Abstract
Recent clinical studies suggest that operational allograft tolerance can be persistent, but long-term surviving allografts may be lost in a subset of patients, sometimes after episodes of infection. In this study, we examined the long-term impact of Listeria monocytogenes (Lm) infection on the quality of tolerance in a mouse model of heart allograft tolerance. Lm infection induced full rejection in 40% of tolerant recipients, with the remaining experiencing a rejection crisis or no palpable change in their allografts. In the surviving allografts on day 8 post-infection, graft-infiltrating cell numbers increased and exhibited a loss in the tolerance gene signature. By day 30 post-infection, the tolerance signature was broadly restored, but with a discernable reduction in the expression of a subset of 234 genes that marked tolerance and was down-regulated at day 8 post-Lm infection. We further demonstrated that the tolerant state after Lm infection was functionally eroded, as rejection of the long-term surviving graft was induced with anti-PD-L1 whereas the same treatment had no effect in non-infected tolerant mice. Collectively, these observations demonstrate that tolerance, even if initially robust, exists as a continuum that can be eroded following by-stander immune responses that accompany certain infections.
INTRODUCTION
Conventional immunosuppression has almost eliminated the early loss of organ allografts to T cell-mediated rejection but chronic rejection remains an unresolved problem, and the need for life-long immunosuppression can cause morbidity and mortality. These limitations have prompted investigators to posit immunological tolerance as a means of achieving long-term allograft survival without the side effects of immunosuppression(1). Successful clinical tolerance can be divided into those that achieve tolerance through hematopoietic stem cell transplantation(2, 3) or when tolerance is revealed after the weaning of conventional immunosuppression(4–6). While the mechanisms by which tolerance is attained by these approaches may be distinct, a common observation is that tolerance in a subset of patients is not stable and allografts can be lost after years of stable function. In the long-term follow-up of patients of combined HLA-haploidentical kidney and bone marrow transplantation, 8 of 10 patients were successfully weaned of immunosuppression(7). However, 2 of the 8 patients were diagnosed with chronic rejection at 5 and 7 years post-transplantation, and 1 patient succumbed to acute cellular rejection 3 weeks after acute pyelonephritis. In a European cohort of 27 kidney transplant recipients that had achieved operational tolerance, 8 lost their grafts after a median of 9 years. Six of the 8 had pre-transplant anti-HLA antibodies and 4 had evidence of active immunologically-driven mechanisms of rejection(6). Furthermore, 4 bacterial and 2 viral infections after immunosuppression withdrawal were recorded for the 8 who lost their grafts, whereas only 2 bacterial and 1 viral infections were noted for the 19 patients with stable operational tolerance. These studies suggest that operational tolerance can be persistent, but may eventually be lost in the setting of pre-transplant donor-specific alloantibody and, potentially, after infections. Currently, there is limited understanding on whether a declining graft function, is due to a loss of immunological tolerance.
Experimental models of tolerance focusing on barriers that prevent tolerance induction have implicated pre-transplant donor-specific antibodies, heterologous and donor-specific memory T cells, and pro-inflammatory cytokines produced in response to infections(8–15). However, many of these agents that prevent the induction of tolerance cannot induce a graft loss after tolerance has been stably established, suggesting that mechanisms maintaining tolerance are robust and capable of containing the pro-rejection signals that are sufficient to prevent tolerance induction. Nevertheless, established tolerance can be overcome if the pro-inflammatory signals are sufficiently potent, and we have previously reported that Lm infection precipitated graft rejection, although in only a subset of tolerant recipients (10–13, 16, 17).
Experimental graft rejection is defined as a complete cessation of heartbeat whereas clinical rejection is defined by pathology long before the graft fully stops functioning. Therefore in this study, we focused on C57BL/6 recipients that exhibited a rejection crisis immediately after Lm infection, as determined by graft enlargement on palpation and a slowing down of the heartbeat but that did not proceed to complete heartbeat cessation, and tested whether the quality of tolerance was eroded after the infection was cleared.
MATERIALS AND METHODS
Mouse transplantation, infection and antibody treatment
C57BL/6 (B6, H-2b) mice as recipients and BALB/c (B/c, H-2d) as donors were purchased from Harlan Laboratories. Mouse abdominal heterotopic cardiac transplantation was performed as previously described(13) and tolerance was induced with anti-CD154 (BioXCell; 0.3–0.5mg/mouse, i.v. on d0, and i.p. on d7, and d14 post-transplantation) and 107 B/c splenocytes (d0). Cardiac grafts were monitored by palpation and the heartbeat speed was graded as normal (too fast to count), slow, or absent. Infection with 3–5 ×106 CFU (i.p.) Lm expressing the Ovalbumin230–359 epitope (Lm-OVA), and enumeration of CFU was quantified by the absorbance at OD600 as previously described(13). Foxp3+CD4+ T cells were depleted with a single i.v. dose of anti-CD25 (PC61 (BioXCell), 0.4 mg/mouse i.v.). Some recipients received anti-PD-L1 (9H10 (BioXCell), 0.5 mg/mouse, then 0.25 mg/mouse q.o.d. for 10 days).
Isolation of graft-infiltrating cells
Hearts from transplanted animals were perfused with sterile HBSS with 1% heparin, cut into small fragments and placed in digestion buffer (HBSS, 0.1% DNAse I (MP Biomedicals), 400 U/mL Collagenase IV (Sigma), 50mM HEPES) for 20 minutes at 37°C. Digested suspensions were collected following 44.5% Nycodenz (Sigma) centrifugation, and cells from the interface stained and analyzed by flow cytometry.
Flow cytometry analysis
Peripheral blood leukocytes, splenocytes, and total graft infiltrating cells (GICs) were stained using monoclonal antibodies specific for mouse CD16/32, CD45.2, CD4, CD8, CD44, PD-1, and Foxp3 (BD Bioscience or BioLegend). Cell viability was determined using Viability Dye (for fixed samples) or DAPI (for unfixed samples) (Invitrogen), and data were analyzed using FlowJo software.
Immunohistochemistry
Grafts were removed and placed in 10% formalin. Sections were stained with haematoxylin and eosin, and the slides were scanned via Cri Pannoramic Scan Whole Slide Scanner at X40, and then patched together to form one single image of the tissue. Pannoramic Viewer (3DHISTECH) was used to view these image files and examined in a blinded manner. Histological sections were scored based on the percentage of infiltration observed in the interstitial tissue and as follows: 0%=0, 1–25% =1, 25–50%=2, 50–75%=3 and 75–100%=4.
Microarray Sample Preparation
RNA from flow-sorted CD45+ cells was extracted using a Qiagen Rneasy Micro Kit, and converted to cDNA using the Ovation Pico/PicoSL WTA Systems enhanced kit (NuGEN). Total RNA per sample varied from between 39–143ng. Samples were analyzed using the Illumina WG-6 gene expression array and normalized for background.
Microarray Analysis
A principal component analysis was performed on the full microarray data using the Partek Genomics Suite (Partek Inco., St. Louis, MO). Differentially expressed genes in the syngeneic (Syn), acute rejecting (AR), stable tolerance (Tol), loss of tolerance after Lm (Lm D8) and restored tolerance (Lm D30) were subject to K-means cluster analysis. Only genes with an average expression of at least double the threshold of detection were analyzed. Significant differential expression is defined as when one group is at least 2-fold higher than at least one other group, taking into account the average standard deviation of the groups (Group1-Average StDev>2*Group2). This resulted in a gene set of 3246 genes. Individual gene expression was then normalized to the median expressing group. The MultiExperiment Viewer (MeV) software was used to perform a Figure of Merit analysis, followed by a 23-cluster K-means cluster analysis. Gene ontology analysis was performed using the Gene Set Enrichment Analysis tool (GSEA; http://www.broadinstitute.org/gsea).
Statistical Methods
The two-tailed Student’s t test, and ANOVA or Kruskal-Wallis tests for multiple comparisons were performed to determine statistical differences between groups (Prism5; GraphPad Software Inc.). Graft mean survival time (MST) and p-values were calculated using the Kaplan-Meier or log rank test (Prism5).
RESULTS
Lm infection of tolerant mice leads to three distinct outcomes
Tolerance was induced in C57BL/6 recipients to BALB/c heart grafts by treatment with anti-CD154 and DST (Figure 1A). Infection with a sub-lethal dose of Lm resulted in the complete rejection of approximately 40% of allografts, where the heartbeat stopped within 14 days post-infection and did not recover. An additional 30% of the allografts underwent a rejection crisis, where the heartbeat slowed down and the heart enlarged at 7–14 days post-infection, while Lm did not detectably impact the remaining 30% of allografts (Figure 1B). In both sets of recipients, the grafts continued to beat for ≥ 60 days post-infection. In this study, we focused on the allografts that were not fully rejected on day 8. Histological examination of these grafts on day 8 post-infection revealed significant cellular infiltration, and the infiltrate remained persistent up to 60 days post-infection despite continued allograft survival (Figure 1C&D).
Figure 1.
Three distinct BALB/c heart graft outcomes following acute Listeria infection of tolerant C57BL/6 recipients at ≥day 60 post-transplantation. (A) Experimental design. (B) Listeria (3–5×106 CFU) infection of tolerant recipients resulted acute graft rejection, a rejection crisis or no significant change in the heartbeat (N=55). (C) Histology of syngeneic (Syn) grafts, tolerant (Tol) BALB/c heart grafts at day >80 post-transplantation, prior to and at 8 days post-Listeria (Lm) infection, and (D) composite histological scores. Each data point represents a single allograft, data are presented as Mean Scores ± standard error of mean, and statistical significance relative to tolerant grafts are indicated as p≤ *0.05 and **0.01.
Gene signature of graft infiltrating cells at tolerance
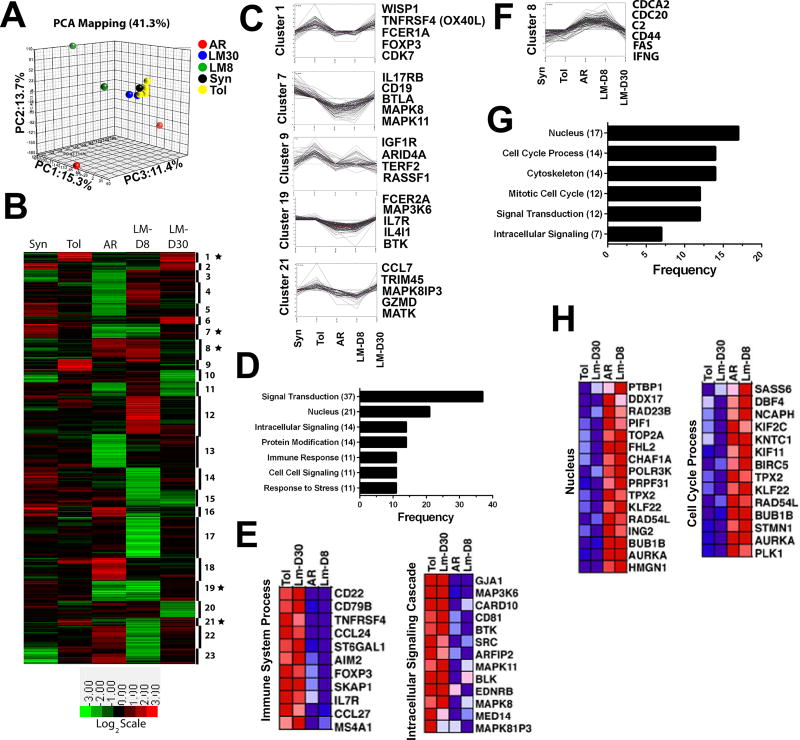
To test whether the quality of the cellular infiltrate at day 8 post-Lm was distinct from that in the allograft at day 30 post-Listeria infection, we performed microarray analyses as previously described(18) on total CD45+ GICs isolated from tolerant grafts and from grafts at day 8 and day 30 post-Listeria infection (Figure 2). Principal component analysis (Figure 2A) and K-means clustering identified a gene signature from tolerant grafts that was distinct from acute rejection (Figure 2B; Cluster 1, 7, 8, 9, 19 and 21), and which was enriched in tolerance for genes involved in cell signaling, protein modification, response to stress and immune response (Figure 2C-E) or enriched in rejection for genes involved in cell cycle, mitosis, and signaling (Figure 2F-H). Notably, genes enriched in the cells infiltrating tolerant grafts included Treg-associated gene (FoxP3), B cell-associated genes (MS4A1(CD20), CD22, CD79B(Igß), BTK, BLK, CD81), chemokine/cytokine/migration genes (CCL24 (MPIF-2/eotaxin-2), IL7R, CCL27(IL-11R-alpha-locus chemokine (ILC)/Skinkine/ESkine/cutaneous T-cell-attracting chemokine (CTACK)), SKAP1 (src kinase-associated phosphoprotein 1 adapter protein) and ST6Gal1 (beta-galactoside alpha-2,6-sialyltransferase 1), costimulatory molecules (TNFRSF4/CD134/4-1BB), and genes in the MAPK signaling cascade (DUSP10; MAP3K6, MAPK8 and MAPK81P3). Some of these genes were upregulated in GIC from tolerant relative to syngeneic grafts, consistent with tolerance being an actively maintained state (Figure 2B; Cluster 1 & 21).
Figure 2.
Microarray analysis of graft infiltrating cells from tolerant and rejecting heart grafts is consistent with a restoration of tolerance at day 30 post-Listeria infection. RNA was isolated from pooled CD45+ graft-infiltrating cells syngeneic (Syn; 0.6 × 104/graft; 5 grafts pooled, n=1), tolerant (Tol; 2.5 × 104/graft; 10 grafts pooled, n=4), acutely rejecting (AR; 11.5 × 104/graft; 4 grafts pooled, n=2), Listeria-infected tolerance d8 (Lm-D8; 6.2 × 104/graft; 4 grafts, n=3), and restored tolerance (Lm-D30; 5.3 × 104/graft; 4 pooled, n=2) mice and microarray analysis was performed. (A) Principal component analysis (PCA) revealed 41.3% of the total variation within the first 3 components. (B) Genes with significant differential expression were analyzed using K-means clustering in 23 clusters. Gene expression is median-centered at the probe level; higher expression is shown in red. The star symbol indicates the clusters associated with tolerance or rejection. (C-E) Genes of interest are shown from clusters, frequency of genes associated with selected Gene Ontology terms and heat map of gene expression wherein expression of genes associated with tolerance (i.e., higher in tolerant and Lm-D30 CD45+ GIC) were enriched compared to acutely rejecting and Lm-D8 mice. (F-H) Genes of interest are shown from clusters, frequency of genes associated with selected Gene Ontology terms and heat map of gene expression wherein expression of genes associated with acute rejection or loss of tolerance (Lm-D8) were distinct from tolerant and restored tolerance (Lm-D30).
Loss of tolerance on day 8 post-infection was associated with down-regulated expression of tolerance-specific genes (Figure 2B; Cluster 1, 7, 9, 19 & 21) and upregulated expression of genes associated with rejection (Figure 2B; Cluster 8). These included genes controlling cell cycle and proliferation, cytoskeleton and intracellular signaling, as well as markers associated with activated or effector T cells including CD44, Fas and IFNγ. Finally, there were considerable differences between the loss-of-tolerance gene signature at day 8 post-infection and the unmodified acute rejection-associated genes (Figure 2A, 2B; Cluster 3, 4, 12, 16, 18, 22, 23).
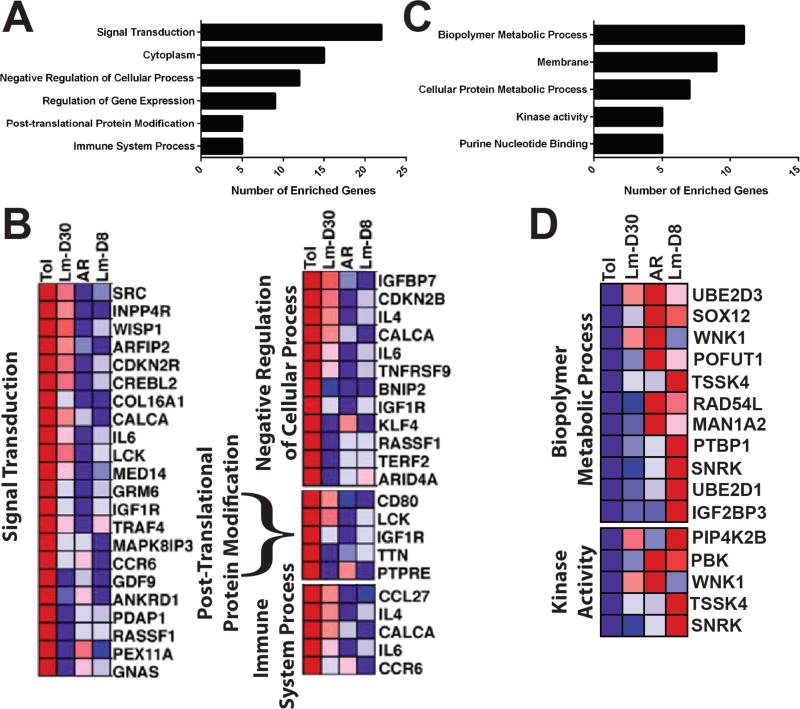
By day 30 post-infection, the gene signature of the CD45+ GICs became more similar to the tolerance signature prior to infection (Figure 2). These observations are consistent with the functional restoration of tolerance, and the acceptance of a donor-matched allograft transplanted without additional immunosuppression, at 7–35 days after the rejection of the first(17). Nevertheless, a closer examination of the gene signature of the GICs at day 30 post-infection of tolerant mice revealed incomplete restoration of the full tolerance gene signature at day 30 post-infection, with the expression levels of 234 genes that were highly expressed in tolerance, down-regulated in rejection (AR or Tol+LmD8) and only partially restored by d30 post-infection (Figure 3A-B). These genes, which included TNFRSF9 (CD137/4-1BB), IL6, IL-4, CCR6, CCL27, KLF4, clustered into signal transduction, post-translational protein modification, negative regulation of cellular processes and immune system process pathways. Additionally, 100 genes which were at least 2-fold higher in AR and Lm-D8 compared to Tol, remained at least 50% higher in the Lm-D30 group, further supporting the notion of an incomplete return to the tolerance signature after rejection (Figure 3C-D). While the gene changes observed likely reflected alterations in cell composition within the graft as well as induced changes within specific cell subsets, they nevertheless provide insights into the complexity of processes involved in the maintenance of quiescence within a tolerant allograft, how some of these processes are altered by infections to result in rejection, and the extent to which they are spontaneously but imperfectly restored with the resolution of infection.
Figure 3.
Microarray analysis of graft infiltrating cells suggests a partial retention of a signature of rejection at day 30 post-Listeria infection. (A-B) Frequency of genes associated with selected Gene Ontology terms and heat map of gene expression derived from the full set of 234 differentially-expressed genes wherein the expression of tolerant genes (Tol) were at least 2-fold higher than in rejection (acute rejection (AR) or loss of tolerance at day 8 post-Listeria infection (Lm-D8)) but ≤ 50% restored at day 30 post-Lm infection (Lm-D30). (C-D) Frequency of genes associated with selected Gene Ontology terms and heat map of gene expression derived from the full set of 100 differentially-expressed genes wherein the expression of tolerant genes was at least 2 fold higher in the AR and Lm-D8 groups as compared to Tol, and was at least 50% higher in the Lm-D30 group than Tol.
Tolerance after Lm infection is maintained despite deletion of CD25+ Tregs
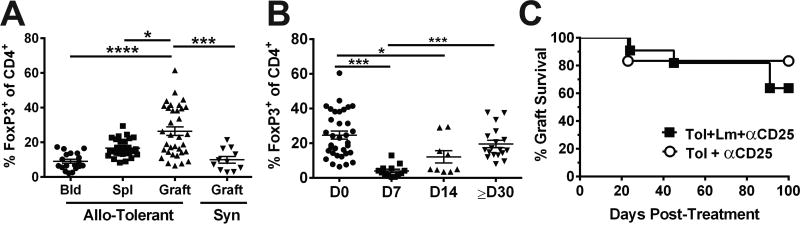
Listeria infection induces a transient increase in circulating IL-6 and IFNß, the combination of these cytokines was sufficient to abrogate established tolerance and the spontaneous recovery of tolerance was facilitated by the return of these cytokines to baseline and by restoration of increased percentages of Tregs within the graft (16, 17). Here we confirm that the frequency of FoxP3+ Tregs in was enriched in the graft, relative to the blood and spleen of tolerant mice, and also relative to syngeneic grafts (Figure 4A; Figure S1 for gating strategy). When grafts were examined on day 8 after infection, the frequency of Tregs was significantly reduced compared to pre-infection levels (Figure 4B), but by day ≥30 post-infection, the percentage of Tregs in the grafts significantly increased to pre-infection levels (Figure 4B). Despite the restoration of Treg frequencies, treatment with anti-CD25 administered on day 14 post-infection was not able to precipitate rejection in recipients that had undergone a rejection crisis. Thus, anti-CD25 treatment was not able to demonstrate a functional erosion of tolerance in this model, in contrast to the ability of the same anti-CD25 treatment to prevent the acceptance of a second BALB/c heart graft transplanted into mice that had fully rejected their first graft after Lm infection(17). The different outcomes with anti-CD25 may be explained by the experimental model, whereby Tregs migrating into the second graft are more susceptible to anti-CD25 depletion compared to Tregs resident within the allograft. Alternatively, Tregs play a less critical role in curtailing rejection in these surviving allografts, and other mechanisms are more essential.
Figure 4.
The survival of tolerant BALB/c allografts in Listeria-infected C57BL/6 recipients is not affected by anti-CD25 treatment. (A) Enriched percentage of Foxp3+ of CD4+ T cells in the tolerant allograft, compared to blood (Bld) and spleen (Spl) in tolerant mice and syngeneic (Syn) grafts at ≥ 60 days post-transplantation. (B) Percentage of Foxp3+ of CD4+ T cells in the tolerant allografts, prior to (D0) and 7, 14 and ≥30 days post-Listeria infection. Each symbol represents a single animal (N=≥7/group), data are presented as mean ± standard error, and statistical significance at p≤ *0.05, **0.01, ***0.001 and ****0.0005. Detailed gating strategy for graft infiltrating CD4+FoxP3+ and CD8+ cells is illustrated in Figure S1. (C) Uninfected (N=6) or day 14 post-infection recipients (N=11) with non-rejected hearts were treated with a single i.v. dose of anti-CD25 (PC61; 400 µg/mouse).
Tolerance after Lm infection is abrogated with anti-PD-L1
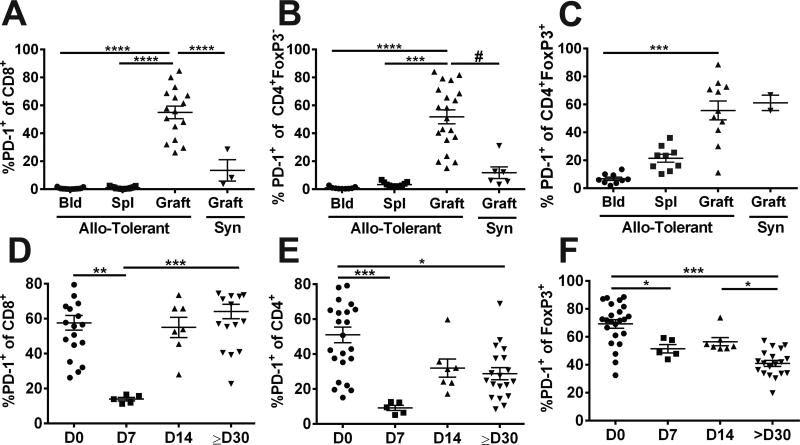
We recently reported that maintenance of tolerance to heart allografts induced with anti-CD154/DST is dependent on multiple mechanisms, and that the reversal of established tolerance required the adoptive transfer of alloreactive T cells in combination with treatment with anti-CD25 and anti-PD-L1(19). We next tested whether PD-1:PDL1 interactions might be restraining the graft-infiltrating cells from driving full rejection of the allografts. Indeed, we found higher frequencies of PD-1-expressing CD8+, and CD4+FoxP3− cells infiltrating the allograft compared to those in the blood or spleen of tolerant recipients, and also relative to syngeneic grafts (Figure 5A&B; Figure S2 for gating strategy). The frequency of PD-1+CD8+ T cells was significantly reduced at day 7 post-infection, and rapidly restored by day 14 (Figure 5D). A similar change in frequency of PD-1-expressing CD4+ FoxP3− cells was observed after Lm infection, although the recovery at ≥30 days post-infection was only partial (Figure 5E). Additionally, the vast majority of Tregs in the allograft were PD-1+ compared to the blood of tolerant recipients and to syngeneic allografts. However, the frequency of PD-1+ Tregs, which was significantly reduced by day 8 post-infection, remained reduced on day 14 and 30 post-infection (Figure 5C&F).
Figure 5.
Listeria infection of tolerant mice results in transient reduction in the percentages of PD1-expressing CD8+ and CD4+Foxp3− cells in the allograft. (A-C) Percentage PD-1+ of CD8+, CD4+Foxp3− and CD4+Foxp3+ cells in the blood, spleen and infiltrating BALB/c allografts in tolerant C57BL/6 mice, or syngeneic grafts at ≥ 60 days post-transplantation. (D-F) Percentages of PD-1+ of CD8+CD4+Foxp3+CD4+Foxp3− and CD4+Foxp3+ cells in the tolerant allografts, prior to (D0) and 7, 14 and ≥30 days post-Listeria infection. Each symbol represents a single animal, data are presented as mean ± standard error, and statistical significance at p≤ *0.05, **0.01, ***0.001 and ****0.0005 by Kruskal-Wallis multiple comparison test, and p≤#0.05 by pair-comparison. Detailed gating strategy for PD-1 expression by each of the T cell subset is illustrated in Figure S2.
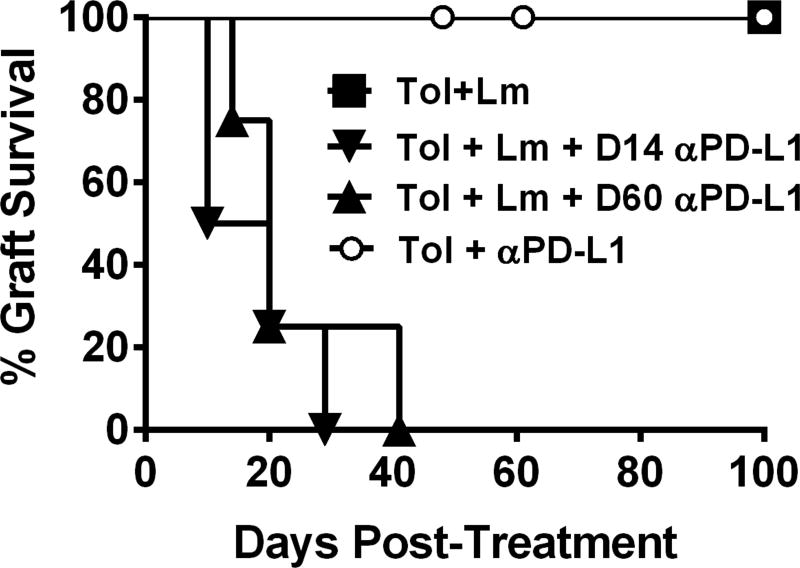
To test whether PD-1:PD-L1 interactions played a role in restraining rejection, tolerant recipients infected with Lm and with surviving heart allografts on day 14 post-Lm infection were treated with anti-PD-L1 (Figure 6). All the grafts in recipients receiving anti-PD-L1 proceeded to fully reject their allograft within 10–40 days, whereas those that did not receive anti-PD-L1 kept maintained a palpable heartbeat long-term. To further test whether susceptibility to anti-PD-L1 was due to unresolved effects of Lm infection, we waited till day 60 post-infection before treating with anti-PD-L1. Again the allografts were rejected, revealing a persistently critical role for PD-L1 in the maintenance of tolerance after recovery from Lm infection and sustained erosion of tolerance. Finally, anti-PD-L1 had no significant effect in tolerant mice that had not been infected with Lm; a finding that suggested a functional erosion in the quality of tolerance only in mice that had undergone a rejection crisis post-Listeria infection.
Figure 6.
Anti-PD-L1 induces allograft rejection in recipients with surviving allografts after Listeria-infection. Uninfected (N=6) and day 14 (N=4) or day 60 post-infection recipients (N=4) with non-rejected hearts were treated were treated with anti-PD-L1 (9H10, 0.5 µg/mouse, then 0.25 µg/mouse, q.o.d. for 10 days).
Discussion
Improvements in conventional immunosuppression have led to better short-term graft outcomes but have not resulted in significantly improved long term-outcomes. The induction of transplantation tolerance has been touted as a means of achieving life-long graft acceptance without the need for continuous immunosuppression. Recent data of long-term follow-up of kidney and liver patients weaned off immunosuppression suggest that a subset of operationally tolerant patients may lose their grafts through potentially immunologically/inflammation-driven processes(6),(20–22). Even in liver transplantation, which is considered more susceptible to transplantation tolerance(23), one of 23 operationally tolerant patients experienced a moderate rejection episode at 5.3 years off immunosuppression(22), and operationally tolerant liver recipients exhibit more fibrosis in the liver allografts that is reduced upon the reintroduction of immunosuppression(20, 21). The cause of acute rejection, low-grade inflammation or indolently progressive fibrosis in operational tolerance is currently not known, and the low numbers of tolerant patients limit mechanistic studies.
In a mouse model of heart allograft tolerance, we previously showed that stable tolerance can be abrogated with Lm infection in a subset of tolerant recipients(16), but remarkably, tolerance spontaneously returned after infection was cleared(17). In this study we focused on the tolerant recipients where Lm infection did not induce full allograft rejection. To gain molecular insights into the dynamic nature of the tolerance and the impact of infection, we performed microarray analyses as previously described(18) on total CD45+GICs isolated from allografts, with the reasoning that these cells would provide an enriched and unbiased signal for immunological tolerance and rejection. We observed that tolerance was associated with upregulated FoxP3, consistent with increased percentages of FoxP3+CD4+ T cells in tolerant allografts relative to syngeneic grafts. The enriched expression of fcer1a gene in tolerance is consistent with mast cells in skin graft tolerance(24–27), while the upregulated expression of TNFRSF4 (OX40) in tolerance may be explained by OX40 in combination with IL-2 promoting Treg function and immune tolerance(28, 29). In addition, B cell-associated and BCR signaling genes enriched in tolerant compared to rejecting allografts are reminiscent of reports on an enriched B cell signature in the peripheral blood of operationally tolerant kidney recipients relative to stable immunosuppressed recipients(4, 5, 30), and raise the possibility that regulatory B cells mediate tolerance within the graft. Also upregulated in tolerance were genes involved in the MAP-kinase and c-Jun N-terminal kinase (JNK) pathway, including MAP3K6 (MAPKKK6/Apoptosis signal-regulating kinase (ASK) 2), MAPk8 (JNK1), MAPK81P3 (mitogen-activated protein kinase 8 interacting protein 3). These upregulated genes may implicate T cell anergy(31, 32) and may be involved in the suppressor function of regulatory T cells(33).
Tolerant grafts at day 8 post-infection partially acquired signatures of rejection that suggest T cell activation, proliferation and acquisition of effector function, such as cell cycle genes, CD44, Fas and IFNγ. We also noted that there were considerable differences in the GIC gene signature from tolerant hearts at day 8 post-Lm infection compared to unmodified rejection (Cluster 3, 4, 12, 16, 18, 22, 23). Because gene signatures of total GICs were examined, these differences could be explained by the presence of both alloreactive and Lm-specific T cells(17) in the allograft during the loss of tolerance but only of alloreactive T cells in acute rejection, as well as differences in the gene signature of alloreactive T cells infiltrating the allografts during the loss of tolerance compared to acute rejection. Future studies that analyze only the alloreactive T cells in these two rejecting scenarios will allow us to definitively identify the gene signature of a “re-awakened” alloreactive T cells response, and the extent to which it differs from the alloreactive T cell response in non-tolerant recipients.
The gene signatures of rejection were reduced by day 30 post-Listeria, and the signature of tolerance became broadly restored. However, a closer examination of the gene profile of the GICs at day 30 post-infection revealed that a set of 234 genes associated with tolerance was imperfectly restored. This subset included immunologically-related genes such as TNFRSF9 (CD137/4-1BB), CCR6, KLF4 and CALCA. 4-1BB has been reported to limit T cell activation thereby creating a more favorable milieu for tolerance(34); CCR6 can recruit Tregs to sites of inflammation(35–37); KLF4 is a negative regulator of memory CD8+ T cell differentiation(38), while CALCA (a neuropeptide calcitonin gene-related peptide (CGRP)) enhances the expression of regulatory macrophage markers(39). These observations support the hypothesis that a rejection crisis eroded the quality of tolerance, even if the grafts recover and continue to function long-term.
That tolerant grafts after a rejection crisis were more susceptible to rejection was functionally demonstrated by the administration of anti-PD-L1 antibodies. PD-1 binding to PD-L1 and PD-L2 triggers co-inhibitory signaling pathways that restrict the strength and duration of immune responses, thereby limiting immune-mediate damage and maintaining T cell tolerance(40–43). Additionally, PD-L1 can interact with CD80 via a reverse signaling pathway to promote T cell anergy(44). Here we confirm and extend these findings by showing that while anti-PD-L1 alone was unable to precipitate rejection in tolerant recipients, it was able to precipitate rejection when administered at 14 or 60 days post-Listeria infection. The former observation is consistent with multiple redundant mechanisms maintaining a robust state of tolerance, including the maintenance of low alloreactive T cell numbers, and suppression by regulatory T cells and PD-L1 interactions(17). After infection, some of these mechanisms may have been eroded, such that PD-L1 interactions are now essential to the maintenance of tolerance, either by constraining conventional T cells from acquiring effector function or by contributing to the function of regulatory T cells(45). Finally, despite the functional relevance of PD-L1 to tolerance maintenance after infection, the gene expression levels of PD-1 in the GIC were too low for meaningful analysis. In contrast, the protein expression of CTLA4, another checkpoint inhibitor, was similar to PD-1 on T cells (Figure S2), and to the CTLA4 gene expression patterns; namely highest in tolerance and reduced in rejection (data not shown). These observations underscore a limitation of the expression profiling approach in that RNA levels may not correlate with protein expression levels.
Our observations of the dynamic nature of established tolerance are consistent with findings reported in tolerant non-human primates and swine(46, 47). In non-human primates, where tolerance was induced with transient mixed bone marrow chimerism, multiple injections of IL-2 precipitated kidney allograft rejection, while cessation of IL-2 administration aborted the rejection process and kidney graft function was spontaneously restored(46). These observations confirm our previous observations that established tolerance is difficult to overcome, and when it is overcome, the reawakening of alloreactivity is transient and tolerance can be spontaneously restored once the proinflammatory cytokines have subsided(17). In pigs tolerant to kidney allografts, the combination of donor-specific transfusion and transplantation of second donor-matched kidney to re-stimulate alloreactivity, in combination with leukapheresis and the removal of tolerant kidney graft to eliminate regulatory T cells and induce homeostatic proliferation, triggered rejection in 8 of 10 recipients. Four fully rejected a second kidney graft, and 4 underwent a rejection crisis but the grafts survived for over 100 days. In the 2 recipients that had undergone a rejection crisis, transplanting a donor-matched skin graft 100 days later precipitated acute rejection, while in the one recipient that received the same tolerance-breaking regimen but did not undergo a rejection crisis, skin grafting did not precipitate rejection(47). The authors concluded, as we have, that a rejection crisis can cause long-term erosion in the quality of the tolerance.
In summary, we used a robust experimental model of transplantation tolerance to demonstrate that tolerance mechanisms can be completely or partially overcome by a Lm infection, resulting either in full rejection or a rejection crisis. Upon clearance of the infection, the tolerant state is restored but is eroded in quality so that it could be overcome by the blockade of PD-L1. We acknowledge that the observations of erosion of tolerance were made in a single model of tolerance with a single type of bacterial infection, and there is an urgent need to quantify host and pathogen variables that result in allograft rejection versus a subclinical erosion of tolerance. Nevertheless, these observations are congruent with pre-clinical and clinical data suggesting that tolerance, even if initially robust, may not be a permanent state but continues to be shaped by ongoing immune processes. Thus continuous vigilance may be required to detect subclinical rejection in tolerant recipients, especially during and after infections, and therapy may be required to restore an eroded state of tolerance.
Supplementary Material
Figure S1. Detailed gating strategy for CD8+, CD4+Foxp3− and CD4+Foxp3+ cells from a tolerant (TOL) spleen or allograft or day 8 post-Listeria infection of tolerant recipient (TOL+LM).
Figure S2. Detailed gating strategy for PD-1 and CTLA-4 expression by each of the T cell subsets isolated from the spleen and graft of tolerant recipient without or on day 8 post-Listeria infection.
Acknowledgments
This work was supported in part by grants (AI072630; R01AI072630; P01AI097113) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. MDD was funded by an AARA supplementary award (R01 AI072630-3S1), and a Respiratory Biology Training Grant (T32 HL07605). M.L.M. was funded by the Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), HHMI Med-into-Grad Program training grant (56006772) and two American Heart Association Midwest pre-doctoral fellowships (13PRE14550022 and 15PRE22180007). JSY received an American Heart Association Midwest post-doctoral fellowship award (15POST25700452) and was funded by a Respiratory Biology Training Grant (T32 HL07605). TMW was supported by an American Heart Association Midwest post-doctoral fellowship award (0920115G).
Abbreviations
- ANOVA
analysis of variance
- DMEM
Dulbecco modified Eagle medium
- DST
donor splenocyte transfusion
- GICs
graft infiltrating cells
- HBSS
Hanks balanced salt solution
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- IFN
Interferon
- Lm
Listeria monocytogenes
- n
number in group
- ns
not significant
- P
probability
- PBS
phosphate-buffered saline
- PMA
phorbol myristate acetate
- Tregs
regulatory T cells
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Kawai T, Leventhal J, Madsen JC, Strober S, Turka LA, Wood KJ. Tolerance: one transplant for life. Transplantation. 2014;98(2):117–121. doi: 10.1097/TP.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leventhal J, Levitsky J, Abecassis MM, Mathew J, Tambur A, Miller J, et al. Spontaneous operational tolerance in kidney transplant recipients. Am J Transplant. 2012;12(5):1350. doi: 10.1111/j.1600-6143.2011.03966.x. author reply 1351-1352. [DOI] [PubMed] [Google Scholar]
- 4.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouard S, Pallier A, Renaudin K, Foucher Y, Danger R, Devys A, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012;12(12):3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7):1599–1611. doi: 10.1111/ajt.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170(8):4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Ahmed E, Wang T, Wang Y, Ochando J, Chong AS, et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182(10):6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6(10):2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed EB, Wang T, Daniels M, Alegre ML, Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant. 2011;11(5):936–946. doi: 10.1111/j.1600-6143.2011.03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Chen L, Ahmed E, Ma L, Yin D, Zhou P, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180(9):5991–5999. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186(1):214–221. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009;182(3):1314–1324. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10(7):1524–1533. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller ML, Daniels MD, Wang T, Chen J, Young J, Xu J, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015;6:7566. doi: 10.1038/ncomms8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335(6069):723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller ML, Daniels MD, Wang TM, Y W, Xu J, Yin DP, et al. Multiple mechanisms of peripheral tolerance maintain cardiac transplant acceptance. Am J Transplant. 2016 doi: 10.1111/ajt.13814. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egawa H, Miyagawa-Hayashino A, Haga H, Teramukai S, Yoshizawa A, Ogawa K, et al. Non-inflammatory centrilobular sinusoidal fibrosis in pediatric liver transplant recipients under tacrolimus withdrawal. Hepatol Res. 2012;42(9):895–903. doi: 10.1111/j.1872-034X.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoshitomi M, Koshiba T, Haga H, Li Y, Zhao X, Cheng D, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87(4):606–614. doi: 10.1097/TP.0b013e318195a7cb. [DOI] [PubMed] [Google Scholar]
- 22.Tryphonopoulos P, Ruiz P, Weppler D, Nishida S, Levi DM, Moon J, et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation. 2010;90(12):1556–1561. doi: 10.1097/TP.0b013e3182003db7. [DOI] [PubMed] [Google Scholar]
- 23.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307(3):283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 24.de Vries VC, Pino-Lagos K, Elgueta R, Noelle RJ. The enigmatic role of mast cells in dominant tolerance. Curr Opin Organ Transplant. 2009;14(4):332–337. doi: 10.1097/MOT.0b013e32832ce87a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries VC, Pino-Lagos K, Nowak EC, Bennett KA, Oliva C, Noelle RJ. Mast cells condition dendritic cells to mediate allograft tolerance. Immunity. 2011;35(4):550–561. doi: 10.1016/j.immuni.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 27.Wasiuk A, Dalton DK, Schpero WL, Stan RV, Conejo-Garcia JR, Noelle RJ. Mast cells impair the development of protective anti-tumor immunity. Cancer Immunol Immunother. 2012;61(12):2273–2282. doi: 10.1007/s00262-012-1276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao X, Gong W, Demirci G, Liu W, Spoerl S, Chu X, et al. New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J Immunol. 2012;188(2):892–901. doi: 10.4049/jimmunol.1101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnear G, Wood KJ, Fallah-Arani F, Jones ND. A diametric role for OX40 in the response of effector/memory CD4+ T cells and regulatory T cells to alloantigen. J Immunol. 2013;191(3):1465–1475. doi: 10.4049/jimmunol.1300553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chesneau M, Pallier A, Braza F, Lacombe G, Le Gallou S, Baron D, et al. Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant. 2014;14(1):144–155. doi: 10.1111/ajt.12508. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9(1):50–55. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janardhan SV, Praveen K, Marks R, Gajewski TF. Evidence implicating the Ras pathway in multiple CD28 costimulatory functions in CD4+ T cells. PLoS One. 2011;6(9):e24931. doi: 10.1371/journal.pone.0024931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adler HS, Kubsch S, Graulich E, Ludwig S, Knop J, Steinbrink K. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells. Blood. 2007;109(10):4351–4359. doi: 10.1182/blood-2006-09-047563. [DOI] [PubMed] [Google Scholar]
- 34.Eun SY, Lee SW, Xu Y, Croft M. 4-1BB ligand signaling to T cells limits T cell activation. J Immunol. 2015;194(1):134–141. doi: 10.4049/jimmunol.1401383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A, et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21(6):974–985. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martinez AC, et al. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol. 2009;39(6):1671–1681. doi: 10.1002/eji.200839123. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Borges CM, Fan MY, Harris JE, Turka LA. Requirement for CD28 in Effector Regulatory T Cell Differentiation, CCR6 Induction, and Skin Homing. J Immunol. 2015;195(9):4154–4161. doi: 10.4049/jimmunol.1500945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamonkin M, Shen Y, Lee PH, Puppi M, Park CS, Lacorazza HD. Differential roles of KLF4 in the development and differentiation of CD8+ T cells. Immunol Lett. 2013;156(1–2):94–101. doi: 10.1016/j.imlet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baliu-Pique M, Jusek G, Holzmann B. Neuroimmunological communication via CGRP promotes the development of a regulatory phenotype in TLR4-stimulated macrophages. Eur J Immunol. 2014;44(12):3708–3716. doi: 10.1002/eji.201444553. [DOI] [PubMed] [Google Scholar]
- 40.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016 doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 41.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174(11):6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179(8):5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Popoola J, Khandwala S, Vadivel N, Vanguri V, Yuan X, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117(5):660–669. doi: 10.1161/CIRCULATIONAHA.107.741025. [DOI] [PubMed] [Google Scholar]
- 44.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada Y, Nadazdin O, Boskovic S, Lee S, Zorn E, Smith RN, et al. Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys. Am J Transplant. 2015;15(12):3055–3066. doi: 10.1111/ajt.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scalea JR, Okumi M, Villani V, Shimizu A, Nishimura H, Gillon BC, et al. Abrogation of renal allograft tolerance in MGH miniature swine: the role of intra-graft and peripheral factors in long-term tolerance. Am J Transplant. 2014;14(9):2001–2010. doi: 10.1111/ajt.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Detailed gating strategy for CD8+, CD4+Foxp3− and CD4+Foxp3+ cells from a tolerant (TOL) spleen or allograft or day 8 post-Listeria infection of tolerant recipient (TOL+LM).
Figure S2. Detailed gating strategy for PD-1 and CTLA-4 expression by each of the T cell subsets isolated from the spleen and graft of tolerant recipient without or on day 8 post-Listeria infection.