Abstract
Objectives:
To assess infants’ cognitive function at the corrected age of 24-36 months, and to identify factors associated with adverse outcome and examine the correlation between Bayley Infants Neurodevelopmental Screener (BINS) score and Gesell Schedule of Child Development (GSCD).
Methods:
This retrospective study was performed on Saudi very low birth-weight (VLBW) infants born in King Khalid University Hospital, Riyadh, Saudi Arabia between 1997 and 2014 by the use of BINS as screening test and GSCD as definitive test.
Results:
Of 561 enrolled infants, 367 (65.4%) continued to follow-up. Three-hundred and fifteen infants (85.6%) had a normal cognitive function. In addition to lower birth weight (beta = -0.003) (p<0.001), male gender (OR =3.9) (p=0.001)and cerebral palsy (OR =33.9) (p<0.001) were the strongest factors associated with poor cognitive outcome. Approximately 75.4% of infants with normal BINS score had normal cognitive function and 7.6% of total infants had sever cognitive impairment.
Conclusion:
The majority of VLBW infants in our center have normal cognitive function at the corrected age of 24-36 months. Male gender, lower birth weight, and cerebral palsy are major predictors of poor outcome. The BINS scores were correlated with GSCD as a valid predictor for future developmental outcome.
The survival rate of very low birth weight (VLBW) infants has dramatically improved over recent decades due to progressive advances in perinatal care. The long-term developmental performance of surviving VLBW infants, as well as their physical growth, has been significantly improved by the use of standardized long-term follow-up tools, which are mainly implemented by specialized early intervention programs. These programs aim to provide proper medical, psycho-social, and developmental monitoring, and support for these infants and their families.1
Premature delivery carries a great risk on newborn infants. Besides the increased mortality rate and increased incidence of morbidities, prematurity is a major risk factor for future neuro developmental delay. Rates of cognitive dysfunction, cerebral palsy, deafness, and blindness are higher in preterm infants. Cognitive impairment had been reported in about 40% of VLBW infants at school age. In comparison with term infants, VLBW infants are more likely to have lower scores in executive functions and suboptimal attentive skills. Language development is also adversely affected in VLBW infants because of the increased risk of impaired receptive and expressive functions which are the major components of language development as well as fine motor and adaptive functions which might be affected as well.2,3 Male gender, lower birth-weight, black race, lower education level of parents, and lower gestational age have been shown to be predictive of global cognitive dysfunction among young children.4 In this study, we investigated the cognitive function outcome at the corrected age of 24-36 months of VLBW Saudi infants, as well as long-term outcomes, such as CP, deafness, and blindness. We also attempted to identify those factors having the greatest impact on the adverse outcome of these patients, and finally, examine predictive value of Bayley Infants Neurodevelopmental Screener (BINS) compared to Gesell Schedule of Child Development (GSCD).
Methods
This retrospective study was performed on all VLBW infants delivered at King Khalid University Hospital (KKUH) in Riyadh, Saudi Arabia, from 1997 to 2014, with a birth weight of 1000-1500 grams. Study was approved by institute review board committee.
Pubmed and google search were used to find prior related researches.
Patients’ data were prospectively collected through the neonatal follow-up program (NFP) of KKUH, which enrolls all at high-risk infants born at KKUH and graduated from the neonatal intensive care unit. Baseline demographic data include: gender, gestational age, birth weight; presence of interventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), sepsis, blindness, deafness, cerebral palsy (CP);, and cognitive score at corrected age of 24 to 36 months by the use of the GSCD. Blindness and deafness were diagnosed by clinical evaluation and brainstem evoked potentials. Throughout follow-up with our NFP, all infants underwent periodic neurodevelopmental evaluation screening by the use of the BINS, which is a brief neurodevelopmental screening test that takes around 10 minutes to perform. It is derived from items in Bayley Scales of Infant Development second edition (BSID II), the score is interpreted as: low risk for future neurodevelopmental delay (normal), moderate risk for future neurodevelopmental delay, and high risk for future neurodevelopmental delay.5 We reported the last collected BINS score, which was performed at the corrected age of 21-24 months. Outborn infants and newborns with congenital syndromes or neurological defects were excluded from the initial recruitment process. All initially illegible infants were included in the analysis for demographic data, comorbidities, deafness, blindness, and CP. For the final analysis, we also excluded those infants who were not evaluated by the GSCDat the corrected age of 24 to 36 months. Scores of ≥85 were considered indicative of normal cognitive function, scores of 71 to 84 were considered indicative of mild to moderate developmental delay, and scores of ≤70 were considered indicative of severe developmental delay or cognitive dysfunction. We divided patients into 2 groups: normal outcome (score ≥85), and abnormal outcome (score <85).
Statistical analysis
Preliminary analyses, including cross-tabulations with Pearson’s chi-square, were computed to assess the simple (bivariate) variate relationships between a given comorbidity and poor cognitive functioning. Independent sample t-tests were used to test for significant differences in continuous demographic variables and cognitive abilities (classified as normal versus abnormal). To assess the complex interaction, primary analyses included a logistic regression that is predicting abnormal cognitive functioning, defined as a cognitive score of <85, with significant predictors. To create the most parsimonious prediction model, multiple iterations were conducted, and only significant predictors were retained in the final model. Lastly, to assess the relationship between BINS scores and cognitive functioning, cross tabulations with Pearson’s chi-square test were conducted. All analyses were conducted in IBM SPSS 25 (IBM, Armonk, NY, USA), and significance was determined at the 0.05 level.
Results
The full sample of infants recruited for this study consisted of 561 infants; however, of these, only 367 (65.4%) completed sufficient follow-up assessment to include primary outcomes. Of the 194 (34.6%) that were lost during follow-up, the average age of the last assessment was 14.57 ± 5.93 months. Comparison of baseline available data between those who completed the study and those who dropped out revealed no significant differences between these groups (all p>0.05). A summary of the demographics of the final sample is given in Tables 1 & 2. Three-hundred and fifteen infants (85.6%) had a normal cognitive function at the corrected age of 24-36 months, and 52 infants (14.2%) had an abnormal cognitive function (6.5% mild-moderate delay, and 7.6% severe cognitive dysfunction). We found that male infants and infants with following comorbidities had a higher proportion of cognitive impairment: IVH, PVL, BPD, sepsis, being blind, being deaf, and CP. Those with normative cognitive ability also had higher gestational age and birth weight.
Table 1.
Categorical demographic variables.
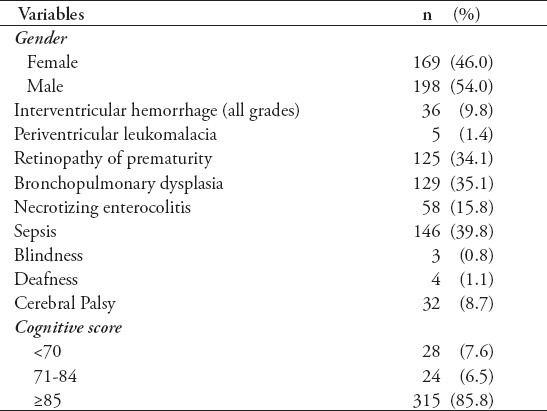
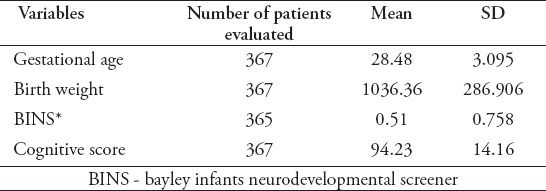
Table 2.
Descriptive of categorical demographics.
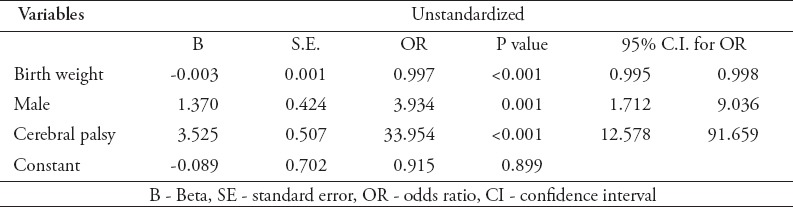
A summary of the final logistic regression is given in Table 3. The overall regression model was significant, x2 (3) = 98.38, p<0.001, Nagelkerke R2 = 0.422. When controlling for all of the significant variables indicated above, only gender, CP, and birth weight were significant predictors of abnormal cognitive functioning. Being male was associated with an almost 4-fold (OR = 3.934) increase in likelihood to having abnormal cognitive functioning. Higher birth weight was associated with lower odds of abnormal cognitive functioning (OR =0.997). Infants who had CP were more than 30-times more likely to have an abnormal cognitive functioning (OR =33.954).
Table 3.
Summary of logistic regression predicting abnormal cognitive functioning.
Lastly, the association between BINS score and cognitive functioning was assessed (Table 4). As shown, a greater proportion of infants with a low BINS score had normative cognitive functioning (75.4%) compared to those with abnormal cognitive abilities (1.9%); conversely, infants with high BINS scores had a larger proportion of abnormal cognitive functioning (82.7%) compared to normative infants (5.1%), x2 (2) = 203.51, p<0.001, Cramer’s V = 0.747.
Table 4.
Associations between Bayley infants neurodevelopmental screener (BINS) scores and cognitive ability.

Discussion
The present study provides information on the neurodevelopmental outcome in a cohort of Saudi VLBW preterm infants born in a tertiary center with the use of a standard developmental test (GSCD). Although the GSCDis an old tool for developmental testing, it nevertheless provides valuable information needed to assess the cognitive function of children born prematurely with VLBW. As seen in this study, all 561 infants who were initially recruited had full demographic data with all morbidities, which is a good source to document incidences of common morbidities known to be peculiar to VLBW infants. The incidences of CP, deafness, and blindness were comparable to international reports, 315 (85.8%) infants had normal cognitive function, and 28 (7.6%) infants had severe cognitive impairment (score <70) at the corrected age of 24-36 months, which is in line with international reports6 (Table 1). We reported higher rates of sepsis, NEC, and BPD, but within internationally reported rates for IVH (all grades), PVL, and ROP (all stages).7 All of the 194 infants (34.6%) who were not evaluated by the GSCDat the corrected age of 24-36 months and, thus, were excluded from the final outcome of the study, were initially evaluated by the BINS at an average age of 14.7 months. This means that all of these excluded infants were initially screened by BINS and had 2-3 evaluations, but did not continue to cognitive evaluation. Review of files and contact with parents of infants who did not continue follow-up revealed the following reasons for droping from the program: parents decision not to continue because their children scored as normal in the BINS evaluation, some families coming from remote areas had difficulty travelling, and some infants died after discharge from the hospital. As published in the literature, we found that bigger infants were less likely to have cognitive impairment, being male was associated with an almost 4-fold greater risk of developing cognitive dysfunction compared to being female. Cerebral palsy had the strongest association with cognitive impairment; infants with CP were almost 34-times more likely be cognitively impaired than infants without CP. In Table 4, in the normal BINS group, 236 infants had normal cognitive function, and only one infant (1 of 237) had an abnormal cognitive function; thus, the probability of having impaired cognitive function with normal BINS score is as low as 0.4%. Infants with an abnormal BINS result (moderate or high-risk at a corrected age of 21-24 months) are more likely to have cognitive impairment at 24-36 months. Of those infants who had a moderate risk for future neurodevelopmental delay by BINS (61 infants), 8 had cognitive impairment; thus, the probability of being developmentally impaired with moderate BINS score is 13.1% whereas the probability of being developmentally impaired with a high-risk BINS score was 73% (43 of 59 infants). Bayley Infants Neurodevelopmental Screener was found to be a good predictor of future neurodevelopmental status in early childhood, which makes it a suitable early intervention tool. Our results were consistent with studies carried out to test the validity of BINS compared to different standard definitive cognitive tests,8-11 however, our study is one of only a few that have tested BINS compared to GSCD.
Study limitations
Limited by being retrospective, and having only around 66% of total infants continuing follow-up until the corrected age of 24-36 months. This study indicates that long term outcome evaluation is mandatory for VLBW infants which should be performed by the use of standard tools for developmental evaluation which is suboptimal in most of Saudi tertiary centers despite of increasing numbers of surviving tiny premature infants.
In conclusion, we found that the majority of VLBW infants (85.6%) had a normal cognitive function at the corrected age of 24-36 months. Cerebral palsy had the strongest association with cognitive impairment. Being male, lower gestational age, and lower birth weight carry a higher risk for future cognitive impairment than other factors. There was the high predictive value of BINS for the future developmental outcome, suggesting that BINS can be used as a valid neurodevelopmental screening tool. We recommend that all high-risk newborn infants should be evaluated by standard developmental tools through the proper early intervention program to detect any deviation from normal development and, hence, start early intervention. We strongly encourage the implementation of NFP, which is the standard program to monitor and evaluate newborns at risk of future neurodevelopmental delay in Saudi Arabia.6 More studies need to be carried out in deferent tertiary centers in Saudi Arabia in order to have a wider national knowledge about the long-term outcome of Saudi VLBW infants.
Acknowledgment
Many thanks to our neonatal follow-up program team, Dr. Amull Faris and Dr. Rozina Banoo for their efforts in data collection and keeping our database updated, Biostatistician John Maddoux for statistical analysis, Dr. Khalid Tirkawi for his support in reviewing the article, and American Manuscript Editors for editing this article.
Footnotes
Authorship entitlement.
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
The international Committee of Medical Journal Editors has recommended the following criteria for authorship; these criteria are still appropriate for those journals that distinguish authors from other contributors.
Authorship credit should be based on 1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) intellectual content; and 3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.
Acquisition of funding, collection of data, or general supervision of the research group, alone, does not justify authorship.
An author should be prepared to explain the order in which authors are listed.
References
- 1.Ballot DE, Potterton J, Chirwa T, Hiiburn N, Cooper PA. Developmental outcome of very low birth weight infants in a developing country. BMC Pediatr. 2012;12:11. doi: 10.1186/1471-2431-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol. 2015;68:134–143. doi: 10.1016/j.jclinepi.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 4.Error!Hyperlink reference not valid.$$$ [Google Scholar]
- 5.Gucuyener K, Ergenekon E, Soysal AS, Aktas A, Derinis O, Koc E, et al. Use of the bayley infant neurodevelopmental screener with premature infants. Brain Dev. 2006;28:104–108. doi: 10.1016/j.braindev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Sobaih B. Neonatal follow-up program: where do we stand. Sudan J Paediatr. 2012;12:21–26. [PMC free article] [PubMed] [Google Scholar]
- 7.John M. Survival and long-term neurodevelopmental outcome of the extremely preterm infant. A Systemic review. Saudi Med J. 2011;32:885–894. [PubMed] [Google Scholar]
- 8.Ann Marie M, George L, Sheila B, Glen P, Eduardo E, Lorette CJ, Norman G, et al. Application of Neurodevelopmental Screening to a Sample of South American Infants: The Bayley Infant Neurodevelopmental Screener (BINS) Infant Behav Dev. 2012;35:280–294. doi: 10.1016/j.infbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guedes DZ, Primi R, Kopelman BI. BINS validation –Bayley neurodevelopmental screener in Brazilian preterm children under risk conditions. Infant Behavior and Development. 2011;34:126–135. doi: 10.1016/j.infbeh.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Naila Z, Humaira M, Dilara B, Asma B, Selina A, Khaleda B, et al. Validation of rapid neurodevelopmental assessment instrument for under-two-year-old children in Bangladesh. Pediatrics. 2010;125:e755–762. doi: 10.1542/peds.2008-3471. [DOI] [PubMed] [Google Scholar]
- 11.Soysal AS, Gucuyener K, Ergenekon E, Turan O, Koc E, Turkylmaz C, et al. The prediction of later neurodevelopmental status of preterm infants at ages 7 to 10 years using the Bayley Infant Neurodevelopmental Screener. J Child Neurol. 2014;29:1349–1355. doi: 10.1177/0883073813520495. [DOI] [PubMed] [Google Scholar]


