Abstract
As our ability to engineer nanoscale materials has developed we can now influence endogenous cellular processes with increasing precision. Consequently, the use of biomaterials to induce and guide the repair and regeneration of tissues is a rapidly developing area. This review focuses on soft tissue engineering, it will discuss the types of biomaterial scaffolds available before exploring physical, chemical and biological modifications to synthetic scaffolds. We will consider how these properties, in combination, can provide a precise design process, with the potential to meet the requirements of the injured and diseased soft tissue niche. Finally, we frame our discussions within clinical trial design and the regulatory framework, the consideration of which is fundamental to the successful translation of new biomaterials.
Keywords: tissue engineering, regenerative soft tissue scaffolds, tendon injury and disease
1. Introduction
Ageing populations and lifestyle changes have resulted in a rapid increase in chronic diseases such as osteoarthritis, heart disease and diabetes [1,2]. Since loss or dysfunction of tissue underpins all such conditions, the replacement of diseased tissue offers new treatments and perhaps even cure. First generation biomaterials designed to replace damaged tissue have been extremely successful [3,4]. Total hip replacements are one of the most cost-effective interventions in medicine [5]. Similarly, artificial heart valves have been a transformative technology [6]. However, the success of many early biomaterials is tempered by a relatively short lifespan and narrow therapeutic application. Unlike living tissue, bioinert materials are unable to respond to everyday physical or biochemical challenges, limiting their longevity and widespread application [7,8]. Consequently, a paradigm shift has occurred and the ability of biomaterials to stimulate a controlled tissue response is now a key design feature [9]. For instance, stimulating new bone formation through hydroxyapatite coating of skeletal prostheses creates a stronger bond between the prosthesis and bone [10]. We are now beginning to produce materials that modify cellular responses with increasing precision, shifting the focus away from simple replacement and towards regeneration. By amplifying the body's natural repair mechanisms, biomaterials are able to recruit native cells and guide endogenous regeneration. In doing so, many of the financial, logistical and legislative hurdles that hamper ex vivo tissue engineering can also be avoided.
Within the field of regenerative medicine, the concept of scaffold-induced endogenous tissue repair has gained increasing popularity. By replicating the native architecture of the extracellular matrix (ECM), scaffolds can support cellular attachment, proliferation, differentiation, influence immune responses and stimulate neoangiogenesis [11–14]. As our ability to engineer nanoscale biomaterials has developed, we are now able to combine mechanical, topographical and biological cues with increasing complexity. This design flexibility allows for the production of materials to suit a range of biological niches and diseases. Indeed, a variety of scaffolds are currently being developed for the treatment of musculoskeletal, cardiac, ophthalmic and gastrointestinal disease [15–18]. This review will draw on research developments within the field of tendon repair as an exemplar of soft tissue injury and disease. We will focus on how materials can be chosen and modified to fulfil the requirements of the tendon niche, concentrating firstly on the mechanical and physical properties, before considering methods of functionalization to enhance the cellular response. Finally, we will consider how regulatory frameworks impact on the design and clinical translation of scaffold technology.
2. Tendon structure
Tendons efficiently transfer tensile forces from the muscles to the skeleton [19]. While the overall cellular content of tendon tissue is low, tenocytes are responsible for the production and maintenance of the ECM, the structure of which is critical to tendon function [20,21]. The most abundant constituent of the ECM is type 1 collagen which constitutes around 60% of the dry mass [22]. Type 1 collagen molecules self-assemble into highly organized fibrils [23]. In turn, these aggregate into fibril bundles and fascicles which are the principal determinant of a tendon's mechanical strength [24,25]. In common with other soft tissues, proteoglycans (PGs) and their attached glycoproteins (GAGs) make up the remaining non-cellular component of tendons and play a critical role in collagen fibrillogenesis and growth factor presentation [26,27].
The insertion of tendon into bone demonstrates a gradual histological transition that is traditionally divided into four zones [28]. Zone 1 (tendon) is characterized by predominantly type I collagen and the PG versican, while zone 2 (fibrocartilage) contains increased amounts of type III collagen and the PG aggrecan [29,30]. The mineralized fibrocartilage in zone 3 contains significant amounts of type II and X collagen with less fibril organization and increasing hydroxyapatite content. Finally, zone 4 (bone) is composed of 30% hydroxyapatite, within a scaffold of type I collagen [31]. A corresponding cellular transition is also observed, from spindle-shaped tenocytes, to rounded fibrochondrocytes within zones 2 and 3 and finally osteoblasts. From a mechanical viewpoint, the graduated changes in mineralization and collagen organization create an intermediate zone that is more compliant than either tendon or bone [32]. This arrangement limits stress concentration and reduces the risk of failure at the tendon–bone interface [33].
Injury to this complex framework, particularly in the ageing skeleton, is a common occurrence. Achilles tendon rupture affects over 11 000 people each year in the UK, and the incidence is increasing [34]. Rotator cuff tendon tears are another debilitating condition affecting around 50% of those over 66 years of age [35]. Injuries to the patellar tendon (e.g. jumper's knee) or tennis elbow represent a further disease burden. Although spontaneous healing can occur, the morphological and mechanical properties of healed tendons never match those of a normal tendon [36,37]. Moreover, many tendon injuries demonstrate little capacity for successful repair and surgical intervention is frequently required. As such, the management of tendon injuries represents a substantial, and growing, economic burden. Over 10 000 rotator cuff repairs are performed annually in the UK and over 200 000 in the United States [38]. Unfortunately, our current surgical techniques result in functionally incompetent scar tissue with over 40% of rotator cuff repairs failing [39].
3. Tendon repair mechanisms
Healing is thought to progress through three overlapping stages of tissue inflammation, cellular proliferation and remodelling [40–43]. After injury, the release of chemotactic and pro-inflammatory molecules attracts neutrophils, monocytes and macrophages to the wound site, with the release of angiogenic factors stimulating the concomitant formation of a primitive vascular network [44]. Tenocytes are gradually recruited, and their rapid proliferation is responsible for the synthesis of a disordered ECM composed mostly of type III collagen [45]. The subsequent remodelling stage is characterized by decreased cellularity and vascularity and a switch from type III to type I collagen synthesis [46,47]. While the extent of remodelling is dependent on anatomical location, species and disease model, in general, there is an incomplete recapitulation of a tendon's ECM structure and mechanical properties [46,48]. However, studies to date have used models that lack pre-existing tendon disease. Since injury often occurs on the background of chronic tendinopathy, there remains a requirement to determine how tendinopathy modifies the biology of tendon healing.
The identity of cells contributing to tendon healing is not yet fully understood but is hypothesized to arise from intrinsic or extrinsic origins. Intrinsic cells are typically defined as the tenocytes within the collagen fascicles, whereas extrinsic populations reside within the surrounding paratenon and perivascular regions. In an attempt to determine the relationship and contribution of these populations to repair, lineage tracing experiments with transgenic ScxGFP+ mice have been undertaken following patellar tendon injury [49]. After one week, the paratenon had thickened and the defect infiltrated by numerous cells that expressed the tenogenic differentiation marker scleraxis (Scx). Concurrently, these infiltrating cells also expressed the paratenon and perivascular cell marker αSMA [49]. Similar findings have also been reported in a murine model of achilles rupture [50]. To determine if the expanded SMA+ population differentiated into ScxGFP+ tenocytes a tamoxifen-inducible SMA9 reporter mouse (αSMA-CreERT2; R26-TdTomato) crossed with ScxGFP+ mice were injured and the percentage of double positive SMA9/Scx cells analysed [51]. At day 7, 12.5% of cells expressed both SMA9+/Scx+ which increased to 65% at day 14, suggesting that amplification of SMA9+ cells are the main contributor of tendon healing in this particular model [51]. However, it is unclear whether the paratendon or perivascular niche is the source of these SMA9+ progenitors. Of note, this pathway of healing does not recapitulate structurally normal tendon with a reduced expression of fibromodulin and decorin, two genes important in normal fibrillogenesis [49]. The authors speculated that altered collagen assembly may be responsible for the impaired biomechanics seen during patellar tendon healing.
In a novel model of achilles transection in the neonatal mouse, there is a near complete recovery of tendon mechanical properties with significantly improved collagen matrix organization compared with adult injury [52]. Lineage tracing with a Scx Cre reporter system (Scx-CreERT2;R26-TdTomato) in adult mice confirmed an abundant proliferation of intrinsic Scx+ cells within the injured tendon stubs, but that these do not contribute to repair, with repair tissue instead expressing αSMA. By contrast, at two and four weeks after neonatal injury the repair site was mostly composed of Scx-lineage tenocytes recruited from the tendon stubs. Further, while adult Scx+ tenocytes were activated they demonstrated abnormal differentiation along chondrogenic lines, suggesting that aberrant differentiation of adult tenocytes may contribute to the failure of intrinsic repair mechanisms [52]. It is tempting to speculate that biomaterials, capable of modulating tenocyte phenotype, might enhance intrinsic tendon healing and thereby improve tendon repair. Unfortunately, to date there have been no studies that have investigated whether cell–material interactions can modify the cellular lineage of repair tissue.
4. Biomaterials for tendon repair
Various surgical approaches have tried, unsuccessfully, to improve the outcome of rotator cuff repair [53]. A more promising strategy is the use of a biomaterial patch or scaffold, surgically sutured at the site of the tendon-to-bone repair, to provide structural and biological support. A clinically useful scaffold must be able to respond positively to the biological and mechanical environment of healing tissue. However, demonstrating biocompatibility of the scaffold is an essential first step. After implantation, the construct must not elicit significant immunogenicity, cytotoxicity or genotoxicity that would result in a severe inflammatory response and rejection of the implant [54]. The scaffold material should ideally be degradable, thereby avoiding the long-term complications of foreign-body reaction (FBR) and fibrous encapsulation reported in the pelvic reconstruction literature [55]. Degradable biomaterials also demonstrate an increased resistance to bacterial infection [56]. Further, because the fundamental objective of tissue engineering is to generate normal tissue, scaffold degradation allows for cellular invasion and ECM deposition. Importantly, the by-products of material degradation must not induce local or systemic toxicity and the degradation kinetics should match the growth rate of the new tissue.
Beyond simply being safe, biomaterials must provide the biological cues required to stimulate the formation of healthy tissue. Modifications to a scaffold's chemistry and architecture enable cellular attachment, proliferation, migration and differentiation. Finally, the mechanical properties of the scaffold must also be considered. From a purely practical perspective, it must be robust enough to withstand handling and implantation. From a functional viewpoint, the scaffold must be able to withstand the physiological loading experienced at the implantation site—allowing the biological repair to proceed without experiencing excessive and disruptive loads. Complicating this, the mechanical integrity must be sufficient throughout the process of tissue regeneration despite scaffold degradation. Compounding this challenge, because the mechanical environment directly influences cellular behaviour [57], the optimal biomechanical environment will change as the repair tissue matures.
5. Biological scaffolds
5.1. Decellularized extracellular matrix
Scaffolds can be broadly grouped into two categories based on the materials used; biological and synthetic. In the field of musculoskeletal bioengineering, the removal of cellular components from xeno- or allografts to produce a decellularized ECM, is a common method of biological scaffold production. It aims to retain proteins and the three-dimensional (3D) architecture of the original tissue to provide instructive chemical and structural signals, but without causing implant rejection [58]. The implantation of demineralized bone matrix (DBM) demonstrates sufficient bioactivity to induce new bone formation in vivo [59], an effect that is maintained even when implanted into muscle [60]. However, in the context of trauma and orthopaedic surgery, the clinical evaluation of DBM has failed to demonstrate efficacy [61]. Despite this, the use of ECM-derived scaffolds have been translated into the tendon arena and are one of the most commonly used methods for augmenting tendon repair [62,63]. Again the promising results reported in animal models [64], have not been borne out in human trials. While observational studies have concluded in favour of porcine small intestinal submucosa (SIS) grafts [65,66]. No discernible difference between augmentation and standard repairs has been reported in more robust studies. For example, Iannotti et al. [67] randomized 15 chronic large-to-massive rotator cuff tears to each treatment arm. Augmentation with SIS did not improve the rate of tendon healing or the clinical outcome scores. Further, several patients developed hypersensitivity reactions, raising concerns over biocompatibility. A similar inflammatory response resulted in Walton et al. [68] having to abort their randomized controlled trial.
No such concerns have yet been raised with decellularized allografts and rates of re-rupture appear lower, but still remain at 15–20% [69,70]. Regardless, no convincing clinical improvement has been reported, highlighting that mechanically stronger and biologically normal repair tissue are not necessarily synonymous. Only one case study has provided histological outcomes following augmented rotator cuff repair [71]. A Graftjacket® matrix, biopsied after three months demonstrated extensive tenocytes and blood vessel infiltration at the graft's peripheral margins. If we are to design more effective scaffolds, there is a need for more rigorous and detailed interrogation of biological as well as clinical outcomes following augmented repair. A corresponding understanding of the macro- and microscale structural properties of scaffolds will allow the identification of designs that facilitate positive biological responses. Unfortunately, a rigorous evaluation of commercially available products has only recently been undertaken [72]. A failure to recapitulate the mechanical and nanoscale structure of human supraspinatus tendon may help to explain their lack of clinical efficacy. Ultimately, the generation of a biologically normal repair is likely to require a more precise control of a scaffold's mechanical, physical and chemical properties, that are each tailored to meet the needs of different tissues and disease niches. While processing methods of ECM scaffolds can selectively remove certain growth factors (GFs) [73] and bias macrophage populations [74], synthetic scaffolds allow for a much more precise ‘bottom-up’ design process.
5.2. Natural hydrogels
Natural hydrogels constitute another major class of biological scaffold. These are water-swollen polymeric networks formed from numerous hydrophilic proteins or polysaccharides [75]. Given that collagen is a key and ubiquitous component of the ECM, it is not surprising that collagen-based hydrogels have been extensively studied for cardiac [76], ophthalmic [77] as well as musculoskeletal applications [78]. However, in the context of tendon repair, rather than being used independently they are often used as a vehicle for cell delivery or as a means of functionalizing a synthetic scaffold [79,80].
In comparison to tissue culture plastic, collagen hydrogels provide a preferential in vitro environment for the culture of fibroblasts and tenocytes [81]. Additionally, human mesenchymal stem cells (hMSCs) cultured onto collagen scaffold demonstrate greater proliferation and differentiation into tenocytes [81], although it is unclear whether endogenous MSC migration contributes to physiological tendon repair.
Few studies have assessed in vivo, the efficacy of unseeded hydrogels in tendon repair. In a rabbit model of achilles tendon rupture, a collagen scaffold has been shown to improve the histological and biomechanical properties of healing tendons over standard repair. Nevertheless, the biomechanical properties remained far below those of a normal achilles tendon [82]. Conversely, a fibrin hydrogel failed to demonstrate any biomechanical or clinical advantage in a rabbit flexor tendon injury model [83].
The poor mechanical properties of hydrogels remain a major limitation and arise from the failure to fully recapitulate natural ECM structure. Cross-linking is commonly employed to improve the mechanical properties but may reduce sites for cellular attachment and can result in toxicity [84] and fibrous encapsulation [85]. Other processing methods include improving fibre anisotropy [86] and the incorporation of other ECM components [87]. However, the mechanical properties remain far below those of native tendon. Hybridization with synthetic polymers is among the most promising strategies [88,89].
5.3. Synthetic scaffolds
Both degradable and non-degradable synthetic scaffolds are currently under development for rotator cuff repair. The latter approach, similar to hernia repair, aims to permanently support the diseased tendon and negates the need for complete tissue regeneration. Teflon (polytetrafluoroethylene) grafts, used in the repair of massive cuff tears, were first reported over 30 years ago [90]. Polycarbonate polyurethane patches are currently under investigation with promising early results. In a non-randomized case series, 90% of patients had an intact repair at 12 months with no adverse events reported [91]. Similar results have been reported for polyethylene terephthalate (Dacron) [92]. However, there remain concerns over a higher infection risk [56] and long-term toxicity. Carbon-fibre implants, once used in the treatment of rotator cuff tears [93], have been shown to shed carbon debris that can elicit a FBR in tissues distant from the initial implantation site [94,95]. A failure to stimulate adequate regeneration, with consequential fatigue of the polymer, has been cited as a plausible causative mechanism [96].
Degradable polyesters, including poly-l-lactic acid (PLLA) [97], poly(lactic-co-glycolic acid) (PLGA) [98], polycaprolactone (PCL) [99,100] and polydioxanone (PDO) [101,102] are among the most promising candidates currently under development. Interestingly, different polymers have diverse effects on the cellular response. Although PLLA, PLGA and polyglycolic acid (PGA) are all poly-α-hydroxyesters, variations in cellular attachment, morphology and proliferation are observed [103].
Differences in the mechanical properties of individual polymers must also be considered. Mathematical modelling has demonstrated that scaffolds with tendon-like properties can improve the biomechanics of rotator cuff repair by offloading the surgical repair and mitigating the poor structural properties of diseased tendon or osteopenic bone [104]. Incorporating scaffolds with inferior or even supra-physiological mechanical properties did not translate into an improved repair [104]. The design of a scaffold with appropriate mechanical properties must also take account of the in vivo degradation kinetics. Lu et al. demonstrated that while a braided PGA scaffold demonstrated the highest initial strength, this advantage was not maintained, with loss of scaffold integrity after just one week. By contrast, braided scaffolds produced from PLLA demonstrated much slower rates of degradation [103]. The ideal polymer is one that matches degradation rate with the speed of tissue ingrowth, such that the mechanical properties of native tissue are mirrored throughout the repair process.
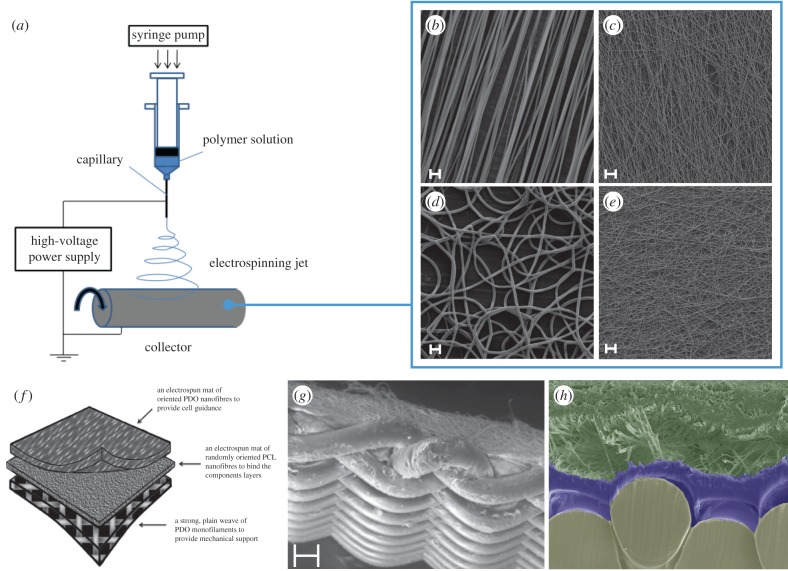
Aside from polymer type, several strategies including changes to porosity, fibre alignment and cross-linking have also been employed to modify the mechanical properties of synthetic scaffolds [105–108]. Many of these techniques often offer only marginal improvements. To overcome this issue, we have developed a bonding technique that enables biologically active layers of PDO to be reinforced with a mechanically robust woven layer (figure 1) [101]. The properties of the woven layer can be adjusted to match the mechanical properties of the rotator cuff.
Figure 1.
Electrospun scaffolds for tendon repair. (a) Application of a high voltage to a polymer solution extrudes an ultrafine jet of polymer that is attracted and collected onto an oppositely charged plate. (b) Scanning electron micrographs (SEM) of electrospun polydioxanone (PDO) fibres in an aligned configuration. Adjustments to the voltage, PDO concentration or collecting plate speed (revolutions per minute), can produce scaffolds with smaller fibre diameters (c), random orientations (d) or both (e). Scale bars are 10 µm. Multi-layered scaffolds from electrospun and woven mats can be produced through a novel bonding technique (f). (g,h) SEM images demonstrating a multi-layered electrospun and woven scaffold in overview and cross-section. Scale bar in (g) is 100 µm. (f–h) Reproduced with permission from Hakimi et al. [101]. Copyright © 2015 Elsevier. (Online version in colour.)
Despite the advantages that degradable polyesters offer, several limitations must still be overcome. Firstly, degradable polymers are broken down through hydrolysis with the generation of lactic and glycolic acid. These physiological compounds are often assumed to be metabolized and cleared without causing toxicity. However, high concentrations have been found to be toxic to both tenocytes [109,110] and osteoblasts [111]. This potential toxicity is influenced by polymer type, quantity, surface area and site of implantation [112]. Therefore, prior clinical use of a specific polymer should not be used as a generalized guarantee of safety. There is a need for greater in vivo research to ensure that, throughout an implants lifespan, these acidic by-products are being effectively cleared from the tendon. Secondly, a problem common to all synthetic scaffolds is that they do not support cell adhesion. Fortunately, numerous strategies are available that can modulate the cellular response to synthetic materials (figure 2).
Figure 2.
Improving cell–material interactions for tendon repair. A synthetic scaffold can undergo numerous modifications that, in isolation or combination, improve bioactivity. Surface chemistry can be modified or bioactive increased through the incorporation of proteins, short peptide sequences (e.g. RGD domain), growth factors or pharmaceutical agents (a–c). Physical modifications to the material's architecture such as fibre anisotropy, fibre diameter, pore size/porosity, degradation rate and stiffness can also be undertaken (d–f). VEGF, vascular endothelial growth factor; FGF-2, fibroblast growth factor-2. (Online version in colour.)
6. Modification of synthetic scaffolds
6.1. Surface hydrophobicity
Engineering of materials with optimal properties for cellular ingrowth and differentiation requires an understanding of the complex relationship between a materials surface properties and cellular response. To date, several variables have been interrogated including hydrophobicity, charge and chemical composition of the material. Of these, surface hydrophobicity has been the most extensively investigated. The exact relationship is influenced by the cell type. For example, hydrophobic surfaces prevent osteoblast adhesion. Progressively greater osteoblast attachment is observed on increasingly hydrophilic materials [113]. By contrast, fibroblasts demonstrate maximal adhesions at moderate levels of wettability, both in the presence and absence of serum [113]. Furthermore, surface hydrophobicity influences fibroblast morphology, proliferation and gene expression [114,115].
On implantation, biomaterials are exposed to the milieu of proteins present in the blood and interstitial fluid, resulting in a surface layer of adsorbed proteins (figure 3a). The adsorption of fibronectin appears to be particularly important for fibroblast adhesion with maximal adsorption again occurring at moderate hydrophobicity [116]. Tamada et al. demonstrated that the removal of fibronectin and vitronectin from culture media resulted in a marked reduction in fibroblast adhesion. Similarly, culturing cells in media that contained only fibronectin resulted in a uniformly high attachment that was independent of hydrophobicity [117]. It is, therefore, apparent that hydrophobicity influences which proteins are competitively adsorbed from the complex assortment of proteins present in serum and ECM. However, it is by no means determinative, factors such as surface charge also influence cell–protein–material interactions.
Figure 3.
Controlling material–protein–cellular interactions. (a) The interaction of cells with biomaterials is governed by the materials surface properties. Surface properties influence protein adsorption, which in turn effects cellular adhesion, proliferation and phenotype. (b) Successful tendon tissue engineering requires us to consider material interaction with multiple cells types (tenocytes, immune cells and endothelial cells). This first requires an understanding of optimal cell ratios and how interactions between cell types influence disease progression and resolution. (Online version in colour.)
6.2. Surface charge
Electrochemically and biologically inert polymers, copolymerized with charged monomers, generate materials with a net negative or positive charge [118]. Both fibroblasts and osteoblasts demonstrated a preferential attachment to positively charged polymers. In fact, the degree of attachment was similar to polymers that had been functionalized with cell adhesive peptide sequences, e.g. Arg-Gly-Asp (RGD) [118]. Positively charged polymers have also been shown to increase fibroblast proliferation, alter morphology and increase collagen production [119,120]. Conversely, it has also been reported that fibroblasts preferentially interact with negatively charged surfaces [115,121]. This discrepancy may be explained by concomitant alterations in hydrophobicity, surface chemistry and topography that techniques used to alter surface charge often induce. In an attempt to determine the relative importance of charge, Qui and colleagues created a novel system. Cells were cultured on two parallel electrodes of indium tin oxide and a voltage applied. Rat marrow stromal cells demonstrated increased adhesion on the positively charged surface [122]. A similar result has also been reported for murine fibroblasts [123].
The mechanisms underpinning the effect of surface charge on cell attachment have not been fully elucidated. Surface charge may facilitate protein adsorption, subsequent integrin binding and ultimately cellular adhesion. By adjusting the number of amine and carboxyl groups, Lin et al. [124] were able to generate different surface potentials, with laminin and fibronectin preferentially binding materials with an overall negative charge. While this might be anticipated for laminin, which carries a positive charge, fibronectin has a net negative potential suggesting that electrostatic interactions may not be the primary determinant of protein adsorption.
6.3. Surface chemistry
Hydrophobicity and charge are themselves determined by the surface chemistry of the material. The most commonly investigated surface chemistries are the carboxyl (−COOH), hydroxyl (−OH), amine (−NH2) and methyl (−CH3) groups [125]. Amine group functionality results in higher quantities of protein adsorption, which include fibronectin, vitronectin and osteopontin [126–128]. This adsorption profile correlated with increased fibroblast adhesion and proliferation [126]. Crucially, it is not only the quantity of protein adsorption that is important but also the protein's structure. Amine functional groups result in a conformational change in fibronectin structure with a subsequent increased availability of adhesional domains that bind α5β1 and αvβ3 integrins [129]. The importance of a protein's conformational profile is further demonstrated by work on hydroxyl surface chemistry. Hydroxyl groups have been shown to adsorb low amounts of plasma proteins [127]. However, as the fraction of hydroxyl groups increase there is a corresponding increase in the availability of α5β1 binding sites resulting in the formation of focal adhesion complexes [127]. Interestingly, this behaviour is seen with osteoblasts but not fibroblasts, again highlighting that a biomaterial's properties must be carefully matched to the intended cellular niche [126]. Rather, surfaces coated with –COOH groups which expose αvβ3 integrin domains within fibronectin, encourage fibroblast attachment and spreading while inhibiting osteoblast differentiation [126,129].
The –CH3 group is a major component of some synthetic polymers, for example, polypropylene, which is employed as a suture material and for meshes used in pelvic surgery. Surface methyl groups abundantly bind fibrinogen but fail to expose suitable binding sites for cellular attachment [129,130]. Instead, non-favourable epitopes may be exposed and trigger an acute inflammatory response. P1 and P2 epitope exposure is responsible for the recruitment of phagocytes to polyethylene and PVC surfaces through the binding of Mac-1 integrin [131]. Similarly, methyl group coated surfaces result in the attraction of Mac-1+ phagocytes [132]. Taken together, these results suggest that methyl functionality may expose immune epitopes and trigger an unfavourable inflammatory response.
Several different surface modification methods have been exploited to change the properties of synthetic polymer scaffolds. The hydrolysis of a polymer with a strong alkali creates –OH and –COOH groups, increases hydrophilicity as well as the roughness of PCL and PLLA surfaces [133,134]. Fibroblasts cultured on NaOH treated polymers display an increased rate of attachment and a spread morphology [135]. Again, the target niche modifies the response—vascular smooth muscle cells [133], osteoblasts and hepatocytes [136], all demonstrate favourable responses to NaOH modifications, while endothelial cells display the opposite [137]. It is likely that this specificity relates to the exact profile of adsorbed proteins. Further work is required to elucidate which protein signatures elicit a favourable biomaterial–cellular interaction, as well as a systematic interrogation of which scaffold modifications can best achieve this. Combining polymer arrays [138] with high-throughput proteomics [139] would allow the rapid screening of polymers and accelerate our understanding of protein–material interactions.
Plasma treatment offers another effective method of surface modification. PLGA treated with oxygen plasma resulted in the generation of hydroxyl groups and an enhanced attachment of murine fibroblasts [140]. Similarly, ammonia plasma treatment of PLLA facilitated a strong cell–biomaterial interaction. The controlled flow of culture media over seeded fibroblasts was used to stimulate a shear stress. Fibroblasts cultures on –NH3 modified surfaces displayed a much lower rate of detachment [141]. However, it must be noted that there remains a real need to establish if modifications to a polymer's surface properties translate into improved tendon healing in vivo.
6.4. Physical properties
The nanoscale structure of the ECM provides a natural network that supports cells and guides their behaviour [142]. Through the formation of focal adhesions, cells are able to transmit mechanical signals into the nucleus, resulting in epigenetic modifications and altered protein expression [143]. Synthetic biomaterials can be modified so as to recapitulate aspects of this complex biophysical environment. A material's dimensionality [144], stiffness [145] and topography [146,147] have all been shown to influence cell behaviour.
As the primary constituent of tendon ECM, collagen fibrils are a critical determinant of a tenocytes physical and mechanical environment. Fibril diameter (50–200 nm) [148,149], anisotrophy [150] and nanotopography created by repeating D-bands [151] all contribute to a tendons biophysical signature. Tendinopathy changes many of these parameters [22] and, in turn, this may contribute to the phenotypic shift that diseased tenocytes undergo [152]. The microscale and nanoscale architecture of a material can be independently adjusted to mimic a healthy tendon niche and thereby manipulate the phenotype of diseased tenocytes.
6.5. Surface roughness
The response of cells to nanoscale roughness is dependent on the cell type. Osteoblasts, cultured on a titanium surface with graduated changes in surface roughness, demonstrate a linear relationship between proliferation and roughness [153]. Human fibroblasts demonstrated the opposite response [153,154]. Similar results have been reported for fibroblasts cultured on polymer surfaces [155,156]. However, it is unclear whether completely smooth surfaces [154,157] or a small degree of roughness [153,155,158] represents the ideal surface for fibroblast attachment and proliferation. Equally, the underpinning mechanisms remain obscure but differences in surface energy [158] and fibrinogen binding [154] have been suggested.
6.6. Surface anisotropy
Collagen anisotropy is fundamental to the function of tendons [159]. If we are to effectively imitate native tendon ECM it follows that a scaffold's microscale architecture must also be highly aligned. Several techniques are available, of which electrospinning is one of the most widely investigated. It provides a relatively easy and cost-effective way of producing a 3D network of aligned ultrafine polymer fibres [160]. Refinement of this technology has enabled the efficient adjustment of fibre alignment (figure 1b–e) [161].
Fibroblasts, seeded onto aligned electrospun PCL fibres orientate and proliferate in the direction of the fibres [162]. An increase in proliferation [163], migration [164], and an upregulation of genes linked to adhesion and actin polymerization is also observed [162]. We have observed similar results for tenocytes cultured on aligned PDO fibres [165]. Fibre orientation has also been found to influence the fate of hMSC differentiation [11]. Likewise, aligned PLLA scaffolds support proliferation and tenogenic differentiation of tendon-derived stem cells [166].
It is hoped that these promising observations will translate into the formation of more competent tissue at the site of surgical repair. Certainly, the quality of newly deposited ECM appears to improve using these strategies. Using second harmonic generation it has been demonstrated that collagen deposition on aligned microfibres displays greater organization [167]. Biomechanical testing found improved tensile properties for orientated scaffolds, a difference that was amplified as appropriately orientated collagen was laid down during prolonged culture. Early in vivo work has also demonstrated improved histological features [166]. However, the vast majority of studies have used ‘healthy’ cells, yet it is recognized that tenocytes isolated from tendon tears demonstrate an altered phenotype [168]. External biomechanical cues also influence the orientation [108] and proliferation rate of diseased rotator cuff tenocytes, but their response at the mRNA level does not precisely mirror that of healthy cells [165]. Clearly, when investigating the effects of biomechanical cues on tendon healing, the targeted tissue niche must always frame our enquires. Site- and disease-specific changes in a fibroblast phenotype [169] will adjust this niche and significantly influence cell–material interactions.
Even finer control over the specific characteristics of scaffolds is possible. How these parameters interact to elicit the most favourable response remains to be fully determined. On randomly orientated PLGA scaffold fibre diameter has no discernible effect on fibroblast proliferation [170]. By contrast, for aligned scaffolds with fibre diameters ranging from 150 to 6000 nm, dermal fibroblast proliferation and collagen expression demonstrated fibre diameter dependency [171,172]. Diseased rotator cuff tenocytes may also be responsive to fibre diameter, with higher rates of proliferation, collagen and GAG production reported for scaffolds with submicrometre fibre diameters [173].
6.7. Scaffold porosity
The spacing between fibres can also provide cells with topographical information. Smaller pore sizes, (less than 150 µm) seem to increase fibroblast adhesion [174,175], an effect that may be driven by an increase in a material's surface area [176], roughness [175] or through modulation of micromechanical properties [174]. However, pore size is also intimately related to overall porosity with large pore size generally resulting in an increased porosity [176]. Higher porosity improves the mass transport of nutrients and increases fibroblast proliferation, migration and enables deeper penetration into the scaffold [177–180]. Overall, the increased proliferative rate associated with large pore, high porosity scaffolds negates the initial advantage offered by small pore sizes [181]. A high porosity is also able to modulate the FBR, possibly through a biasing of the macrophage population towards an M2 phenotype [182]. Further, computational modelling has demonstrated that adequate vascularization of a construct requires large pore sizes of 160–270 µm [183]. The degree of connectivity between pores is also important. Scaffolds that demonstrate higher degrees of interconnectivity, but constant porosity, facilitate increased migration of fibroblasts into the scaffold [184].
6.8. Substrate stiffness
The micromechanical properties of an implant must also be given consideration. The ability to detect and respond to the stiffness of the ECM has been demonstrated in a variety of cell types including endothelial, smooth muscle cells and fibroblasts [185–187]. Stiffer substrates promote the formation of stable focal adhesions, which subsequently results in cytoskeletal re-organization, increased proliferation, migration and altered cell shape [187,188]. Contractility and tensional forces generated by fibroblasts also increase with matrix stiffness [189]. This may have implications for the organization of newly produced ECM. Fibrillinogenesis of fibronectin is dependent on appropriate levels of cytoskeletal tension [187]. Additionally, microarray analysis has demonstrated that construct stiffness modulates cellular phenotype, with an increased expression of ECM genes and a decreased expression of matrix-degrading enzymes, characterizing fibroblasts grown in less compliant environments [190]. Tenocytes demonstrate a similar responsiveness to substrate stiffness [191].
Despite the well-accepted role that substrate stiffness plays in fibroblast biology, to date, this has seen only limited translation into the tissue engineering arena [192–194]. Unfortunately, with many of the methodologies used it is often difficult to separate the influence of matrix stiffness from the concurrent changes in porosity and surface chemistry. There remains a need to establish the ideal stiffness for tendon engineering. However, to promote the invasion of cells from surrounding tissue it is likely that the construct will need to be stiffer than native tissue. Fibroblasts cultured on materials with a graduated stiffness preferentially migrate towards the stiffer surface, a phenomenon termed ‘durotaxis’ [195]. It is also likely that dynamic changes in stiffness are important. During cutaneous wound repair, the mechanical stimulus is removed as repair tissue matures. Persistent mechanical loading of fibroblasts creates pathological conditions and is implicated in contracture and hypertrophic scar formation [196]. If we wish to generate normal, functional tissue, then this presents a further argument against the use of non-degradable constructs in tendon repair.
7. Bioactive functionalization of scaffolds
Apart from modifying the chemical and physical properties of biomaterials, biological adjustments can also be undertaken to provide optimal conditions for repair. Scaffolds can be incorporated with a range of bioactive molecules in the form of whole proteins, short peptide sequences, GFs or even pharmaceuticals. We will review the biomolecules that are currently finding application in the field of tendon tissue engineering.
7.1. Protein and peptide incorporation
In native tissue, cells attach to the substratum through the interaction of cell surface receptors, such as integrins, with short peptide sequences contained within matrix proteins. These interactions provide cues that prevent apoptosis, enable migration and stimulate differentiation. Therefore, the addition of adhesion proteins has the capacity to modulate cellular phenotype. The commonest method for their incorporation into polymer scaffolds is the simple mixing of the solutions before processing. Alternatives include the immersion of a scaffold in a protein solution and, more recently, coaxial electrospinning which produces fibres with a distinct inner-core and outer-shell [197]. The coating of PLGA with fibronectin or collagen results in an increased adhesion strength for human embryonic tenocytes [197]. However, the coating of various electrospun polymers with fibronectin did not translate into an improvement in fibroblast proliferation or tenogenic differentiation of mesenchymal stem cells [198,199]. In this respect, the method of protein incorporation may be important. Zhang et al. [200] showed that dermal fibroblasts seeded onto a coaxially spun PCL–collagen matrix, demonstrated an increased proliferative and migratory profile compared to cells grown on collagen-soaked scaffolds. Similarly, covalent attachment improves the quantity of surface-bound fibronectin with a corresponding improvement fibroblast attachment and proliferation [201]. Interestingly, the effect of fibronectin binding on human dermal fibroblast function is modified by the cytokine environment, presenting a challenge for accurate in vitro optimization of scaffold design [202].
The addition of short peptide sequences is an alternative strategy. The well-known RGD motif is found in various ECM proteins including fibronectin, collagen I and laminin [203]. In bone healing, RGD enhances the attachment, differentiation and mineralization of osteoblasts [204], but with variable results when coated implants have been implanted in vivo [205–207]. Much more limited data exist on the effect of short peptide sequences on tendon healing. The addition of RGD to a PEG hydrogel promoted fibroblast attachment and cell spreading in a concentration-dependent manner [208]. Kim et al. [209] covalently bonded RGD peptides onto an electrospun PLGA matrix and demonstrated that an improved fibroblast attachment was independent of concurrent changes in the hydrophobicity. Human hamstring tenocytes respond similarly, with an increased expression of matrix proteins, collagen I and decorin [210]. To our knowledge no in vivo studies have yet investigated the efficacy of RGD peptides in tendon or ligament healing, although coated sutures for cuff repair have been patented [210]. It must also be remembered that the coating of fibres with bioactive molecules can only provide a transient stimulus, which is quickly lost as the polymer surface degrades. The incorporation of binding sites into the polymer backbone would provide a more sustained stimulus and is an exciting research area [210].
7.2. Growth factors
Growth factors are small cell-signalling polypeptides that are secreted and held within the ECM, playing a crucial role in chemotaxis, proliferation and matrix synthesis. During rotator cuff tendon healing the expression of several GFs changes. Basic fibroblast growth factor (FGF-2), transforming growth factor-beta 1 (TGF-β1), platelet-derived growth factor-beta (PDGF-B), insulin-like growth factor 1 (IGF-1), bone morphogenic protein 12 (BMP-12), BMP-13, BMP-14 and connective tissue growth factor (CTGF) are all upregulated in animal models of tendon healing [211,212]. Administration of these factors to the site of tendon injury is hypothesized to improve the healing process. Impregnation with GFs enables a focused delivery that may also act synergistically with a scaffold's physical and chemical properties.
7.2.1. Fibroblast growth factor family
The FGF family consists of 22 different polypeptides with FGF-1 and FGF-2 being the most abundant in adult tissue [213]. FGF-2 facilitates angiogenesis, regulates fibroblast proliferation, controls matrix synthesis and is critical to cutaneous wound healing [214,215]. The in vitro supplementation of FGF-2 to rat patellar tenocytes increases proliferation [216]. Similar effects have been demonstrated for human rotator cuff cells but with a dose-dependent reduction in collagen I and III synthesis [217,218].
The local application of FGF-2 to rat supraspinatus repairs resulted in an early improvement in histological scores at the enthesis and a corresponding improvement in load-to-failure [219]. Similarly, the injection of FGF-2 into rat patellar tendon defects showed no added benefit beyond 7 days [219]. Interestingly, when the same group incorporated FGF-2 into a dermal matrix this effect was modified, with improvements noted only after six weeks [220]. A study on FGF-2 loaded hydrogels for rat rotator cuff repair demonstrated similar findings with improvements in collagen orientation and vascularity not manifesting until one month after surgery [221]. However, in contrast to these studies no convincing improvement was demonstrated for an FGF-2 impregnated collagen sponge, implanted into an ovine model of rotator cuff injury [222]. Dosing discrepancies, which were an order of magnitude different, may be responsible. Indeed, a study on ligament repairs in rabbits found that at high doses FGF-2 became detrimental to the healing process [223].
7.2.2. Transforming growth factor β
During wound healing, TGF-β is secreted by both immune and stromal cells. Three mammalian isoforms exist (β1, β2 and β3), each with distinct functions [224]. A wide variety of animal tendon injury models have shown that the gene and protein expression of all isoforms increase in disease [225]. Their role in human tendinopathy is less clear with TGF-β1 increasing, decreasing or remaining unchanged in rotator cuff tears, patellar and achilles tendinopathy, respectively [226–228].
The inhibition of TGF-β signalling with neutralizing antibodies impairs the healing of rat rotator cuff repairs [229]. In response to exogenous TGF-β1, tendon cells isolated from healing rat patellar tendons demonstrate a persistent reduction in the expression of the ECM proteins decorin and biglycan, and a temporary reduction in Col1A1 and Col1A3 [220]. However, when TGF-β1 was loaded into hydrogels an improved load-to-failure was recorded for rat supraspinatus repairs [229]. This was attributed to an inhibition of matrix degrading proteases and not to any improvements in the histological organization of repair tissue. TGF-β3 has been implicated in embryological development of the enthesis as well as the formation of more organized repair tissue in cutaneous injury models [230]. Kovacevic and colleagues reported that incorporation of TGF-β3 into an osteoconductive calcium-phosphate matrix resulted in a more favourable collagen I/III ratio and an improved strength of infraspinatus tendon repair in rats [229].
7.2.3. Platelet-derived growth factor
PDGF-BB acts as a chemotactic and mitogen factor for tenocytes, osteoblasts and mesenchymal stem cells. In a rat model of rotator cuff repair, collagen scaffolds were loaded with incremental doses of PDGF. A dose-dependent increase in fibroblast proliferation and angiogenesis was seen, but this did not translate into an improvement in collagen organization or biomechanical properties compared with standard suture repair [231]. Several other studies have reported improvements in histological features but found no advantage on biomechanical testing [232–234]. A similar study in sheep, using much larger doses, found that the incorporation of 75 and 150 µg of PDGF-BB improved collagen orientation, collagen density and interdigitation at the bone–tendon interface. Corresponding biomechanical improvements were seen. However, the delivery of 500 µg proved detrimental with histological scores and biomechanical outcomes below those of controls [235]. Similar findings have been reported for FGF-2 and taken together underscores that correct dosing, timing and delivery of GFs must be systematically interrogated.
7.2.4. Vascular endothelial growth factor A
The role of vascularity in tendon healing is unclear. A watershed area of critical hypovascularity is often cited as being responsible for supraspinatus tears. It follows that increased vascularity may improve cuff healing. However, a watershed area may simply be a histological artefact that more recent studies have failed to confirm [236]. Nonetheless, vascularity of the cuff is changed in disease, increasing in tendinopathy before a sequential reduction occurs as the size of tear progresses [236,237]. Furthermore, the inhibition of angiogenesis prevents ligamentous healing [238].
VEGF, a potent angiogenic protein, is secreted by tenocytes in increased amounts in tendon disease [239,240]. However, the utility of exogenous VEGF in tendon healing is largely unknown and no studies have evaluated the role of VEGF in cuff repair. In vitro, VEGF does not alter collagen synthesis but does alter the expression of other GFs, including TGF-β [241]. In a murine transection model of achilles rupture, the injection of a proteolysis-resistant VEGF splice variant increased the ultimate tensile strength and mechanical stress of spontaneously healing tendons [242]. In a separate study, in which VEGF injections were combined with suture repair no sustained biomechanical benefit was found [242]. The in situ duration of VEGF stimulation is, therefore, likely to be important. In a study on anterior cruciate ligament (ACL) reconstruction, the authors observed that by using a hyaluranon carrier to retard the release of VEGF, an improvement in allograft vascularity and biomechanical properties occurred [242]. Conversely, the simple soaking of allografts in VEGF did not benefit the experimental group in an ovine model of ACL reconstruction [242]. Other methodologies, such as the transplantation of endothelial progenitor cells, that provide a more sustained stimulus for angiogenesis have also been shown to improve ligamentous healing [238]. Whether these findings can be translated into the arena of tendon healing is a developing research area.
7.2.5. Insulin-like growth factor-1
IGF-1 is an important mediator of cutaneous wound healing that is upregulated following tendon injury [243–245]. A beneficial role for IGF-1 is supported by in vitro studies on tendon and ligament explants, which have demonstrated increased proliferation, migration and collagen synthesis [246–248]. The injection of IGF-1 following collagenase induced tendon injury in horses resulted in increased proliferation and total collagen content, although no biomechanical improvement was seen [249]. Similarly, in an achilles transection model, IGF-1 injections improved the functional score, but with no corresponding histological or biomechanical improvement [250]. Conversely, daily injection of IGF-1 following rat medial collateral ligament transection was reported to improve all histological and biomechanical outcomes [251]. The effect of IGF-1 administration has also been investigated among healthy Danish volunteers. Local injection into the patellar tendon resulted in an increase in collagen synthesis, but with a highly variable response between patients [252]. To date, only one study has investigated the incorporation of IGF-1 into a scaffold. They used transfected tenocytes as the delivery vehicle for IGF-1, seeded onto a PGA scaffold implanted at the site of rotator cuff repair in rats. At two weeks an improvement in histological scores and biomechanical characteristics was seen. This improvement was much greater than for a separate group in which PDGF-β transfected cells were seeded onto scaffolds, lending support to IGF-1, rather than a non-specific cellular response, being responsible for the effect [253].
The application of GFs provides a powerful tool for controlling cellular function, but several challenges must be overcome. Research to date has shown us that a single factor will not be sufficient, instead it is likely that the reprogramming of diseased tendon cells will require a combination of factors. The permutations are significant but the application of high-throughput arrays that enables the rapid screening of cell–material interactions is a promising area that may accelerate the discovery process [254]. Secondly, the temporal relationship of each growth factor must be considered. We should be aiming to recreate the natural symphony that is seen during tissue development, when different factors become active at different times and in different locations. Controlled-release strategies are being developed that allow the delivery of multiple factors, each with distinct kinetics. Their utility has already been proven in the context of angiogenesis [254]. Finally, the correct dosing of each factor is critical to determining both efficacy and safety. The use of supra-physiological concentrations of rhBMP-2 during spinal fusion has been linked to numerous adverse effects including heterotopic ossification and neurological sequelae [254]. By optimizing the kinetic release profile, lower concentrations can achieve maximal effects. Systems that mimic natural mechanisms of growth factor binding and release are promising. For example, heparin incorporation offers a method for slowing the release profile and enhancing FGF-induced blood vessel formation [254]. Since local proteolytic activity is responsible for the release of glycosaminoglycan-entrapped factors, a degree of spatial control is also possible [254].
7.3. Bone–tendon interface
Unlike achilles tendon ruptures, which most commonly occur within the mid-body of the tendon, rotator cuff tears often occur at the bone–tendon interface. The successful restoration of intra-tendinous ECM and tenocyte biology is itself insufficient, there must also be recapitulation of the tissue transition that occurs at the enthesis. The biology of osseointegration is poorly understood but it has striking similarities to the process of endochondral ossification [255]. For this reason, the application of factors capable of triggering ossification has been proposed as a means of restoring the enthesis. As well as being important stimulators of osteogenesis, BMPs which are part of the TGF-β superfamily, also promote matrix production by tenocytes [256]. Seeherman et al. examined the role of BMP-12 in an ovine model of rotator cuff injury [257]. A collagen sponge containing BMP-12 was applied to the bone–tendon interface at the time of repair. When compared with the use of the carrier alone, the histological features of the enthesis were improved but still easily distinguishable from normal controls. A doubling in the maximal load and stiffness of the repair was seen, but again this remained significantly below that of healthy supraspinatus tendon. Taken together, this suggests that rather than stimulating tissue regeneration BMP-12 resulted in the formation of stronger scar tissue, a result that echoes studies that have used TGF-β as the bioactive factor. Similar results have been reported for BMP-2 and BMP-13 in a rabbit model of rotator cuff tears [258,259].
Given the heterogeneous cell types found at bone–tendon interfaces, a homogeneous scaffold is unlikely to provide the ideal niche for fibroblasts, chondrocytes and osteoblasts alike. Instead, it is hypothesized that biomaterials with gradients in chemical composition, nanoscale structure and mechanical properties, closely mimicking the gradual transition of entheseal ECM, will provide environments more conducive to the repair of damaged entheseal tissues. The use of microspheres containing recombinant BMP-2 and IGF-1 has allowed the generation of growth factor gradients on silk scaffolds. hMSCs cultured on these scaffolds exhibited osteogenic and chondrogenic differentiation in areas of high BMP-2 but low IGF-1 concentrations [260]. In a novel approach, zonal organization of osteoblasts and fibroblasts was achieved by seeding fibroblasts onto scaffolds with a spatial gradient of retrovirus that encoded the osteogenic transcription factor Runx2 [261]. Alternatively, gradients of calcium phosphate have been found to positively correlate with the expression of the osteoblast differentiation markers by MSCs [262]. Since calcium phosphate content also affects scaffold stiffness [263], it remains unclear whether regional differences in MSC differentiation were driven by the chemical or mechanical environment. Another solution is the production of multi-phased scaffolds in which each phase has properties that are optimized for specific cell populations. Spalazzi et al. [264] designed a tri-phasic scaffold combined of either a polyglactin mesh, PLGA microspheres or PLGA/bioactive glass microspheres. Following subcutaneous implantation, a fibrous, fibrocartilaginous or mineralized ECM was laid down in each of the distinct phases, but only after pre-seeding of each phase with fibroblasts, chondrocytes or osteoblasts, respectively. By contrast, acellular scaffolds did not demonstrate a transitional fibrocartilaginous zone, forming only fibrous and mineralized ECM.
8. Regulating the immune response
To date, tissue engineers have largely focused on how materials can modulate cell populations that are directly involved in the formation of new tissue. There has been limited research on how biomaterials affect other host processes such as inflammation and angiogenesis, the manipulation of which is likely a key determinate of successful translation (figure 3b).
The implantation of biomaterials triggers a FBR, a process that, if chronic, is characterized by inflammatory cell recruitment, angiogenesis and tissue fibrosis [265]. Such a reaction is detrimental to medical implants and may compromise integration and mechanical strength [266]. There is also an increasing recognition of the role of inflammation in human tendinopathy, with diseased tissue demonstrating an increased numbers of macrophages, mast cells, as well activation of pro-inflammatory signalling pathways [267]. However, immune cells have also been shown to be crucial to the repair process for many tissues [268,269]. Rather than simply avoiding an immune response, biomaterials that are designed to modulate the response might improve the quality of tissue repair.
Macrophages, a key regulator of the FBR [270], can be directly influenced by biomaterial design. PEG-RGD hydrogels manufactured with increasing stiffness resulted in an increased production of pro-inflammatory cytokines and a thicker fibrous capsule [271]. Topographical cues have also been shown to have significant effects on macrophage phenotype. Substrates with 20 µm wide lines of fibronectin promoted differentiation of murine macrophages towards an ‘M2’ phenotype, an effect that was lost with 50 µm wide micro-patterning [272]. Human macrophages demonstrate a similar responsiveness to surface topography [273].
The chemical composition of biomaterials is also influential. The cytokine expression of adherent macrophages is dependent on a material's surface chemistry, with hydrophilic surfaces reported to cause downregulation of pro-inflammation genes and apoptosis of foreign-body giant cells [274,275].
Dendritic cells (DCs) are a key component of both innate and adaptive immune responses and can also possess immunoregulatory capacities through induction of T-cell tolerance and expansion of regulatory T cells [276]. The biofunctionalization of scaffolds may be able to modulate this process, for example, alginate and hyaluronic acid coated surfaces appear to restrain DC maturation and induce T-cell tolerance [277]. Similarly, albumin stimulated DCs have been shown to induce a Th2 response, whilst collagen and vitronectin were shown to stimulate DCs to produce high levels of IL-12 that correlated with a Th1 type response [278]. This opens up the challenge of understanding the role and relative importance of each immune population during tendon healing. Additionally, we must better elucidate how manipulations of the immune compartment influence the FBR and subsequent scaffold integration.
The role of other cells types must also be explored. Angiogenesis is intimately linked to inflammation [279]. Indeed, increased vascularity is a key histological feature of many inflammatory diseases and high levels of VEGF and other pro-angiogenic factors have been documented in chronic inflammatory as well as rotator cuff disease [279,280].
The interplay between inflammation and angiogenesis is complex. While inflammatory mediators can promote new vessel formation, angiogenesis can, in turn, sustain inflammation. The expression of chemokines and adhesion molecules by activated endothelial cells recruits more immune cells to the site of inflammation. Fibroblast–endothelial cell interactions are critical to this process [281,282]. Yet, the vascular bed is not just a passive transport network. Through the production of angiocrine factors, endothelial cells regulate the homeostasis and repair of cardiac tissue, bone and skeletal muscle [283,284]. While there is evidence to suggest that the healing of murine ligaments can be improved by artificially increasing vessel formation, other authors have found an association between neovascularization and FBRs [238,270,285]. There remains a need to establish the role of endothelial cells in the progression and treatment of tendon disease.
9. The regulatory framework and scaffold design
In Europe, the legal instruments that govern the commercial development of regenerative biomaterials include the recent Medical Devices Regulation (MDR) [286], pharmaceutical regulations [287] and the Advanced Therapy Medicinal Products (ATMP) regulation [288]. A biomaterials design, constituents and mechanism of action will determine which legislative framework must be satisfied. In turn, this will significantly impact upon the duration, cost and likelihood of successful translation.
In general terms, three distinct scenarios can be envisaged: a regenerative scaffold that induces endogenous repair solely through topographical and mechanical stimulation of cells, a regenerative scaffold with additional bioactive functionalization and a scaffold pre-seeded with viable cells (figure 4). In order to decide whether a product can be considered a medical device the key point for consideration is the method by which the principal intended action is achieved. To be classified within the remit of the MDR, the principle action cannot be through pharmacological, immunological or metabolic means. That is to say, that medical devices may contain medicinal substances, for example GFs, so long as their action is ancillary. In cases of doubt, the provisions of the medicinal products directive will apply [289], placing a clear incentive on manufactures of functionalized scaffolds to carefully delineate their products primary mode of action.
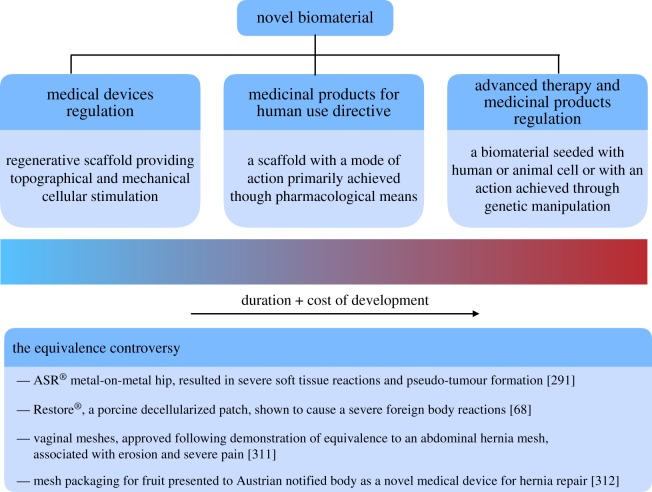
Figure 4.
Biomedical regulatory framework in the European Union (EU). (a) The legislative pathways governing the approval of novel biomaterials for clinical applications. (b) Summary of recent medical devices, approved following demonstration of equivalence and subsequent safety concerns. (Online version in colour.)
Medical device approval in each EU country is overseen by a competent authority, such as the Medicines and Healthcare Products Regulatory Authority (MHRA) in the UK. Under the current framework devices are classified into the following categories based on risk: (i) class I (low risk), (ii) class IIa and IIb (moderate risk) and (iii) class III (high risk). Indeed, this is similar to the US Food and Drug Administration's (FDA) classification system. While low-risk devices (e.g. surgical gloves) can be declared directly to the competent authority, all other products must be approved via the notified body—a designated but independent company that specializes in evaluating medical devices. The current classification system places particular emphasis on whether an implanted device demonstrates biological activity, which includes biodegradation, as well as duration of application. Presumably, this has arisen from concerns that bioactivity may induce local or systemic toxicity. Consequently, within the context of regenerative medicine, it is difficult to envisage a device being classified as anything other than high risk. Such class III products require the notified body to undertake a more significant review, including greater scrutiny of available pre-market clinical data.
Unlike pharmacological compounds, supporting clinical data can be obtained not only through clinical trials but also by demonstrating equivalence to existing medical devices. This has been a point of significant controversy, both in Europe and the USA where a similar process exists—the 510(k) pathway (figure 4). For example, in 2005 a metal-on-metal hip, the ASR® was introduced into the US market following approval by the FDA via the 510(k) process. The ASR® used a metal cup from a different device (ASR Hip Resurfacing system) and fitted this onto a standard femoral implant. The manufacturer was able to argue that the new implant was substantially equivalent and was given market clearance without any requirement for clinical testing. It subsequently became clear that the ASR® hip replacement had a significantly higher rate of revision but not before almost 100 000 had already been implanted worldwide [290]. Furthermore, an analysis of all high-risk device recalls in the USA has demonstrated that 71% have been approved via the 510(k) pathway [291].
Perhaps unsurprisingly, under the new MDR demonstrating equivalence has been significantly curtailed. A manufacturer must be able to demonstrate significant similarities in the products designs and performance as well as identical biological and clinical characteristics. Further, the manufacturer must have significant access to all data relating to the device to which they claim equivalence. Given that there is no requirement for pre-market evaluation data to be made publicly available [292], proving equivalence seems to have been limited to modifications of an existing device by the original manufacturer.
Therefore, it is highly likely that within the field of regenerative medicine and biomaterial design, clinical trials will become commonplace. However, the specific requirements for pre-marketing studies are vague, and contrast sharply with the clear legislative requirements surrounding the conduct of trials for medicinal products. While the new MDR now requires clinical trials to demonstrate efficacy as well as safety, the design of such trials is not defined. Unlike pharmaceuticals, in which randomized controlled trials (RCTs) are the standard [293], no binding guidelines on study designs have yet been placed on medical device manufactures or the notified bodies [294]. While logistical challenges and ethical issues have been sighted as justification for using non-controlled observational studies [295], recent randomized, placebo controlled, orthopaedic trials are making this position less tenable for designers of orthopaedic implants [296].
It is likely that future amendments will bring the clinical evaluation of medical devices even closer to that of pharmaceutical testing. However, in the current legislative climate an implantable scaffold, whose primary regenerative action is mediated through their physical structure, would not require a randomized controlled trial to prove efficacy. Once again, this emphasizes the importance of optimizing a regenerative scaffold's physical design before supplementary bioactive functionalization is considered.
Of note, special attention has been given by the MDR to nanomaterials, for which there is felt to be scientific uncertainty regarding their risk. Such devices are required to be subject to ‘the most stringent conformity assessment procedures' [286]. A nanomaterial is defined based on an external size of 1–100 nm for nanoparticles or less than 1 nm for nanoscale structures such as carbon nanotubes. Thus, an electrospun, 3D printed or otherwise produced scaffold is unlikely to be classified as a nanomaterial unless augmented with nanoparticles. This would suggest that rather than using nanoparticles to modulate surface roughness or act as drug carriers, alternative methods of manufacture should first be explored.
Finally, ATMPs constitute a heterogeneous class of innovative health technologies. It encompasses gene therapy, somatic cell therapy and engineered cells or tissues, administered alone or in combination with a medical device such as a scaffold. The Committee for Advanced Therapies (CAT) of the European Medicines Agency provides a centralized regulatory oversight for all such products. This includes quality and safety standards for donation, procurement and testing of human cells under the EU Tissue and Cell Directive [297] and the conduct of clinical trials in accordance with EU Directive 2001/20/EC [293]. Additionally, any base scaffold would require CE marking under the MDR [298]. The fact that only six ATMPs have successfully completed this centralized marketing authorization implies a burdensome procedure, and likely contributes to their significant cost. For example, Glybera® a gene therapy for lipoprotein lipase deficiency was priced at 1.1 million euros per patient [299].
10. Clinical trial design
The introduction of new techniques and technologies has traditionally occurred on the basis of weak scientific evidence. We must move beyond established pathways of surgical innovation, and instead strive to undertake robust and meaningful clinical trials (figure 5).
Figure 5.
Design of clinical trials for novel scaffold technology. A proposed clinical trial pathway, built around the IDEAL framework [300], that provides a robust means of assessing the clinical utility of new biomaterials. (Online version in colour.)
The current research evaluating the efficacy of commercially available scaffolds for rotator cuff repair is dominated by small, retrospective, single centre case reports and series. A situation that is seen throughout the surgical innovation literature [301,302] and one that predisposes to a significant risk of bias. Only four RCTs, comparing scaffold augmented rotator cuff repair with standard repair, have been undertaken and serve to highlight the pitfalls of relying on weaker methodologies [67,68,70,303]. Despite several case series reporting promising results for Restore® [304,305], randomized trials have not only failed to show any benefit but also demonstrated an excess risk of adverse events [67,68,303].
Unlike the clearly defined phases of pharmaceutical trials, there is no widely accepted roadmap for surgical innovations. To this end, the IDEAL framework has been developed to help overcome our reliance on poor quality clinical data and describes five key stages: idea, development, exploration, assessments and long-term study [300,306,307]. Importantly, these recommendations suggest the abandonment of retrospective case series and instead propose that all first-in-man studies are prospectively registered, with clearly defined a priori outcomes and that all cases are reported in a sequential, transparent manner. As more patients are recruited to undergo the procedure, and as more surgeons and institutions participate, the new device can move through the development and exploratory phases, but with these key methodological elements persisting.
Ultimately, an interventions relative benefit must be assessed, with RCTs providing the gold standard for comparisons of efficacy and effectiveness [308]. Further, placebo controls are particularly important when the available outcomes depend on subjective patient-reported ratings. Such measures are particularly susceptible to bias from the placebo effect, making it difficult to ascertain the true efficacy of the crucial surgical element. While this remains a divisive issue, there is growing acceptance that placebo controls are not only desirable but an ethical imperative—saving patients from potentially harmful but ineffective interventions, whilst also helping to re-allocate funding to more effective procedures [309]. The timing of such randomized trials must also be considered. During the development phase of a scaffold for tendon repair, procedural refinement and operator learning curves may limit the generalizability of early RCTs. Conversely, waiting until development has settled can result in a loss of clinical equipoise, making trials impossible to conduct. This is a difficult balancing act but with a move towards the conduct of early randomized trials [307,308]. To this end, expertise-based RCTs provide an elegant solution to the challenges of expertise bias and clinical equipoise. In this design, patients are randomized to either surgeon A or surgeon B. Each holds experience for one intervention and is solely responsible for performing their procedure of choice [310].
A robust RCT will establish the benefit of a new medical device but, in the field of orthopaedic surgery, the measured outcomes are often short term (1–2 years). Safety concerns may take many years before becoming evident. For instance, during the first 12 months, the revision rate for metal-on-metal total hip replacements was not clearly different from that of other hip replacements [310]. Long-term evaluations are needed to assess a devices ongoing safety and effectiveness. To this end, the European Union requires all medical device manufacturers to collect data on quality, performance and safety, throughout a devices lifetime. Manufacturers of class III devices must now submit periodic reports detailing the findings of their surveillance [286]. This is envisaged to facilitate a contemporaneous assessment of a devices risk–benefit ratio. However, within both the EU and USA the identification of safety concerns remains the main focus of post-marketing surveillance [307]. The long-term study of a device's effectiveness is conspicuously absent and there remains a need to improve the routine monitoring of medical devices. The traditional passive reporting of safety concerns should be universally replaced by well-designed, large observational studies that routinely record data on safety but also effectiveness. National patient registries can help fulfil this requirement and should be extended beyond their current focus on arthroplasty into the arena of tendon repair.
11. Conclusion and future perspectives
Biomaterials form a major component of tissue engineering strategies. Rather than acting as a simple structural support, modern materials both provide physical and biochemical signals that orchestrate cellular behaviour and can also act as delivery vehicles for drugs and small molecules. If the full potential of these new technologies is to be realized, several challenges remain. It is now clear that the cellular response to polymer chemistry, nanoscale architecture and bioactive functionalization is heavily dependent on the target niche, type and stage of disease being targeted. It follows that scaffolds must be tailor-made to meet the specific demands of each tissue and disease. The successful development of tissue engineering scaffolds will require the use of high-throughput preclinical platforms which are capable of assessing large combinations of scaffold design. Biomaterials for soft tissue repair must also be capable of not only influencing stromal cell behaviour but also modulating immune cell responses and promoting angiogenesis. Critical to this will be an improved understanding of cell biology and the interactive role of GFs, immune cells and blood vessels during soft tissue healing. Finally, the translation of new therapies will require rigorously designed early phase clinical trials governed by a regulatory framework that is fit for purpose.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
M.B. has been generously supported by the Royal College of Surgeons and the Dunhill Medical Trust.
References
- 1.Lozano R, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 380, 2095–2128. ( 10.1016/S0140-6736(12)61728-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abajobir AA, et al. 2017. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet 390, 1211–1259. ( 10.1016/S0140-6736(17)32154-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng C, Ballantyne J, Brenkel I. 2007. Quality of life and functional outcome after primary total hip replacement. A five-year follow-up. J. Bone Joint Surg. Br. 89, 868–873. ( 10.1302/0301-620X.89B7.18482) [DOI] [PubMed] [Google Scholar]
- 4.Laupacis A, Bourne R, Rorabeck C, Feen D, Wong C, Tugwell P, Leslie K, Bullas R. 1993. The effect of elective total hip replacement on health-related quality of life. J. Bone Joint Surg 75, 1619–1626. ( 10.2106/00004623-199311000-00006) [DOI] [PubMed] [Google Scholar]
- 5.WHO. 1998. The bone and joint decade 2000–2010. Inaugural Meeting 17 and 18 April 1998, Lund, Sweden. Acta Orthop. Scand. Suppl. 281, 67–86. [PubMed] [Google Scholar]
- 6.Walter PJ, Mohan R, Amsel BJ. 1992. Quality of life after heart valve replacement. J. Heart Valve Dis. 1, 34–41. [PubMed] [Google Scholar]
- 7.Labek G, Thaler M, Janda W, Agreiter M, Stockl B. 2011. Revision rates after total joint replacement. cumulative results from worldwide joint register datasets. J. Bone Joint Surg Br. 93, 293–297. ( 10.1302/0301-620X.93B3.25467) [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield P, Wheatley DJ, Prescott RJ, Miller HC. 2004. Twelve-year comparison of a Bjork–Shiley mechanical heart valve with porcine bioprostheses. Heart 89, 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hench LL, Polak JM. 2002. Third-generation biomedical materials. Science 295, 1014–1017. ( 10.1126/science.1067404) [DOI] [PubMed] [Google Scholar]
- 10.Sandén B, Olerud PC, Larsson S, Petrén-Mallmin M, Olerud C. 2002. Hydroxyapatite coating improves fixation of pedicle screws: a clinical study. J. Bone Joint Surg [Br] 8484, 387–391. ( 10.1302/0301-620X.84B3.0840387) [DOI] [PubMed] [Google Scholar]
- 11.Rowland DCL, Aquilina T, Klein A, Hakimi O, Alexis-Mouthuy P, Carr AJ, Snelling SJB. 2016. A comparative evaluation of the effect of polymer chemistry and fiber orientation on mesenchymal stem cell differentiation. J. Biomed. Mater. Res. Part A 104, 2843–2853. ( 10.1002/jbm.a.35829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz S, Rammelt S, Scharnweber D, Simon JC. 2011. Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32, 6692–6709. ( 10.1016/j.biomaterials.2011.05.078) [DOI] [PubMed] [Google Scholar]
- 13.Xiao X, Wang W, Liu D, Zhang H, Gao P, Geng L, Yuan Y, Lu J, Wang Z. 2015. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3 K/Akt pathways. Sci. Rep. 5, 9409 ( 10.1038/srep09409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Patra PK, Warner SB, Bhowmick S. 2007. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 13, 579–587. ( 10.1089/ten.2006.0205) [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Wang S, Zhang R. 2017. Composite poly(lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. Int. J. Biol. Macromol. 103, 1130–1137. ( 10.1016/j.ijbiomac.2017.05.101) [DOI] [PubMed] [Google Scholar]
- 16.Gallagher AG, Alorabi JA, Wellings DA, Lace R, Horsburgh MJ, Williams RL. 2016. A novel peptide hydrogel for an antimicrobial bandage contact lens. Adv. Healthc. Mater. 5, 2013–2018. ( 10.1002/adhm.201600258) [DOI] [PubMed] [Google Scholar]
- 17.Horejs CM, et al. 2014. Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proc. Natl Acad. Sci. USA 111, 5908–5913. ( 10.1073/pnas.1403139111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan F, Tare RS, Kanczler JM, Oreffo ROC, Bradley M. 2010. Strategies for cell manipulation and skeletal tissue engineering using high-throughput polymer blend formulation and microarray techniques. Biomaterials 31, 2216–2228. ( 10.1016/j.biomaterials.2009.11.101) [DOI] [PubMed] [Google Scholar]
- 19.Wang JHC. 2006. Mechanobiology of tendon. J. Biomech. 39, 1563–1582. ( 10.1016/j.jbiomech.2005.05.011) [DOI] [PubMed] [Google Scholar]
- 20.Benjamin M, Kaiser E, Milz S. 2008. Structure–function relationships in tendons: a review. J. Anat. 212, 211–228. ( 10.1111/j.1469-7580.2008.00864.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyer RF, Enna CD. 1976. Ultrastructural features of adult human tendon. Cell Tissue Res. 259, 247–259. ( 10.1007/BF00215881) [DOI] [PubMed] [Google Scholar]
- 22.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. 1994. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 53, 359–366. ( 10.1136/ard.53.6.359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadler KE, Holmes DF, Trotter JA, Chapman JA. 1996. Collagen fibril formation. J. Biochem. 316, 1–11. ( 10.1042/bj3160001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. 1998. Fibrillar structure and mechanical properties of collagen. J. Struct. Biol. 122, 119–122. ( 10.1006/jsbi.1998.3966) [DOI] [PubMed] [Google Scholar]
- 25.Svensson RB, Hansen P, Hassenkam T, Haraldsson BT, Aagaard P, Kovanen V, Krogsgaard M, Kjaer M, Magnusson SP. 2012. Mechanical properties of human patellar tendon at the hierarchical levels of tendon and fibril. J. Appl. Physiol. 112, 419–426. ( 10.1152/japplphysiol.01172.2011) [DOI] [PubMed] [Google Scholar]
- 26.Reese SP, Underwood CJ, Weiss JA. 2013. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix. Biol. 32, 414–423. ( 10.1016/j.matbio.2013.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shute J. 2012. Glycosaminoglycan and chemokine/growth factor interactions. In Heparin—a century of progress (ed. Lever R.), pp. 307–324. Berlin, Germany: Springer. [Google Scholar]
- 28.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. 2002. The skeletal attachment of tendons–tendon ‘entheses’. Comp. Biochem. Physiol. Part A 133, 931–945. ( 10.1016/S1095-6433(02)00138-1) [DOI] [PubMed] [Google Scholar]
- 29.Waggett AD, Ralphs JR, Kwan APL, Woodnutt D, Benjamin M. 1998. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix. Biol. 16, 457–470. ( 10.1016/S0945-053X(98)90017-8) [DOI] [PubMed] [Google Scholar]
- 30.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. 2003. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res. 21, 413–419. ( 10.1016/S0736-0266(03)0057-3) [DOI] [PubMed] [Google Scholar]
- 31.Feng X. 2009. Chemical and biochemical basis of cell–bone matrix interaction in health and disease Xu. Curr. Chem. Biol. 3, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, Thomopoulos S. 2009. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys. J. 97, 976–985. ( 10.1016/j.bpj.2009.05.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liua Y, Thomopoulosb S, Birmanc V, Lid J, Genin GM. 2014. Bi-material attachment through a compliant interfacial system at the tendon-to-bone insertion site. Mech. Mater. 44, 83–92. ( 10.1016/j.mechmat.2011.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. 1999. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin. J. Sport Med. 9, 157–160. ( 10.1097/00042752-199907000-00007) [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. 2006. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J. Bone Joint Surg. Am. 88, 1699–1704. ( 10.2106/JBJS.E.00835) [DOI] [PubMed] [Google Scholar]
- 36.Bruns J, Kampen J, Kahrs J, Plitz W. 2000. Achilles tendon rupture: experimental results on spontaneous repair in a sheep-model. Knee Surgery, Sport Traumatol. Arthrosc. 8, 364–369. ( 10.1007/s001670000149) [DOI] [PubMed] [Google Scholar]
- 37.Maffulli N, Barrass V, Ewen SW. 2000. Light microscopic histology of achilles tendon ruptures. A comparison with unruptured tendons. Am. J. Sports Med. 28, 857–863. ( 10.1177/03635465000280061401) [DOI] [PubMed] [Google Scholar]
- 38.Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. 2014. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. Bone Joint J. 96-B, 70–74. ( 10.1302/0301-620X.96B1.32556) [DOI] [PubMed] [Google Scholar]
- 39.Carr A, et al. 2017. Effectiveness of open and arthroscopic rotator cuff repair (UKUFF). Bone Joint J 99-B, 107–115. ( 10.1302/0301-620X.99B1.BJJ-2016-0424.R1) [DOI] [PubMed] [Google Scholar]
- 40.Flynn JE, Graham JH. 1965. Healing of tendon wounds. Am. J. Surg. 109, 315–324. ( 10.1016/S0002-9610(65)80080-0) [DOI] [PubMed] [Google Scholar]
- 41.Peacock E. 1965. Physiology of tendon repair. Am. J. Surg. 109, 283–286. ( 10.1016/S0002-9610(65)80075-7) [DOI] [PubMed] [Google Scholar]
- 42.Gelberman RH, Vandeberg JS, Manske PR, Akeson WH. 1985. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J. Hand Surg. Am. 10, 776–784. ( 10.1016/S0363-5023(85)80151-9) [DOI] [PubMed] [Google Scholar]
- 43.Gelberman RH, Manske PR, Akeson WH, Woo SL, Amiel D. 1986. Flexor tendon repair. J. Orthop. Res. 4, 119–128. ( 10.1002/jor.1100040116) [DOI] [PubMed] [Google Scholar]
- 44.Fenwick S, Hazleman B, Riley G. 2002. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 4, 252–260. ( 10.1186/ar416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gelberman RH, Vandeberg JS, Lundborg GN, Akeson WH. 1983. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J. Bone Joint Surg. Am. 65, 70–80. ( 10.2106/00004623-198365010-00010) [DOI] [PubMed] [Google Scholar]
- 46.Williams IF, Craig AS, Parry DAD, Goodship AE, Shah J, Silver IA. 1985. Development of collagen fibril organization and collagen crimp patterns during tendon healing. Int. J. Biol. Macromol. 7, 275–282. ( 10.1016/0141-8130(85)90025-X) [DOI] [Google Scholar]
- 47.Voleti PB, Buckley MR, Soslowsky LJ. 2012. Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng. 14, 47–71. ( 10.1146/annurev-bioeng-071811-150122) [DOI] [PubMed] [Google Scholar]
- 48.Postacchini F, De Martino C. 1980. Regeneration of rabbit calcaneal tendon maturation of collagen and elastic fibers following partial tenotomy. Connect. Tissue Res. 8, 41–47. ( 10.3109/03008208009152120) [DOI] [PubMed] [Google Scholar]
- 49.Dyment NA, et al. 2013. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PLoS ONE 8, e59944 ( 10.1371/journal.pone.0059944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gumucio JP, Phan AC, Ruehlmann DG, Noah AC, Mendias CL. 2014. Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J. Appl. Physiol. 117, 1287–1291. ( 10.1152/japplphysiol.00720.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW. 2014. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS ONE 9, e96113 ( 10.1371/journal.pone.0096113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, Andarawis-Puri N, Huang AH. 2017. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci. Rep. 7, 1–14. ( 10.1038/s41598-016-0028-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coghlan J, Buchbinder R, Green S, Johnston R, Bell S. 2008. Surgery for rotator cuff disease (Review). Cochrane Database Syst. Rev. 23, CD005619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson JM. 2001. Biological responses to materials. Annu. Rev. Mater. Res. 31, 81–110. ( 10.1146/annurev.matsci.31.1.81) [DOI] [Google Scholar]
- 55.Kavvadias T, Kaemmer D, Klinge U, Kuschel S, Schuessler B. 2009. Foreign body reaction in vaginally eroded and noneroded polypropylene suburethral slings in the female: a case series. Int. Urogynecol. J. 20, 1473–1476. ( 10.1007/s00192-009-0974-y) [DOI] [PubMed] [Google Scholar]
- 56.Daghighi S, Sjollema J, van der Mei HC, Busscher HJ, Rochford ETJ. 2013. Infection resistance of degradable versus non-degradable biomaterials: an assessment of the potential mechanisms. Biomaterials 34, 8013–8017. ( 10.1016/j.biomaterials.2013.07.044) [DOI] [PubMed] [Google Scholar]
- 57.Bayer ML, Schjerling P, Herchenhan A, Zeltz C, Heinemeier KM, Christensen L, Krogsgaard M, Gullberg D, Kjaer M. 2014. Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS ONE 9, e86078 ( 10.1371/journal.pone.0086078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badylak SF, Freytes DO, Gilbert TW. 2009. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 5, 1–13. ( 10.1016/j.actbio.2008.09.013) [DOI] [PubMed] [Google Scholar]
- 59.Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. 2004. Osteoinductivity of commercially available demineralized bone matrix. preparations in a spine fusion model. J. Bone Joint Surg. Am. 86-A, 2243–2250. ( 10.2106/00004623-200410000-00016) [DOI] [PubMed] [Google Scholar]
- 60.Urist M. 1965. Bone: formation by autoinduction. Science 150, 893–899. ( 10.1126/science.150.3698.893) [DOI] [PubMed] [Google Scholar]
- 61.van der Stok J, Hartholt KA, Schoenmakers DAL, Arts JJC. 2017. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery: a systematic review. Bone Joint Res. 6, 423–432. ( 10.1302/2046-3758.67.BJR-2017-0027.R1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Derwin K, Badylak S, Steinmann S, Iannotti J. 2010. Extracellular matrix scaffold devices for rotator cuff repair. J. Shoulder Elb. Surg. 19, 467–476. ( 10.1016/j.jse.2009.10.020) [DOI] [PubMed] [Google Scholar]
- 63.Ono Y, Davalos Herrera D, Woodmass J, Boorman R, Thornton G, Lo I. 2017. Graft augmentation versus bridging for large to massive rotator cuff tears: a systematic review. Arthrosc. J. Arthrosc. Relat. Surg. 33, 673–680. ( 10.1016/j.arthro.2016.08.030) [DOI] [PubMed] [Google Scholar]
- 64.Güngörmüş C, Kolankaya D, Aydin E. 2015. Histopathological and biomechanical evaluation of tenocyte seeded allografts on rat Achilles tendon regeneration. Biomaterials 51, 108–118. ( 10.1016/j.biomaterials.2015.01.077) [DOI] [PubMed] [Google Scholar]
- 65.Metcalf MH, Savoie FH, Kellum B. 2002. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper. Tech. Orthop. 12, 204–208. ( 10.1053/otor.2002.36298) [DOI] [Google Scholar]
- 66.Giannotti S, et al. 2014. Study of the porcine dermal collagen repair patch in morpho-functional recovery of the rotator cuff after minimum follow-up of 2.5 years. Surg. Technol. Int. 24, 348–352. [PubMed] [Google Scholar]
- 67.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. 2006. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. J. Bone Joint Surg. Am. 88, 1238–1244. ( 10.2106/JBJS.E.00524) [DOI] [PubMed] [Google Scholar]
- 68.Walton JR. 2007. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J. Bone Joint Surg. 89, 786–791. ( 10.2106/00004623-200704000-00013) [DOI] [PubMed] [Google Scholar]
- 69.Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, Park YS, Park JK. 2009. Increase in cell migration and angiogenesis in a composite silk scaffold for tissue-engineered ligaments. J. Orthop. Res. 27, 495–503. ( 10.1002/jor.20752) [DOI] [PubMed] [Google Scholar]
- 70.Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. 2012. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 28, 8–15. ( 10.1016/j.arthro.2011.06.038) [DOI] [PubMed] [Google Scholar]
- 71.Snyder SJ, Arnoczky SP, Bond JL, Dopirak R. 2009. Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket Matrix. Arthrosc. J. Arthrosc. Relat. Surg. 25, 329–333. ( 10.1016/j.arthro.2008.05.023) [DOI] [PubMed] [Google Scholar]
- 72.Smith RDJ, Zargar N, Brown CP, Nagra NS, Dakin SG, Snelling SJB, Hakimi O, Carr A. 2017. Characterizing the macro and micro mechanical properties of scaffolds for rotator cuff repair. J. Shoulder Elb. Surg. 26, 2038–2046. ( 10.1016/j.jse.2017.06.035) [DOI] [PubMed] [Google Scholar]
- 73.Reing J, et al. 2010. The effects of processing methods upon mechanical and biological properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31, 8626–8633. ( 10.1016/j.biomaterials.2010.07.083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keane TJ, Londono R, Turner NJ, Badylak SF. 2012. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33, 1771–1781. ( 10.1016/j.biomaterials.2011.10.054) [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, Marchant RE. 2011. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 8, 607–626. ( 10.1586/erd.11.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dai W, Wold LE, Dow JS, Kloner RA. 2005. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats a novel approach to preserve cardiac function after myocardial infarction. J. Am. Coll. Cardiol. 46, 714–719. ( 10.1016/j.jacc.2005.04.056) [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, et al. 2006. a simple, cross-linked collagen tissue substitute for corneal implantation. Invest. Opthalmol. Vis. Sci. 47, 1869 ( 10.1167/iovs.05-1339) [DOI] [PubMed] [Google Scholar]
- 78.Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, Li S, Deng Y, He N. 2017. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 5, 17014 ( 10.1038/boneres.2017.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Awad HA, Butler DL, Boivin GP, Smith FNL, Malaviya P, Huibregtse B, Caplan AI. 1999. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 5, 267–277. ( 10.1089/ten.1999.5.267) [DOI] [PubMed] [Google Scholar]
- 80.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. 1998. Use of mesenchymal stem cells in a collagen matrix for achilles tendon repair. J. Orthop. Res. 16, 406–413. ( 10.1002/jor.1100160403) [DOI] [PubMed] [Google Scholar]
- 81.Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS. 2013. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 34, 9295–9306. ( 10.1016/j.biomaterials.2013.08.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moshiri A, Oryan A, Meimandi-Parizi A. 2013. Role of tissue-engineered artificial tendon in healing of a large achilles tendon defect model in rabbits. J. Am. Coll. Surg. 217, 421–441.e8. ( 10.1016/j.jamcollsurg.2013.03.025) [DOI] [PubMed] [Google Scholar]
- 83.He M, Gan AWT, Lim AYT, Goh JCH, Hui JHP, Lee EH, Chong AKS. 2013. The effect of fibrin glue on tendon healing and adhesion formation in a rabbit model of flexor tendon injury and repair. J. Plast. Surg. Hand Surg. 47, 509–512. [DOI] [PubMed] [Google Scholar]
- 84.Sung H-W, Huang R-N, Huang LLH, Tsai C-C. 1999. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 10, 63–78. ( 10.1163/156856299X00289) [DOI] [PubMed] [Google Scholar]
- 85.Wood R. 1989. Development of a reconstituted collagen tendon prosthesis. J. Bone Joint Surg. 71A, 1183–1191. [PubMed] [Google Scholar]
- 86.Yunoki S, Hatayama H, Ebisawa M, Kondo E, Yasuda K. 2015. A novel fabrication method to create a thick collagen bundle composed of uniaxially aligned fibrils: an essential technology for the development of artificial tendon/ligament matrices. J. Biomed. Mater. Res. Part A 103, 3054–3065. ( 10.1002/jbm.a.35440) [DOI] [PubMed] [Google Scholar]
- 87.Stuart K, Panitch A. 2009. Characterization of gels composed of blends of collagen l, collagen III, and chondroitin sulfate. Biomacromolecules 10, 25–31. ( 10.1021/bm800888u) [DOI] [PubMed] [Google Scholar]
- 88.Yang G, Lin H, Rothrauff BB, Yu S, Tuan RS. 2016. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 35, 68–76. ( 10.1016/j.actbio.2016.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ide A, Sakane M, Chen G, Shimojo H, Ushida T, Tateishi T, Wadano Y, Miyanaga Y. 2001. Collagen hybridization with poly(l-lactic acid) braid promotes ligament cell migration. Mater. Sci. Eng. C 17, 95–99. ( 10.1016/S0928-4931(01)00315-0) [DOI] [Google Scholar]
- 90.Ozaki J, Fujimoto S, Masuhara K, Tamai S, Yoshimoto S. 1986. Reconstruction of chronic massive rotator cuff tears with synthetic materials. Clin. Orthop. Relat. Res. 202, 173–183. ( 10.1097/00003086-198601000-00022) [DOI] [PubMed] [Google Scholar]
- 91.Encalada-Diaz I, Cole BJ, MacGillivray JD, Ruiz-Suarez M, Kercher JS, Friel NA, Valero-Gonzalez F. 2011. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months' follow-up. J. Shoulder Elb. Surg. 20, 788–794. ( 10.1016/j.jse.2010.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nada A, Debnath U, Robinson D, Jordan C. 2010. Treatment of massive rotator-cuff tears with a polyester ligament (Dacron) augmentation: clinical outcome. J. Bone Joint Surg. Br. 92, 1397–1402. ( 10.1302/0301-620X.92B10.24299) [DOI] [PubMed] [Google Scholar]
- 93.Visuri T, Kiviluoto O, Eskelin M. 1991. Carbon fiber for repair of the rotator cuff. A 4-year follow-up of 14 cases. Acta Orthop. Scand. 62, 356–359. ( 10.3109/17453679108994469) [DOI] [PubMed] [Google Scholar]
- 94.King J, Bulstrode C. 1985. Polylactate-coated carbon fiber in extra-articular reconstruction of the unstable knee. Clin. Orthop. Relat. Res. 196, 139–142. ( 10.1097/00003086-198506000-00019) [DOI] [PubMed] [Google Scholar]
- 95.Szabó G, Barabás J, Bogdán S, Németh Z, Sebők B, Kiss G. 2015. Long-term clinical and experimental/surface analytical studies of carbon/carbon maxillofacial implants. Maxillofac Plast. Reconstr. Surg. 37, 34 ( 10.1186/s40902-015-0031-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amis A, Kempson S, Campbell J, Miller J. 1988. Anterior cruciate ligament replacement. Biocompatibility and biomechanics of polyester and carbon fibre in rabbits. J. Bone Joint Sur. Br. 70, 628–634. ( 10.1302/0301-620X.70B4.3403613) [DOI] [PubMed] [Google Scholar]
- 97.Inui A, et al. 2010. Potency of double-layered poly l-lactic acid scaffold in tissue engineering of tendon tissue. Int. Orthop. 34, 1327–1332. ( 10.1007/s00264-009-0917-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cooper J, Lu H, Ko F, Freeman J, Laurencin C. 2005. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials 26, 1523–1532. ( 10.1016/j.biomaterials.2004.05.014) [DOI] [PubMed] [Google Scholar]
- 99.Orr S, Chainani A, Hippensteel K, Kishan A, Gilchrist C, Garrigues NW, Ruch D, Guilak F, Little D. 2015. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 24, 117–126. ( 10.1016/j.actbio.2015.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beason D, Connizzo B, Dourte L, Mauck R, Soslowsky L, Steinberg D, Bernstein J, Bernstein J. 2012. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J. Shoulder Elb. Surg. 21, 245–250. ( 10.1016/j.jse.2011.10.021) [DOI] [PubMed] [Google Scholar]
- 101.Hakimi O, Mouthuy P-A, Zargar N, Lostis E, Morrey M, Carr A. 2015. A layered electrospun and woven surgical scaffold to enhance endogenous tendon repair. Acta Biomater. 26, 124–135. ( 10.1016/j.actbio.2015.08.007) [DOI] [PubMed] [Google Scholar]
- 102.Mouthuy P-A, Zargar N, Hakimi O, Carr A. 2014. Polydioxanone electrospun filaments to mimic tendon hierarchical architecture. Br. J. Sports Med. 48(Suppl. 2), A48 ( 10.1136/bjsports-2014-094114.73) [DOI] [Google Scholar]
- 103.Lu H, Cooper J, Manuel S, Freeman J, Attawia M, Ko F, Laurencin C. 2005. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials 26, 4805–4816. ( 10.1016/j.biomaterials.2004.11.050) [DOI] [PubMed] [Google Scholar]
- 104.Aurora A, McCarron J, van den Bogert A, Gatica J, Iannotti J, Derwin K. 2012. The biomechanical role of scaffolds in augmented rotator cuff tendon repairs. J. Shoulder Elb. Surg. 21, 1064–1071. ( 10.1016/j.jse.2011.05.014) [DOI] [PubMed] [Google Scholar]
- 105.Camposeo A, Greenfeld I, Tantussi F, Pagliara S, Mo M, Fuso F, Allegrini M, Zussman E, Pisignano D. 2013. Local mechanical properties of electrospun fibers correlate to their internal nanostructure. Nano Lett. 13, 5056–5062. ( 10.1021/nl4033439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maria L, Costa M, Henrique L, Mattoso C. 2013. Electrospinning of PCL/natural rubber blends. J. Mater. Sci. 48, 8501–8508. ( 10.1007/s10853-013-7332-7) [DOI] [Google Scholar]
- 107.Merkle V, Zeng L, Teng W, Slepian M, Wu X. 2013. Gelatin shells strengthen polyvinyl alcohol core-shell nanofibers. Polymer (Guildf) 54, 6003–6007. ( 10.1016/j.polymer.2013.08.056) [DOI] [Google Scholar]
- 108.Moffat K, Kwei AS-P, Spalazzi J, Doty S, Levine W, Lu HH. 2009. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng. Part A 15, 115–126. ( 10.1089/ten.tea.2008.0014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hakimi O, Chaudhury S, Murphy R, Carr A. 2012. Differential growth on sutures of tendon cells derived from torn human rotator cuff. J. Biomed. Mater. Res. Part B Appl. Biomater. 100B, 685–692. ( 10.1002/jbm.b.31993) [DOI] [PubMed] [Google Scholar]
- 110.Hakimi O, Murphy R, Stachewicz U, Hislop S, Carr A. 2012. An electrospun polydioxanone patch for the localisation of biological therapies during tendon repair. Eur. Cells Mater. 24, 344–357. ( 10.22203/eCM.v024a25) [DOI] [PubMed] [Google Scholar]
- 111.Meyer F, Wardale J, Best S, Cameron R, Rushton N, Brooks R. 2012. Effects of lactic acid and glycolic acid on human osteoblasts: a way to understand PLGA involvement in PLGA/calcium phosphate composite failure. J. Orthop. Res. 30, 864–871. ( 10.1002/jor.22019) [DOI] [PubMed] [Google Scholar]
- 112.Taylor M, Daniels A, Andriano K, Heller J. 1994. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J. Appl. Biomater. 5, 151–157. ( 10.1002/jab.770050208) [DOI] [PubMed] [Google Scholar]
- 113.Wei J, Igarashi T, Okumori N, Igarashi T, Maetani T, Liu B, Yoshinari M. 2009. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 4, 45002 ( 10.1088/1748-6041/4/4/045002) [DOI] [PubMed] [Google Scholar]
- 114.Kim S, Ha H, Ko Y, Yoon S, Rhee J, Kim S, Lee H, Khang G, Kim S. 2007. Correlation of proliferation, morphology and biological responses of fibroblasts on LDPE with different surface wettability. J. Biomater. Sci. Polym. Edn. 18, 609–622. ( 10.1163/156856207780852514) [DOI] [PubMed] [Google Scholar]
- 115.Webb K, Hlady V, Tresco PA. 1998. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 41, 422–430. ( 10.1002/(SICI)1097-4636(19980905)41:3%3C422::AID-JBM12%3E3.0.CO;2-K) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu LC, Siedlecki CA. 2007. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 28, 3273–3283. ( 10.1016/j.biomaterials.2007.03.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tamada Y, Ikada Y. 1993. Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J. Colloid Interface Sci. 155, 334–339. ( 10.1006/jcis.1993.1044) [DOI] [Google Scholar]
- 118.Schneider GB, English A, Abraham M, Zaharias R, Stanford C, Keller J. 2004. The effect of hydrogel charge density on cell attachment. Biomaterials 25, 3023–3028. ( 10.1016/j.biomaterials.2003.09.084) [DOI] [PubMed] [Google Scholar]
- 119.Rosa MD, et al. 2004. Cationic polyelectrolyte hydrogel fosters fibroblast spreading, proliferation, and extracellular matrix production: implications for tissue engineering. J. Cell. Physiol. 198, 133–143. ( 10.1002/jcp.10397) [DOI] [PubMed] [Google Scholar]
- 120.Chang H, Kao W, You Y, Chu Y, Chu K, Chen P, Wu C, Lee Y, Shyue J. 2016. Effect of surface potential on epithelial cell adhesion, proliferation and morphology. Colloids Surfaces B Biointerfaces 141, 179–186. ( 10.1016/j.colsurfb.2016.01.049) [DOI] [PubMed] [Google Scholar]
- 121.Castro P, Bertotti M, Naves A, Catalani L, Cornejo D, Bloisi G, Petri D. 2017. Hybrid magnetic scaffolds: the role of scaffolds charge on the cell proliferation and Ca2+ ions permeation. Colloids Surfaces B Biointerfaces 156, 388–396. ( 10.1016/j.colsurfb.2017.05.046) [DOI] [PubMed] [Google Scholar]
- 122.Qiu Q, Sayer M, Kawaja M, Shen X, Davies J. 1998. Attachment, morphology, and protein expression of rat marrow stromal cells cultured on charged substrate surfaces. J. Biomed. Mater. Res. 42, 117–127. ( 10.1002/(SICI)1097-4636(199810)42:1%3C117::AID-JBM15%3E3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 123.Hamdan M, Blanco L, Khraisat A, Tresguerres I. 2006. Influence of titanium surface charge on fibroblast adhesion. Implant Dent. 8, 32–38. ( 10.2310/j.6480.2005.00028.x) [DOI] [PubMed] [Google Scholar]
- 124.Lin J, et al. 2014. Effect of surface potential on extracellular matrix protein adsorption. Langmuir 30, 10 328–10 335. ( 10.1021/la5020362) [DOI] [PubMed] [Google Scholar]
- 125.Thevenot P, Hu W, Tang L. 2008. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 8, 270–280. ( 10.2174/156802608783790901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Faucheux N, Schweiss R, Lützow K, Werner C, Groth T. 2004. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 25, 2721–2730. ( 10.1016/j.biomaterials.2003.09.069) [DOI] [PubMed] [Google Scholar]
- 127.Llopis-Hernández V, Rico P, Ballester-Beltrán J, Moratal D, Salmerón-Sánchez M. 2011. Role of surface chemistry in protein remodeling at the cell–material interface. PLoS ONE 6, e19610 ( 10.1371/journal.pone.0019610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu L, Chen S, Giachelli C, Ratner B, Jiang S. 2005. Controlling osteopontin orientation on surfaces to modulate endothelial cell adhesion. J. Biomed. Mater. Res. Part A 74, 23–31. ( 10.1002/jbm.a.30221) [DOI] [PubMed] [Google Scholar]
- 129.Keselowsky B, Collard D, García A. 2004. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 25, 5947–5954. ( 10.1016/j.biomaterials.2004.01.062) [DOI] [PubMed] [Google Scholar]
- 130.Tegoulia V, Rao W, Kalambur A, Rabolt J, Cooper S. 2001. Surface properties, fibrinogen adsorption, and cellular interactions of a novel phosphorylcholine-containing self-assembled monolayer on gold. Langmuir 17, 4396–4404. ( 10.1021/la001790t) [DOI] [Google Scholar]
- 131.Hu W-J, Eaton J, Tang L. 2001. Molecular basis of biomaterial-mediated foreign body reactions. Blood 98, 1231–1238. ( 10.1182/blood.V98.4.1231) [DOI] [PubMed] [Google Scholar]
- 132.Barbosa J, Madureira P, Barbosa M, Guas A. 2005. The attraction of Mac-1+ phagocytes during acute inflammation by methyl-coated self-assembled monolayers. Biomaterials 26, 3021–3027. ( 10.1016/j.biomaterials.2004.09.002) [DOI] [PubMed] [Google Scholar]
- 133.Gao J, Laura N, Langer R. 1998. Surface modification of polyglycolic acid meshes increases the seeding density and spreading of smooth muscle cells. J. Biomed. Mater. Res. 42, 417–424. ( 10.1002/(SICI)1097-4636(19981205)42:3%3C417::AID-JBM11%3E3.0.CO;2-D) [DOI] [PubMed] [Google Scholar]
- 134.Yeo A, Wong W, Khoo H, Teoh S. 2010. Surface modification of PCL-TCP scaffolds improve interfacial mechanical interlock and enhance early bone formation: an in vitro and in vivo characterization. J. Biomed. Mater. Res. Part A 92, 311–321. ( 10.1002/jbm.a.32366) [DOI] [PubMed] [Google Scholar]
- 135.Jiao Y-P, Cui F-Z. 2007. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2, R24–R37. ( 10.1088/1748-6041/2/4/R02) [DOI] [PubMed] [Google Scholar]
- 136.Nam YS, Yoon JJ, Lee JG, Park TG. 1999. Adhesion behaviours of hepatocytes cultured onto biodegradable polymer surface modified by alkali hydrolysis process. J. Biomater. Sci. Polym. Ed. 10, 1145–1158. ( 10.1163/156856299X00801) [DOI] [PubMed] [Google Scholar]
- 137.Miller D, Thapa A, Haberstroh K, Webster T. 2004. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials 25, 53–61. ( 10.1016/S0142-9612(03)00471-X) [DOI] [PubMed] [Google Scholar]
- 138.Benoit D, Schwartz M, Durney A, Anseth K. 2008. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 7, 816–823. ( 10.1038/nmat2269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nilsson T, Mann M, Aebersold R, Yates J, Bairoch A, Bergeron J. 2010. Mass spectrometry in high-throughput proteomics: ready for the big time. Nat. Methods 7, 681–685. ( 10.1038/nmeth0910-681) [DOI] [PubMed] [Google Scholar]
- 140.Wan Y, Qu X, Lu J, Zhu C, Wan L, Yang J, Bei J, Wang S. 2004. Characterization of surface property of poly(lactide-co-glycolide) after oxygen plasma treatment. Biomaterials 25, 4777–4783. ( 10.1016/j.biomaterials.2003.11.051) [DOI] [PubMed] [Google Scholar]
- 141.Wan Y, Yang J, Yang J, Bei J, Wang S. 2003. Cell adhesion on gaseous plasma modified poly-(l-lactide) surface under shear stress field. Biomaterials 24, 3757–3764. ( 10.1016/S0142-9612(03)00251-5) [DOI] [PubMed] [Google Scholar]
- 142.Crowder S, Leonardo V, Whittaker T, Papathanasiou P, Stevens M. 2016. Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell 18, 39–52. ( 10.1016/j.stem.2015.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Downing T, et al. 2013. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 12, 1154–1162. ( 10.1038/nmat3777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mabry K, Payne S, Anseth K. 2016. Microarray analyses to quantify advantages of 2D and 3D hydrogel culture systems in maintaining the native valvular interstitial cell phenotype. Biomaterials 74, 31–41. ( 10.1016/j.biomaterials.2015.09.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Engler A, Sen S, Sweeney H, Discher D. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. ( 10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 146.Dalby M, Gadegaard N, Wilkinson C. 2008. The response of fibroblasts to hexagonal nanotopography fabricated by electron beam lithography. J. Biomed. Mater. Res. Part A 84, 973–979. ( 10.1002/jbm.a.31409) [DOI] [PubMed] [Google Scholar]
- 147.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC. 2007. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 6, 997–1003. ( 10.1038/nmat2013) [DOI] [PubMed] [Google Scholar]
- 148.Magnusson SP, Qvortrup K, Larsen JO, Rosager S, Hanson P, Aagaard P, Krogsgaard M, Kjaer M. 2002. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix. Biol. 21, 369–377. ( 10.1016/S0945-053X(02)00011-2) [DOI] [PubMed] [Google Scholar]
- 149.Wang V, Wang F, McNickle A, Friel N, Yanke A, Chubinskaya S, Romeo A, Verma N, Cole B. 2010. Medial versus lateral supraspinatus tendon properties: implications for double-row rotator cuff repair. Am. J. Sports Med. 38, 2456–2463. ( 10.1177/0363546510376817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franchi M, Trirè A, Quaranta M, Orsini E, Ottani V. 2007. Collagen structure of tendon relates to function. Sci. World J. 7, 404–420. ( 10.1100/tsw.2007.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fang M, Holl M. 2013. Variation in type I collagen fibril nanomorphology: the significance and origin. Bonekey Rep. 2, 1–7. ( 10.1038/bonekey.2013.128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chaudhury S, Xia Z, Thakkar D, Hakimi O, Carr AJ. 2016. Gene expression profiles of changes underlying different-sized human rotator cuff tendon tears. J. Shoulder Elb. Surg. 25, 1561–1570. ( 10.1016/j.jse.2016.02.037) [DOI] [PubMed] [Google Scholar]
- 153.Kunzler T, Drobek T, Schuler M, Spencer N. 2007. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 28, 2175–2182. ( 10.1016/j.biomaterials.2007.01.019) [DOI] [PubMed] [Google Scholar]
- 154.Dolatshahi-Pirouz A, Pennisi CP, Skeldal S, Foss M, Chevallier J, Zachar V, Andreasen P, Yoshida K, Besenbacher F. 2009. The influence of glancing angle deposited nano-rough platinum surfaces on the adsorption of fibrinogen and the proliferation of primary human fibroblasts. Nanotechnology 20, 95101 ( 10.1088/0957-4484/20/9/095101) [DOI] [PubMed] [Google Scholar]
- 155.Dalby M, Riehle M, Johnstone H, Affrossman S, Curtis A. 2002. Polymer-demixed nanotopography: control of fibroblast spreading and proliferation. Tissue Eng. 8, 1099–1108. ( 10.1089/107632702320934191) [DOI] [PubMed] [Google Scholar]
- 156.Prasad B, Brook M, Smith T, Zhao S, Chen Y, Sheardown H, D'souza R, Rochev Y. 2010. Controlling cellular activity by manipulating silicone surface roughness. Colloids Surfaces B Biointerfaces 78, 237–242. ( 10.1016/j.colsurfb.2010.03.006) [DOI] [PubMed] [Google Scholar]
- 157.Pennisi C, Sevcencu C, Dolatshahi-Pirouz A, Foss M, Hansen J, Larsen A, Zachar V, Besenbacher F, Yoshida K. 2009. Responses of fibroblasts and glial cells to nanostructured platinum surfaces. Nanotechnology 20, 385103 ( 10.1088/0957-4484/20/38/385103) [DOI] [PubMed] [Google Scholar]
- 158.Ranella A, Barberoglou M, Bakogianni S, Fotakis C, Stratakis E. 2010. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 6, 2711–2720. ( 10.1016/j.actbio.2010.01.016) [DOI] [PubMed] [Google Scholar]
- 159.Lynch H. 2003. Effect of fiber orientation and strain rate on the nonlinear uniaxial tensile material properties of tendon. J. Biomech. Eng. 125, 726 ( 10.1115/1.1614819) [DOI] [PubMed] [Google Scholar]
- 160.Sankar S, Sharma CS, Rath SN, Ramakrishna S. 2018. Electrospun nanofibres to mimic natural hierarchical structure of tissues: application in musculoskeletal regeneration. J. Tissue Eng. Regen. Med. 12, e604–e619. ( 10.1002/term.2335) [DOI] [PubMed] [Google Scholar]
- 161.Pan H, Li L, Hu L, Cui X. 2006. Continuous aligned polymer fibers produced by a modified electrospinning method. Polymer (Guildf) 47, 4901–4904. ( 10.1016/j.polymer.2006.05.012) [DOI] [Google Scholar]
- 162.Fee T, Surianarayanan S, Downs C, Zhou Y, Berry J. 2016. Nanofiber alignment regulates NIH3T3 cell orientation and cytoskeletal gene expression on electrospun PCL + gelatin nanofibers. PLoS ONE 11, e0154806 ( 10.1371/journal.pone.0154806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Full SM, Delman C, Gluck JM, Abdmaulen R, Shemin RJ, Heydarkhan-Hagvall S. 2015. Effect of fiber orientation of collagen-based electrospun meshes on human fibroblasts for ligament tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 103, 39–46. ( 10.1002/jbm.b.33153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mi HY, Salick MR, Jing X, Crone WC, Peng XF, Turng LS. 2015. Electrospinning of unidirectionally and orthogonally aligned thermoplastic polyurethane nanofibers: fiber orientation and cell migration. J. Biomed. Mater. Res. Part A 103, 593–603. ( 10.1002/jbm.a.35208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kendal A, Snelling S, Dakin S, Stace E, Mouthuy P-A, Carr A. 2017. Resorbable electrospun polydioxanone fibres modify the behaviour of cells from both healthy and diseased human tendons. Eur. Cells Mater. 33, 169–182. ( 10.22203/eCM.v033a13) [DOI] [PubMed] [Google Scholar]
- 166.Yin Z, Chen X, Chen J, Shen W, Hieu Nguyen T, Gao L, Ouyang H. 2010. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 31, 2163–2175. ( 10.1016/j.biomaterials.2009.11.083) [DOI] [PubMed] [Google Scholar]
- 167.Delaine-Smith R, Green N, Matcher S, MacNeil S, Reilly G. 2014. Monitoring fibrous scaffold guidance of three-dimensional collagen organisation using minimally-invasive second harmonic generation. PLoS ONE 9, e89761 ( 10.1371/journal.pone.0089761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dakin SG, et al. 2015. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 7, 311ra173 ( 10.1126/scitranslmed.aac4269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Naylor A, Filer A, Buckley C. 2013. The role of stromal cells in the persistence of chronic inflammation. Clin. Exp. Immunol. 171, 30–35. ( 10.1111/j.1365-2249.2012.04634.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bashur C, Dahlgren L, Goldstein A. 2006. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials 27, 5681–5688. ( 10.1016/j.biomaterials.2006.07.005) [DOI] [PubMed] [Google Scholar]
- 171.Kumbar S, Nukavarapu S, James R, Nair L, Laurencin C. 2008. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 29, 4100–4107. ( 10.1016/j.biomaterials.2008.06.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hsia H, Nair M, Mintz R, Corbett S. 2011. The fiber diameter of synthetic bioresorbable extracellular matrix influences human fibroblast morphology and fibronectin matrix assembly. Plast. Reconstr. Surg. 127, 2312–2320. ( 10.1097/PRS.0b013e3182139fa4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Erisken C, Zhang X, Moffat K, Levine W, Lu H. 2013. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng. Part A 19, 519–528. ( 10.1089/ten.tea.2012.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grier W, Iyoha E, Harley B. 2017. The influence of pore size and stiffness on tenocyte bioactivity and transcriptomic stability in collagen–GAG scaffolds. J. Mech. Behav. Biomed. Mater. 65, 295–305. ( 10.1016/j.jmbbm.2016.08.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang J, Shi G, Bei J, Wang S, Cao Y, Shang Q, Yang G, Wang W. 2002. Fabrication and surface modification of macroporous poly(l-lactic acid) and poly(l-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J. Biomed. Mater. Res. 62, 438–446. ( 10.1002/jbm.10318) [DOI] [PubMed] [Google Scholar]
- 176.Tang Y, Wong C, Wang H, Sutti A, Kirkland M, Wang X, Lin T. 2011. Three-dimensional tissue scaffolds from interbonded poly(ε-caprolactone) fibrous matrices with controlled porosity. Tissue Eng. Part C Methods 17, 209–218. ( 10.1089/ten.tec.2010.0223) [DOI] [PubMed] [Google Scholar]
- 177.Butler S, Kohles S, Thielke R, Chen C, Vanderby R. 1997. Interstitial fluid flow in tendons or ligaments: a porous medium finite element simulation. Med. Biol. Eng. Comput. 35, 742–746. ( 10.1007/BF02510987) [DOI] [PubMed] [Google Scholar]
- 178.Rnjak-Kovacina J, Wise S, Li Z, Maitz PK, Young C, Wang Y, Weiss A. 2011. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 32, 6729–6736. ( 10.1016/j.biomaterials.2011.05.065) [DOI] [PubMed] [Google Scholar]
- 179.Mandal B, Kundu S. 2009. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 30, 2956–2965. ( 10.1016/j.biomaterials.2009.02.006) [DOI] [PubMed] [Google Scholar]
- 180.Gu B, Park S, Kim M, Kang C, Kim J, Kim C. 2013. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 97, 65–73. ( 10.1016/j.carbpol.2013.04.060) [DOI] [PubMed] [Google Scholar]
- 181.Murphy C, O'Brien F. 2010. Understanding the effect of mean pore size on cell activity in collagen–glycosaminoglycan scaffolds. Cell Adhes. Migr. 4, 377–381. ( 10.4161/cam.4.3.11747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. 2014. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 42, 1508–1516. ( 10.1007/s10439-013-0933-0) [DOI] [PubMed] [Google Scholar]
- 183.Artel A, Mehdizadeh H, Chiu Y-C, Brey EM, Cinar A. 2011. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A 17, 2133–2141. ( 10.1089/ten.tea.2010.0571) [DOI] [PubMed] [Google Scholar]
- 184.Zhang Q, Luo H, Zhang Y, Zhou Y, Ye Z, Tan W, Lang M. 2013. Fabrication of three-dimensional poly(ε-caprolactone) scaffolds with hierarchical pore structures for tissue engineering. Mater. Sci. Eng. C 33, 2094–2103. ( 10.1016/j.msec.2013.01.025) [DOI] [PubMed] [Google Scholar]
- 185.Califano JP, Reinhart-King CA. 2008. A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell. Mol. Bioeng. 1, 122–132. ( 10.1007/s12195-008-0022-x) [DOI] [Google Scholar]
- 186.Engler A, Sheehan M, Sweeney H, Discher D. 2003. Substrate compliance vs ligand density in cell on gel responses. Eur. Cells Mater. 6(suppl. 1), 7–8. [Google Scholar]
- 187.Pelham RJ, Wang Y. 1997. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Proc. Natl Acad. Sci. USA 94, 13 661–13 665. ( 10.1073/pnas.94.25.13661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rosenfeldt H, Grinnell F. 2000. Fibroblast quiescence and the disruption of ERK signaling in mechanically unloaded collagen matrices. J. Biol. Chem. 275, 3088–3092. ( 10.1074/jbc.275.5.3088) [DOI] [PubMed] [Google Scholar]
- 189.Yeung T, et al. 2005. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 60, 24–34. ( 10.1002/cm.20041) [DOI] [PubMed] [Google Scholar]
- 190.Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B. 2001. Fibroblasts in mechanically stressed collagen lattices assume a ‘synthetic’ phenotype. J. Biol. Chem. 276, 36 575–36 585. ( 10.1074/jbc.M101602200) [DOI] [PubMed] [Google Scholar]
- 191.Maeda E, Sugimoto M, Ohashi T. 2013. Cytoskeletal tension modulates MMP-1 gene expression from tenocytes on micropillar substrates. J. Biomech. 46, 991–997. ( 10.1016/j.jbiomech.2012.11.056) [DOI] [PubMed] [Google Scholar]
- 192.Branco da Cunha C, Klumpers D, Li W, Koshy S, Weaver J, Chaudhuri O, Granja P, Mooney D. 2014. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 35, 8927–8936. ( 10.1016/j.biomaterials.2014.06.047) [DOI] [PubMed] [Google Scholar]
- 193.Mabry KM, Lawrence R, Anseth K. 2015. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 49, 47–56. ( 10.1016/j.biomaterials.2015.01.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jeong J, et al. 2013. Stiffness-modulated water retention and neovascularization of dermal fibroblast-encapsulating collagen gel. Tissue Eng. Part A 19, 1275–1284. ( 10.1089/ten.tea.2012.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lo C-M, Wang H-B, Dembo M, Wang Y. 2000. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152. ( 10.1016/S0006-3495(00)76279-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grinnell F. 2003. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 13, 264–269. ( 10.1016/S0962-8924(03)00057-6) [DOI] [PubMed] [Google Scholar]
- 197.Kwon I, Matsuda T. 2005. Co-electrospun nanofiber fabrics of poly(l-lactide-co-epsilon-caprolactone) with type I collagen or heparin. Biomacromolecules 6, 2096–2105. ( 10.1021/bm050086u) [DOI] [PubMed] [Google Scholar]
- 198.Sharifi-Aghdam M, Faridi-Majidi R, Derakhshan M, Chegeni A, Azami M. 2017. Preparation of collagen/polyurethane/knitted silk as a composite scaffold for tendon tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 231, 652–662. ( 10.1177/0954411917697751) [DOI] [PubMed] [Google Scholar]
- 199.Chainani A, Hippensteel K, Kishan A, Garrigues N, Ruch D, Guilak F, Little D. 2013. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. Part A 19, 2594–2604. ( 10.1089/ten.tea.2013.0165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang Y, Venugopal J, Huang Z, Lim C, Ramakrishna S. 2005. Characterization of the surface biocompatibility of the electrospun PCL–collagen nanofibers using fibroblasts. Biomacromolecules 6, 2583–2589. ( 10.1021/bm050314k) [DOI] [PubMed] [Google Scholar]
- 201.Nuttelman C, Mortisen D, Henry S, Anseth K. 2001. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 57, 217–223. ( 10.1002/1097-4636(200111)57:2%3C217::AID-JBM1161%3E3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 202.Sapudom J, Rubner S, Martin S, Thoenes S, Anderegg U, Pompe T. 2015. The interplay of fibronectin functionalization and TGF-β1 presence on fibroblast proliferation, differentiation and migration in 3D matrices. Biomater. Sci. 3, 1291–1301. ( 10.1039/C5BM00140D) [DOI] [PubMed] [Google Scholar]
- 203.van der Flier A, Arnoud S. 2001. Function and interactions of integrins. Cell Tissue Res. 305, 285–298. ( 10.1007/s004410100417) [DOI] [PubMed] [Google Scholar]
- 204.Kantlehner M, et al. 2000. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem 1, 107–114. ( 10.1002/1439-7633(20000818)1:2%3C107::AID-CBIC107%3E3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 205.Hennessy K, Clem W, Phipps M, Sawyer A, Shaikh F, Bellis S. 2008. Biomaterials the effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials 29, 3075–3083. ( 10.1016/j.biomaterials.2008.04.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. 2006. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 27, 5561–5571. ( 10.1016/j.biomaterials.2006.06.034) [DOI] [PubMed] [Google Scholar]
- 207.Bitschnau A, et al. 2008. Comparison of new bone formation, implant integration, and biocompatibility between RGD-hydroxyapatite and pure hydroxyapatite coating for cementless joint prostheses—an experimental study in rabbits. J. Biomed. Mater. Res. Part B 88B, 66–74. ( 10.1002/jbm.b.31150) [DOI] [PubMed] [Google Scholar]
- 208.Shu X, Ghosh K, Liu Y, Palumbo F, Luo Y, Clark R, Prestwich G. 2004. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J. Biomed. Mater. Res. 68A, 365–375. ( 10.1002/jbm.a.20002) [DOI] [PubMed] [Google Scholar]
- 209.Kim T, Park T. 2006. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(d,l-lactic-co-glycolic acid) naofiber Mesh. Tissue Eng. 12, 221–233. ( 10.1089/ten.2006.12.221) [DOI] [PubMed] [Google Scholar]
- 210.Kardestuncer T, McCarthy M, Karageorgiou V, Kaplan D, Gronowicz G. 2006. RGD-tethered silk substrate stimulates the differentiation of human tendon cells. Clin. Orthop. Relat. Res. 448, 234–239. ( 10.1097/01.blo.0000205879.50834.fe) [DOI] [PubMed] [Google Scholar]
- 211.Dahlgren L, Mohammed H, Nixon A. 2005. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J. Orthop. Res. 23, 84–92. ( 10.1016/j.orthres.2004.05.007) [DOI] [PubMed] [Google Scholar]
- 212.Würgler-Hauri C, Dourte L, Baradet T, Williams G, Soslowsky L. 2007. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J. Shoulder Elb. Surg. 16(5 Suppl.), 198–203. ( 10.1016/j.jse.2007.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ornitz DM, Itoh N. 2001. Fibroblast growth factors. Genome Biol. 2, reviews3005.1 ( 10.1186/gb-2001-2-3-reviews3005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ortega S, Ittmann M, Tsang S, Ehrlich M, Basilico C. 1998. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc. Natl Acad. Sci. USA 95, 5672–5677. ( 10.1073/pnas.95.10.5672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tsuboi R, Rifkin D. 1990. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J. Exp. Med. 172, 245–251. ( 10.1084/jem.172.1.245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chan BP, Chan KM, Maffulli N, Webb S, Lee KKH. 1997. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clin. Orthop. Relat. Res. 342, 239–247. ( 10.1097/00003086-199709000-00031) [DOI] [PubMed] [Google Scholar]
- 217.Thomopoulos S, Das R, Sakiyama-Elbert S, Silva M, Charlton N, Gelberman R. 2010. BFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann. Biomed. Eng. 38, 225–234. ( 10.1007/s10439-009-9844-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Takahasih S, Nakajima M, Kobayashi M, Wakabayashi I, Miyakoshi N, Minagawa H. 2002. Effect of recombinant basic fibroblast growth factor (bFGF) on fibroblast-like cells from human rotator cuff tendon. Tohoku J. Exp. Med. 198, 207–214. ( 10.1620/tjem.198.207) [DOI] [PubMed] [Google Scholar]
- 219.Ide J, Kikukawa K, Hirose J, Iyama Ki, Sakamoto H, Fujimoto T, Mizuta H. 2009. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J. Shoulder Elb. Surg. 18, 391–398. ( 10.1016/j.jse.2009.01.013) [DOI] [PubMed] [Google Scholar]
- 220.Ide J, Kikukawa K, Hirose J, Iyama Ki, Sakamoto H, Mizuta H. 2009. The effects of fibroblast growth factor-2 on rotator cuff reconstruction with acellular dermal matrix grafts. Arthrosc. J. Arthrosc. Relat. Surg. 25, 608–616. ( 10.1016/j.arthro.2008.11.011) [DOI] [PubMed] [Google Scholar]
- 221.Tokunaga T, Shukunami C, Okamoto N, Taniwaki T, Oka K, Sakamoto H, Ide J, Mizuta H, Hiraki Y. 2015. FGF-2 stimulates the growth of tenogenic progenitor cells to facilitate the generation of Tenomodulin-positive tenocytes in a rat rotator cuff healing model. Am. J. Sports Med. 43, 2411–2422. ( 10.1177/0363546515597488) [DOI] [PubMed] [Google Scholar]
- 222.Peterson D, et al. 2015. Evaluation of a collagen-coated, resorbable fiber scaffold loaded with a peptide basic fibroblast growth factor mimetic in a sheep model of rotator cuff repair. J. Shoulder Elb. Surg. 24, 1764–1773. ( 10.1016/j.jse.2015.06.009) [DOI] [PubMed] [Google Scholar]
- 223.Fukui N, Katsuragawa Y, Sakai H, Oda H, Nakamura K. 1998. Effect of local application of basic fibroblast growth factor on ligament healing in rabbits. Rev. Rhum. Engl. Ed. 65, 406–414. [PubMed] [Google Scholar]
- 224.Böttinger E, Letterio J, Roberts A. 1997. Biology of TGF-β in knockout and transgenic mouse models. Kidney Int. 51, 1355–1360. ( 10.1038/ki.1997.185) [DOI] [PubMed] [Google Scholar]
- 225.Morita W, Snelling S, Dakin S, Carr A. 2016. Profibrotic mediators in tendon disease: a systematic review. Arthritis Res. Ther. 18, 269 ( 10.1186/s13075-016-1165-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Goodier H, Carr A, Snelling S, Roche L, Wheway K, Watkins B, Dakin S. 2016. Comparison of transforming growth factor beta expression in healthy and diseased human tendon. Arthritis Res. Ther. 18, 48 ( 10.1186/s13075-016-0947-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fu S, Wang W, Pau H, Wong Y, Chan K, Rolf C. 2002. Increased expression of transforming growth factor beta 1 in patellar tendinosis. Clin. Orthop. Relat. Res. 400, 174–183. ( 10.1097/00003086-200207000-00022) [DOI] [PubMed] [Google Scholar]
- 228.Fenwick S, Curry V, Harrall R, Hazleman B, Hackney R, Riley G. 2001. Expression of transforming growth factor-beta isoforms and their receptors in chronic tendinosis. J. Anat. 199, 231–240. ( 10.1046/j.1469-7580.2001.19930231.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim H, Galatz L, Das R, Havlioglu N, Rothermich S, Thomopoulos S. 2011. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect. Tissue Res. 52, 87–98. ( 10.3109/03008207.2010.483026) [DOI] [PubMed] [Google Scholar]
- 230.Shah M, Foreman D, Ferguson M. 1995. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 108, 985–1002. [DOI] [PubMed] [Google Scholar]
- 231.Kovacevic D, Gulotta L, Ying L, Ehteshami J, Deng X, Rodeo S. 2015. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin. Orthop. Relat. Res. 473, 1644–1654. ( 10.1007/s11999-014-4020-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Uggen C, Dines J, McGarry M, Grande D, Lee T, Limpisvasti O. 2010. The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model. Arthroscopy 26, 1456–1462. ( 10.1016/j.arthro.2010.02.025) [DOI] [PubMed] [Google Scholar]
- 233.Min H, Kwon O, Oh S, Lee J. 2016. Platelet-derived growth factor-BB-immobilized asymmetrically porous membrane for enhanced rotator cuff tendon healing. Tissue Eng. Regen. Med. 13, 568–578. ( 10.1007/s13770-016-9120-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cummings S, et al. 2012. Effect of recombinant human platelet-derived growth factor-BB-coated sutures on Achilles tendon healing in a rat model: a histological and biomechanical study. J. Tissue Eng. 3, 2041731412453577 ( 10.1177/2041731412453577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hee C, et al. 2011. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am. J. Sports Med. 39, 1630–1638. ( 10.1177/0363546511404942) [DOI] [PubMed] [Google Scholar]
- 236.Hegedus E, Cook C, Brennan M, Wyland D, Garrison J, Driesner D. 2010. Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. Br. J. Sports Med. 44, 838–847. ( 10.1136/bjsm.2008.053769) [DOI] [PubMed] [Google Scholar]
- 237.Millar N, Hueber A, Reilly J, Xu Y, Fazzi U, Murrell G, McInnes I. 2010. Inflammation is present in early human tendinopathy. Am. J. Sports Med. 38, 2085–2091. ( 10.1177/0363546510372613) [DOI] [PubMed] [Google Scholar]
- 238.Tei K, et al. 2008. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem. Cells 26, 819–830. ( 10.1634/stemcells.2007-0671) [DOI] [PubMed] [Google Scholar]
- 239.Pufe T, Petersen W, Tillmann B, Mentlein R. 2001. The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons. Virchows Arch. 439, 579–585. ( 10.1007/s004280100422) [DOI] [PubMed] [Google Scholar]
- 240.Sejersen M, Frost P, Hansen T, Deutch S, Svendsen S. 2015. Proteomics perspectives in rotator cuff research a systematic review of gene expression and protein composition in human tendinopathy. PLoS ONE 10, e0119974 ( 10.1371/journal.pone.0119974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang X, Liu P, Tang J. 2005. Tendon healing in vitro: modification of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming growth factor beta but minimally affects expression of collagen genes. J. Hand Surg. Am. 30, 222–229. ( 10.1016/j.jhsa.2004.09.002) [DOI] [PubMed] [Google Scholar]
- 242.Kaux J-F, et al. 2014. Vascular endothelial growth factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles Ligaments Tendons J. 4, 24–28. ( 10.11138/mltj/2014.4.1.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Suh D, Hunt T, Spencer M. 1992. Insulin-like growth factor-I reverses the impairment of wound healing induced by corticosteroids in rats. Endocrinology 131, 2399–2403. ( 10.1210/endo.131.5.1425438) [DOI] [PubMed] [Google Scholar]
- 244.Berglund M, Hart D, Reno C, Wiig M. 2011. Growth factor and protease expression during different phases of healing after rabbit deep flexor tendon repair. J. Orthop. Res. 29, 886–892. ( 10.1002/jor.21330) [DOI] [PubMed] [Google Scholar]
- 245.Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. 2006. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J. Shoulder Elb. Surg. 15, 371–377. ( 10.1016/j.jse.2005.09.003) [DOI] [PubMed] [Google Scholar]
- 246.Herchenhan A, Bayer M, Eliasson P, Magnusson P, Kjaer M. 2015. Insulin-like growth factor I enhances collagen synthesis in engineered human tendon tissue. Growth Horm. IGF Res. 25, 13–19. ( 10.1016/j.ghir.2014.09.001) [DOI] [PubMed] [Google Scholar]
- 247.Caliari S, Harley B. 2013. Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen–GAG scaffolds. Tissue Eng. Part A 19, 1100–1112. ( 10.1089/ten.tea.2012.0497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jann H, Stein L, Slater D. 1999. In vitro effects of epidermal growth factor or insulin-like growth factor on tenoblast migration on absorbable suture material. Vet. Surg. 28, 268–278. ( 10.1053/jvet.1999.0268) [DOI] [PubMed] [Google Scholar]
- 249.Dahlgren L, Van Der Meulen M, Bertram J, Starrak G, Nixon A. 2002. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J. Orthop. Res. 20, 910–919. ( 10.1016/S0736-0266(02)00009-8) [DOI] [PubMed] [Google Scholar]
- 250.Kurtz C, Loebig T, Anderson D, DeMeo P, Campbell P. 1999. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am. J. Sports Med. 27, 363–369. ( 10.1177/03635465990270031701) [DOI] [PubMed] [Google Scholar]
- 251.Provenzano P, Alejandro-Osorio A, Grorud K, Martinez D, Vailas A, Grindeland R, Vanderby R. 2007. Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: evaluation of loaded and unloaded ligaments. BMC Physiol. 7, 2 ( 10.1186/1472-6793-7-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hansen M, Boesen A, Holm L, Flyvbjerg A, Langberg H, Kjaer M. 2013. Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scand. J. Med. Sci. Sport 23, 614–619. [DOI] [PubMed] [Google Scholar]
- 253.Dines J, Grande D, Dines D. 2007. Tissue engineering and rotator cuff tendon healing. J. Shoulder Elb. Surg. 16(5 Suppl.), 204–207. ( 10.1016/j.jse.2007.03.004) [DOI] [PubMed] [Google Scholar]
- 254.Carragee E, Hurwitz E, Weiner B. 2011. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 11, 471–491. ( 10.1016/j.spinee.2011.04.023) [DOI] [PubMed] [Google Scholar]
- 255.Schwartz A, Pasteris J, Genin G, Daulton T, Thomopoulos S. 2012. Mineral distributions at the developing tendon enthesis. PLoS ONE 7, e48630 ( 10.1371/journal.pone.0048630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pauly S, Klatte F, Strobel C, Schmidmaier G, Greiner S, Scheibel M, Wildemann B. 2012. BMP-2 and BMP-7 affect human rotator cuff tendon cells in vitro. J. Shoulder Elb. Surg. 21, 464–473. ( 10.1016/j.jse.2011.01.015) [DOI] [PubMed] [Google Scholar]
- 257.Seeherman H, Archambault J, Rodeo S, Turner S, Zekas L, D'Augusta D, Jian Li X, Smith E, Wozney J. 2008. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J. Bone Joint Surg. 90, 2206–2219. ( 10.2106/JBJS.G.00742) [DOI] [PubMed] [Google Scholar]
- 258.Murray D, Kubiak E, Jazrawi L, Araghi A, Kummer F, Loebenberg M, Zuckerman J. 2007. The effect of cartilage-derived morphogenetic protein 2 on initial healing of a rotator cuff defect in a rat model. J. Shoulder Elb. Surg. 16, 251–254. ( 10.1016/j.jse.2006.07.002) [DOI] [PubMed] [Google Scholar]
- 259.Lee K, Lee J, Kim Y, Shim Y, Jang J, Lee K. 2016. Effective healing of chronic rotator cuff injury using recombinant bone morphogenetic protein-2 coated dermal patch in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 105B, 1840–1846. [DOI] [PubMed] [Google Scholar]
- 260.Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. 2009. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control Release 134, 81–90. ( 10.1016/j.jconrel.2008.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mohan N, Detamore MS. 2012. Engineering graded tissue interfaces. In Structural interfaces and attachments in biology (eds Thomopoulos S, Birman V, Genin GM), pp. 299–322. New York, NY: Springer. [Google Scholar]
- 262.Erisken C, Kalyon DM, Wang H. 2008. Functionally graded electrospun polycaprolactone and β-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials 29, 4065–4073. ( 10.1016/j.biomaterials.2008.06.022) [DOI] [PubMed] [Google Scholar]
- 263.Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y. 2009. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 9, 2763–2768. ( 10.1021/nl901582f) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. 2008. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J. Biomed. Mater. Res. Part A 86, 1–12. ( 10.1002/jbm.a.32073) [DOI] [PubMed] [Google Scholar]
- 265.Anderson J, Rodriguez A, Chang D. 2008. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100. ( 10.1016/j.smim.2007.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhao QH, et al. 1993. Human plasma α2-macroglobulin promotes in vitro oxidative stress cracking of pellethane 2363-80A: in vivo and in vitro correlations. J. Biomed. Mater. Res. 27, 379–388. ( 10.1002/jbm.820270311) [DOI] [PubMed] [Google Scholar]
- 267.Dean BJF, Gettings P, Dakin SG, Carr AJ. 2016. Are inflammatory cells increased in painful human tendinopathy? A systematic review. Br. J. Sports Med. 50, 216–220. ( 10.1136/bjsports-2015-094754) [DOI] [PubMed] [Google Scholar]
- 268.Tidball J, Wehling-Henricks M. 2007. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 578, 327–336. ( 10.1113/jphysiol.2006.118265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mirza R, DiPietro L, Koh T. 2009. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 175, 2454–2462. ( 10.2353/ajpath.2009.090248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Dondossola E, Holzapfel B, Alexander S, Filippini S, Hutmacher D, Friedl P. 2016. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 1, 7 ( 10.1038/s41551-016-0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Blakney A, Swartzlander M, Bryant S. 2012. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. Part A 100A, 1375–1386. ( 10.1002/jbm.a.34104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.McWhorter F, Wang T, Nguyen P, Chung T, Liu WF. 2013. Modulation of macrophage phenotype by cell shape. Proc. Natl Acad. Sci. USA 110, 17 253–17 258. ( 10.1073/pnas.1308887110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bota PC, Collie AM, Puolakkainen P, Vernon R, Sage EH, Ratner B, Stayton P. 2010. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J. Biomed. Mater. Res. Part A 95A, 649–657. ( 10.1002/jbm.a.32893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Brodbeck W, Voskerician G, Ziats N, Nakayama Y, Matsuda T, Anderson J. 2003. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J. Biomed. Mater. Res. A 64, 320–329. ( 10.1002/jbm.a.10425) [DOI] [PubMed] [Google Scholar]
- 275.Brodbeck W, Patel J, Voskerician G, Christenson E, Shive M, Nakayama Y, Matsuda T, Ziats N, Anderson J. 2002. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc. Natl Acad. Sci. USA 99, 10 287–10 292. ( 10.1073/pnas.162124199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Frick J-S, Grünebach F, Autenrieth I. 2010. Immunomodulation by semi-mature dendritic cells: a novel role of Toll-like receptors and interleukin-6. Int. J. Med. Microbiol. 300, 19–24. ( 10.1016/j.ijmm.2009.08.010) [DOI] [PubMed] [Google Scholar]
- 277.Babensee J, Paranjpe A. 2005. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J. Biomed. Mater. Res. Part A 74A, 503–510. ( 10.1002/jbm.a.30429) [DOI] [PubMed] [Google Scholar]
- 278.Acharya A, Dolgova N, Clare-Salzler M, Keselowsky B. 2008. Adhesive substrate-modulation of adaptive immune responses. Biomaterials 29, 4736–4750. ( 10.1016/j.biomaterials.2008.08.040) [DOI] [PubMed] [Google Scholar]
- 279.Costa C, Incio J, Soares R. 2007. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis 10, 149–166. ( 10.1007/s10456-007-9074-0) [DOI] [PubMed] [Google Scholar]
- 280.Dean B, Franklin S, Murphy R, Javaid M, Carr A. 2014. Glucocorticoids induce specific ion-channel-mediated toxicity in human rotator cuff tendon: a mechanism underpinning the ultimately deleterious effect of steroid injection in tendinopathy? Br. J. Sports Med. 48, 1620–1626. ( 10.1136/bjsports-2013-093178) [DOI] [PubMed] [Google Scholar]
- 281.Filer A, et al. 2017. Identification of a transitional fibroblast function in very early rheumatoid arthritis. Ann. Rheum. Dis. 76, 2105–2112. ( 10.1136/annrheumdis-2017-211286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McGettrick H, Smith E, Filer A, Kissane S, Salmon M, Buckley C, Rainger G, Nash G. 2009. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur. J. Immunol. 39, 113–125. ( 10.1002/eji.200838232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rafii S, Butler J, Ding B-S. 2016. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325. ( 10.1038/nature17040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kusumbe A, Ramasamy S, Adams R. 2014. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328. ( 10.1038/nature13145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Takayama K, et al. 2015. The effect of blocking angiogenesis on anterior cruciate ligament healing following stem cell transplantation. Biomaterials 60, 9–19. ( 10.1016/j.biomaterials.2015.03.036) [DOI] [PubMed] [Google Scholar]
- 286.European Parliament and The Council of the European Union. 2017. Regulation 2017/745 on medical devices. Off. J. Eur. Union 60, L117/1. [Google Scholar]
- 287.European Parliament and The Council of the European Union. 2000. Directive 2001/83/EC concerning medicinal products for human use. Off. J. Eur. Communities 44, L311/67. [Google Scholar]
- 288.European Parliament and The Council of the European Union. 2007. Regulation 1394/2007 on advanced therapy medicinal products. Off. J. Eur. Union L324/121.
- 289.Medicines and Healthcare products Regulatory Agency. Borderlines between medical devices and medicinal products. How to distinguish between products that are regulated as medical devices and those regulated as medicinal products. See https://www.gov.uk/government/publications/borderlines-between-medical-devices-and-medicinal-products.
- 290.Curfman G, Redberg R. 2011. Medical devices—balancing regulation and innovation. N Engl. J. Med. 365, 975–977. ( 10.1056/NEJMp1109094) [DOI] [PubMed] [Google Scholar]
- 291.Zuckerman D, Brown P, Nissen S. 2011. Medical device recalls and the FDA approval process. Arch. Intern. Med. 171, 1006–1011. ( 10.1001/archinternmed.2011.30) [DOI] [PubMed] [Google Scholar]
- 292.Hatswell A, Baio G, Berlin J, Irs A, Freemantle N. 2016. Regulatory approval of pharmaceuticals without a randomised controlled study: analysis of EMA and FDA approvals 1999–2014. BMJ Open 6, e011666 ( 10.1136/bmjopen-2016-011666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.The European Parliament and the Council of the European Union. 2001. Directive 2001/20/EC on the implementatoin of good clinical practice in the conduct of clinical trails on medicinal products for human use. Off. J. Eur. Union L121.
- 294.European Commission. 2016. Clinical evaluation: a guide for manufacturers and notified bodies under Directives 93/42/EEC and 90/385/EEC. MEDDEV. 2.7.1 Rev.4. See http://ec.europa.eu/DocsRoom/documents/17522/attachments/1/translations/.
- 295.Parvizi N, Woods K. 2014. Regulation of medicines and medical devices: contrasts and similarities. Clin. Med. (Northfield Il) 14, 6–12. ( 10.7861/clinmedicine.14-1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Beard D, et al. 2015. The CSAW Study (Can Shoulder Arthroscopy Work?) — a placebo-controlled surgical intervention trial assessing the clinical and cost effectiveness of arthroscopic subacromial decompression for shoulder pain: study protocol for a randomised controlled trial. Trials 16, 210 ( 10.1186/s13063-015-0725-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.The European Parliament and the Council of the European Union. 2004. Directive 2004/23/EC on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 47, L 102/48. [Google Scholar]
- 298.Hourd P, Medcalf N, Segal J, Williams D. 2015. A 3D bioprinting exemplar of the consequences of the regulatory requirements on customized processes. Regen. Med. 10, 863–883. ( 10.2217/rme.15.52) [DOI] [PubMed] [Google Scholar]
- 299.Hanna E, Rémuzat C, Auquier P, Toumi M. 2016. Advanced therapy medicinal products: current and future perspectives. J. Mark Access Heal Policy 4, 31036 ( 10.3402/jmahp.v4.31036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McCulloch P, Cook J, Altman D, Heneghan C, Diener M. 2013. IDEAL framework for surgical innovation 1: the idea and development stages. BMJ 346, f3012 ( 10.1136/bmj.f3012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Currie A, Brigic A, Blencowe N, Potter S, Faiz O, Kennedy R, Blazeby J. 2015. Systematic review of surgical innovation reporting in laparoendoscopic colonic polyp resection. Br. J. Surg. 102, e108–e116. ( 10.1002/bjs.9675) [DOI] [PubMed] [Google Scholar]
- 302.Nikkels C, Vervoort A, Mol B, Hehenkamp W, Huirne J, Brölmann H. 2017. IDEAL framework in surgical innovation applied on laparoscopic niche repair. Eur. J. Obstet. Gynecol. Reprod. Biol. 215, 247–253. ( 10.1016/j.ejogrb.2017.06.027) [DOI] [PubMed] [Google Scholar]
- 303.Bryant D, Holtby R, Willits K, Litchfield R, Drosdowech D, Spouge A, White D, Guyatt G. 2016. A randomized clinical trial to compare the effectiveness of rotator cuff repair with or without augmentation using porcine small intestine submucosa for patients with moderate to large rotator cuff tears: a pilot study. J. Shoulder Elb. Surg. 25, 1623–1633. ( 10.1016/j.jse.2016.06.006) [DOI] [PubMed] [Google Scholar]
- 304.Metcalf M, Kellum B, Savoie F. 2002. Use of porcine small intestine submucosa xenograft for chronic rotator cuff tears: initial results. Arthroscopy 18, 41–42. ( 10.1016/S0749-8063(07)60048-6) [DOI] [Google Scholar]
- 305.Sclamberg S, Tibone J, Itamura J, Kasraeian S. 2004. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J. Shoulder Elb. Surg. 13, 538–541. ( 10.1016/j.jse.2004.03.005) [DOI] [PubMed] [Google Scholar]
- 306.Ergina P, Barkun J, McCulloch P, Cook J, Altman D. 2013. IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ 346, f3011 ( 10.1136/bmj.f3011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cook JA, McCulloch P, Blazeby JM, Beard DJ, Marinac-Dabic D, Sedrakyan A. 2013. IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ 346, f2820 ( 10.1136/bmj.f2820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Cook J. 2009. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 10, 9 ( 10.1186/1745-6215-10-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Savulescu J, Wartolowska K, Carr A. 2016. Randomised placebo-controlled trials of surgery: ethical analysis and guidelines. J. Med. Ethics 42, 776–783. ( 10.1136/medethics-2015-103333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Smith A, Dieppe P, Vernon K, Porter M, Blom A. 2012. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the national joint registry of england and wales. Lancet 379, 1199–1204. ( 10.1016/S0140-6736(12)60353-5) [DOI] [PubMed] [Google Scholar]
- 311.Lenzer J. 2009. Watching over the medical device industry. BMJ 338, b2321 ( 10.1136/bmj.b2321) [DOI] [PubMed] [Google Scholar]
- 312.Rogers L.2015. Scandal of fruit netting ‘approved as surgical implant.’ The Sunday Times, 11 January. See: www.thetimes.co.uk/article/scandal-of-fruit-netting-approved-as-surgical-implant-dvcqd2rt9mr .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.