Abstract
BACKGROUND
Despite improvements in the management of severely injured patients, development of multiple organ dysfunction syndrome (MODS) remains a morbid complication of traumatic shock. One of the key attributes of MODS is a profound bioenergetics crisis, for which the mediators and mechanisms are poorly understood. We hypothesized that metabolic uncoupling using an experimental phosphoinositol-3 kinase (PI3-K) inhibitor, LY294002 (LY), may prevent mitochondrial abnormalities that lead to the generation of mitochondrial DNA (mtDNA) damage and the release of mtDNA damage-associated molecular patterns (DAMPs).
METHODS
Sixteen swine were studied using LY, a nonselective PI3-K inhibitor. Animals were assigned to trauma only (TO, n = 3), LY drug only (LYO, n = 3), and experimental (n = 10), trauma + drug (LY + T) groups. Both trauma groups underwent laparotomy, 35% hemorrhage, severe ischemia-reperfusion injury, and protocolized resuscitation. A battery of hemodynamic, laboratory, histological, and bioenergetics parameters were monitored. Mitochondrial DNA damage was determined in lung, liver, and kidney using Southern blot analyses, whereas plasma mtDNA DAMP analysis used polymerase chain reaction amplification of a 200-bp sequence of the mtDNA D-loop region.
RESULTS
Relative to control animals, H + I/R (hemorrhage and ischemia/reperfusion) produced severe, time-dependent decrements in hepatic, renal, cardiovascular, and pulmonary function accompanied by severe acidosis and lactate accumulation indicative of bioenergetics insufficiency. The H-I/R animals displayed prominent oxidative mtDNA damage in all organs studied, with the most prominent damage in the liver. Mitochondrial DNA damage was accompanied by accumulation of mtDNA DAMPs in plasma. Pretreatment of H + I/R animals with LY resulted in profound metabolic suppression, with approximately 50% decreases in O2 consumption and CO2 production. In addition, it prevented organ and bioenergetics dysfunction and was associated with a significant decrease in plasma mtDNA DAMPs to the levels of control animals.
CONCLUSIONS
These findings show that H + I/R injury in anesthetized swine is accompanied by MODS and by significant mitochondrial bioenergetics dysfunction, including oxidative mtDNA damage and accumulation in plasma of mtDNA DAMPs. Suppression of these changes with the PI3-K inhibitor LY indicates that pharmacologically induced metabolic uncoupling may comprise a new pharmacologic strategy to prevent mtDNA damage and DAMP release and prevent or treat trauma-related MODS.
LEVEL OF EVIDENCE
Therapeutic study, level III.
Keywords: Damage-associated molecular pattern accumulation, hemorrhage, LY294002, mitochondrial DNA, trauma
Exsanguinating hemorrhage continues to be the most common cause of preventable death both on the battlefield and in civilian trauma settings.1,2 Although recent advances in casualty care, resuscitation, and damage control principles have decreased overall mortality in traumatically injured populations, especially in combat,3 these advances have likely led to increases in two significant, delayed complications, namely, acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS).4 Thus far, effective pharmacotherapeutics have not been developed to treat or prevent either of these syndromes. One proposed reason for this deficiency is the lack of specific therapeutic targets, the inherent difficulty being due to the vast number of involved signaling pathways and potential drug targets that are activated or dysfunctional in human trauma victims.5
Multiple lines of evidence implicate prolonged oxygen debt, inflammation, and resultant metabolic derangements following ischemia, reperfusion, and resuscitation in death from multiorgan failure.6–8 Indeed, all of these characteristics of multiorgan failure in the setting of severe injury are accompanied by a profound oxidant stress and a “bioenergetic crisis” attributed to mitochondrial dysfunction and attendant cytotoxicity. Furthermore, there is evidence that mitochondrial DNA (mtDNA) damage, which is a direct consequence of reactive oxygen species (ROS) stress, leads to cell death via multiple endogenous pathways.9–12 Oxidatively damaged mtDNA, if not quickly repaired, seems to be rapidly degraded into small proinflammatory fragments, termed mtDNA damage-associated molecular patterns (DAMPs).12,13 These DAMPs are released from damaged and dying cells, accumulate in the circulation, and then act on both local and distant targets, which induce further inflammation and cellular dysfunction via cytokine, ROS-mediated, and other pathways.14–16 This elevation in mtDNA-associated DAMPs has been implicated in the development of ARDS and MODS in critical illness and after injury and may lead to worse patient outcomes and mortality.17–19 There is also some evidence in animal models that enhancing the repair or clearance of oxidatively damaged mtDNA and preventing the associated DAMPs can decrease ischemia-reperfusion injury or ventilator-induced lung injury and may be protective against the development of ARDS and MODS.20–24 Collectively, these considerations support the idea that reversing mitochondrial dysfunction and improving mitochondrial biogenesis following shock states may be possible and may lead to improved survival following severe trauma.25–27
One potential pharmacologic strategy to decrease DAMP production and release that has not been well studied is that of metabolic uncoupling. Theoretically, decreasing the metabolic demand during and after injury would decrease the amount of ROS released, thereby reducing the amount of tissue or organ mtDNA damage and subsequent elaboration of mtDNA DAMPs and their sequelae. We have shown previously that LY294002 (LY), an experimental phosphoinositide-3 kinase (PI3-K) inhibitor, when given in a porcine model of hemorrhage, ischemia, and reperfusion and resuscitation (HIRR) leads to profound metabolic suppression, with approximately 50% decreases in oxygen consumption (VO2) and carbon dioxide production (VCO2) (published online ahead of print, Journal of Trauma, JT-D-16-04594). Thus, the objectives of this study were to examine whether significant mtDNA damage and elevated circulating mtDNA DAMPs are present in this model of severe hemorrhagic shock and reperfusion injury and determine whether administration of this novel PI3-K inhibitor (LY) is associated with any alteration in these sequelae of mtDNA damage.
METHODS
This study was performed at an American Association for Laboratory Animal Science–accredited animal research facility following protocol approval by the Institutional Animal Care and Use Committee. Sixteen adult Yorkshire swine (Sus scrofa) weighing 30 kg to 55 kg were housed for 7 days, under the supervision of licensed veterinary staff. Diet was ad libitum, and no additional treatments were performed other than usual care and feeding.
Three arms were utilized in this study: Group 1, trauma only (TO, n = 3) received HIRR only; Group 2, LY only (LYO, n = 3), received no HIRR and LY dissolved in dimethyl sulfoxide (DMSO); and Group 3, LY plus trauma (LY + T, n = 10), received HIRR followed by LY. Hemodynamic (HD) and biosamples were analyzed at baseline and at 30 minutes and 2, 4, and 6 hours after cross-clamp removal. Complete blood count and chemistry panels (Heska, Loveland, CO) and arterial blood gas (iStat; Abaxis, Union City, CA) measurements were also performed. Metabolic parameters were measured via metabolic cart (MGC Diagnostics Corp., St. Paul, MN). Tissue mtDNA damage and the presence and levels of circulating mtDNA DAMPs were assessed as described below.
Preparation and Invasive Monitoring
On the day of study, all animals received an intramuscular cocktail of ketamine, tiletamine HCl and zolazepam HCl (Telazol), and xylazine dosed at 2.2 mg/kg to 2.5 mg/kg for sedation and maintained on sevoflurane (2–3%) gaseous anesthetic and oxygen for maintenance anesthesia following endotracheal intubation (6–7.5 Fr tube). Ventilatory parameters were adjusted to maintain normophysiologic baseline blood gas parameters.
Central neck cut-down was performed; the right common carotid artery was cannulated with a 7 Fr Micropuncture kit, and the internal jugular vein was cannulated with 9.5 Fr Cordis catheter to measure invasive arterial and venous pressures. A Swan-Ganz catheter was placed. Invasive HD measures included mean arterial pressure (MAP), central venous pressure, pulmonary artery pressures, pulmonary wedge pressures, cardiac index, and cardiac output. A percutaneous external jugular venous catheter was placed in the experimental animals for drug administration.
A midline laparotomy with placement of a transcystic Foley catheter was performed, and liver tissue collected. The inferior vena cava was cannulated for the controlled hemorrhage portion, followed by dissection of the supraceliac aorta with placement of vessel loops in preparation for cross clamp.
Controlled HIRR Phases
The study consisted of three sequential procedural phases to produce a physiologic state of systemic acidosis and coagulopathy: a volume-controlled hemorrhage phase, an ischemia phase, and a reperfusion plus resuscitation phase. These phases excellently model the sequence of initial wounding, hemorrhage, and organ hypoperfusion followed by hemorrhage control, restoration of perfusion, and resuscitation, which is common in human trauma.28,29 Hemorrhage was accomplished by removing 35% circulating blood volume through the inferior vena cava catheter at rate of physiologic tolerance of MAPs 40 mm Hg or greater. Directly after, the supraceliac aorta was cross clamped for 45 minutes plus 5 minutes allowed for clamp removal. Clamp removal time was considered Time 0. Reperfusion and resuscitation was then instituted utilizing lactated Ringer’s solution and epinephrine to maintain MAPs of at least 25 mm Hg. No attempt was made to resuscitate animals to normal physiology.
LY294002 Drug Preparation and Dosing
LY294002 (Cayman Chemical [Ann Arbor, MI] and Med Chem Express [Monmouth Junction, NJ]) was obtained and stored under recommended conditions provided by the vendor. Selected animals received 20mg/kg of drug dissolved in DMSO (Sigma Life Science, St. Louis, MO) facilitated by bench-side vortex agitation at a ratio of 30 mg drug/mL DMSO. Dissolved drug was injected over the last 30 minutes of cross-clamp time as a single, one-time injection in the animals undergoing HIRR. Injection completion time was used as Time 0 for the LYO group.
Study End Points
Primary study end points were mtDNA damage in select tissues as well as mtDNA DAMP levels in plasma. Secondary end points included HD parameters (i.e., MAP, CO) plus metabolic and inflammatory burden measured by lactate levels, base deficit, and cytokines, as well as metabolic suppression measured by respiratory quotient and oxygen/carbon dioxide consumption/utilization. After study completion, animals were killed, and tissue samples (lung, liver, and kidney) were obtained for histological mtDNA damage analysis. Serum samples were also obtained at baseline and at study completion for analysis of levels of circulating mtDNA DAMPs.
Mitochondrial DNA and DAMP Analysis
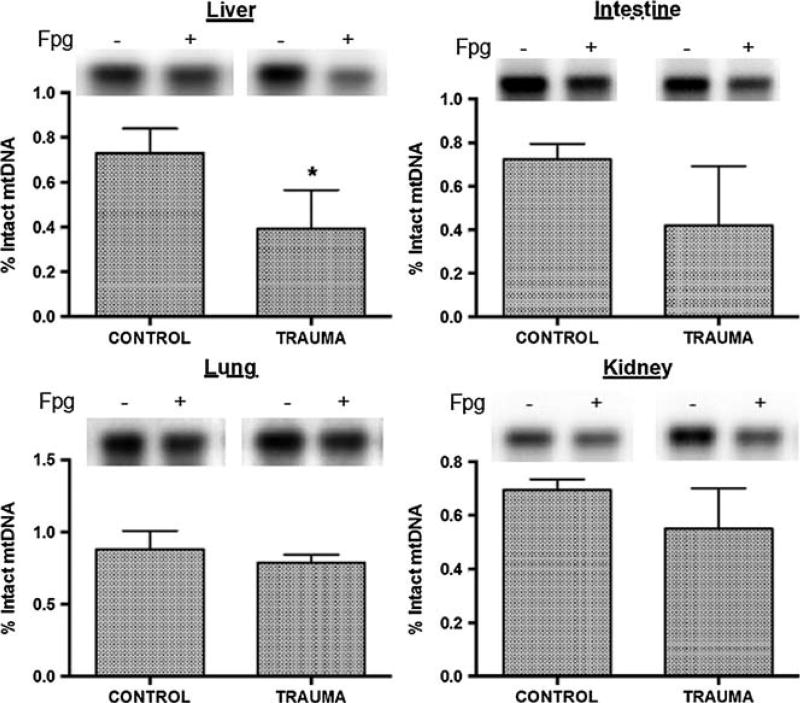
Mitochondrial DNA damage was determined in liver, intestine, lung, and kidney tissues harvested at study completion. Biopsies of liver, intestine, lung, and kidney tissue were snap frozen in liquid nitrogen and stored at −80°C. Samples were then powdered with a mortar and pestle, and oxidative mtDNA damage assessed using a quantitative Southern blot assay as previously described.12,30,31 In brief, purified DNA was digested with PpuMI and AhdI restriction enzymes (New England Biolabs, Beverly, MA) overnight at 37°C. This resulted in cutting mtDNA into two fragments, with one being a 2.7-kb sequence containing the D-loop region. Digested DNA samples were precipitated, dissolved in TE buffer, and precisely quantified on the Bio-Rad Versa Fluor fluorometer (Bio-Rad Laboratories, Hercules, CA) using Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, Carlsbad, CA). To reveal oxidative base modifications, DNA was treated with formamidopyrimidine glycosylase (Fpg; New England Biolabs), a bacterial DNA repair enzyme that cleaves DNA at sites of oxidized purines. Samples containing 500 ng DNA were treated with 8 U of Fpg in 20 μL of reaction volume at 37°C for 1 hour. Subsequently, Fpg-treated and untreated samples were incubated with 0.1 N NaOH for 15 minutes at 37°C and resolved in 0.6% agarose alkaline gel. After electrophoresis and DNA transfer to a nylon membrane (Roche Diagnostics, Sigma, St. Louis, MO), the membrane was hybridized with a polymerase chain reaction (PCR)–generated probe to the corresponding D-loop region of mtDNA. The probe, labeled with a DIG-labeling kit (Roche Diagnostics, Sigma), was generated with porcine mtDNA sequences used as the template. After cross linking, the membranes were washed and processed according to the manufacturer’s suggestions. Hybridization bands were detected with Amersham Hyperfilm ECL (GE Healthcare, Piscataway, NJ) and a Gel Logic 1500 Imaging System (Kodak, Rochester, NY). Single-strand breaks formed at the sites of oxidized purines by Fpg treatment resulted in decreased Southern blot hybridization band intensity. Accordingly, we depicted the proportional oxidative base damage in the mtDNA D-loop region as the percent intact mtDNA calculated from the band density determined in Fpg-treated specimens relative to the band intensity in specimens not treated with the DNA glycosylase.
Quantitative real-time PCR was used to detect selected 200-bp sequences of the mitochondrial D-loop sequence in porcine serum, also as described previously.31,32 Primers were developed using Beacon Designer software. Quantitative reverse transcriptase–PCR was done using the HotStart-IT SYBR Green One Step quantitative reverse transcriptase–PCR kit (USB) following manufacturer’s instructions. An iCycler with the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories) was used to analyze each sample. The amplification efficiency of each primer set was determined by calculating the slope of the calibration curve, which was constructed by performing PCR with serial dilutions of a known concentration of template DNA.
Data Analysis
Data are presented as means ± SE and were analyzed using SPSS version 22 (IBM, Chicago, IL). Between-subject and within-subject time point parameters were compared using Bonferroni repeated-measures analysis and regression analysis with a model for estimated marginal means. These parameters included physiologic, laboratory, and metabolic cart readings. Changes in mtDNA damage and circulating mtDNA DAMPs were evaluated using unpaired t tests and one-way analyses of variance, respectively.
RESULTS
Baseline HD, laboratory, and metabolic cart values were similar between study groups (Table 1) with two notable differences. Baseline MAP in LYO was lower than LY + T (75 ± 4 vs. 110 ± 21 mm Hg, p = 0.015). Hematocrit in LYO was also significantly lower at baseline when compared with LY + T (23 ± 1 vs. 28 ± 3, p = 0.04).
TABLE 1.
Baseline Hemodynamic, Metabolic, and Laboratory Values
| Variable | TO | LYO | LY + T | P |
|---|---|---|---|---|
| n | 3 | 3 | 10 | |
| Hemodynamics | ||||
| HR, beats/min | 112 ± 9 | 121 ± 13 | 119 ± 5 | 0.54 |
| MAP, mm Hg | 96 ± 11 | 75 ± 4 | 110 ± 6 | 0.02 |
| CVP, mm Hg | 1.7 ± 1.2 | 5.3 ± 3.8 | 4.4 ± 4.2 | 0.26 |
| CI (CO/BSA) | 5.2 ± 0.5 | 4.5 ± 1.0 | 4.5 ± 0.3 | 0.31 |
| PCWP, mm Hg | 6.7 ± 1.5 | 4.3 ± 1.5 | 7.8 ± 3.5 | 0.10 |
| Temperature, °F | 100.1 ± 0.7 | 100.3 ± 2.7 | 101.7 ± 0.4 | 0.08 |
| Metabolism | ||||
| VO2, mL/min | 225 ± 55 | 242 ± 70 | 235 ± 28 | 0.48 |
| VCO2, mL/min | 242 ± 36 | 228 ± 56 | 236 ± 23 | 0.61 |
| RQ (VCO2/VO2) | 0.96 ± 0.06 | 0.95 ± 0.06 | 1.0 ± 0.07 | 0.27 |
| Physiologic | ||||
| Hct | 26 ± 1.8 | 23 ± 1 | 28 ± 1 | 0.04 |
| PTT | 47 ± 7 | 46 ± 5 | 40 ± 4 | 0.47 |
| INR | 0.96 ± 0.03 | 0.95 ± 0.02 | 0.96 ± 0.03 | 0.28 |
| Fibrinogen | 204 ± 19 | 189 ± 26 | 198 ± 11 | 0.63 |
| pH | 7.6 ± 0.41 | 7.6 ± 0.02 | 7.5 ± 0.23 | 0.48 |
| Lactate | 1.8 ± 0.4 | 2.9 ± 0.9 | 2.6 ± 0.24 | 0.12 |
| Organ function | ||||
| Cr | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.28 |
| ALT | 39 ± 4 | 36 ± 5 | 38 ± 8 | 0.60 |
p Values represent the lowest p for any intergroup comparison.
ALT, alanine transferase; BSA, body surface area; Cr, creatinine; HR, heart rate; CVP, central venous pressure, CI, cardiac index; Hct, hematocrit; INR, international normalized ratio; PCWP, pulmonary capillary wedge pressure; PTT, prothrombin time; RQ, respiratory quotient.
Mitochondrial DNA and DAMP Accumulation
Oxidative base damage in mtDNA from liver, intestine, lung, and kidney was determined in tissue samples taken at baseline and 6 hours after HIRR. As shown in Figure 1, oxidative mtDNA damage was significantly increased after HIRR in liver versus control animals, with similar trends (0.15 > p > 0.1) suggesting elevated damage in intestinal and renal tissue. Examination of harvested lung tissue did not show any statistically significant evidence of tissue mtDNA damage.
Figure 1.
Representative Southern blot samples in Fpg untreated (−) versus treated (+) DNA. Decrease in signal denotes an increase in DNA damage in the represented tissue. Also percent intact mtDNA in representative tissue samples. Asterisk denotes statistical significance.
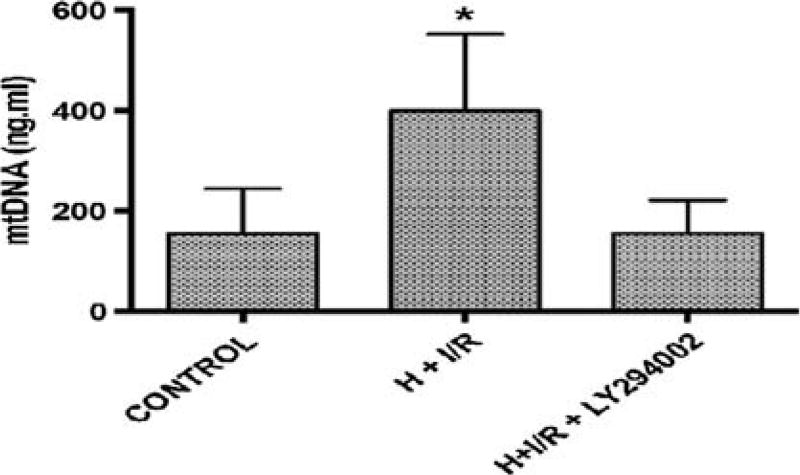
The significant mtDNA damage in liver and trends toward damage in intestine and kidney tissues, all organs composed of multiple cell types of differing sensitivities to oxidant stress, implied that mtDNA DAMPs could accumulate in the circulation after hemorrhage and reperfusion injury. Accordingly, we measured circulating serum mtDNA DAMP levels again at baseline, after HIRR, and after HIRR in animals treated with LY. As shown in Figure 2, relative to baseline values, a 200-bp fragment of the mtDNA D-loop region significantly increased in serum after 6 hours of resuscitation in the HIRR in animals not treated with LY. However, in animals receiving the PI3-K inhibitor, no elevations in mtDNA DAMPs were observed after HIRR, and were similar to levels found in control (uninjured) animals.
Figure 2.
Mitochondrial DNA DAMP accumulation, control versus traumatically injured animals versus traumatically injured animals treated with PI3-K inhibition. Asterisk denotes statistical significance.
Hemodynamic and Physiologic Outcomes
LY + T showed significant decreases in multiple physiologic parameters when compared with other groups, which sustained until study end point. All HIRR animals showed reproducible changes that mimic the shock state following traumatic injury (Table 2). Mean arterial pressure at 6 hours in LY + T versus TO (32 ± 6 vs. 41 ± 6 mm Hg, p = 0.026) and LYO (32 ± 6 vs. 56 ± 7 mm Hg, p < 0.001) was significantly lower. Similarly, at 6 hours, heart rate in LY + T was lower when compared with TO (76 ± 12 vs. 113 ± 39, p = 0.031) and LYO (76 ± 12 vs. 118 ± 44, p = 0.016). Cardiac index was also lower in LY + T when compared with TO (1.9 ± 0.8 vs. 4.8 ± 2.7, p = 0.01) and LYO (1.9 ± 0.8 vs. 4.5 ± 2.4, p = 0.02) at 6 hours. Temperature in LY + T approached significance when compared with LYO (90.9 ± 2.4 vs. 95.2 ± 6.4°F, p = 0.08) and was found to be similar to the TO group (90.9 ± 2.4 vs. 93.8 ± 5.1°F, p = 0.22).
TABLE 2.
Hemorrhagic Shock Model Mimics Human Shock Physiology
| Variable | TO Baseline | TO 30 min | LY + T Baseline | LY + T 30 min |
|---|---|---|---|---|
| HR, beats/min | 112 ± 9 | 115 ± 11 | 119 ± 5 | 97 ± 6 |
| MAP, mm Hg | 96 ± 11 | 43 ± 5 | 110 ± 6 | 34 ± 3 |
| CI (CO/BSA) | 5.2 ± 0.5 | 4.4 ± 0.6 | 4.5 ± 0.3 | 2.6 ± 0.3 |
| Temperature, °F | 100.1 ± 0.7 | 97.1 ± 0.9 | 101.7 ± 0.4 | 94.9 ± 0.5 |
| Hct | 26 ± 1.8 | 18 ± 2.3 | 28 ± 1 | 17 ± 1.3 |
| PTT | 47 ± 7 | 23 ± 9 | 40 ± 4 | 27 ± 5 |
| INR | 0.96 ± 0.03 | 1.14 ± 0.07 | 0.99 ± 0.02 | 1.24 ± 0.04 |
| Fibrinogen | 204 ± 19 | 106 ± 14 | 198 ± 11 | 100 ± 8 |
| pH | 7.6 ± 0.41 | 7.25 ± 0.4 | 7.5 ± 0.23 | 6.96 ± 0.2 |
| Lactate | 1.8 ± 0.4 | 11.2 ± 0.7 | 2.6 ± 0.24 | 10.5 ± 0.4 |
| Base deficit | 10.7 ± 1.8 | −11.7 ± 2.2 | 7.3 ± 1 | −12.3 ± 1.2 |
Values at baseline and again at 30 minutes after cross-clamp removal in animal groups undergoing hemorrhage, ischemia, and reperfusion.
Abbreviations are as defined in footnote to Table 1.
Hematocrit in the traumatically injured animals decreased significantly. This parameter was significantly lower in TO and LY + T when compared with LYO (17 ± 5.2 and 18 ± 2.4 vs. 25 ± 1.5, p = 0.017 and 0.009) at 6 hours. International normalized ratio was found to be higher in LY + T only when compared with LYO (1.34 ± 0.16 vs. 1.07 ± 0.04, p = 0.039) at 6 hours. Fibrinogen level in LY + T at 6 hours was also lower when compared with LYO (100 ± 19 vs. 152 ± 10, p = 0.009). The groups undergoing HIRR showed decreases in pH initially especially in LY + T (7.54 baseline to 6.96 at 30 minutes). This was significantly different only when the LY + T group was compared with LYO at 2 hours (7.36 ± 0.27 vs. 7.49 ± 0.49, p = 0.034) There was no significant difference in serum pH levels at the other time points when the three groups were compared.
Metabolic Outcomes
Metabolic cart data showed significant changes in the groups that received LY, confirming the production of significant metabolic depression with administration of the PI3-K inhibitor. Both LY groups had initial decreases in oxygen consumption (VO2) and carbon dioxide production (VCO2). In LYO, this initial decrease was nearly reversed by the study end point, thought to be secondary to drug metabolism and washout. LY + T, however, showed decreases in these parameters consistent with major metabolic suppression that was sustained throughout resuscitation. At 6 hours, LY + T showed significantly lower VO2 when compared with TO and LYO (119 ± 32 vs. 229 ± 93 and 224 ± 99 mL/min, p = 0.012 and 0.014). Similarly, VCO2 in LY + T at 6 hours was significantly lower when compared with TO and LYO (114 ± 26 vs. 196 ± 42, and 195 ± 88 mL/min, p = 0.043 and 0.034). Respiratory quotient (VCO2/VO2) was found to be significantly different only when TO was compared with LY + Tat the 6-hour time point (0.82 ± 0.07 vs. 0.98 ± 0.11, p = 0.013).
DISCUSSION
While improvements in trauma care over the last few decades have significantly reduced mortality rates from exsanguinating hemorrhage and the immediate effects of traumatic injury, there still remains a significant portion of severely injured patients who go on to develop and succumb to the more downstream effects of trauma. These “intermediate” or “late” deaths typically occur days to weeks after the initial injury and are attributed to either single- or multiple-organ system failures seen in the intensive care unit. There has been a widely recognized increase in associated morbidity and mortality with single-organ failure syndromes, such as ARDS or acute renal failure, and then an exponential increase in adverse outcomes when multiple organ systems fail (MODS). This current findings show that HIRR injury in anesthetized swine is accompanied by MODS and by significant mtDNA damage and accumulation in plasma of mtDNA DAMPs. Accumulation of damaged mitochondrial products has the potential to lead to cell death and organ failure via multiple pathways, many of which are not well understood.9–12
The area of study of mtDNA and its potential contribution to local and systemic inflammation and organ injury or failure syndromes is still in its relative infancy. Mitochondrial DNA is the unique DNA found in the mitochondria of eukaryotic cells and represents only a small part of the total cellular DNA, which is primarily located in the cell nucleus. Mitochondrial DNA is intimately involved in the cellular respiration and bioenergetics functions, particularly in the conversion of nutritional intake to cellular energy in the form of adenosine triphosphate. Significant injury or damage to the mitochondria, including the mtDNA, can rapidly lead to cellular dysfunction and death if not reversed or repaired. This can result in the release of mtDNA fragments into the local and systemic circulation, and thus elevated levels of mtDNA damage and circulating mtDNA DAMPs can provide a proxy for the amount and severity of cellular or organ injury.18,25,32
These circulating mtDNA DAMPs not only are an indicator of the degree of cellular injury and death, but also have been shown to have significant proinflammatory and oxidative properties that can provoke further deleterious effects. Much of the deleterious effects of the systemic release of mtDNA fragments are thought to be secondary to their proposed origin. The “endosymbiotic theory” proposes that mitochondria are actually of bacterial origin and represent the evolutionary consequence of bacteria that were engulfed and incorporated into the machinery of early eukaryotic cells. Their origin from the original bacterial circular DNA is thought to largely explain the rapid and severe inflammatory and oxidative responses that occur when these “foreign” DNA fragments are released into the systemic circulation. Circulating mtDNA DAMPs have been implicated in a wide range of disease processes including sepsis, myocardial infarction, ARDS, severe pneumonia, and trauma.9,10,14,15,19,20,32 A recent study by Simmons et al.32 demonstrated that elevated levels of mtDNA DAMPs correlated with the severity of injury and systemic illness among a cohort of trauma patients and that the degree of elevation also correlated with worse clinical outcomes. Similar findings were reported from a series including both trauma and severe sepsis patients.17 There is also now a growing body of evidence that demonstrates that mtDNA damage and circulating mtDNA DAMPs may represent an opportune target for intervention. Several series have demonstrated that mitochondrial dysfunction and subsequent apoptosis after mtDNA damage can be reversed with administration of novel mtDNA repair enzymes.10,12 Additional series have shown that administration of these mtDNA repair agents and administration of drugs that degrade circulating mtDNA are able to prevent or reverse ventilator-induced lung injury, severe pneumonia or ARDS, and myocardial infarction.12,21,23,24 Our study results suggest that there are likely multiple potential pathways and mechanisms that could be leveraged to either minimize or mitigate the adverse effects of mtDNA damage and systemic release, with the potential for benefit in preventing or reducing the severity of posttraumatic organ failure syndromes.
While much recent literature implicates mtDNA damage and DAMP accumulation in the development of these processes,9,11,19,20 there have been few advances in the treatment of these syndromes outside prolonged supportive therapy. Forcing suppression of these changes with pharmacologic agents, such as the PI3-K inhibitor examined in this study, could indicate that pharmacologically induced metabolic uncoupling may comprise a new and relatively simple strategy to minimize or even prevent mtDNA damage and DAMP release and prevent or treat trauma-related organ failure syndromes. Although the data from this study are only preliminary and cannot identify the exact mechanisms of action of the LY compound that resulted in a decrease in mtDNA damage and circulating plasma mtDNA DAMPs, it suggests a real possibility for pharmacologic interventions. In addition and most importantly, it suggests a potential therapeutic role for specifically targeted therapies that can be administered after the initial injury and hemorrhage, which would obviously carry more promise for real-world utility than other interventions that have shown efficacy only when given before the initial injury.
Phosphoinositol-3 kinases are a family of lipid kinases that have downstream regulatory effects on many cellular functions and pathways originally associated with viral oncogenes.33 Targeting of certain arms in the PI3-K pathway for treatment in cancer is the subject of much current research.34,35 Some of that research links adaptations or dysfunction of the PI3-K pathway to mitochondrial dysfunction in the development of certain cancers and may give some insight into the downstream effects of PI3-K inhibition on mitochondrial damage.36,37 This study further implicates this pathway in the development of mtDNA damage and DAMP accumulation in trauma, and the suppression of this pathway may prove to be beneficial in the traumatically injured patient to help curb the development of secondary injury from trauma.
The liver also appears to play a primary role in posttraumatic inflammation, intestinal ischemia-reperfusion injury, and the subsequent development of both hepatic and distant organ dysfuction.38–40 Our model showed a significant reduction in liver mtDNA damage and DAMP accumulation. Targeting reversal of liver dysfunction following traumatic injury, especially in the presence of severe intestinal ischemia-reperfusion injury, may prove to be beneficial as this organ is likely one of the key mediators of MODS thought to be led by “gut dysfunction” in this setting.
There are several limitations to this study that should be addressed. Primarily, this pilot study utilized a porcine model; thus, the findings may not translate to human subjects. In addition, there were certain baseline differences in hemodynamics and initial laboratory values between two groups that may have had an effect on the intergroup comparison. Metabolic suppression and bioenergetic uncoupling, however, are based on within-groups comparisons with each animal serving as its own control. Obviously additional study is needed as the side effect profile of PI3-K inhibition and optimal dosing of the compounds used in this protocol have not been established in humans. Further research should additionally focus on potential adverse short-and long-term outcomes from PI3-K inhibition and the apparent metabolic depression that results. Whether inducing metabolic uncoupling and the short-term, 6-hour decrease in mtDNA damage and DAMP accumulation will translate into longer-term effects and decreases in the development of ARDS and MODS is also unknown because this was a nonsurvival model. No blood products were administered during the course of this study, and thus, the interaction with damaged mtDNA and DAMPs, which are thought to be present in stored, donated blood, cannot be commented on and may lead to different physiologic and metabolic effects from those shown in this study.
We suggest that administration of LY, possibly as a consequence of its metabolic suppression effect, leads to global bioenergetic uncoupling with a reduction in oxidant stress and attendant suppression of mtDNA damage and mtDNA DAMP release into the circulation. When taken in combination with the existing literature on circulating DAMPs and mtDNA DAMPs, this type of therapy could have the potential to reduce or even prevent subsequent local or distant organ injury, dysfunction, and eventual failure. In addition, there were no short-term adverse impacts of the induced metabolic suppression in regard to systemic markers of perfusion and acidosis (lactate) or in end-organ perfusion or damage. Much further research will be necessary to delineate the exact pathways that lead to the effects shown in this study, as well as refine delivery method, timing, dosage, and interactions with other therapies. Future research should also focus on larger, survival-type studies to determine the longer-term implications of PI3-K inhibition in traumatic injury.
Acknowledgments
This work was funded by a DARPA research grant under the Biochronicity Project. The results and opinions expressed in this article are those of the authors, and do not reflect the opinions or official policy of the US Army or the Department of Defense.
Footnotes
AUTHORSHIP
All authors meet authorship criteria for this article as described below. All authors have seen and approved the final manuscript as submitted. The senior authors (M.J.M. and M.G.) had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M.J.M., G.E.B., K.K.S., D.M.M., J.D.S., and M.G. conceived and designed the study. G.E.B., K.K.S., M.J.M., D.M.M., V.P., G.C., O.G., and M.R. performed acquisition of data. G.E.B., K.K.S., M.J.M., O.G., J.D.S., and M.G. analyzed and interpreted the data. G.E.B., K.K.S., M.J.M., and M.G. drafted the manuscript. M.J.M. and M.G. performed critical revision of the manuscript. M.J.M., K.K.S., G.E.B., and M.G. provided statistical expertise. M.J.M., K.K.S., G.E.B., D.M.M. G.C., and O.G. provided administrative, technical, or material support. M.J.M. and M.G. provided supervision. Both M.J.M. and Dr. M.G. served as the senior supervisors of their respective research laboratories and contributed equally as senior authors of this article.
DISCLOSURE
The authors declare no conflicts of interest.
References
- 1.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, et al. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245(6):986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoencamp R, Vermetten E, Tan EC, Putter H, Leenen LP, Hamming JF. Systematic review of the prevalence and characteristics of battle casualties from NATO coalition forces in Iraq and Afghanistan. Injury. 2014;45(7):1028–1034. doi: 10.1016/j.injury.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Martin M, Oh J, Currier H, Tai N, Beekley A, Eckert M, Holcomb J. An analysis of in-hospital deaths at a modern combat support hospital. J Trauma. 2009;66(Suppl 4):S51–S60. doi: 10.1097/TA.0b013e31819d86ad. discussion S-1. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadopoulos D, Siempis T, Theodorakou E, Tsoulfas G. Hepatic ischemia and reperfusion injury and trauma: current concepts. Arch Trauma Res. 2013;2(2):63–70. doi: 10.5812/atr.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satterly SA, Martin M, Wingerd M, Hempel J, Hoffer Z, Stallings JD. Flutamide fails to reduce resuscitation requirements in a porcine ischemia-reperfusion model. J Surg Res. 2013;184(1):472–479. doi: 10.1016/j.jss.2013.04.083. [DOI] [PubMed] [Google Scholar]
- 8.Waxman K. Shock: ischemia, reperfusion, and inflammation. New Horiz. 1996;4(2):153–160. [PubMed] [Google Scholar]
- 9.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1300–L1308. doi: 10.1152/ajplung.2001.280.6.L1300. [DOI] [PubMed] [Google Scholar]
- 10.Rachek LI, Grishko VI, Ledoux SP, Wilson GL. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic Biol Med. 2006;40(5):754–762. doi: 10.1016/j.freeradbiomed.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi AB, Gillespie MN. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L530–L535. doi: 10.1152/ajplung.00255.2004. [DOI] [PubMed] [Google Scholar]
- 12.Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells. Free Radic Biol Med. 2011;50(9):1107–1113. doi: 10.1016/j.freeradbiomed.2010.10.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37(8):2539–2548. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10(12):e1001577. doi: 10.1371/journal.pmed.1001577. discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care. 2013;28(6):1027–1031. doi: 10.1016/j.jcrc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Sursal T, Stearns-Kurosawa DJ, Itagaki K, Oh SY, Sun S, Kurosawa S, Hauser CJ. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013;39(1):55–62. doi: 10.1097/SHK.0b013e318276f4ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma. 2010;24(9):534–538. doi: 10.1097/BOT.0b013e3181ec4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MV, Yang X-M, Cui L, Wilson GL, Alexeyev M, Gillespie MN, Downey JM. Is Mitochondrial DNA fragmentation a major contributor to necrosis in myocardial infarction? Circulation. 2014;130(Suppl):A0000. [Google Scholar]
- 21.Yang XM, Cui L, Wilson GL, Alexeyev M, Gillespie MN, Downey JM, Cohen MV. Mitochondrially targeted endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Res Cardiol. 2015;110(2):3. doi: 10.1007/s00395-014-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebb SA, Decoux A, Waggoner A, Wilson GL, Gillespie MN. Mitochondrial DNA damage mediates hyperoxic dysmorphogenesis in rat fetal lung explants. Neonatology. 2013;103(2):91–97. doi: 10.1159/000342632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC. Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol Lung Cell Mol Physiol. 2013;304(4):L287–L297. doi: 10.1152/ajplung.00071.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Wilson GL, Gillespie MN, Parker JC. Mitochondrial targeted endonuclease III DNA repair enzyme protects against ventilator induced lung injury in mice. Pharmaceuticals (Basel) 2014;7(8):894–912. doi: 10.3390/ph7080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carre JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182(6):745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Athale J, Ulrich A, Chou Macgarvey N, Bartz RR, Welty-Wolf KE, Suliman HB, Piantadosi CA. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med. 2012;53(8):1584–1594. doi: 10.1016/j.freeradbiomed.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med. 2012;53(11):2043–2053. doi: 10.1016/j.freeradbiomed.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann RK, Brounts LR, Lesperance KE, Eckert MJ, Lesperance RN, Beekley AC, Sebesta JA, Martin MJ. Hypoxemic versus normoxemic reperfusion in a large animal model of severe ischemia-reperfusion injury. J Surg Res. 2011;166(2):194–198. doi: 10.1016/j.jss.2010.05.052. [DOI] [PubMed] [Google Scholar]
- 29.Lesperance RN, Lehmann RK, Harold DM, Beekley AC, Sebesta JA, Martin MJ. Recombinant factor VIIa is effective at reversing coagulopathy in a lactic acidosis model. J Trauma Acute Care Surg. 2012;72(1):123–129. doi: 10.1097/TA.0b013e318224e24a. [DOI] [PubMed] [Google Scholar]
- 30.Pastukh VM, Gorodnya OM, Gillespie MN, Ruchko MV. Regulation of mitochondrial genome replication by hypoxia: the role of DNA oxidation in D-loop region. Free Radic Biol Med. 2016;96:78–88. doi: 10.1016/j.freeradbiomed.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuck JL, Obiako BO, Gorodnya OM, Pastukh VM, Kua J, Simmons JD, Gillespie MN. Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am J Physiol Lung Cell Mol Physiol. 2015;308(10):L1078–L1085. doi: 10.1152/ajplung.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591–596. doi: 10.1097/SLA.0b013e3182a4ea46. discussion 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784(1):159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Wempe SL, Gamarra-Luques CD, Telleria CM. Synergistic lethality of mifepristone and LY294002 in ovarian cancer cells. Cancer Growth Metastasis. 2013;6:1–13. doi: 10.4137/CGM.S11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujiwara M, Izuishi K, Sano T, Hossain MA, Kimura S, Masaki T, Suzuki Y. Modulating effect of the PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic cancer cells. J Exp Clin Cancer Res. 2008;27:76. doi: 10.1186/1756-9966-27-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao M, Wang J, Wang W, Liu J, Wong CW. Phosphatidylinositol 3-kinase affects mitochondrial function in part through inducing peroxisome proliferator-activated receptor γ coactivator-1β expression. Br J Pharmacol. 2011;162(4):1000–1008. doi: 10.1111/j.1476-5381.2010.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jane EP, Premkumar DR, Morales A, Foster KA, Pollack IF. Inhibition of phosphatidylinositol 3-kinase/AKT signaling by NVP-BKM120 promotes ABT-737–induced toxicity in a caspase-dependent manner through mitochondrial dysfunction and DNA damage response in established and primary cultured glioblastoma cells. J Pharmacol Exp Ther. 2014;350(1):22–35. doi: 10.1124/jpet.114.212910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia-reperfusion. Pathophysiology. 2001;8(1):11–20. doi: 10.1016/s0928-4680(01)00063-3. [DOI] [PubMed] [Google Scholar]
- 39.Collange O, Charles AL, Lavaux T, Noll E, Bouitbir J, Zoll J, Chakfé N, Mertes M, Geny B. Compartmentalization of inflammatory response following gut ischemia reperfusion. Eur J Vasc Endovasc Surg. 2015;49(1):60–65. doi: 10.1016/j.ejvs.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Poggetti RS, Moore EE, Moore FA, Koike K, Banerjee A. Gut ischemia/reperfusion-induced liver dysfunction occurs despite sustained oxygen consumption. J Surg Res. 1992;52(5):436–442. doi: 10.1016/0022-4804(92)90308-m. [DOI] [PubMed] [Google Scholar]