an unconstrained smoothing algorithm. The generated surface contained a large number of triangular faces (approx. 7 × 106). To maintain fidelity, the number of faces was progressively reduced with further implementation of best isotropic vertex placement until a ‘feasible’ triangle number of approximately 0.25 × 106 was achieved. The volume enclosed by the bone surface was then filled with linear four node tetrahedral mesh elements. Thus, we created models with adaptive multi-resolution grids, in which the geometry of the trabecular bone was preserved. All these procedures were performed using commercial software (Amira 5.4.3, FEI, Hillsboro, USA).
2.4.1. Loads and boundary conditions
Loading and boundary conditions aimed to replicate previously reported strain-gauge in vivo loading experiments in mouse tibia [19]. A compression load was introduced through the knee with an inclination relative to the bone axis of 10° [27]. The load was applied on a reference node, which was kinematically coupled to the nodes on the surface of the proximal tibial plateau (relative displacements were constrained, relative rotations were kept free). The reference node was allowed to move exclusively in the direction of the applied load. Surface nodes on the distal part of the bone were allowed to move in the anterior–posterior direction but not in the medial–lateral (malleoli constrain this movement) and to rotate around the medial–lateral axis of the tibia, which is the axis of the articulatio talocruralis (hinge joint).
Two different load cases were investigated:
(1) Load-matched load case: an identical compression load of −11 N was applied to all groups.
(2) Strain-matched load case: a compression load that induced 900 microstrains (μɛ) at the anteromedial aspect of the tibia midshaft, as we have previously measured using in vivo strain gauging [19] was applied. Pflanz et al. [19] determined that compression loads of −7, −7, −12.9 and −14.5 N induce 900 µɛ at the strain gauge (SG) site in 10 week LC, 26 week LC, 10 week Sost KO and 26 week Sost KO mice, respectively. The applied axial loads were not scaled for mouse body weight since the aim was to ‘replicate’ the in vivo experiment. These load levels triggered an osteogenic response in all four groups [19]. For this load case, we studied the effect of variations of ±10° in the load axis due to potential misalignments (see electronic supplementary material, data S3).
Boundary conditions and the load were introduced using the same commercial software that we used for the analysis and post-processing (Abaqus 6.12.2, Dassault Systemés Simulia, Providence, USA).
2.4.2. Material properties
Bone material properties were considered linear elastic and isotropic. The Poisson's ratio was set to 0.35 [28] for all models. Heterogeneous models were created in which Young's modulus (E) assigned to each element was estimated using a power-law relationship between the linear attenuation coefficient (μ) and stiffness [28,29]. For a thorough description of the bone material properties assignation, we refer to the electronic supplementary material, data S4. The code for the assignation of μ to the tetrahedral elements and the code for the final model-assembly are included as electronic supplementary material, data S6.
In addition, to isolate the influence of bone geometry on the predicted strains, we developed homogeneous models with an identical Young's modulus of 12 GPa for all elements in all groups. In 10 and 26 week old LC and in 10 week old Sost KO, the proximal tibio-fibular junction was not completely mineralized. Therefore, a linear elastic material with a Young's modulus of 1.6 MPa and Poisson's ratio of 0.2 was used to model this non-mineralized region, according to values found in the literature for mouse cartilage [30]. This approach has been followed by other groups developing FEMs of the tibia of young C57Bl/6 mice [31].
In the 10 week old LC, we additionally found that the mineralization at the growth plate was so low that there was a disconnection between the epiphysis and metaphysis in the meshed models. We remeshed the model including the growth plate and manually assigned to this region the same material properties that we used for the cartilage of the tibio-fibular junction.
2.4.3. Data analysis
Heterogeneous FEMs (n = 1/age/genotype) were validated by comparing the predicted strains at the strain gauge site (strain-matched load case) to the strains measured in the in vivo loading experiments previously reported [19]. The strain gauge position was, after removal of the metallic parts, still visible in the microCT scans. From the microCT scans, we measured that the strain gauges had been positioned, on average, at 41% of the whole tibial length measured from the proximal end. The corresponding predicted strain gauge value for each bone was calculated by averaging the strain in the direction of the strain gauge at its mounting position (ɛxx).
We calculated the structural stiffness of the bone in all groups by dividing the load in the bone axis direction by the displacement of the reference node in this same direction.
For each element within the heterogeneous and homogeneous FEMs, the maximum absolute value between the maximal (tensile, ɛmax) and minimal (compressive, ɛmin) principal strains was calculated. Thereafter, the mean values of the tensile (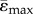 ) and compressive (
) and compressive (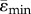 ) strains in each cross-section along the bone length were determined. Elements for which abs(ɛmax) > abs(ɛmin) were used to calculate
) strains in each cross-section along the bone length were determined. Elements for which abs(ɛmax) > abs(ɛmin) were used to calculate 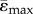 and s.d.(ɛMax), elements for which abs(ɛMax) ≤ abs(ɛMin) were used to calculate
and s.d.(ɛMax), elements for which abs(ɛMax) ≤ abs(ɛMin) were used to calculate 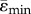 and s.d.(ɛmin), where s.d. stands for standard deviation. We focused on the CS of the midshaft since it is the centre of the region of interest (ROI) in which bone formation and resorption in response to loading has been previously evaluated in this mouse model [19].
and s.d.(ɛmin), where s.d. stands for standard deviation. We focused on the CS of the midshaft since it is the centre of the region of interest (ROI) in which bone formation and resorption in response to loading has been previously evaluated in this mouse model [19].
3. Results
3.1. Bone morphology
The ANOVAs performed on the morphological parameters of the cortical bone showed that both genotype and age have a significant effect on the Ct.Ar, Imin and Imax (figure 1) at the tibial midshaft. The t-tests revealed significant genotype related differences in Ct.Ar, Imin and Imax for both the 10 week and the 26 week old animals. Ct.Ar, Imin and Imax were 84%, 171% and 161% higher in the 10 week Sost KO than in the 10 week old LC. The genotype related differences were even more dramatic in the adult animals with 131% higher Ct.Ar, 249% higher Imin and 223% higher Imax in 26 week Sost KO compared with the age-matched LC (p < 0.0001 for all three variables and both ages). Skeletal maturation led to a significantly greater Ct.Ar, Imin and Imax in the tibial midshaft of the Sost KO and LC mice (figure 1).
Figure 1.
Morphometric parameters of the cortical bone at the tibial midshaft: cortical area (Ct.Ar) and maximal and minimal moments of inertia (Imax, Imin). ANOVA. Main effects: (a) genotype, (b) age; Interactions: (c) genotype × age. p < 0.05. The percentages indicate the increase of the morphometric parameters with maturation in each genotype.
Regarding bone curvature, the ANOVA showed no significant difference either in the CML or the CAP at the midshaft between Sost KO and their age-matched LCs (figure 2). In contrast, animal age was found to have a significant effect on the CAP and CML. The t-tests revealed that adult LC mice had significantly greater CAP (+142%) and CML (+32%) than young LC mice, but this age-related effect on whole bone curvature was not measured in the Sost KO mice (table 1).
Table 1.
Mean ± s.d. of all parameters used to describe bone morphology at the cortical midshaft. + indicates age related differences and * significant differences between genotypes.
| 10 week old |
26 week old |
|||
|---|---|---|---|---|
| LC | Sost KO | LC | Sost KO | |
| Ct.Ar (mm2) | 0.520 ± 0.040 | 0.963 ± 0.057* | 0.599 ± 0.027+ | 1.380 ± 0.047*+ |
| Imin (mm4) | 0.048 ± 0.006 | 0.130 ± 0.012* | 0.060 ± 0.005+ | 0.210 ± 0.010*+ |
| Imax (mm4) | 0.062 ± 0.011 | 0.161 ± 0.017* | 0.080 ± 0.009+ | 0.259 ± 0.020*+ |
| CML (mm) | 0.12 ± 0.06 | 0.14 ± 0.05 | 0.29 ± 0.10+ | 0.16 ± 0.12 |
| CAP (mm) | 0.51 ± 0.06 | 0.55 ± 0.09 | 0.67 ± 0.06+ | 0.65 ± 0.11 |
| TL (mm) | 16.47 ± 0.15 | 17.05 ± 0.14* | 17.23 ± 0.07+ | 17.65 ± 0.12*+ |
*p < 0.05 compared to age-matched LC.
+p < 0.05 compared to young mice with same genotype.
As expected, the tibiae of both Sost KO and LC mice were significantly longer at the age of 26 weeks than at the age of 10 weeks (table 1). Both young and adult Sost KO mice showed a significantly greater tibial length than aged-matched LCs (TL = +3.5% for the 10 week old and TL = +2.4% for the 26 week old mice).
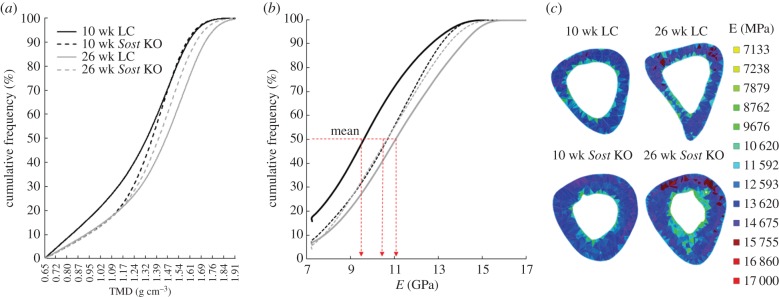
3.2. Bone tissue mineral density and bone stiffness
The 10 week old LC mouse showed higher bone volume with low TMD values compared with the 26 week old LC (figure 3a). The 10 and 26 week old Sost KO mice showed similar TMD distributions, which were between the values obtained for the 10 and 26 week old LC mice. Accordingly, higher amounts of bone volume were characterized by a low Young's modulus in the 10 week old compared to the 26 week old LC heterogeneous model (figure 3b,c). Age-related differences in the mean distribution of tissue stiffness were not observed between 10 and 26 week Sost mice (figure 3b), however regional differences were observed. For example, a larger tissue volume presented high stiffness at the midshaft in the 26 week old compared with 10 week old Sost mice (figure 3c). The mean Young's modulus of the heterogeneous FEMs was similar between 10 and 26 week old Sost KO animals (10.67 GPa for the 10 week old Sost KO, 10.79 GPa for the 26 week old Sost KO). A lower mean Young's modulus was determined for the 10 week old compared with the 26 week LC mouse (9.87 GPa for the 10 week old LC, and 11.16 GPa for the 26 week old LC).
Figure 3.
(a) TMD cumulative frequency for the bony voxels of the segmented scans. (b) E cumulative frequency for the bony elements of the FEMs. (c) E distribution within the central section of the midshaft for all four FEMs.
3.3. Mechanical strains within the tibiae of load-matched Sost knockout and littermate control mice
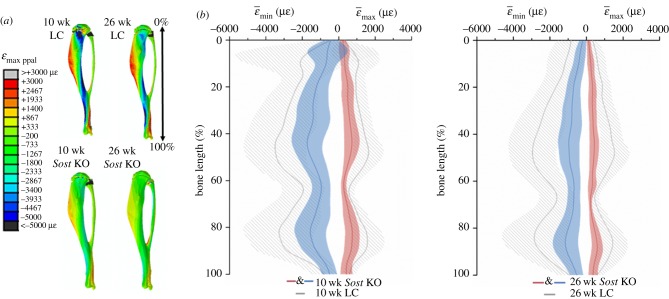
When comparing the age-matched genotypes using homogeneous FEMs under the same compression load, the diminished strains, due to increased bone mass in Sost deficient mice becomes visible (figure 4a). Tensile strains were on average 52% lower in the 10 week old Sost KO than in the age-matched LC, whereas they were 63% lower (in absolute value) for the adult Sost KO than for the adult LC (figure 4b). The compressive strains were on average 50% lower in the 10 week old Sost KO than in the age-matched LC, whereas they were 62% lower for the adult Sost KO than for the adult LC. Interestingly, these differences were more pronounced in the diaphyseal midshaft than in the rest of the bone for both tension and compression. Maturation led to lower strains in both the LC and the Sost KO mice, with the decrease being much more pronounced in the Sost KO than in the LC.
Figure 4.
Maximal principal strain (ɛmax ppal = max (|ɛmin|, |ɛmax|) distribution (a) and mean tensile and compressive strains (b) along the length of the tibia. Homogeneous models with identical material properties (E = 12 GPa) under an 11 N compressive load (load-matched load case). Lines represent 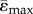 and
and 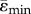 . Shaded regions correspond to ±s.d. (ɛmax) and ±s.d. (ɛmin), where s.d. stands for standard deviation, which reflects the range of ɛmax for tension and ɛmin for compression within the cross-section.
. Shaded regions correspond to ±s.d. (ɛmax) and ±s.d. (ɛmin), where s.d. stands for standard deviation, which reflects the range of ɛmax for tension and ɛmin for compression within the cross-section.
Heterogeneous FEMs, to compare the age-matched genotypes under the same compression load, led to similar strain distribution within the tibia when compared to the strains obtained with the homogeneous models (electronic supplementary material, data S5).
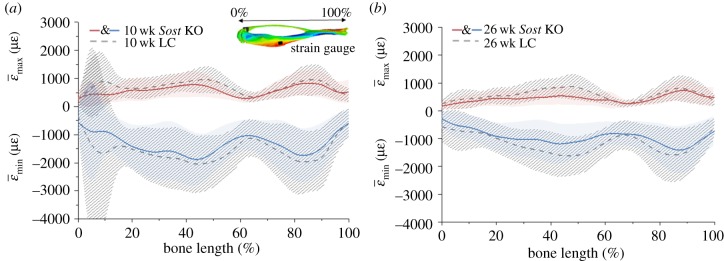
3.4. Mechanical strains within the tibiae of strain-matched Sost knockout and littermate control mice
The predicted strains at the strain gauge site by heterogeneous FEMs were: 1085 µɛ young Sost KO, 1010 µɛ young LC, 870 µɛ adult Sost KO and 862 µɛ adult LC. The whole-bone stiffness was: 290 N mm−1 in the young Sost KO, 122 N mm−1 in the young LC, 409 N mm−1 in the adult Sost KO and 165 N mm−1 in the adult LC.
When comparing age-matched mice using heterogeneous FEMs, mean tensile and compressive predicted strains in cortical bone were higher for LC than for Sost KO mice along the whole tibia (figure 5) except for its distal end, where the strains were slightly higher in the Sost KO.
Figure 5.
Mean tensile and compressive strains along the length of the tibia for the heterogeneous FEMs of the 10 (a) and 26 (b) week old female Sost KO and LC mice. Strain-matched load case. Lines represent 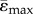 and
and 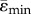 . Shaded regions correspond to ±s.d. (ɛmax) and ±s.d. (ɛmin), where s.d. stands for standard deviation, which reflects the range of ɛmax for tension and ɛmin for compression within the cross-section.
. Shaded regions correspond to ±s.d. (ɛmax) and ±s.d. (ɛmin), where s.d. stands for standard deviation, which reflects the range of ɛmax for tension and ɛmin for compression within the cross-section.
At the tibial midshaft region (47.5% to 52.5% of tibial length), large differences between genotypes in both ages were found, with higher strains in LC mice compared with the age-matched Sost KO (table 2). Establishing a comparison between ages, adult animals showed lower strains than young mice with the same genotype for both tension (28% for the Sost KO and 5% for the LC) and compression (31% for the Sost KO and 16% for the LC).
Table 2.
Mean compressive (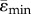 ) and tensile strains (
) and tensile strains (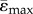 ) at the tibial midshaft region (47.5% to 52.5% of tibial length). Strain-matched load case.
) at the tibial midshaft region (47.5% to 52.5% of tibial length). Strain-matched load case.
| 10 week old |
26 week old |
|||
|---|---|---|---|---|
| LC | Sost KO | LC | Sost KO | |
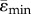 (µɛ) (µɛ) |
−1968 ± 893 | −1653 ± 752 | −1663 ± 668 | −1140 ± 611 |
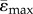 (µɛ) (µɛ) |
921 ± 460 | 706 ± 381 | 874 ± 460 | 505 ± 285 |
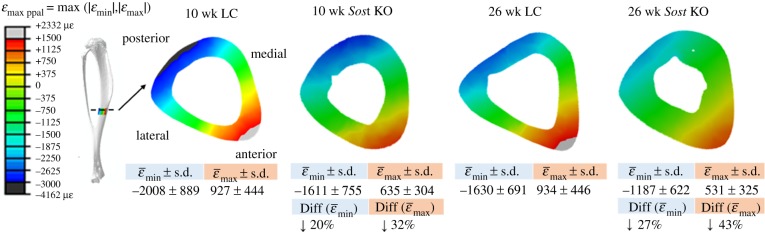
Although predicted strains at the strain gauge site were similar between aged-matched genotypes, maximum tensile strains at the central section of the tibial midshaft were considerably higher in the LC compared to Sost KO mice, in both age groups (figure 6).
Figure 6.
Strain distribution in the cross section located in the middle of the tibia. Heterogeneous models, strain-matched load case. Percentages indicate the strain difference between Sost KO and their age matched LC (Sost KO − LC)/LC) × 100%.
4. Discussion
In this study, we investigated the long-term effects of sclerostin deficiency in bone morphology, material properties and the mechanical environment within the tibia in young and adult mice. We found that long-term sclerostin deficiency leads to changes in bone morphology, which result in decreased mechanical strains within the cortical bone. The decreased strains can be observed even at the mid-shaft region, where we previously reported an increased response to mechanical loading in female Sost KO mice [19]. In agreement with Morse et al. [14], we showed the tibiae of the Sost KO to have a notably greater cortical area and moment of inertia than those from the LC mice.
Although we recognize that the assessment of volumetric TMD using polychromatic microCT has limitations [32], we aimed to consider the effects of regional differences in TMD on the strain environment. Two studies [10,11] report significantly increased TMD in the trabecular bone of the distal metaphysis of the femora of 17 week old female Sost KO compared with WT. Contrarily, Ross and colleagues [13] found no significant differences on the global bone mineralization, measured with bSEM, between the Sost KO and the WT mice. Similar to this finding [13] but not to other data [10,11], we did not observe dramatic differences between the TMD distribution in the adult Sost KO and the aged-matched LC mice. It should be mentioned that even though the TMD was assessed locally to calculate an estimated Young's modulus, the average values that we report are for the whole bone and no distinction between small compartments of either cortical or trabecular bone have been analysed separately, as the above referenced studies did [10,11].
The differences in the TMD distribution between the young Sost KO and LC mice were more pronounced than between adult mice, but we think that they rather rely on the phenotypic singularities and the apparently delayed maturation that we found in the LC. We found that the LC mice had a larger growth plate (non-mineralized tissue) in the proximal tibia compared to what we have observed previously in C57Bl/6 mice [28]. Since canonical Wnt signalling promotes maturation of chondrocytes, which express sclerostin themselves when terminally differentiated, it has been speculated that lack of sclerostin may favour increased differentiation towards hypertrophic chondrocytes which results in a larger hypertrophic zone in the growth plate [23]. We cannot explain the reasons for the abnormal growth plate in the LC mice but they could be related to an insufficient production of sclerostin by heterozygote mothers, which has influence on the endochondral ossification of their homozygote +/+ offspring. A genetic drift is extremely unlikely since breeding programmes at animal facilities are designed to avoid them.
Bone curvature was considerably higher in the anterior–posterior direction (CAP) compared to the medial–lateral direction (CML) in both LC and Sost KO mice. This finding has also been previously shown in young growing (6 to 16 week old) female C57Bl/6 mice [17]. CAP plays a key role in the amount of bending engendered by a compressive load and thus it is one of the major contributors to the tensile strains at the anterior part of the tibia. No significant difference either in anterior–posterior or medial–lateral direction were found at the midshaft between Sost KO and their age-matched LCs.
We used both homogeneous and heterogeneous FEMs to characterize the mechanical environment within the bone due to controlled external loading. Homogeneous models allowed us to isolate the influence of bone geometry on the induced strains, while heterogeneous models gave us insight into how the TMD was influencing the strains. When introducing an identical compressive load to all four models (load-matched experiment) the strains in the tibiae of the Sost deficient were dramatically lower than in their age-matched LC for both the heterogeneous and homogeneous models. Interestingly, these differences in the strains were more pronounced at the diaphyseal midshaft than the rest of the bone in both tension and compression. The region of the midshaft is commonly used to characterize cortical bone, so further studies assessing bone formation in Sost KO mice and using littermate mice as controls should consider this fact. Also the most distal part of the bone (80–90% bone-length) was a region of pronounced differences between the Sost KO animals and their age-matched LC.
Previous studies examining the anabolic response to mechanical loading in Sost KO mice did not use LC mice, but rather WT controls. These mice should display more variability in terms of basal Sost expression, due to differing genetic backgrounds. In the present study, we ensured the animals were from the same breeding pair to avoid such variability. The use of LC is even more important considering the growth plate anomaly we observed in Sost+/+ mice. Additionally, none of the studies so far published included FEMs to examine the strain distribution during in vivo loading, but only relied on strain gauging at a single site on the tibia [14] or on extrapolations based on the ulnar strains of a different mice strain [20]. This work shows that strain gauge-based strain-matched experiments do not ensure comparable strains along the tibiae of Sost KO and LC mice and that in silico models are needed to assess the strain environment.
The present work reveals that the use of heterogeneous FEMs leads to small differences in the strain distribution within the tibia when compared to the strains obtained with the homogeneous models. The homogeneous models predicted higher strains than the heterogeneous models in the mid-diaphysis for both ages and phenotypes, both in tension and compression, although the Young's modulus (E) used for the homogeneous models (12 GPa) is higher than the average E of the corresponding heterogeneous model (figure 3b). This can be explained since the TMD at the mid-diaphysis is higher than in other regions of the bone and so is the E assuming the power law relationship between TMD and E used in this work (figure 3c). We conclude that for tissue level models like the ones presented here, homogeneous models provide results that are accurate enough. Nevertheless, the choice of the E should not be made just under consideration of the average E for the whole bone but also considering the local stiffness of the region of interest and its relationship to the average stiffness.
Heterogeneous FEMs predicted similar strain levels to those we measured in strain gauge experiments at the midshaft of Sost KO and LC mice for the strain-matched load case [19]. The whole bone stiffness of the young Sost KO was 137% higher than the whole bone stiffness of the young LC, while the adult Sost KO had a 147% higher whole bone stiffness than the adult LC. Yang et al. [31] reported that the whole bone stiffness of their heterogeneous FEM of a 16 week old WT mouse was approximately 180 N mm−1, which is in good agreement with the 122 N mm−1 for the 10 week LC and the 165 N mm−1 for the 26 week old LC presented here. The same strain level at the gauge site did not ensure comparable strains along the whole tibial length in Sost KO and LC mice. In both age groups, FEMs predicted lower mechanical strains along the length of the tibiae in Sost KO compared to LC mice.
Morse et al. [14] reported significant higher increase of BV in the cortical midshaft of 10 week old Sost KO mice compared to WT after a strain-matched loading regime. The load levels determined by Morse and their colleagues do not match our strain gauge experiments [19]. They applied a compression load of −12.5 N to engender 1200 µɛ in the midshaft of 10 week old Sost KO mice, while we determined that −12.9 N engenders 900 µɛ. Discrepancies could be due to strain gauge positioning, since the strain gradients in the strain gauge region are high. Differences between the two studies in the bone anabolic response to loading could also be due to control mice used.
Interestingly, Morse et al. [14] report the increase in cortical BV to be the highest around 2.12 mm below the growth plate, which will correspond to the 14% of the total tibial length (TL), while it remains small around the midshaft. They speculate that higher strains in the metaphyseal region could be the origin of the higher increase on cortical BV occurring with loading. Our results (10 week old Sost KO, strain-matched load case) contradict this hypothesis since 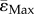 at 14% of TL and 50% of TL are similar and
at 14% of TL and 50% of TL are similar and 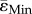 is higher (more negative) at the midshaft than at the cortical bone in the metaphysis.
is higher (more negative) at the midshaft than at the cortical bone in the metaphysis.
We have previously shown that the cortical adaptive formation response in the midshaft was enhanced in female Sost KO compared to LC mice [19]. Loading-induced gains in mineralizing surface were enhanced in Sost KO mice, although the FEMs in the current study predict lower mean compressive (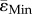 ) and tensile strains (
) and tensile strains (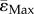 ) in female Sost KO compared to LC mice. Our histomorphometric data reveal that bone formation (MS/BS, MAR) is higher in the endosteal surface than in the periosteal, for both Sost KO and LC mice. Sost KO mice showed an increased bone formation in response to loading (interlimb difference) at both the endosteal and periosteal surface, although the effect is most pronounced at the periosteum surface. A qualitative analysis of the strains in the loaded limb at the tibial midshaft (figure 6) reveals that strains in the periosteum of the Sost KO mice are lower than in their age matched LCs for both tension and compression. This is particularly surprising since our previous studies in WT mice have shown an enhanced bone formation response at the endosteal surface compared to the periosteal surface despite increased strains at the periosteal surface [33]. Nevertheless, in the present study we are not able to elucidate if the enhanced anabolic response to loading is because the strains in the endosteum remain under a certain threshold or due to biological, non-mechanically related factors. We cannot conclude that a lower strain-threshold triggers an osteogenic response in Sost KO. For that, a correlation between formation events and the strain environment in the surrounding area, as performed in [21], must be determined.
) in female Sost KO compared to LC mice. Our histomorphometric data reveal that bone formation (MS/BS, MAR) is higher in the endosteal surface than in the periosteal, for both Sost KO and LC mice. Sost KO mice showed an increased bone formation in response to loading (interlimb difference) at both the endosteal and periosteal surface, although the effect is most pronounced at the periosteum surface. A qualitative analysis of the strains in the loaded limb at the tibial midshaft (figure 6) reveals that strains in the periosteum of the Sost KO mice are lower than in their age matched LCs for both tension and compression. This is particularly surprising since our previous studies in WT mice have shown an enhanced bone formation response at the endosteal surface compared to the periosteal surface despite increased strains at the periosteal surface [33]. Nevertheless, in the present study we are not able to elucidate if the enhanced anabolic response to loading is because the strains in the endosteum remain under a certain threshold or due to biological, non-mechanically related factors. We cannot conclude that a lower strain-threshold triggers an osteogenic response in Sost KO. For that, a correlation between formation events and the strain environment in the surrounding area, as performed in [21], must be determined.
Our previous study [19] showed that ablation of the Sost gene in mice resulted in an increased expression of another Wnt inhibitor, Dkk1, possibly due to a feedback mechanism intended to compensate for the loss of Sost. Similar to other reports (Holguin et al. [22]) Dkk1 expression was significantly downregulated with loading (loaded versus control nonloaded limb). The enhanced bone formation response to loading observed in Sost KO mice compared to LC mice suggest that Dkk1 was not sufficient to compensate for the loss of Sost. In addition to altered Wnt signalling, other mechanosensitive signalling pathways are likely activated in the absence of Sost.
It is known that there is a loss of mechanosensation occurring with ageing in both humans and mice [1,22] and it is known as well, that physiologic sclerostin levels increase with age in humans, causing a decrease in Wnt canonical signalling. Thus, clinical trials are underway to determine if sclerostin neutralizing antibodies can maintain Wnt-signalling activation in patients with low bone mass. We also showed that the anabolic response to loading was reduced at maturation in both LC and Sost KO mice coincident with age-dependent expression of Wnt target genes in Sost KO and LC mice as well as Sost gene expression in LC mice [19]. The strain-matched load case reveals that both the mean maximal principal strain (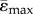 ) and mean minimal principal strain (
) and mean minimal principal strain (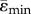 ) are lower in the tibial midshaft of the adult mice compared to the young mice, so we cannot exclude that lower tensions are, at least in part, responsible for the reduced anabolic response of the adult mice.
) are lower in the tibial midshaft of the adult mice compared to the young mice, so we cannot exclude that lower tensions are, at least in part, responsible for the reduced anabolic response of the adult mice.
Skeletal maturation led to a significant increase in Ct.Ar, Imin and Imax in the Sost KO animals. In contrast to LC mice, where a significant increase in CAP was observed in adult compared to young mice, the CAP did not increase significantly with maturation in Sost KO mice (table 1). With their greater area and moments of inertia and non-increased bone curvature, Sost KO mice present all possible geometrical features that lead to low strains in their cortical midshaft. These results likely explain why the strains in the central portion of the adult Sost KO tibia FEM are remarkably lower than in the central portion of the young Sost KO tibia FEM, although the strains at the strain gauge site are similar. Interestingly, our histomorphometric data revealed that the greater load-induced bone gain observed in both young and adult Sost KO mice was achieved through higher bone formation at both the periosteal and endocortical surface in the adult Sost KO, whereas just the periosteal surface showed a higher response to loading in young Sost KO compared to young LC mice. These findings support the hypothesis that lower strain thresholds trigger an osteogenic response in Sost KO even in adult mice, but, as we have already mentioned, a correlation between formation events and the strain environment must be performed to test this hypothesis.
This work has limitations. Variability of the tibial morphologic parameters between specimens within the same age and phenotype was small (figures 1 and 2) therefore one FEM per phenotype and age was created. Razi et al. [28] and Yang et al. [29] showed that small differences in bone morphology between female C57BL/6 J mice of the same age led to very similar strain distributions. The models predicted very high compressive strains in the cortical bone of the metaphyseal region of the young and adult LC animals. The increased size of the growth plate in the LC, compared to Sost KO and age-matched WT female C57BL/6 J [28], will likely lead to an overestimation of the strains predicted by the models in this region, especially in the cartilage–bony element interface. A more accurate modelling of this interface and a mesh with very small elements in this region are required to evaluate with precision the strain environment of the cortical bone of the metaphysis. In this study we did not investigate the mechanical environment in the tibia of elderly Sost KO mice. This was motivated by several reasons including the increased mortality observed in elderly Sost KO mice in our breeding programme, which would have required an excessive number of animals. Also, the load levels necessary to reach osteogenic mechanical strain levels for corresponding experimental studies in elderly Sost KO mice would have damaged the joints and thus were not performed [19].
We investigated the effect of Sost deficiency and skeletal maturation on whole bone morphology and the local mechanical strain environment induced within the bone under external controlled in vivo loading. Our key findings were: (i) the differences in Ct.Ar, Imin and Imax between age-matched Sost KO compared to LC mice were much more dramatic in the adult mice, suggesting that the phenotypic changes in bone mass observed in Sost deficient mice follow an increasing trend until they reach adulthood. This fact is reflected in the mechanical environment within the bone: differences occurring with maturation were higher for the Sost KO than for the LC mice. (ii) TMD is a minor contributor to the strain distribution in tibia cortical bone compared to bone geometry in Sost KO mice. This finding is in agreement with what has been already shown for C57Bl/6 mice [28,29]. (iii) The strains in the tibiae of the Sost deficient mice are dramatically lower than in age-matched LC mice under an externally applied compressive load. These differences are especially pronounced in the diaphyseal midshaft, a region that is commonly used to assess anabolic and catabolic changes on cortical bone. (iv) Matching the strain level at a single point on the bone surface of the tibial mid-diaphysis, where a strain gauge can be placed in vivo, does not ensure comparable strains along the entire length of the tibia in age-matched Sost KO and LC mice. It also does not ensure comparable strains along the tibial length of young and adult animals within the same genotype, with discrepancies observed to be greater for the Sost KO than for the LC mice.
It is important that we understand how long-term sclerostin inhibition alters the strains environment in bone and furthermore how bone anabolic and catabolic processes are affected by it. This knowledge is crucial to determine in which grade a multimodal therapy including sclerostin-antibody and mechanical stimuli could have additive or synergistic effects on bone formation. Sclerostin neutralizing antibodies are being tested in ongoing clinical trials to target osteoporosis [23,24], and other low bone mass disorders such as osteogenesis imperfecta [25]. Thus, maturity-dependent differences in bone strains should be considered when conceiving those multimodal therapies. Sost KO mice are a widely used model to study the effects of long-term sclerostin inhibition. Data from the current study show decreased strains in both tension and compression at the cortical midshaft of young and adult Sost KO mice compared to age-matched LC even in strain-matched load conditions. Surprisingly, our previous work has shown an increase of load induced bone formation surface relative to total surface (MS/BS) in young and adult Sost KO mice compared to age-matched LC in this region. Together these data suggest that even physical activity which engenders low strain magnitudes, in combination with sclerostin inhibition, may be a promising anabolic therapy, although age-dependent mechano-responsiveness must be considered.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
All animal experiments described were carried out according to the policies and procedures approved by the local legal research animal welfare representative (LaGeSo Berlin, G0021/11).
Data accessibility
In compliance with the EPSRC's open access initiative, the datasets and code for analysis are included in the main text of the manuscript or in the electronic supplementary material, data section (see S1–S6).
Authors' contributions
L.A.: laboratory work, methods development, data analysis and interpretation, article writing. M.C.: methods development, article revising. D.P.: laboratory work, article revising. I.K.: study conception, article revising. M.K.: study conception, article revising. G.N.D.: study conception, article revising. B.M.W.: study conception, data analysis and interpretation, article writing. S.C.: changes in study design, data interpretation, article writing. All authors gave final approval for publication.
Competing interests
The initial four male Sost KO mice that underwent embryo transfer were provided by Novartis. Two authors (I.K. and M.K.) are employed by Novartis. No funding was received for this project by Novartis Ltd.
Funding
This study was partially supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung: TP5/DIMEOs), the German Research Foundation (Deutsche Forschungsgemeinschaft; WI-3761/4-1, CH-1123/4-1, DU-298/21-1), Shriners Hospitals for Children, and the Réseau de recherche en santé buccodentaire et osseuse recruitment aid programme.
References
- 1.Bonewald LF, Johnson ML. 2008. Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615. ( 10.1016/j.bone.2007.12.224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin C, et al. 2009. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J. Bone Miner. Res. 24, 1651–1661. ( 10.1359/jbmr.090411) [DOI] [PubMed] [Google Scholar]
- 3.Issack PS, Helfet DL, Lane JM. 2008. Role of Wnt signaling in bone remodeling and repair. HSS J. 4, 66–70. ( 10.1007/s11420-007-9072-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang KS, Robling AG. 2015. New insights into Wnt-Lrp5/6-β-catenin signaling in mechanotransduction. Front. Endocrinol. 5, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balemans W, et al. 2001. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543. ( 10.1093/hmg/10.5.537) [DOI] [PubMed] [Google Scholar]
- 6.Brunkow ME, et al. 2001. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68, 577–589. ( 10.1086/318811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Lierop AH, Appelman-Dijkstra NM, Papapoulos SE. 2017. Sclerostin deficiency in humans. Bone 96, 51–62. ( 10.1016/j.bone.2016.10.010) [DOI] [PubMed] [Google Scholar]
- 8.Gardner JC, van Bezooijen RL, Mervis B, Hamdy NA, Lowik CW, Hamersma H, Beighton P, Papapoulos SE. 2005. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J. Clin. Endocrinol. Metab. 90, 6392–6395. ( 10.1210/jc.2005-1235) [DOI] [PubMed] [Google Scholar]
- 9.van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE. 2011. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J. Bone Miner. Res. 26, 2804–2811. ( 10.1002/jbmr.474) [DOI] [PubMed] [Google Scholar]
- 10.Li X, et al. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 23, 860–869. ( 10.1359/jbmr.080216) [DOI] [PubMed] [Google Scholar]
- 11.Kramer I, Loots GG, Studer A, Keller H, Kneissel M. 2010. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 25, 178–189. ( 10.1359/jbmr.090730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassler N, et al. 2014. Sclerostin deficiency is linked to altered bone composition. J. Bone Miner. Res. 29, 2144–2151. ( 10.1002/jbmr.2259) [DOI] [PubMed] [Google Scholar]
- 13.Ross RD, Mashiatulla M, Robling AG, Miller LM, Sumner DR. 2016. Bone matrix composition following PTH treatment is not dependent on sclerostin status. Calcif. Tissue Int. 98, 149–157. ( 10.1007/s00223-015-0074-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse A, McDonald MM, Kelly NH, Melville KM, Schindeler A, Kramer I, Kneissel M, Van Der Meulen MCH, Little DG. 2014. Mechanical load increases in bone formation via a sclerostin-independent pathway. J. Bone Miner. Res. 29, 2456–2467. ( 10.1002/jbmr.2278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin CT, Lanyon LE. 1984. Regulation of bone formation by applied dynamic loads. J. Bone Joint Surg. Am. 66, 397–402. ( 10.2106/00004623-198466030-00012) [DOI] [PubMed] [Google Scholar]
- 16.Duffy MP, Jacobs CR. 2015. Seeing the unseen: cell strain and mechanosensing. Biophys. J. 108, 1583–1584. ( 10.1016/j.bpj.2015.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Main RP, Lynch ME, van der Meulen MC. 2010. In vivo tibial stiffness is maintained by whole bone morphology and cross-sectional geometry in growing female mice. J. Biomech. 43, 2689–2694. ( 10.1016/j.jbiomech.2010.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu X, et al. 2012. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50, 209–217. ( 10.1016/j.bone.2011.10.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pflanz D, et al. 2017. Sost deficiency led to a greater cortical bone formation response to mechanical loading and altered gene expression. Sci. Rep. 7, 9435 ( 10.1038/s41598-017-09653-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robling AG, Kang KS, Bullock WA, Foster WH, Murugesh D, Loots GG, Genetos DC. 2016. Sost, independent of the non-coding enhancer ECR5, is required for bone mechanoadaptation. Bone 92, 180–188. ( 10.1016/j.bone.2016.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkhold AI, Razi H, Duda GN, Checa S, Willie BM. 2017. Tomography-based quantification of regional differences in cortical bone surface remodeling and mechano-response. Calcif. Tissue Int. 100, 255–270. ( 10.1007/s00223-016-0217-4) [DOI] [PubMed] [Google Scholar]
- 22.Holguin N, Brodt MD, Silva MJ. 2016. Activation of wnt signaling by mechanical loading is impaired in the bone of old mice. J. Bone Miner. Res. 31, 2215–2226. ( 10.1002/jbmr.2900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosman F, et al. 2016. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543. ( 10.1056/NEJMoa1607948) [DOI] [PubMed] [Google Scholar]
- 24.McClung MR, et al. 2014. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420. ( 10.1056/NEJMoa1305224) [DOI] [PubMed] [Google Scholar]
- 25.Glorieux FH, et al. 2017. BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: results of a randomized phase 2a trial. J. Bone Miner. Res. 32, 1496–1504. ( 10.1002/jbmr.3143) [DOI] [PubMed] [Google Scholar]
- 26.Doube M, Kłosowski MM, Arganda-Carreras I, Cordeliéres F, Dougherty RP, Jackson J, Schmid B, Hutchinson JR, Shefelbine SJ. 2010. BoneJ: free and extensible bone image analysis in ImageJ. Bone 47, 1076–1079. ( 10.1016/j.bone.2010.08.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willie BM, et al. 2013. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone 55, 335–346. ( 10.1016/j.bone.2013.04.023) [DOI] [PubMed] [Google Scholar]
- 28.Razi H, et al. 2015. Skeletal maturity leads to a reduction in the strain magnitudes induced within the bone: a murine tibia study. ActaBiomaterialia 13, 301–310. ( 10.1016/j.actbio.2014.11.021) [DOI] [PubMed] [Google Scholar]
- 29.Yang H, et al. 2017. Examining tissue composition, whole-bone morphology and mechanical behavior of Gorab(Prx1) mice tibiae: a mouse model of premature aging. J. Biomech. 65, 145–153. ( 10.1016/j.jbiomech.2017.10.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao L, Youn I, Guilak F, Setton LA. 2006. Compressive properties of mouse articular cartilage determined in a novel micro-indentation test method and biphasic finite element model. J. Biomech. Eng. 128, 766–771. ( 10.1115/1.2246237) [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Butz KD, Duffy D, Niebur GL, Nauman EA, Main RP. 2014. Characterization of cancellous and cortical bone strain in the in vivo mouse tibial loading model using microCT-based finite element analysis. Bone 66, 131–139. ( 10.1016/j.bone.2014.05.019) [DOI] [PubMed] [Google Scholar]
- 32.Zou W, Hunter N, Swain MV. 2011. Application of polychromatic µCT for mineral density determination. J. Dent. Res. 90, 18–30. ( 10.1177/0022034510378429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkhold AI, Razi H, Duda GN, Weinkamer R, Checa S, Willie BM. 2016. The periosteal bone surface is less mechano-responsive than the endocortical. Sci. Rep. 6, 23480 ( 10.1038/srep23480) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In compliance with the EPSRC's open access initiative, the datasets and code for analysis are included in the main text of the manuscript or in the electronic supplementary material, data section (see S1–S6).