Abstract
Voltage-gated proton channels are unique ion channels, membrane proteins that allow protons but no other ions to cross cell membranes. They are found in diverse species, from unicellular marine life to humans. In all cells, their function requires that they open and conduct current only under certain conditions, typically when the electrochemical gradient for protons is outwards. Consequently, these proteins behave like rectifiers, conducting protons out of cells. Their activity has electrical consequences and also changes the pH on both sides of the membrane. Here we summarize what is known about the way these proteins sense the membrane potential and the pH inside and outside the cell. Currently, it is hypothesized that membrane potential is sensed by permanently charged arginines (with very high pKa) within the protein, which results in parts of the protein moving to produce a conduction pathway. The mechanism of pH sensing appears to involve titratable side chains of particular amino acids. For this purpose their pKa needs to be within the operational pH range. We propose a ‘counter-charge’ model for pH sensing in which electrostatic interactions within the protein are selectively disrupted by protonation of internally or externally accessible groups.
Keywords: ion channels, pH, proton conduction, voltage gating, proton transport
1. Introduction
The focus of this review is how the voltage-gated proton channel, HV1, senses voltage and pH. HV1 is a unique ion channel, a membrane protein that allows protons (H+) but no other ions to cross cell membranes. Its existence was first postulated in 1972 by Hastings and co-workers [1], who proposed that it triggered the flash in bioluminescent dinoflagellates, a role that was recently confirmed [2,3]. Proof of the existence of HV1 was produced a decade later by Thomas and Meech with their 1982 voltage-clamp study of snail neurons [4]. Nearly a quarter of a century later, the gene for voltage-gated proton channels was finally identified [5,6]. Subsequently, proton currents have been identified in cells from 15 species, and HVCN1 genes (that code for HV1) in another 11 species have been confirmed by expression in heterologous systems and voltage clamp. To date, only one gene per species has been found, although, in several cases, truncated isoforms have been identified [7–9]. An astonishing variety of functions have been identified in these phylogenetically disparate species, many of which are listed in table 1. Involvement of HV1 in human health is extensive [48], but beyond the scope of this review.
Table 1.
Types of functions proposed for HV1 in different cells. Functions proposed for HV1 in various cells are sorted into the four main effects of HV1 activity, in some cases arbitrarily. NOX is NADPH oxidase. BCR is B cell receptor. Vm is membrane potential.
| cell | ↑pHi | ↓pHo | regulate Vm | charge compensation |
|---|---|---|---|---|
| dinoflagellates | trigger bioluminescent flash [1,3]; feeding [2] | proton action potential [3] | ||
| coccolithophores | calcification [10] | |||
| insect | acid extrusion [11] | |||
| snail neurons | acid extrusion [4,12,13] | acidification of confined spaces [14] | ROS production for host defence [15] | |
| amphibian oocyte | maturation, fertilization [16,17] | Vm oscillations [18] | ||
| zebra fish | neutrophils [19] | |||
| respiratory epithelium | acid extrusion [20] | optimize pH of airway surface fluid [21]; CO2 extrusion [22] | facilitate DUOX1 activity [23] | |
| skeletal myotubes | acid extrusion [24] | |||
| phagocyte | optimize pHi for NOX [25–27] | phagosome pH and volume [28,29] | regulate Vm [28,30]; avoid apoptosis [31] | prevent NOX self-inhibition at high potentials [32–34] |
| microglia | optimize pHi for NOX [35]; volume regulation [36] | ROS production [35,37] | ||
| basophil | histamine secretion [38] | |||
| cardiac myocytes | CO2 elimination [39] | |||
| cardiac fibroblasts | regulate Vm [40] | |||
| osteoclasts | acid extrusion [41] | regulate Vm [41] | ||
| sperm | alkaline pHi triggers capacitation [42] | ROS production by NOX5 mediates motility [43] | ||
| cancer cells | tumour growth [44] | metastasis [44] | ||
| B lymphocyte | ROS production in BCR signalling [7,45] | |||
| malignant B cells | short isoform promotes proliferation [8] | |||
| kidney | acid extrusion [46] | Na+-dependent ROS production [47] |
The protein at the focus of this chapter is the voltage-gated proton channel, HV1. Being ‘voltage-gated’ means that it can sense voltage, specifically the electrical potential difference across a cell membrane. As indicated by its name, the voltage-gated proton channel is an ion channel that conducts protons selectively when it is opened by depolarizing transmembrane voltages (making the membrane potential—the difference in voltage inside the cell compared with outside—more positive). HV1 channels open in response to depolarization, and they close with hyperpolarization (more negative membrane potentials). How this occurs will be discussed. A crucial and unique property of the HV1 channel is that its voltage sensitivity is modulated profoundly by the pH. Therefore, a second focus of this review is how the protein senses and responds to the pH. The aim of this review is to describe the current state of understanding of the gating mechanism of HV1. Gating is a quintessential property of all ion channels—a channel without gating is simply a pernicious shunt that would rapidly dissipate the membrane potential as well as the concentration gradient of any ions to which it is permeable. Ion gradients are required to drive transport of substances into or out of the cell, to generate energy from food and to conduct electrical impulses in excitable cells. By ‘gating’ we mean that the channel exists in at least two distinct functional states, ‘closed’ and ‘open’. When the channel is closed, it does not conduct current. To be precise, one K+ channel was shown to conduct detectably when closed, but the ‘closed’ channel current was more than 105 smaller current than the open channel current [49]. When a channel is open, it conducts current in the form of ions, usually at a constant rate. Ion channels differ from other transporters in being completely passive, conducting ions according to their electrochemical gradient. The chemical gradient drives ions from the side with higher concentration towards the side with lower concentration. The size and direction of ionic current is also sensitive to the electrical potential across the membrane, which can drive current in either direction, either supported or opposed by the chemical gradient. The membrane potential at which the electrical and concentration gradients balance is the Nernst potential [50]. For example, the Nernst potential for H+ (EH) is
where R is the gas constant, T is the absolute temperature, F is Faraday's constant, [H+] is the proton concentration, and the subscripts o and i mean outside and inside the cell, respectively. If the membrane potential is positive to EH, protons will be driven out of the cell; at voltages negative to EH, they will enter the cell. The Nernst potential is useful experimentally to establish the ion selectivity of a channel. Current through a proton-selective channel will reverse at EH regardless of the presence of other ions.
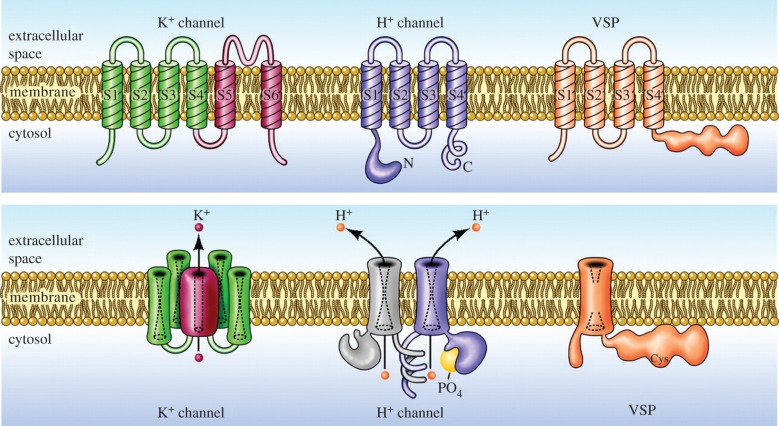
The topology of HV1 is illustrated by the cartoon in figure 1, which emphasizes the similarities and differences between molecules that contain voltage-sensing domains (VSDs). The VSD contains a number of charged amino acids that are thought to move when changes in membrane potential alter the electric field within the membrane. Most of these charges are Arg, or occasionally Lys, which are located every third position in the S4 helix and are thought to face the pore [61]. Voltage-gated K+ and Na+ channels have up to seven charged groups in S4, the VSP has four and HV1 has only three. In K+ channels, applied voltage causes the four VSDs to move and pull on parts of the central pore domain (S5 and S6), causing an opening to appear through which K+ as well as water molecules can pass. Ions are hydrophilic and can diffuse through water rapidly, but they avoid entering hydrophobic regions of cell membranes or of membrane proteins. Most ion channels are thought to have narrow hydrophilic regions where ions and water must move in single file. The conduction pathway of HV1 is within its VSD, which comprises the entire transmembrane region (S1–S4), whereas the VSDs in other membrane proteins serve mainly to sense voltage, and then in response they open a separate pore (e.g. the K+ channel) or turn-on an enzyme (e.g. the voltage-sensing phosphatase, VSP). As indicated schematically by the dashed hourglass shapes in figure 1, each VSD contains aqueous vestibules with a narrow constriction at the middle of the membrane. The constriction in HV1 conducts protons, but in K+ channels or the VSP they normally do not conduct at all. Intriguingly, if the K+ pore domain is removed altogether, the K+ channel VSD in isolation forms a proton-conducting channel [62]. Even when the molecules are still intact and attached to their pore domains, the VSDs of both voltage-gated K+ [63–65] and Na+ channels [66,67] can be induced to conduct protons by mutating particular Arg in the S4 segment to histidine (His). This mutation would result in four proton-conducting VSDs surrounding the central K+-conducting pore. His are well known for their ability to transfer protons [68,69], as they do in the M2 influenza A viral proton channel [70] and in carbonic anhydrase II [71]. Similarly, when Arg205 (the first—outermost—of three Arg (arginines) in S4)1 in hHV1 is replaced by His, inward proton current is detectable [72]. All of these ‘gating pore’ currents support the idea that the VSD resembles an hourglass with aqueous vestibules separated by a narrow hydrophobic region. In the guise of hydronium ions, protons can reach most places that water can. Although aquaporin channels normally conduct water at a high rate but exclude protons, showing that this is not a firm rule, point mutations can enable proton conduction even through aquaporin [73,74]. Presumably, a single His at the centre of a VSD can, perhaps with a bit of wiggling around, access both external and internal solutions and transfer a proton across this narrow bridge. This is what HV1 normally does whenever it opens, except without the benefit of His. In hHV1, the proton is transferred by the carboxyl group of Asp112 [75,76] and perhaps other acidic groups [77], as occurs in numerous proton pathways in other pumps and enzymes [69,78–81].
Figure 1.
Architectural features of voltage-gated K+ channels, HV1 channels and voltage-sensing phosphatases (VSP). The top row shows monomeric subunits of the complete molecule in the lower row. K+ channels are homotetramers with six transmembrane helices per monomer. Segments S1–S4 form the voltage-sensing domain (VSD) and S5–S6 form the conduction pathway. In the complete assembled channel (below), four VSDs (each comprising S1–S4) surround a single central pore through which K+ permeates. Dashed lines indicate central aqueous regions inside each VSD. HV1 resembles an isolated VSD with only four TM segments and no explicit pore domain [5,6], but functions without accessory proteins [51]. It forms a dimer, largely due to coiled-coil interaction in the C terminus, but each protomer has its own conduction pathway [52–54]. Phosphorylation of Thr29 in the N terminus [8,55] greatly enhances HV1 activity [56], especially in phagocytes [57]. The VSP lacks conduction altogether, but senses voltage and modulates phosphatatse activity accordingly [58,59]. Reprinted with permission from DeCoursey [60] (Copyright © 2010 American Physiological Society).
We will identify HV1 from different species with prefixes, hHV1 = human, mHV1 = mouse, otherwise two letters for genus and species, e.g. CiHV1 = Ciona intestinalis. Although there are some apparent differences [82], the functional similarities among HV1 from widely disparate species are remarkable.
2. HV1 exhibits cooperative gating
As shown in figure 1, HV1 is a dimer, but each protomer has its own conduction pathway. The channel is voltage-gated, meaning that it opens when the membrane potential is depolarized, i.e. made more positive. Compared to most voltage-gated ion channels, HV1 opens extremely slowly (figure 2), at least in mammals. Voltage-gated Na+ channels, for example, open within a millisecond or so, triggering an action potential in nerve or muscle cells. K+ channels in the same cells open only slightly more slowly, to repolarize the membrane. Although in snail neurons where proton currents were first identified, HV1 are as fast as other channels [4,12], mammalian HV1 are approximately 103 slower. When HV1 is forced to function as a monomer, it continues to exhibit the main properties of the dimer, but it opens five to seven times faster [52,83,84], as seen in figures 2a,b.
Figure 2.
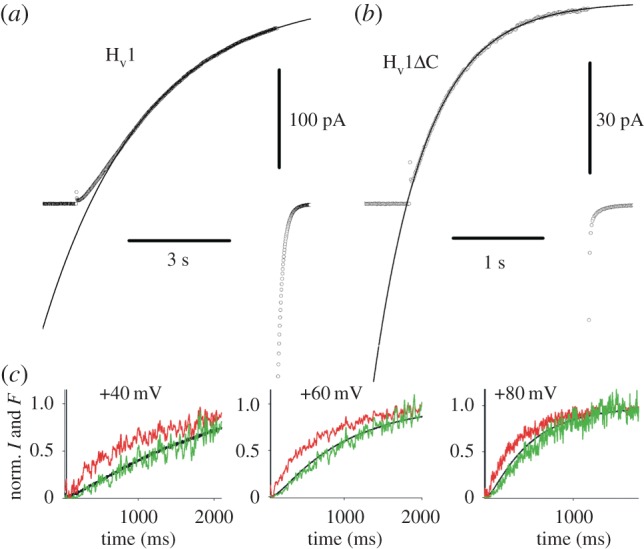
Cooperative gating of HV1. The HV1 dimer behaves as expected of a classical Hodgkin–Huxley n2 system. The WT channel in most species is a dimer in which both protomers must undergo a conformational change before either conducts. This manifests as sigmoidal activation kinetics in hHV1 (a). Truncation of the C terminus eliminates coiled-coil interaction and results in each monomer apparently functioning independently. In monomeric constructs (b), activation is exponential and is five to seven times faster than in the dimer [52,83,84]. Lines show single exponential fits; both currents were recorded at +50 mV at a symmetrical pH of 7.5. In (c), the red trace is the fluorescence signal from a tag attached to S4 in dimeric CiHV1, showing the exponential time course of its movement. The black trace shows the current, with its sigmoid turn-on. The green trace is the square of the red fluorescence signal, matching the current in the classical Hodgkin–Huxley manner, in which both protomers must activate before current is observed. (a,b) Reprinted with permission from Musset et al. [83] (Copyright © 2010 The Physiological Society) and (c) Reprinted with permission from Gonzalez et al. [85] (Copyright © 2010 Nature Publishing Group).
There is agreement that the two protomers in the HV1 dimer gate cooperatively, but not on precisely what ‘cooperative gating’ means. One definition of cooperative gating of HV1 is that a voltage-sensitive conformational change (generally envisaged as outward movement of the S4 helix) must occur in each protomer before either one can conduct [85]. Figure 2c illustrates that HV1 is well described by this type of cooperative gating, analogous to that proposed by Hodgkin and Huxley for squid axon Na+ and K+ channels [86]. In the latter channels, three to four identical ‘particles’ (what we now would call VSDs) were proposed, and molecular biology eventually confirmed that there are four subunits in both types of (tetrameric) channel [87]. An alternative proposal is that strong positive cooperativity exists in HV1 such that opening of one protomer greatly accelerates the opening of the other [88], analogous to the cooperative binding of oxygen to the four haem groups in a haemoglobin molecule. The gating model discussed below in §5.2 illustrates a possible mechanism for ΔpH dependent gating, which will be discussed later. In this model, the channel gates cooperatively, and the final concerted opening process results from protonation of both protomers at internal locations [89].
3. What is the difference between open and closed HV1 channels?
A straightforward, if reductionistic, way to dissect the physical process of gating would be to compare the structures of open and closed HV1 channels, which would reveal which parts must move and by how much. This is not possible at present, because only one crystal structure exists and this is presumed to be in a closed state (i.e. the membrane potential in the crystal is effectively 0 mV and mHV1 is closed at 0 mV). The molecule crystallized was a chimeric protein that includes parts of mouse HV1 (mHV1) spliced together with parts of two other proteins [90]. In addition, electron paramagnetic resonance (EPR) spectroscopy data exist for the human HV1, hHV1, also in a presumed closed state [91]. A growing number of homology models have been produced that often reflect the preconceived notions of their creators [72,77,83,92–98]. These are based mostly on homology with the VSDs of other voltage-gated ion channels [99]. The main differences are with regard to the extent of movement of the S4 helix during gating. A different approach by Li et al. [91] was to use the crystal structures of the ‘down’ and ‘up’ states of a voltage-sensing phosphatase [100] as templates. To create a homology model, a starting conformation is selected based on structures of homologous proteins, which is allowed to relax using molecular dynamics (MD) simulations. Sometimes multiple possible templates are assumed and statistical analysis or other criteria reveal the more probable model [93,94,99]. Accurate homology models would provide a starting point for understanding gating. However, the value of structural information, preferably structures for both closed and open channel proteins, cannot be overstated.
The question how open and closed channels differ is more difficult and subtle for HV1 than it is for other kinds of ion channels, because protons can and do traverse pathways that other ions cannot [68,69,101–107]. Normal ions require a pore wide enough to accommodate them, typically accompanied by water; often there is a ‘single-file’ region within which ions and water molecules cannot pass each other [87,108–110]. Proton pathways through proteins consist of hydrogen-bonded chains that may include any combination of water and side chains of certain amino acids [68,78,80,101,111–113]. Protons transfer across hydrogen bonds between waters or titratable groups; exclusion of other ions can be achieved by packing the protein to preclude water or foreign ion permeation [69]. There need not be any ‘pore’ as such; in principle, a channel might conduct protons but not water. In contrast with other ion channels, it is not obvious a priori that conducting and non-conducting states of proton channels would differ in any predictable and easily observable way. For example, the M2 proton channel of influenza A virus ‘opens’ to conduct H+ with high [114,115], albeit not perfect, selectivity [116–118] when the third of four His residues in a ring is protonated [119–124]; this produces only a subtle conformational change—a slight expansion of the ring due to electrostatic repulsion, which is nevertheless sufficient to permit H+ shuttling.
Protons (and to a lesser extent OH–) diffuse through water approximately five times faster than other ions [125] because the proton alone can move by a Grotthuss hopping mechanism [126,127]. Other ions must diffuse around waters, whereas protons can save time and distance by virtually hopping ‘through’ waters because, crucially, the identity of the proton may change with each transfer [128]. Water contains 110 M hydrogen atoms, each of which is interchangeable with the excess proton in H3O+. Because the mechanism of proton transfer in water is highly efficient, proton pathways through proteins typically comprise mostly water [103].
The crystal structure of closed HV1 revealed two hydrophobic regions in the permeation pathway [90]. The presumption was that these hydrophobic zones would be smaller or absent in the open (conducting) state. However, most homology models of the open state of HV1 also predict distinctly hydrophobic regions in the HV1 permeation pathway [94,96,98]. It is instructive that the mean hydration profile calculated for a series of hHV1 mutants was indistinguishable for constructs found experimentally to be proton-selective, anion-permeable or non-conducting [95]. In addition, an MD simulation of presumed closed and open states of hHV1 revealed no clear wet/dry transitions [98]. Nevertheless, the conductance of hHV1 mutants in which Asp at position 112 was replaced with various neutral amino acids decreased with hydrophobicity of the substituent, to zero (undetectable) for the two most hydrophobic amino acids Val and Ile (fig. 9 in [129]). Evidently, a sufficiently hydrophobic region can occlude even H+ conduction, but whether HV1 gating uses a tunable hydrophobic constriction [96] is not clear at present. Introduction of His into the S4 helix as R205H results in proton leakage in closed hHV1 channels [72]. This result suggests that H+ conduction is occluded at only a single constriction in the closed state.
4. What evidence supports molecular movement during gating of HV1 channels?
4.1. What evidence supports molecular movement during gating of other voltage-gated ion channels?
In part because of its relative novelty, most people who study HV1 today previously or contemporaneously worked on other ion channels. Consequently, we all have preconceptions of how voltage-gated channels work, and we tend to project onto HV1 the properties and mechanisms that apply to other (more extensively studied) channels. Several types of experimental evidence provide information about the extent of molecular movement of channels: gating currents, accessibility studies, FRET (fluorescence resonance energy transfer) measurements, and structural studies including X-ray crystallography and EPR. Similar experiments performed on HV1 are discussed in § 4.3. Most such studies indicate substantial movement of the S4 transmembrane segment during gating of other voltage-gated ion channels [110,130–139]. The S4 helix is believed to be the main voltage-sensing element of voltage-gated ion channels, because it has a series of cationic residues (mostly Arg with occasionally Lys) along its inner wall, spaced at every third position so that they line the pore [61,140]. It is widely believed that voltage gating occurs when S4 moves outwards, with a twist [141]. Recent studies conclude that four Arg move from intracellular towards extracellular positions in the Shaker K+ channel [134,142–145]. Because the cationic Arg are thought to interact with acidic residues in other parts of the VSD [146–147], one may quantify S4 movement by the number of discrete ‘clicks’ each Arg moves, being stabilized sequentially by the negatively charged groups as S4 ratchets outwards. Tao et al. [143] proposed that each gating charge moves through a ‘gating charge transfer center’ where it interacts with two acidic groups. S4 appears to move slightly less in Na+ channels [148,149], and even less in CiVSP, just one click [100]. The default starting point of our imagination is, therefore, our view of how other VSD-containing molecules move during gating.
We now step back to the foundation of modern ion channel research, Hodgkin & Huxley [86]. Based on their pioneering application of the voltage-clamp technique, they proposed that the pathway for ionic currents could be activated by the movement of a large quantity of charge across the membrane. They measured ionic currents using voltage clamp, and from the maximum current (I) at each voltage (V) and Ohm's Law they calculated the conductance (inversely related to resistance) G = 1/R = I/V. Assuming that current through a single type of channel has been isolated, the conductance is roughly proportional to the fraction of channels that open at each voltage. The G–V relationship thus shows the probability of channel opening as a function of voltage. Hodgkin and Huxley commented on the extreme steepness of the G–V relationship, from which they calculated that the equivalent of six elementary charges (e0) must cross the membrane for each conduction site (now called a ‘channel’). Later, more sophisticated estimates increased the gating charge for voltage-gated Na+, K+ and even Ca2+ channels to 12–14 e0 per channel [150–154]. The gating currents predicted by Hodgkin and Huxley to reflect the movement of charges within the membrane have been detected [155–158]. A crucial discovery was that replacing each of four Arg in S4 individually with His produced a proton-selective pathway through the Shaker K+ channel VSD [63–65]. Each mutant behaved as though protons (carried on a hydronium ion) could approach the His, bind, and then be translocated to the other side. This provided strong evidence that only a quite narrow region of the VSD is inaccessible to aqueous solution; and that the VSD is hourglass-shaped with large aqueous vestibules. If the first Arg is replaced by an amino acid smaller than His, a non-selective cation current is seen [159]. Intriguingly, even without Arg mutation, the isolated Shaker VSD (i.e. with the pore domain S5–S6 removed) conducts cations, with a strong preference for protons [62]. The short region between the vestibules has been called variously a hydrophobic gasket [91,160], hydrophobic plug [62,96,161,162,] or hydrophobic barrier [163]. The importance for voltage gating is that most of the transmembrane electric field drops across this hydrophobic region. The aqueous vestibules are low-resistance pathways in series with the high-resistance hydrophobic gasket. Consequently, if a charge moves or switches its accessibility from one side of the short hydrophobic region to the other, the result is electrically indistinguishable from the charge crossing the entire membrane.
4.2. Gating charge movement
Gating mechanisms can be constrained by measuring the amount of charge that moves when the channel opens, as ‘gating current’. Unfortunately, this measurement is more difficult for HV1 than for other channels [82]. Direct measurement of gating currents using voltage clamp requires eliminating the permeant ion, which is impossible for H+, or blocking the current by occlusion, but all known potent inhibitors of HV1 modify gating and exhibit state dependence [164,165]. A recent approach is to measure HV1 gating currents in a non-conducting mutant [166,167]. Very rough estimates of gating charge can be obtained from the slope of a Boltzmann function fit to the gH–V relationship; a more reliable estimate can be obtained from its limiting slope at large negative voltages [168,169].
The channel at the focus of this review, HV1, has only three Arg in its S4 helix [5,6], which remains true for confirmed HV1 in all species thus far [2,3,10,11,19,170–172]. It is, however, a dimer that operates cooperatively [52–54,83,85,88,173,174]. If all three Arg moved effectively across the entire membrane electical field when HV1 opened, one would predict a gating charge of 6 e0 for the dimer. Remarkably, this is precisely the value that was obtained from limiting slope measurements a decade before the gene was identified [175,176]! Tetrameric voltage-gated ion channels have four VSDs (figure 1), each moving approximately three charges for a total of 12–14 e0; the two VSDs of the HV1 dimer together move half this charge. Similar values have been measured in heterologously expressed proton channels: 6 e0 for hHV1, [177], 6 e0 for CiHv1 [178], 5.5 for HtHV1 [172] and 4 e0 for mHV1 [84]. Consistent with the cooperative gating mechanism, monomeric constructs exhibit gating charge just half of those values: 3 e0 for CiHv1 [85] and 2 e0 for mHV1 [84]. Finally, mutation of each of the three Arg in S4 to Asn reduced the gating charge assessed by the limiting slope method [178]. However, despite everything working out so neatly, it is not clear that all of the gating charge movement in HV1 results from S4 movement (as will be seen shortly.
4.3. Accessibility of various parts of the HV1 protein to aqueous solution
Membrane proteins are proteins embedded in the plasma or organelle membranes of cells. The accessibility of specific locations on a protein gives clues to its gross topology. The parts of the protein that are in contact with the aqueous solutions on either side of the membrane should be accessible to water-soluble probe molecules. Sites buried within the protein or that abut the membrane are not likely to be accessible. In a commonly used technique called ‘cysteine scanning mutagenesis' or ‘Cys scanning’, individual amino acids are replaced with Cys, and then probed with MTS (methanethiosulfonate) reagents [133,135]. If a Cys is accessible, MTS reagents may react with it and alter channel function. Under voltage clamp, the sidedness and state dependence (i.e. whether accessibility differs when the channel is open or closed) of MTS action can be determined. The ‘PEGylation protection’ assay also uses Cys scanning, but requires western blots which cannot be done in vivo and thus reveals accessibility only of presumed closed channels (because there is no membrane potential) and does not distinguish sidedness [179–181]. Cys scanning and MTS modification of CiHV1 channels in open or closed states clearly show changes in accessibility consistent with outward S4 movement of roughly one click [85,178]. Accessibility changes in the S1 segment are consistent with inward movement of S1 or simply widening of the internal vestibule of hHV1 [182]. Inward movement of S1, which has two to three negatively charged groups (Asp and Glu), and outward movement of S4, with its three cationic Arg, could both contribute to measured gating charge movement.
Accessibility of specific locations in the protein can be assessed in other ways. Introducing a pair of Cys or His residues and then probing with metals (Cd2+ or Zn2+) under voltage clamp can reveal state-dependent interactions (i.e. the metal binds preferentially in open or closed channels) [183,184]. When the three Arg in S4 of hHV1 (figure 3) were individually replaced with His and probed with Zn2+ in the open state, the outermost two, R1 and R2, were accessible to the external solution, but R3, Arg211 was not. R2 appeared also to be internally accessible, presumably in closed channels, but the innermost Arg, R3, was accessible only from the internal solution and was clearly accessible even in the open state [94,95]. These data were interpreted as indicating that a one-click outward movement of S4 was sufficient to result in hHV1 opening. In the closed crystal structure of HV1 [90], the Asp112 in the middle of S1 that is crucial to proton selectivity [75] interacts with the first Arg in S4 (R1 or Arg205). In our model, Asp112 interacts with the second Arg of S4, Arg208 in the open channel [94,95]. Statistical analysis of extensive MD simulations of the open hHV1 model to compare the assumptions that Asp112 interacted either with R2 or R3 consistently supported the stability of the R2 interaction [94]. If the R211H mutation can be taken at face value, models in which the third Arg moves all the way into the external vestibule must be ruled out. As with all mutations, the interpretation of R211H assumes that the molecule behaves essentially identically to wild-type (WT). It is also evident that neutralizing the cationic Arg in S4 may alter the extent of S4 movement [178]. His is a conditionally conservative replacement for Arg in that it might be cationic, but its pKa in solution is 6.5 and this could be altered by the local environment within the protein, and thus its protonation state even at pH 6.0 is not clear. Another note of caution is that the hHV1 molecule is highly dynamic, even more than other VSDs [91], and this mobility might manifest as the molecule sampling a wide range of conformations. Thus accessibility by any criterion will have a statistical component. However, that R3 is not externally accessible in spite of the high molecular mobility strengthens the argument that S4 outward movement is limited.
Figure 3.
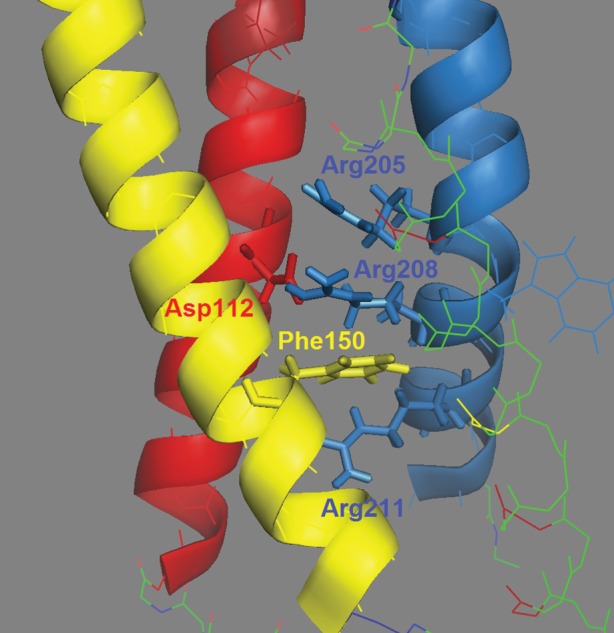
Side view of the open human HV1 channel, with the external end up. Transmembrane helices are colour-coded: S1 = red, S2 = yellow, S4 = blue and S3 is shown as lines to be unobtrusive. Key amino acids are labelled and shown with side chains as sticks. Asp112 is crucial for selectivity; Phe150 demarcates inner and outer aqueous vestibules and the three Arg in S4 sense voltage. Figure is based on the model of Li et al. [91]. Note that Asp112 interacts with Asp208 [94], and Arg211 is below Phe150, and thus is exposed to the inner vestibule. Drawn with PyMol.
5. What is the mechanism of ΔpH-dependent gating?
5.1. What is ΔpH-dependent gating?
One of the most distinctive properties of HV1 is ΔpH-dependent gating [89]. This feature occurs universally in all species studied thus far and is essential to all of its functions [48]. The biological significance of ΔpH dependence is that HV1 acts to extrude acid from cells (figure 4). The channel is regulated by pH so that (with rare exceptions) it only opens when doing so will result in outward H+ current. This functional ‘rectification’ is due almost entirely to the pH dependence of gating, and does not reflect rectification of the open channel current. Under symmetrical pH conditions (pHo = pHi) the open HV1 channel conducts outward current somewhat better than inward, but by a factor of less than 2 [89]. Four types of consequences of HV1 activity can be listed (table 1), although some proposed functions do not fit neatly into these categories or have uncertain mechanisms. H+ efflux will change pH on both sides of the membrane, depending on the situation, pHo or pHi may be more critical. One could subdivide these further: in the face of an acid load, H+ efflux serves to keep pHi constant, but increasing pHi is a signal for sperm capacitation [42]. In a number of cells, the electrical consequences of HV1 activity are crucial (table 1). The best studied example is charge compensation during the phagocyte ‘respiratory burst’, i.e. NADPH oxidase (NOX) activity. NOX is electrogenic [32,34,57,185] and produces massive depolarization in neutrophils [186–188]. HV1 compensates for the electron efflux through NOX, limiting the extent of depolarization [28,30,33,34,189]. Without HV1, the NOX-induced depolarization would rapidly produce self-inhibition [28,33,34]. Another cell that uses the electrical manifestations of HV1 activity is the dinoflagellate, in which an HV1-mediated action potential triggers the bioluminescent flash [2,3].
Figure 4.
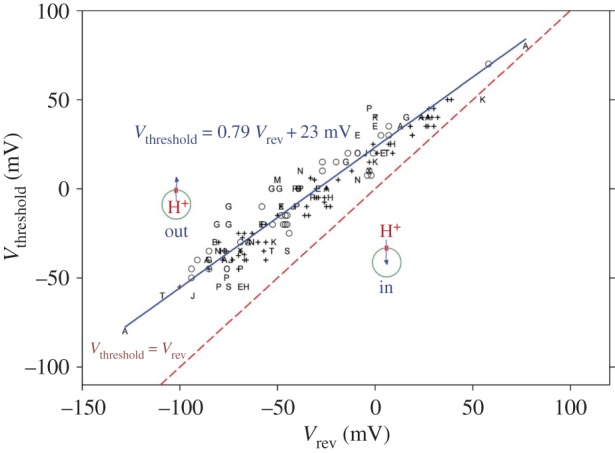
The ΔpH dependence of gating ensures that, in most species, HV1 channels open only when doing so will result in acid extrusion. Each symbol or letter indicates a different cell type or species, defined in [68]. The blue line shows the relationship that results in H+ channels in almost all cells opening at their threshold voltage, Vthreshold, at voltages positive to the Nernst potential (or the experimentally measured reversal potential, Vrev), shown as the red dashed line. At more positive voltages (above the blue line) more channels would open, extruding more protons. Reprinted with permission from DeCoursey & Hosler [69], with modification (Copyright © 2014 Rockefeller University Press).
5.2. How does ΔpH-dependent gating work?
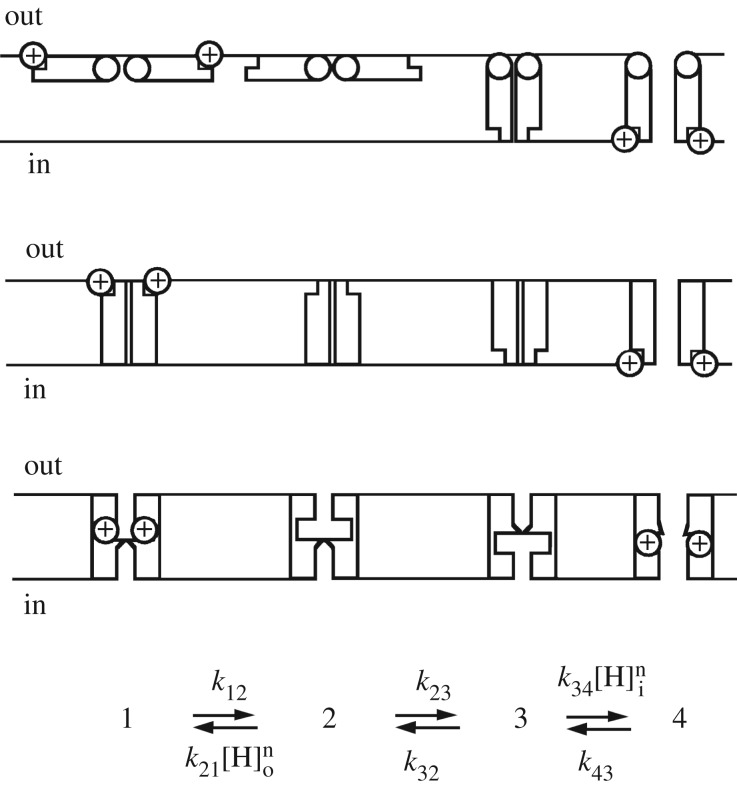
Increasing pHo or decreasing pHi shifts the position of the gH–V relationship negatively by 40 mV per unit change in pH [89]. How does the channel sense pH, or more specifically, the pH gradient, ΔpH? Then, how does the channel transduce this perception into channel opening? Many enzymes are pH-sensitive, and they generally sense pH via protonatable groups. In a survey of 35 arbitrarily selected proteins, pH sensing was impaired by mutation of His in 20, Glu in 15, Asp in 7, Arg in 6, Lys in 6 and Gly in 3, and pH sensing frequently involved multiple amino acids [190]. It is difficult to envisage a pH-sensing mechanism that does not involve titratable amino acid side chains, although one exhaustive study of hHV1 found that mutation of several dozen individual titratable residues failed to eliminate or even attenuate ΔpH-dependent gating [92]. These authors concluded somewhat cryptically that ‘interactions between water molecules and S4 arginines may underlie coupling between voltage- and pH-gradient sensing’. The only explicit model to explain ΔpH-dependent gating postulated that one or more protonatable groups sense pH as shown in figure 5. This model accounts for the ΔpH dependence of gating by means of titratable groups on the channel that stabilize the closed or open conformation when protonated from the outside or the inside, respectively [89]. A crucial aspect of this model is a requirement for alternating access of the titratable groups; they are accessible to the external or internal solution but not at the same time, and accessibility changes occur only in the deprotonated condition. The voltage dependence may result from movement of charges through the membrane electrical field during the conformational change (states 2 ↔ 3) or from voltage-dependent binding or unbinding of protons to the titratable groups or both. The latter possibility was called a ‘proton well’ by Mitchell [191]. This model predicts the 40 mV shift of the gH–V relationship and qualitatively reproduces pH effects on gating kinetics [89]. Measurement of the pH dependence of gating transitions [89,192] provides a basis for refining such a model.
Figure 5.
A four-state ‘butterfly’ model that explains the main features of ΔpH-dependent gating is shown with three possible physical representations. Channel opening occurs from left to right, with state 4 the only conducting state. Top row shows two ‘wings’ that cross the membrane exposing the sites to the opposite solution. The middle row depicts equivalent but distinct internal and external sites, of which only those on one side are accessible. The bottom row shows sites within the pore whose accessibility switches due to a subtle conformational change. Protonation from the external solution stabilizes the deepest closed (non-conducting) configuration (state 1). Deprotonation (state 1 → 2) is required before a conformational change switches the accessibility of the titratable groups to face inwardly (state 2 → 3). Finally, protonation from the inside (state 3 → 4) stabilizes the open channel (state 4). Because no single amino acid substitution abolishes ΔpH dependence [92], multiple groups are probably involved. Reprinted with permission from Cherny et al. [89] (Copyright © 1995 Rockefeller University Press).
Recent indirect evidence indirectly supports a model for ΔpH-dependent gating that involves titratable sites. The WT hHV1 was shown to exhibit saturation of ΔpH dependence at pHi or pHo higher than 8.0 [193], which might be expected if the ambient pH were approaching the pKa of one or more titratable groups. More surprisingly HtHV1, a proton channel from the snail Helisoma trivolvis, was identified whose gH–V relationship shifted only 20 mV or less when pHi was varied, despite normal or even hyper-normal responses to changes in pHo (greater than 50 mV per unit) [172]. One key difference between the sequences of snail and human HV1 was in the S2 and S3 intracellular linker. When His168 in human HV1 was replaced with glutamine, which occupies that position in HtHV1, the mutant human channel behaved like the snail, with greatly weakened pHi sensitivity [194]! A shortened isoform of HV1 in human sperm, lacking the first 68 amino acids of the intracellular N terminus also has subnormal pHi sensing [9]. Selective impairment in pHi sensing is consistent with distinct internal and external pH sensors, as opposed to a centrally located sensor that samples pH on both sides of the membrane. Additional evidence that distinct external and internal sensors exist is that mutation of an unusual tryptophan in the hHV1 pore, Trp207, modifies pHo sensing without affecting pHi sensing [193].
5.3. The counter-charge model for ΔpH-dependent gating
The identification of the gene for the voltage-gated proton channel HV1 [5,6] revealed its surprising homology with the VSD of K+, Na+ and Ca2+ channels (figure 1). Despite several distinct differences, for example, HV1 contains only three Arg residues in S4, the overall arrangement is similar. All VSDs have four transmembrane helices, with a series of cationic Arg or Lys in S4 that are thought to sense voltage, and several conserved acidic amino acids in S1–S3 that are thought to interact with the cationic residues to stabilize closed, open or intermediate states. The basic groups in S4 are thought to move outwards during channel opening, passing through a ‘hydrophobic gasket’ [91,100,195,196,] or ‘charge transfer centre’ [143] that includes an extremely highly conserved Phe (Phe150 in hHV1), which is the delimiter between inner and outer vestibules that access internal and external aqueous solutions, respectively. Furthermore, Cys scanning indicates that the general movement of S4 relative to the other domains (S1–S3) in proton channels [85] is qualitatively similar to the movement that occurs in other voltage-gated ion channels [63,133–135]. Thus, one or more Arg residues are accessible to the internal solution in the closed state, but move outwards past a short constriction (depicted in figure 6 as the highly conserved Phe150), to become accessible to the external solution in the open state. We assume that the Arg in S4 contribute to the voltage dependence of gating [178], as they do in other ion channels [130,131,140,152,154]. The high pKa of Arg means that it will remain positively charged under almost all conditions, a desirable property for a voltage-sensing element. One of the unique features of voltage-gated proton channels is that their voltage-dependent gating is strictly regulated by the pH gradient, ΔpH. Specifically, increasing pHo or decreasing pHi by one unit shifts the gH–V relationship by −40 mV [89]. This regulation results in the proton channel opening only when the electrochemical gradient is outwards (figure 4), such that opening will result in acid extrusion from cells [89]. This property is observed in all voltage-gated proton channels identified to date, and is crucial to the physiological roles of this channel [60,68].
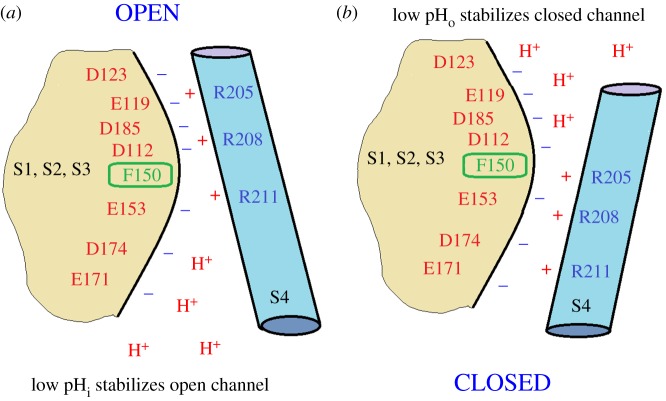
Figure 6.
Cartoon illustrating the ‘counter-charge model’ for ΔpH-dependent gating of HV1. The premise is that both open and closed states of the channel are stabilized by interhelical electrostatic interactions. The main charged groups on the S4 helix, which is thought to move outwards during channel opening, are three Arg (blue). The S1–S3 segments have a number of acidic groups. Internal protons (low pHi) will tend to protonate acidic groups (a), preventing them from engaging in electrostatic interactions, thereby destabilizing the closed state and promoting opening. Conversely, external protons (low pHo) will protonate externally accessible acidic groups, destabilizing the open state and promoting channel closing. Positions of groups are highly schematic! The hydrophobic gasket that demarcates internal and external accessibility is depicted as a highly conserved Phe150 [143].
The model in figure 6 illustrates a hypothetical mechanism for the ΔpH dependence of gating. In this model, electrostatic interactions between the Arg in S4 and acidic residues in other transmembrane segments regulate the ΔpH dependence of gating. This kind of charge–pair interaction has been proposed to occur within the VSD during gating of other voltage-gated ion channels, stabilizing closed or open states [146,147,197]. An additional twist added to this strategy by HV1 is that charge–pair interactions can be inhibited by protonating the acidic member of the pair. In this way, pH naturally exerts the effects that are predicted more generally by the model in figure 5. Protonation of acidic groups that are accessible to the internal solution in the closed state destabilizes their interaction with Arg, promoting channel opening. Conversely, protonation of groups that are externally accessible in the open state destabilizes the open state by eliminating their interaction with Arg residues, thus promoting channel closing. Several acidic residues unique to proton channels (i.e. lacking homology with the VSD of other channels), such as Asp112 and Asp185 [82], may contribute to this mechanism, in addition to acidic residues homologous to those thought to interact electrostatically with S4 Arg residues in K+ channels [146,147,198–200]. Given that no single point mutation abolishes ΔpH dependence [82,92], the mechanism that produces the ΔpH-dependent gating crucial to proton channel physiology is evidently robust and incorporates redundancy.
Strong experimental support for this type of model exists [82,92]. Neutralizing acidic residues that are internally accessible and interact with Arg preferentially in the closed state should destabilize the closed state and promote the open state. In other words, neutral mutants of internal acidic amino acids should shift the gH–V relationship negatively. Two key residues whose neutralization promotes the open state are Asp174 and Glu153 [82,92,96,201]. Conversely, neutralizing acidic residues that are externally accessible and interact with Arg preferentially in the open state should destabilize the open state and promote channel closing. Replacing acidic amino acids with neutral ones should shift the gH–V relationship positively, and this has been reported for Asp112, Asp123 and Asp185 [82,92,96].
Despite the simplicity and intuitive appeal of the counter-charge model, an explicit quantitative model has not been published, and other types of models can be envisaged. Understanding the mechanism of ΔpH-dependent gating of HV1 remains an elusive pimpernel.
6. Physiological modulators of gating
Several physiological molecules modulate HV1 gating, in each case increasing the sensitivity to voltage as well as altering the kinetics of the response. The best characterized response is a constellation of four profound changes called the ‘enhanced gating mode’, which occurs in phagocytes during the ‘respiratory burst’ when phagocytosed bacteria are killed or agonists like chemotactic peptides are applied [57,189,202]. The proton current increases, activation (channel opening) becomes much faster, deactivation (channel closing) much slower and the gH–V relationship shifts negatively by 40 mV [57,60,68,202,203]. In most cases, the signalling pathway involves protein kinase C (PKC) [56,60,68,204–206], which phosphorylates the hHV1 molecule at Thr29 in the intracellular N terminus [8,55]. Another type of gating enhancer is arachidonic acid [207] and other unsaturated long-chain fatty acids [42,208]. Arachidonic acid can increase H+ currents directly [206–212], but can also act indirectly by activating PKC [206]. The actual physical mechanism by which gating enhancers enhance gating is unknown. The PKC phosphorylation site Thr29, for example, is located in the mostly disordered intracellular N terminal region, and how it manages to influence gating can only be speculated.
7. Summary
Given the uncertainties in interpreting data and the inaccessibility of the events and structures responsible for gating and conduction in hHV1, what conclusions can we draw? To some extent, gating and conduction are not such clearly separable processes as they are in other channels. Protons both carry current and also tightly regulate when the channel will open or close. Gating at minimum requires rearrangement between conformations that permit or prevent selective H+ conduction. That gating is regulated by both voltage and pH constrains possible mechanisms. Although little molecular movement is required to effect gating by a priori considerations, many types of evidence support some movement occurring, especially of the S4 helix. The dynamic nature of hHV1 revealed by EPR means that there is extensive motion [91], but the nature of the motion is unspecified. That the selectivity filter retains function when repositioned from position 112 to 116 in the S1 helix (WT Asp112 to V116D) means that there is some leeway in creating an open and H+-selective conducting state, but the fact that moving Asp to other locations failed to produce H+ current means that H+-selective conduction has fairly stringent requirements [95]. That many point mutations cause loss of selectivity or abolish function altogether [82] indicates that there are a number of places in the HV1 molecule where arbitrary changes are not allowed. Evidently, it is easier to impair function than to explain it. The more exotic mechanism of ΔpH-dependent gating most probably involves titratable sites, but if so, these must exhibit redundancy, because ΔpH dependence is not eliminated by single point mutations [82,92].
Acknowledgements
I appreciate helpful comments on the manuscript by Boris Musset.
Endnote
Amino acids in proteins are numbered starting from the N terminus. Because there are differences in the primary sequences in different species, the numbering of equivalent positions differs. The first Arg in S4 in human HV1 is at position 205, but in mouse it is 201.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
This work was supported by NIH grants GM102336, GM121462, and NSF grant MCB-1242985.
References
- 1.Fogel M, Hastings JW. 1972. Bioluminescence: mechanism and mode of control of scintillon activity. Proc. Natl Acad. Sci. USA 69, 690–693. ( 10.1073/pnas.69.3.690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith SME, Morgan D, Musset B, Cherny VV, Place AR, Hastings JW, DeCoursey TE. 2011. Voltage-gated proton channel in a dinoflagellate. Proc. Natl Acad. Sci. USA 108, 18 162–18 167. ( 10.1073/pnas.1115405108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez JD, et al. 2017. Identification of a vacuolar proton channel that triggers the bioluminescent flash in dinoflagellates. PLoS ONE 12, e0171594 ( 10.1371/journal.pone.0171594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas RC, Meech RW. 1982. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299, 826–828. ( 10.1038/299826a0) [DOI] [PubMed] [Google Scholar]
- 5.Ramsey IS, Moran MM, Chong JA, Clapham DE. 2006. A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216. ( 10.1038/nature04700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki M, Takagi M, Okamura Y. 2006. A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592. ( 10.1126/science.1122352) [DOI] [PubMed] [Google Scholar]
- 7.Capasso M, et al. 2010. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat. Immunol. 11, 265–272. ( 10.1038/ni.1843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hondares E, et al. 2014. Enhanced activation of an amino-terminally truncated isoform of the voltage-gated proton channel HVCN1 enriched in malignant B cells. Proc. Natl Acad. Sci. USA 111, 18 078–18 083. ( 10.1073/pnas.1411390111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger TK, et al. 2017. Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating. J. Physiol. 595, 1533–1546. ( 10.1113/JP273189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor AR, Chrachri A, Wheeler G, Goddard H, Brownlee C.. 2011. A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLoS Biol. 9, e1001085 ( 10.1371/journal.pbio.1001085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaves G, Derst C, Franzen A, Mashimo Y, Machida R, Musset B. 2016. Identification of an HV1 voltage-gated proton channel in insects. FEBS J. 283, 1453–1464. ( 10.1111/febs.13680) [DOI] [PubMed] [Google Scholar]
- 12.Byerly L, Meech R, Moody W Jr. 1984. Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J. Physiol. 351, 199–216. ( 10.1113/jphysiol.1984.sp015241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahaut-Smith MP. 1989. Separation of hydrogen ion currents in intact molluscan neurones. J. Exp. Biol. 145, 439–454. [DOI] [PubMed] [Google Scholar]
- 14.Thomas RC. 1988. Changes in the surface pH of voltage-clamped snail neurones apparently caused by H+ fluxes through a channel. J. Physiol. 398, 313–327. ( 10.1113/jphysiol.1988.sp017044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright BJ, Bickham-Wright U, Yoshino TP, Jackson MB.. 2017. H+ channels in embryonic Biomphalaria glabrata cell membranes: putative roles in snail host–schistosome interactions. PLoS Negl Trop Dis. 11, e0005467 ( 10.1371/journal.pntd.0005467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barish ME, Baud C. 1984. A voltage-gated hydrogen ion current in the oocyte membrane of the axolotl, Ambystoma. J. Physiol. 352, 243–263. ( 10.1113/jphysiol.1984.sp015289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud C, Barish ME. 1984. Changes in membrane hydrogen and sodium conductances during progesterone-induced maturation of Ambystoma oocytes. Dev. Biol. 105, 423–434. ( 10.1016/0012-1606(84)90299-9) [DOI] [PubMed] [Google Scholar]
- 18.Humez S, Collin T, Matifat F, Guilbault P, Fournier F. 1996. InsP3-dependent Ca2+ oscillations linked to activation of voltage-dependent H+ conductance in Rana esculenta oocytes. Cell. Signal. 8, 375–379. ( 10.1016/0898-6568(96)00082-4) [DOI] [PubMed] [Google Scholar]
- 19.Ratanayotha A, Kawai T, Higashijima SI, Okamura Y.. 2017. Molecular and functional characterization of the voltage-gated proton channel in zebrafish neutrophils. Physiol. Rep. 5, e13345 ( 10.14814/phy2.13345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy R, Cherny VV, Morgan D, DeCoursey TE. 2005. Voltage-gated proton channels help regulate pHi in rat alveolar epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 288, L398–L408. ( 10.1152/ajplung.00299.2004) [DOI] [PubMed] [Google Scholar]
- 21.Iovannisci D, Illek B, Fischer H. 2010. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J. Gen. Physiol. 136, 35–46. ( 10.1085/jgp.200910379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeCoursey TE. 2000. Hypothesis: do voltage-gated H+ channels in alveolar epithelial cells contribute to CO2 elimination by the lung? Am. J. Physiol. Cell Physiol. 278, C1–C10. ( 10.1152/ajpcell.2000.278.1.C1) [DOI] [PubMed] [Google Scholar]
- 23.Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, Ballard PL. 2007. Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1506–L1514. ( 10.1152/ajplung.00029.2007) [DOI] [PubMed] [Google Scholar]
- 24.Bernheim L, Krause RM, Baroffio A, Hamann M, Kaelin A, Bader CR. 1993. A voltage-dependent proton current in cultured human skeletal muscle myotubes. J. Physiol. 470, 313–333. ( 10.1113/jphysiol.1993.sp019860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson LM, Chappell JB, Jones OTG. 1988. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem. J. 251, 563–567. ( 10.1042/bj2510563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan D, Cherny VV, Murphy R, Katz BZ, DeCoursey TE. 2005. The pH dependence of NADPH oxidase in human eosinophils. J. Physiol. 569, 419–431. ( 10.1113/jphysiol.2005.094748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan D, Capasso M, Musset B, Cherny VV, Ríos E, Dyer MJS, DeCoursey TE. 2009. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc. Natl Acad. Sci. USA 106, 18 022–18 027. ( 10.1073/pnas.0905565106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy R, DeCoursey TE. 2006. Charge compensation during the phagocyte respiratory burst. Biochim. Biophys. Acta 1757, 996–1011. ( 10.1016/j.bbabio.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 29.El Chemaly A, Nunes P, Jimaja W, Castelbou C, Demaurex N. 2014. Hv1 proton channels differentially regulate the pH of neutrophil and macrophage phagosomes by sustaining the production of phagosomal ROS that inhibit the delivery of vacuolar ATPases. J. Leukoc. Biol. 95, 827–839. ( 10.1189/jlb.0513251) [DOI] [PubMed] [Google Scholar]
- 30.El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N. 2010. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J. Exp. Med. 207, 129–139. ( 10.1084/jem.20091837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X, Mose E, Zimmermann N.. 2013. Proton channel HVCN1 is required for effector functions of mouse eosinophils. BMC Immunol. 14, 24 ( 10.1186/1471-2172-14-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson LM, Chappell JB, Jones OTG. 1987. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246, 325–329. ( 10.1042/bj2460325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson LM, Chappell JB, Jones OTG. 1988. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 255, 285–290. [PMC free article] [PubMed] [Google Scholar]
- 34.DeCoursey TE, Morgan D, Cherny VV. 2003. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422, 531–534. ( 10.1038/nature01523) [DOI] [PubMed] [Google Scholar]
- 35.Eder C, DeCoursey TE. 2001. Voltage-gated proton channels in microglia. Prog. Neurobiol. 64, 277–305. ( 10.1016/S0301-0082(00)00062-9) [DOI] [PubMed] [Google Scholar]
- 36.Morihata H, Nakamura F, Tsutada T, Kuno M. 2000. Potentiation of a voltage-gated proton current in acidosis-induced swelling of rat microglia. J. Neurosci. 20, 7220–7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu LJ, Wu G, Sharif MR, Baker A, Jia Y, Fahey FH, Luo HR, Feener EP, Clapham DE. 2012. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat. Neurosci. 15, 565–573. ( 10.1038/nn.3059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musset B, Morgan D, Cherny VV, MacGlashan DW Jr, Thomas LL, Ríos E, DeCoursey TE. 2008. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc. Natl Acad. Sci. USA 105, 11 020–11 025. ( 10.1073/pnas.0800886105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vairamani K, Wang HS, Medvedovic M, Lorenz JN, Shull GE.. 2017. RNA SEQ analysis Indicates that the AE3 Cl−/HCO3− exchanger contributes to active transport-mediated CO2 disposal in heart. Sci. Rep. 7, 7264 ( 10.1038/s41598-017-07585-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Chemaly A, Guinamard R, Demion M, Fares N, Jebara V, Faivre JF, Bois P. 2006. A voltage-activated proton current in human cardiac fibroblasts. Biochem. Biophys. Res. Commun. 340, 512–516. ( 10.1016/j.bbrc.2005.12.038) [DOI] [PubMed] [Google Scholar]
- 41.Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, Kuno M. 2003. Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J. Bone Miner. Res. 18, 2069–2076. ( 10.1359/jbmr.2003.18.11.2069) [DOI] [PubMed] [Google Scholar]
- 42.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. 2010. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337. ( 10.1016/j.cell.2009.12.053) [DOI] [PubMed] [Google Scholar]
- 43.Musset B, et al. 2012. NOX5 in human spermatozoa: expression, function and regulation. J. Biol. Chem. 287, 9376–9388. ( 10.1074/jbc.M111.314955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Li SJ, Wu X, Che Y, Li Q. 2012. Clinicopathological and biological significance of human voltage-gated proton channel Hv1 over-expression in breast cancer. J. Biol. Chem. 287, 13 877–13 888. ( 10.1074/jbc.M112.345280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schilling T, Gratopp A, DeCoursey TE, Eder C. 2002. Voltage-activated proton currents in human lymphocytes. J. Physiol. 545, 93–105. ( 10.1113/jphysiol.2002.028878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu X, Sackin H. 1995. Effect of pH on potassium and proton conductance in renal proximal tubule. Am. J. Physiol. 269, F289–F308. ( 10.1152/ajprenal.1995.269.3.F289) [DOI] [PubMed] [Google Scholar]
- 47.Jin C, et al. 2014. HV1 acts as a sodium sensor and promotes superoxide production in medullary thick ascending limb of Dahl salt-sensitive rats. Hypertension 64, 541–550. ( 10.1161/HYPERTENSIONAHA.114.03549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeCoursey TE. 2013. Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the HV family. Physiol. Rev. 93, 599–652. ( 10.1152/physrev.00011.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soler-Llavina GJ, Holmgren M, Swartz KJ. 2003. Defining the conductance of the closed state in a voltage-gated K+ channel. Neuron 38, 61–67. ( 10.1016/S0896-6273(03)00157-0) [DOI] [PubMed] [Google Scholar]
- 50.Nernst W. 1888. Zur Kinetik der in Lösung befindlichen Körper: Theorie der Diffusion. Z. Phys. Chem. 2, 613–637. [Google Scholar]
- 51.Lee SY, Letts JA, MacKinnon R. 2009. Functional reconstitution of purified human Hv1 H+ channels. J. Mol. Biol. 387, 1055–1060. ( 10.1016/j.jmb.2009.02.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. 2008. Multimeric nature of voltage-gated proton channels. Proc. Natl Acad. Sci. USA 105, 9111–9116. ( 10.1073/pnas.0801553105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SY, Letts JA, Mackinnon R. 2008. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl Acad. Sci. USA 105, 7692–7695. ( 10.1073/pnas.0803277105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tombola F, Ulbrich MH, Isacoff EY. 2008. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58, 546–556. ( 10.1016/j.neuron.2008.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Musset B, Capasso M, Cherny VV, Morgan D, Bhamrah M, Dyer MJS, DeCoursey TE. 2010. Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes. J. Biol. Chem. 285, 5117–5121. ( 10.1074/jbc.C109.082727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan D, Cherny VV, Finnegan A, Bollinger J, Gelb MH, DeCoursey TE. 2007. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2α activity. J. Physiol. 579, 327–344. ( 10.1113/jphysiol.2006.124248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeCoursey TE, Cherny VV, Zhou W, Thomas LL. 2000. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc. Natl Acad. Sci. USA 97, 6885–6889. ( 10.1073/pnas.100047297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243. ( 10.1038/nature03650) [DOI] [PubMed] [Google Scholar]
- 59.Murata Y, Okamura Y. 2007. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J. Physiol. 583, 875–889. ( 10.1113/jphysiol.2007.134775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeCoursey TE. 2010. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology 25, 27–40. ( 10.1152/physiol.00039.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noda M, et al. 1984. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature 312, 121–127. ( 10.1038/312121a0) [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Blunck R.. 2016. The isolated voltage sensing domain of the Shaker potassium channel forms a voltage-gated cation channel. Elife 5, e18130 ( 10.7554/eLife.18130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Starace DM, Stefani E, Bezanilla F. 1997. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron 19, 1319–1327. ( 10.1016/S0896-6273(00)80422-5) [DOI] [PubMed] [Google Scholar]
- 64.Starace DM, Bezanilla F. 2001. Histidine scanning mutagenesis of basic residues of the S4 segment of the Shaker K+ channel. J. Gen. Physiol. 117, 469–490. ( 10.1085/jgp.117.5.469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Starace DM, Bezanilla F. 2004. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427, 548–553. ( 10.1038/nature02270) [DOI] [PubMed] [Google Scholar]
- 66.Struyk AF, Cannon SC. 2007. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J. Gen. Physiol. 130, 11–20. ( 10.1085/jgp.200709755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gosselin-Badaroudine P, Delemotte L, Moreau A, Klein ML, Chahine M. 2012. Gating pore currents and the resting state of Nav1.4 voltage sensor domains. Proc. Natl Acad. Sci. USA 109, 19 250–19 255. ( 10.1073/pnas.1217990109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeCoursey TE. 2003. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579. ( 10.1152/physrev.00028.2002) [DOI] [PubMed] [Google Scholar]
- 69.DeCoursey TE, Hosler J.. 2014. Philosophy of voltage-gated proton channels. J. R. Soc. Interf. 11, 20130799 ( 10.1098/rsif.2013.0799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong M, DeGrado WF. 2012. Structural basis for proton conduction and inhibition by the influenza M2 protein. Protein Sci. 21, 1620–1633. ( 10.1002/pro.2158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu CK, Silverman DN, Forsman C, Jonsson BH, Lindskog S. 1989. Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochemistry 28, 7913–7918. ( 10.1021/bi00445a054) [DOI] [PubMed] [Google Scholar]
- 72.Randolph AL, Mokrab Y, Bennett AL, Sansom MS, Ramsey IS.. 2016. Proton currents constrain structural models of voltage sensor activation. Elife 5, e18017 ( 10.7554/eLife.18017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. 2006. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc. Natl Acad. Sci. USA 103, 269–274. ( 10.1073/pnas.0507225103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Chen H, Steinbronn C, Wu B, Beitz E, Zeuthen T, Voth GA. 2011. Enhancement of proton conductance by mutations of the selectivity filter of aquaporin-1. J. Mol. Biol. 407, 607–620. ( 10.1016/j.jmb.2011.01.036) [DOI] [PubMed] [Google Scholar]
- 75.Musset B, Smith SME, Rajan S, Morgan D, Cherny VV, DeCoursey TE. 2011. Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature 480, 273–277. ( 10.1038/nature10557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dudev T, Musset B, Morgan D, Cherny VV, Smith SM, Mazmanian K, DeCoursey TE, Lim C.. 2015. Selectivity mechanism of the voltage-gated proton channel, HV1. Sci. Rep. 5, 10320 ( 10.1038/srep10320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Keulen SC, Gianti E, Carnevale V, Klein ML, Rothlisberger U, Delemotte L. 2017. Does proton conduction in the voltage-gated H+ channel hHv1 involve Grotthuss-like hopping via acidic residues? J. Phys. Chem. B 121, 3340–3351. ( 10.1021/acs.jpcb.6b08339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagle JF, Tristram-Nagle S. 1983. Hydrogen bonded chain mechanisms for proton conduction and proton pumping. J. Membr. Biol. 74, 1–14. ( 10.1007/BF01870590) [DOI] [PubMed] [Google Scholar]
- 79.Chen K, Hirst J, Camba R, Bonagura CA, Stout CD, Burgess BK, Armstrong FA. 2000. Atomically defined mechanism for proton transfer to a buried redox centre in a protein. Nature 405, 814–817. ( 10.1038/35015610) [DOI] [PubMed] [Google Scholar]
- 80.Hosler JP, Ferguson-Miller S, Mills DA. 2006. Energy transduction: proton transfer through the respiratory complexes. Annu. Rev. Biochem. 75, 165–187. ( 10.1146/annurev.biochem.75.062003.101730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varanasi L, Hosler JP. 2012. Subunit III-depleted cytochrome c oxidase provides insight into the process of proton uptake by proteins. Biochim. Biophys. Acta 1817, 545–551. ( 10.1016/j.bbabio.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeCoursey TE, Morgan D, Musset B, Cherny VV. 2016. Insights into the structure and function of HV1 from a meta-analysis of mutation studies. J. Gen. Physiol. 148, 97–118. ( 10.1085/jgp.201611619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Musset B, Smith SME, Rajan S, Cherny VV, Sujai S, Morgan D, DeCoursey TE. 2010. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J. Physiol. 588, 1435–1449. ( 10.1113/jphysiol.2010.188318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujiwara Y, Kurokawa T, Takeshita K, Kobayashi M, Okochi Y, Nakagawa A, Okamura Y.. 2012. The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H+ channel Hv1. Nat. Commun. 3, 816 ( 10.1038/ncomms1823) [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez C, Koch HP, Drum BM, Larsson HP. 2010. Strong cooperativity between subunits in voltage-gated proton channels. Nat. Struct. Mol. Biol. 17, 51–56. ( 10.1038/nsmb.1739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodgkin AL, Huxley AF. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544. ( 10.1113/jphysiol.1952.sp004764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hille B. 2001. Ion channels of excitable membranes, 3rd edn Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 88.Tombola F, Ulbrich MH, Kohout SC, Isacoff EY. 2010. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Mol. Biol. 17, 44–50. ( 10.1038/nsmb.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cherny VV, Markin VS, DeCoursey TE. 1995. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 105, 861–896. ( 10.1085/jgp.105.6.861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takeshita K, et al. 2014. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21, 352–357. ( 10.1038/nsmb.2783) [DOI] [PubMed] [Google Scholar]
- 91.Li Q, Shen R, Treger JS, Wanderling SS, Milewski W, Siwowska K, Bezanilla F, Perozo E. 2015. Resting state of the human proton channel dimer in a lipid bilayer. Proc. Natl Acad. Sci. USA 112, E5926–E5935. ( 10.1073/pnas.1515043112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramsey IS, Mokrab Y, Carvacho I, Sands ZA, Sansom MSP, Clapham DE. 2010. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17, 869–875. ( 10.1038/nsmb.1826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wood ML, Schow EV, Freites JA, White SH, Tombola F, Tobias DJ. 2012. Water wires in atomistic models of the Hv1 proton channel. Biochim. Biophys. Acta 1818, 286–293. ( 10.1016/j.bbamem.2011.07.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulleperuma K, Smith SME, Morgan D, Musset B, Holyoake J, Chakrabarti N, Cherny VV, DeCoursey TE, Pomès R. 2013. Construction and validation of a homology model of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 141, 445–465. ( 10.1085/jgp.201210856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morgan D, Musset B, Kulleperuma K, Smith SME, Rajan S, Cherny VV, Pomès R, DeCoursey TE. 2013. Peregrination of the selectivity filter delineates the pore of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 142, 625–640. ( 10.1085/jgp.201311045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chamberlin A, Qiu F, Rebolledo S, Wang Y, Noskov SY, Larsson HP. 2014. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl Acad. Sci. USA 111, E273–E282. ( 10.1073/pnas.1318018111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chamberlin A, Qiu F, Wang Y, Noskov SY, Larsson HP. 2015. Mapping the gating and permeation pathways in the voltage-gated proton channel Hv1. J. Mol. Biol. 427, 131–145. ( 10.1016/j.jmb.2014.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gianti E, Delemotte L, Klein ML, Carnevale V. 2016. On the role of water density fluctuations in the inhibition of a proton channel. Proc. Natl Acad. Sci. USA 113, E8359–E8368. ( 10.1073/pnas.1609964114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pupo A, Baez-Nieto D, Martinez A, Latorre R, González C. 2014. Proton channel models: filling the gap between experimental data and the structural rationale. Channels 8, 180–192. ( 10.4161/chan.28665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Q, et al. 2014. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 21, 244–252. ( 10.1038/nsmb.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagle JF, Morowitz HJ. 1978. Molecular mechanisms for proton transport in membranes. Proc. Natl Acad. Sci. USA 75, 298–302. ( 10.1073/pnas.75.1.298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer E. 1992. Internal water molecules and H-bonding in biological macromolecules: a review of structural features with functional implications. Protein Sci. 1, 1543–1562. ( 10.1002/pro.5560011203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wraight CA. 2006. Chance and design—proton transfer in water, channels and bioenergetic proteins. Biochim. Biophys. Acta 1757, 886–912. ( 10.1016/j.bbabio.2006.06.017) [DOI] [PubMed] [Google Scholar]
- 104.Swanson JM, Maupin CM, Chen H, Petersen MK, Xu J, Wu Y, Voth GA. 2007. Proton solvation and transport in aqueous and biomolecular systems: insights from computer simulations. J. Phys. Chem. B 111, 4300–4314. ( 10.1021/jp070104x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ishikita H, Saito K. 2013. Proton transfer reactions and hydrogen-bond networks in protein environments. J. R. Soc. Interf. 11, 20130518 ( 10.1098/rsif.2013.0518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rich PR, Maréchal A. 2013. Functions of the hydrophilic channels in protonmotive cytochrome c oxidase. J. R. Soc. Interf. 10, 20130183 ( 10.1098/rsif.2013.0183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng Y, Swanson JM, Kang SG, Zhou R, Voth GA. 2015. Hydrated excess protons can create their own water wires. J. Phys. Chem. B 119, 9212–9218. ( 10.1021/jp5095118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodgkin AL, Keynes RD. 1955. The potassium permeability of a giant nerve fibre. J. Physiol. 128, 61–88. ( 10.1113/jphysiol.1955.sp005291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hille B, Schwarz W. 1978. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 72, 409–442. ( 10.1085/jgp.72.4.409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77. ( 10.1126/science.280.5360.69) [DOI] [PubMed] [Google Scholar]
- 111.Schulten Z, Schulten K. 1985. A model for the resistance of the proton channel formed by the proteolipid of ATPase. Eur. Biophys. J. 11, 149–155. ( 10.1007/BF00257393) [DOI] [PubMed] [Google Scholar]
- 112.Brandsburg-Zabary S, Fried O, Marantz Y, Nachliel E, Gutman M. 2000. Biophysical aspects of intra-protein proton transfer. Biochim. Biophys. Acta 1458, 120–134. ( 10.1016/S0005-2728(00)00063-3) [DOI] [PubMed] [Google Scholar]
- 113.Brzezinski P, Gennis RB. 2008. Cytochrome c oxidase: exciting progress and remaining mysteries. J. Bioenerg. Biomembr. 40, 521–531. ( 10.1007/s10863-008-9181-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. 1996. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J. Physiol. 494, 329–336. ( 10.1113/jphysiol.1996.sp021495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin TI, Schroeder C. 2001. Definitive assignment of proton selectivity and attoampere unitary current to the M2 ion channel protein of influenza A virus. J. Virol. 75, 3647–3656. ( 10.1128/JVI.75.8.3647-3656.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moffat JC, Vijayvergiya V, Gao PF, Cross TA, Woodbury DJ, Busath DD. 2008. Proton transport through influenza A virus M2 protein reconstituted in vesicles. Biophys. J. 94, 434–445. ( 10.1529/biophysj.107.109082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leiding T, Wang J, Martinsson J, DeGrado WF, Årsköld SP. 2010. Proton and cation transport activity of the M2 proton channel from influenza A virus. Proc. Natl Acad. Sci. USA 107, 15 409–15 414. ( 10.1073/pnas.1009997107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peterson E, Ryser T, Funk S, Inouye D, Sharma M, Qin H, Cross TA, Busath DD. 2011. Functional reconstitution of influenza A M2(22–62). Biochim. Biophys. Acta 1808, 516–521. ( 10.1016/j.bbamem.2010.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma M, Yi M, Dong H, Qin H, Peterson E, Busath D, Zhou H, Cross T. 2010. Insight into the mechanism of the influenza A proton channel from structure in a lipid bilayer. Science 330, 509–512. ( 10.1126/science.1191750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wei C, Pohorille A. 2013. Activation and proton transport mechanism in influenza A M2 channel. Biophys. J. 105, 2036–2045. ( 10.1016/j.bpj.2013.08.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liang R, Li H, Swanson JM, Voth GA. 2014. Multiscale simulation reveals a multifaceted mechanism of proton permeation through the influenza A M2 proton channel. Proc. Natl Acad. Sci. USA 111, 9396–9401. ( 10.1073/pnas.1601982113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liao SY, Yang Y, Tietze D, Hong M. 2015. The influenza M2 cytoplasmic tail changes the proton-exchange equilibria and the backbone conformation of the transmembrane histidine residue to facilitate proton conduction. J. Am. Chem. Soc. 137, 6067–6077. ( 10.1021/jacs.5b02510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miao Y, Fu R, Zhou HX, Cross TA. 2015. Dynamic short hydrogen bonds in histidine tetrad of full-Length M2 proton channel reveal tetrameric structural heterogeneity and functional mechanism. Structure 23, 2300–2308. ( 10.1016/j.str.2015.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liang R, Swanson JMJ, Madsen JJ, Hong M, DeGrado WF, Voth GA. 2016. Acid activation mechanism of the influenza A M2 proton channel. Proc. Natl Acad. Sci. USA 113, E6955–E6964. ( 10.1073/pnas.1615471113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robinson RA, Stokes RH.. 1959. Electrolyte solutions. London, UK: Butterworths. [Google Scholar]
- 126.de Grotthuss CJT. 1806. Mémoire sur la décomposition de l'eau et des corps qu'elle tient en dissolution à l'aide de l'électricité galvanique. Ann. Chim. LVIII, 54–74. [Google Scholar]
- 127.de Grotthuss CJT. 2006. Memoir on the decomposition of water and of the bodies that it holds in solution by means of galvanic electricity. 1805. Biochim. Biophys. Acta 1757, 871–875. ( 10.1016/j.bbabio.2006.07.004) [DOI] [PubMed] [Google Scholar]
- 128.Danneel H. 1905. Notiz über Ionengeschwindigkeiten. Zeitschrift für Elektrochemie und angewandte physikalische Chemie 11, 249–252. ( 10.1002/bbpc.19050111603) [DOI] [Google Scholar]
- 129.DeCoursey TE. 2015. The voltage-gated proton channel: a riddle, wrapped in a mystery, inside an enigma. Biochemistry 54, 3250–3268. ( 10.1021/acs.biochem.5b00353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liman ER, Hess P, Weaver F, Koren G. 1991. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature 353, 752–756. ( 10.1038/353752a0) [DOI] [PubMed] [Google Scholar]
- 131.Papazian DM, Timpe LC, Jan YN, Jan LY. 1991. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature 349, 305–310. ( 10.1038/349305a0) [DOI] [PubMed] [Google Scholar]
- 132.Perozo E, Santacruz-Toloza L, Stefani E, Bezanilla F, Papazian DM. 1994. S4 mutations alter gating currents of Shaker K channels. Biophys. J. 66, 345–354. ( 10.1016/S0006-3495(94)80783-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang N, Horn R. 1995. Evidence for voltage-dependent S4 movement in sodium channels. Neuron 15, 213–218. ( 10.1016/0896-6273(95)90078-0) [DOI] [PubMed] [Google Scholar]
- 134.Larsson HP, Baker OS, Dhillon DS, Isacoff EY. 1996. Transmembrane movement of the Shaker K+ channel S4. Neuron 16, 387–397. ( 10.1016/S0896-6273(00)80056-2) [DOI] [PubMed] [Google Scholar]
- 135.Yang N, George AL Jr, Horn R. 1996. Molecular basis of charge movement in voltage-gated sodium channels. Neuron 16, 113–122. ( 10.1016/S0896-6273(00)80028-8) [DOI] [PubMed] [Google Scholar]
- 136.Yusaf SP, Wray D, Sivaprasadarao A. 1996. Measurement of the movement of the S4 segment during the activation of a voltage-gated potassium channel. Pflügers Arch. 433, 91–97. ( 10.1007/s004240050253) [DOI] [PubMed] [Google Scholar]
- 137.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. 2003. X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41. ( 10.1038/nature01580) [DOI] [PubMed] [Google Scholar]
- 138.Ruta V, Chen J, MacKinnon R. 2005. Calibrated measurement of gating-charge arginine displacement in the KvAP voltage-dependent K+ channel. Cell 123, 463–475. ( 10.1016/j.cell.2005.08.041) [DOI] [PubMed] [Google Scholar]
- 139.Posson DJ, Selvin PR. 2008. Extent of voltage sensor movement during gating of shaker K+ channels. Neuron 59, 98–109. ( 10.1016/j.neuron.2008.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stühmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S. 1989. Structural parts involved in activation and inactivation of the sodium channel. Nature 339, 597–603. ( 10.1038/339597a0) [DOI] [PubMed] [Google Scholar]
- 141.Swartz KJ. 2008. Sensing voltage across lipid membranes. Nature 456, 891–897. ( 10.1038/nature07620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cha A, Snyder GE, Selvin PR, Bezanilla F. 1999. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature 402, 809–813. ( 10.1038/45552) [DOI] [PubMed] [Google Scholar]
- 143.Tao X, Lee A, Limapichat W, Dougherty DA, MacKinnon R. 2010. A gating charge transfer center in voltage sensors. Science 328, 67–73. ( 10.1126/science.1185954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin MC, Hsieh JY, Mock AF, Papazian DM. 2011. R1 in the Shaker S4 occupies the gating charge transfer center in the resting state. J. Gen. Physiol. 138, 155–163. ( 10.1085/jgp.201110642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Henrion U, Renhorn J, Börjesson SI, Nelson EM, Schwaiger CS, Bjelkmar P, Wallner B, Lindahl E, Elinder F. 2012. Tracking a complete voltage-sensor cycle with metal-ion bridges. Proc. Natl Acad. Sci. USA 109, 8552–8557. ( 10.1073/pnas.1116938109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Papazian DM, Shao XM, Seoh SA, Mock AF, Huang Y, Wainstock DH. 1995. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron 14, 1293–1301. ( 10.1016/0896-6273(95)90276-7) [DOI] [PubMed] [Google Scholar]
- 147.Tiwari-Woodruff SK, Schulteis CT, Mock AF, Papazian DM. 1997. Electrostatic interactions between transmembrane segments mediate folding of Shaker K+ channel subunits. Biophys. J. 72, 1489–1500. ( 10.1016/S0006-3495(97)78797-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan CY, Scheuer T, Baker D, Catterall WA. 2012. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl Acad. Sci. USA 109, E93–E102. ( 10.1073/pnas.1118434109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gamal El-Din TM, Scheuer T, Catterall WA. 2014. Tracking S4 movement by gating pore currents in the bacterial sodium channel NaChBac. J. Gen. Physiol. 144, 147–157. ( 10.1085/jgp.201411210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. 1992. The size of gating charge in wild-type and mutant Shaker potassium channels. Science 255, 1712–1715. ( 10.1126/science.1553560) [DOI] [PubMed] [Google Scholar]
- 151.Hirschberg B, Rovner A, Lieberman M, Patlak J. 1995. Transfer of twelve charges is needed to open skeletal muscle Na+ channels. J. Gen. Physiol. 106, 1053–1068. ( 10.1085/jgp.106.6.1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aggarwal SK, MacKinnon R. 1996. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron 16, 1169–1177. ( 10.1016/S0896-6273(00)80143-9) [DOI] [PubMed] [Google Scholar]
- 153.Noceti F, Baldelli P, Wei X, Qin N, Toro L, Birnbaumer L, Stefani E. 1996. Effective gating charges per channel in voltage-dependent K+ and Ca2+ channels. J. Gen. Physiol. 108, 143–155. ( 10.1085/jgp.108.3.143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seoh SA, Sigg D, Papazian DM, Bezanilla F. 1996. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron 16, 1159–1167. ( 10.1016/S0896-6273(00)80142-7) [DOI] [PubMed] [Google Scholar]
- 155.Armstrong CM, Bezanilla F. 1973. Currents related to movement of the gating particles of the sodium channels. Nature 242, 459–461. ( 10.1038/242459a0) [DOI] [PubMed] [Google Scholar]
- 156.Schneider MF, Chandler WK. 1973. Voltage dependent charge movement of skeletal muscle: a possible step in excitation–contraction coupling. Nature 242, 244–246. ( 10.1038/242244a0) [DOI] [PubMed] [Google Scholar]
- 157.Armstrong CM, Bezanilla F. 1974. Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 63, 533–552. ( 10.1085/jgp.63.5.533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Keynes RD, Rojas E. 1974. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J. Physiol. 239, 393–434. ( 10.1113/jphysiol.1974.sp010575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tombola F, Pathak MM, Isacoff EY. 2005. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron 45, 379–388. ( 10.1016/j.neuron.2004.12.047) [DOI] [PubMed] [Google Scholar]
- 160.Horn R. 2005. How ion channels sense membrane potential. Proc. Natl Acad. Sci. USA 102, 4929–4930. ( 10.1073/pnas.0501640102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Campos FV, Chanda B, Roux B, Bezanilla F. 2007. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl Acad. Sci. USA 104, 7904–7909. ( 10.1073/pnas.0702638104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Okamura Y, Fujiwara Y, Sakata S. 2015. Gating mechanisms of voltage-gated proton channels. Annu. Rev. Biochem. 84, 685–709. ( 10.1146/annurev-biochem-060614-034307) [DOI] [PubMed] [Google Scholar]
- 163.Chanda B, Bezanilla F. 2008. A common pathway for charge transport through voltage-sensing domains. Neuron 57, 345–351. ( 10.1016/j.neuron.2008.01.015) [DOI] [PubMed] [Google Scholar]
- 164.Cherny VV, DeCoursey TE. 1999. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J. Gen. Physiol. 114, 819–838. ( 10.1085/jgp.114.6.819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hong L, Pathak MM, Kim IH, Ta D, Tombola F. 2013. Voltage-sensing domain of voltage-gated proton channel Hv1 shares mechanism of block with pore domains. Neuron 77, 274–287. ( 10.1016/j.neuron.2012.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carmona EM, Baez-Nieto D, Pupo A, Castillo K, Alvarez O, Neely A, Latorre R, Gonzalez C.. 2018. Properties of the voltage gated proton channel gating currents. Biophys. J. 114, 546a ( 10.1016/j.bpj.2017.11.2986) [DOI] [Google Scholar]
- 167.De La Rosa V, Ramsey I.. 2018. Gating currents in Hv1 proton channels. Biophys. J. 114, 124a ( 10.1016/j.bpj.2017.11.705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Almers W. 1978. Gating currents and charge movements in excitable membranes. Rev. Physiol. Biochem. Pharmacol. 82, 96–190. ( 10.1007/BFb0030498) [DOI] [PubMed] [Google Scholar]
- 169.Sigg D, Bezanilla F. 1997. Total charge movement per channel. The relation between gating charge displacement and the voltage sensitivity of activation. J. Gen. Physiol. 109, 27–39. ( 10.1085/jgp.109.1.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kang BE, Baker BJ.. 2016. Pado, a fluorescent protein with proton channel activity can optically monitor membrane potential, intracellular pH, and map gap junctions. Sci. Rep. 6, 23865 ( 10.1038/srep23865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sakata S, et al. 2016. Comparison between mouse and sea urchin orthologs of voltage-gated proton channel suggests role of S3 segment in activation gating. Biochim. Biophys. Acta 1858, 2972–2983. ( 10.1016/j.bbamem.2016.09.008) [DOI] [PubMed] [Google Scholar]
- 172.Thomas S, Cherny VV, Morgan D, Artinian LR, Rehder V, Smith SME, DeCoursey TE.. In press. Exotic properties of a voltage gated proton channel in the snail Helisoma trivolvis. J. Gen. Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Musset B, Smith SME, Rajan S, Cherny VV, Morgan D, DeCoursey TE. 2010. Oligomerization of the voltage gated proton channel. Channels 4, 260–265. ( 10.4161/chan.4.4.12789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smith SME, DeCoursey TE. 2013. Consequences of dimerization of the voltage-gated proton channel. Prog. Mol. Biol. Transl. Sci. 117, 335–360. ( 10.1016/B978-0-12-386931-9.00012-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.DeCoursey TE, Cherny VV. 1996. Effects of buffer concentration on voltage-gated H+ currents: does diffusion limit the conductance? Biophys. J. 71, 182–193. ( 10.1016/S0006-3495(96)79215-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.DeCoursey TE, Cherny VV. 1997. Deuterium isotope effects on permeation and gating of proton channels in rat alveolar epithelium. J. Gen. Physiol. 109, 415–434. ( 10.1085/jgp.109.4.415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Musset B, Cherny VV, Morgan D, Okamura Y, Ramsey IS, Clapham DE, DeCoursey TE. 2008. Detailed comparison of expressed and native voltage-gated proton channel currents. J. Physiol. 586, 2477–2486. ( 10.1113/jphysiol.2007.149427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gonzalez C, Rebolledo S, Perez ME, Larsson HP. 2013. Molecular mechanism of voltage sensing in voltage-gated proton channels. J. Gen. Physiol. 141, 275–285. ( 10.1085/jgp.201210857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu J, Deutsch C. 2001. Pegylation: a method for assessing topological accessibilities in Kv1.3. Biochemistry 40, 13 288–13 301. ( 10.1021/bi0107647) [DOI] [PubMed] [Google Scholar]
- 180.Sakata S, Kurokawa T, Norholm MH, Takagi M, Okochi Y, von Heijne G, Okamura Y. 2010. Functionality of the voltage-gated proton channel truncated in S4. Proc. Natl Acad. Sci. USA 107, 2313–2318. ( 10.1073/pnas.0911868107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kurokawa T, Okamura Y. 2013. Mapping of sites facing aqueous environment of voltage-gated proton channel at resting state: a study with PEGylation protection. Biochim. Biophys. Acta 1838, 382–387. ( 10.1016/j.bbamem.2013.10.001) [DOI] [PubMed] [Google Scholar]
- 182.Mony L, Berger TK, Isacoff EY. 2015. A specialized molecular motion opens the Hv1 voltage-gated proton channel. Nat. Struct. Mol. Biol. 22, 283–290. ( 10.1038/nsmb.2978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yellen G, Sodickson D, Chen TY, Jurman ME. 1994. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys. J. 66, 1068–1075. ( 10.1016/S0006-3495(94)80888-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Holmgren M, Shin KS, Yellen G. 1998. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron 21, 617–621. ( 10.1016/S0896-6273(00)80571-1) [DOI] [PubMed] [Google Scholar]
- 185.Schrenzel J, Serrander L, Bánfi B, Nüsse O, Fouyouzi R, Lew DP, Demaurex N, Krause KH. 1998. Electron currents generated by the human phagocyte NADPH oxidase. Nature 392, 734–737. ( 10.1038/33725) [DOI] [PubMed] [Google Scholar]
- 186.Geiszt M, Kapus A, Nemet K, Farkas L, Ligeti E. 1997. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes. Alterations in chronic granulomatous disease. J. Biol. Chem. 272, 26 471–26 478. ( 10.1074/jbc.272.42.26471) [DOI] [PubMed] [Google Scholar]
- 187.Jankowski A, Grinstein S. 1999. A noninvasive fluorimetric procedure for measurement of membrane potential. Quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J. Biol. Chem. 274, 26 098–26 104. ( 10.1074/jbc.274.37.26098) [DOI] [PubMed] [Google Scholar]
- 188.Rada BK, Geiszt M, Káldi K, Tímár C, Ligeti E. 2004. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood 104, 2947–2953. ( 10.1182/blood-2004-03-1005) [DOI] [PubMed] [Google Scholar]
- 189.Bánfi B, Schrenzel J, Nüsse O, Lew DP, Ligeti E, Krause KH, Demaurex N. 1999. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J. Exp. Med. 190, 183–194. ( 10.1084/jem.190.2.183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.DeCoursey TE. 2008. Voltage-gated proton channels: what's next? J. Physiol. 586, 5305–5324. ( 10.1113/jphysiol.2008.161703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mitchell P, Moyle J. 1974. The mechanism of proton translocation in reversible proton-translocating adenosine triphosphatases. Biochem. Soc. Spec. Publ. 4, 91–111. ( 10.1042/bst0020463) [DOI] [Google Scholar]
- 192.Villalba-Galea CA. 2014. Hv1 proton channel opening is preceded by a voltage-independent transition. Biophys. J. 107, 1564–1572. ( 10.1016/j.bpj.2014.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cherny VV, Morgan D, Musset B, Chaves G, Smith SME, DeCoursey TE. 2015. Tryptophan 207 is crucial to the unique properties of the human voltage-gated proton channel, hHV1. J. Gen. Physiol. 146, 343–356. ( 10.1085/jgp.201511456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cherny VV, Morgan D, Thomas S, Smith SME, DeCoursey TE. In press. Histidine168 is crucial to ΔpH dependent gating of the human voltage gated proton channel, hHV1. J. Gen. Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lacroix JJ, Hyde HC, Campos FV, Bezanilla F. 2014. Moving gating charges through the gating pore in a Kv channel voltage sensor. Proc. Natl Acad. Sci. USA 111, E1950–E1959. ( 10.1073/pnas.1406161111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.DeCoursey TE. 2015. Structural revelations of the human proton channel. Proc. Natl Acad. Sci. USA 112, 13 430–13 431. ( 10.1073/pnas.1518486112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Catterall WA. 2010. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron 67, 915–928. ( 10.1016/j.neuron.2010.08.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tiwari-Woodruff SK, Lin MA, Schulteis CT, Papazian DM. 2000. Voltage-dependent structural interactions in the Shaker K+ channel. J. Gen. Physiol. 115, 123–138. ( 10.1085/jgp.115.2.123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khalili-Araghi F, Jogini V, Yarov-Yarovoy V, Tajkhorshid E, Roux B, Schulten K. 2010. Calculation of the gating charge for the Kv1.2 voltage-activated potassium channel. Biophys. J. 98, 2189–2198. ( 10.1016/j.bpj.2010.02.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu D, Delaloye K, Zaydman MA, Nekouzadeh A, Rudy Y, Cui J. 2010. State-dependent electrostatic interactions of S4 arginines with E1 in S2 during Kv7.1 activation. J. Gen. Physiol. 135, 595–606. ( 10.1085/jgp.201010408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hong L, Kim IH, Tombola F. 2014. Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc. Natl Acad. Sci. USA 111, 9971–9976. ( 10.1073/pnas.1324012111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.DeCoursey TE, Cherny VV, DeCoursey AG, Xu W, Thomas LL. 2001. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J. Physiol. 535, 767–781. ( 10.1111/j.1469-7793.2001.00767.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Musset B, Cherny VV, Morgan D, DeCoursey TE. 2009. The intimate and mysterious relationship between proton channels and NADPH oxidase. FEBS Lett. 583, 7–12. ( 10.1016/j.febslet.2008.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nanda A, Grinstein S. 1991. Protein kinase C activates an H+ (equivalent) conductance in the plasma membrane of human neutrophils. Proc. Natl Acad. Sci. USA 88, 10 816–10 820. ( 10.1073/pnas.88.23.10816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bankers-Fulbright JL, Kita H, Gleich GJ, O'Grady SM. 2001. Regulation of human eosinophil NADPH oxidase activity: a central role for PKCδ. J. Cell. Physiol. 189, 306–315. ( 10.1002/jcp.10022) [DOI] [PubMed] [Google Scholar]
- 206.Cherny VV, Henderson LM, Xu W, Thomas LL, DeCoursey TE. 2001. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J. Physiol. 535, 783–794. ( 10.1111/j.1469-7793.2001.00783.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.DeCoursey TE, Cherny VV. 1993. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys. J. 65, 1590–1598. ( 10.1016/S0006-3495(93)81198-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kapus A, Romanek R, Grinstein S. 1994. Arachidonic acid stimulates the plasma membrane H+ conductance of macrophages. J. Biol. Chem. 269, 4736–4745. [PubMed] [Google Scholar]
- 209.Henderson LM, Chappell JB. 1992. The NADPH-oxidase-associated H+ channel is opened by arachidonate. Biochem. J. 283, 171–175. ( 10.1042/bj2830171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gordienko DV, Tare M, Parveen S, Fenech CJ, Robinson C, Bolton TB. 1996. Voltage-activated proton current in eosinophils from human blood. J. Physiol. 496, 299–316. ( 10.1113/jphysiol.1996.sp021686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schrenzel J, Lew DP, Krause KH. 1996. Proton currents in human eosinophils. Am. J. Physiol. 271, C1861–C1871. ( 10.1152/ajpcell.1996.271.6.C1861) [DOI] [PubMed] [Google Scholar]
- 212.Kawanabe A, Okamura Y. 2016. Effects of unsaturated fatty acids on the kinetics of voltage-gated proton channels heterologously expressed in cultured cells. J. Physiol. 594, 595–610. ( 10.1113/JP271274) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.