Abstract
Background
Inhaled corticosteroids (ICS) offer targeted treatment for bronchopulmonary dysplasia (BPD) with minimal systemic effects compared to systemic steroids. However, dosing of ICS in the management of infants at high-risk of developing BPD is not well established. The objective of this study was to determine an effective dose of ICS for the treatment of ventilator-dependent infants to facilitate extubation or reduce fractional inspired oxygen concentration.
Methods
Forty-one infants born at < 32 weeks gestational age (GA) or < 1250 g who were ventilator-dependent at 10–28 days postnatal age were included. A non-randomized dose-ranging trial was performed using aerosolized inhaled beclomethasone with hydrofluoralkane propellant (HFA-BDP). Four dosing groups (200, 400, 600 and 800 μg twice daily for 1 week) with 11, 11, 10 and 9 infants in each group, respectively, were studied. The primary outcome was therapeutic efficacy (successful extubation or reduction in FiO2 of > 75% from baseline) in ≥60% of infants in the group. Oxygen requirements, complications and long-term neurodevelopmental outcomes were also assessed.
Results
The median age at enrollment was 22 (10–28) postnatal days. The primary outcome, therapeutic efficacy as defined above, was not achieved in any group. However, there was a significant reduction in post-treatment FiO2 at a dose of 800 μg bid. No obvious trends were seen in long-term neurodevelopmental outcomes.
Conclusions
Therapeutic efficacy was not achieved with all studied doses of ICS. A significant reduction in oxygen requirements was noted in ventilator-dependent preterm infants at 10–28 days of age when given 800 μg of HFA-BDP bid. Larger randomized trials of ICS are required to determine efficacy for the management of infants at high-risk for development of BPD.
Trial registration
This clinical trial was registered retrospectively on clinicaltrials.gov. The registration number is NCT03503994.
Keywords: Infant-newborn, Preterm, Inhaled steroid, Metered dose inhaler, Bronchopulmonary dysplasia
Background
While many short-term morbidities associated with extreme prematurity have declined over the last two decades, the incidence of bronchopulmonary dysplasia (BPD) has increased to a rate of approximately 45% in neonates < 28 weeks gestational age (GA) and birth weight (BW) < 1500 g [1, 2]. Neonates with BPD are at increased risk for adverse short-and long-term neurodevelopmental and respiratory outcomes that often persist into adulthood [3, 4].
There is a growing body of pathological and biochemical evidence that implicates inflammation in its pathogenesis [5–7]. This is further supported by randomized controlled trials (RCTs) that demonstrate the efficacy of systemic corticosteroids in facilitating extubation and reducing BPD [8, 9]. However, several short- and long-term adverse effects associated with the use of systemic corticosteroids have been described [8–10], the most concerning of which is their effect on neurodevelopment, specifically an increased rate of cerebral palsy (CP) [11].
Inhaled corticosteroids (ICS) are an attractive alternative to systemic steroids because of these concerns. Earlier systematic reviews had not found any benefit in using ICS for the prevention or treatment of BPD [12]. However, a recent systematic review showed a significant reduction in death or BPD at 36 weeks’ corrected GA (CGA) (risk ratio = 0.86, 95% confidence interval 0.75, 0.99), BPD (RR = 0.77, 95% CI 0.65, 0.91), and use of systemic steroids (RR = 0.87, 95% CI 0.76, 0.98) in infants treated with ICS [13].
Despite growing evidence of the effectiveness of ICS for BPD, uncertainty remains over treatment timing, effective dose, and long-term effects. There is also variation in the delivery systems used for delivery of ICS. These concerns continue to be echoed in a recent review by Nelin et al. [14]. Given that the long-term neurodevelopmental impact of ICS were unknown at the time of this study and many infants are able to wean from ventilation without steroids, we conducted an escalating-dose ranging study of late ICS (i.e. administered after the first week of life) delivered by a metered dose inhaler (MDI) utilizing a specially designed valved delivery system to determine the minimum effective dose necessary to achieve extubation or reduction in oxygen requirements and the long-term neurodevelopmental impact of increasing doses of ICS.
Methods
The study was conducted in the NICUs of Mount Sinai Hospital (MSH), and Sunnybrook Health Sciences Centre (SHSC), Toronto, Ontario and Izaak Walton Killam (IWK) Health Centre, Halifax, Nova Scotia, Canada from March 2002 to October 2006. The Mount Sinai Hospital Research Ethics Board, the Sunnybrook Health Sciences Centre Research Ethics Board and the Izaak Walton Killam Research Ethics Board approved this study.
Study population
Neonates with BW < 1250 g and GA < 32 weeks with need for mechanical ventilation (defined as conventional ventilation with a rate > 15 breaths/min or high frequency oscillatory ventilation) and fractional inspired oxygen concentration (FiO2) of > 0.30 but < 0.60), postnatal age of 10–28 days and stable ventilator requirements over the 48–72 h prior to enrollment were included. Baseline demographics, including GA, BW, Apgar scores at 1 and 5 min of life, and presence of respiratory distress syndrome (FiO2 > 0.30 or significant work of breathing and/or the need for surfactant therapy) were collected (Table 1). Neonates with actual or suspected sepsis, congenital cardiorespiratory malformation, patent ductus arteriosus, any stage of necrotizing enterocolitis (NEC), gastrointestinal hemorrhage, perforation or treatment with systemic dexamethasone were excluded. These criteria were chosen in order to avoid confounding with other coexisting conditions that may result in inability to wean ventilation or necessitating high FiO2.
Table 1.
Demographic characteristics of the study population
| Variablea | 200 μg bid (n = 11) |
400 μg bid (n = 11) |
600 μg bid (n = 10) |
800 μg bid (n = 9) |
p-value |
|---|---|---|---|---|---|
| Gestational age (weeks), mean (SD) | 25.7 (1.3) | 25.6 (1.2) | 25.7 (1.6) | 25.4 (1.6) | 0.97 |
| Birth weight (grams), mean (SD) | 788 (224) | 802 (231) | 749 (209) | 754 (188) | 0.91 |
| Apgar score at 1 min, median (range) | 5 (1, 7) | 6 (2, 8) | 5 (1,9) | 3 (2, 9) | 0.33 |
| Apgar score at 5 min, mean (range) | 8 (4, 9) | 8 (4, 9) | 8 (5, 10) | 7 (3, 8) | 0.81 |
| Respiratory distress syndrome, % | 100 | 100 | 90 | 100 | 0.37 |
a bid = twice a day, μg microgram, n number, SD Standard deviation
Drug regimen and delivery
Groups of neonates were treated with escalating doses (200, 400, 600 and 800 μg twice daily [15]) of hydrofluoralkane beclomethasone dipropionate (HFA-BDP) until efficacy or significant side effects were observed. If the baby was extubated within 7 days after dosing had commenced, administration of ICS was discontinued at that point. Ventilatory settings were temporarily adjusted to achieve an expiratory tidal volume of 7 ml/kg (based on current weight) with a respiratory rate of 30 breaths/minute and positive end expiratory pressure (PEEP) of 5–8 cm H2O. Arterial oxygen saturation was maintained within the target range of 88–92%. Following drug administration, the delivery system was removed and baby returned to the previous ventilatory settings. Infants who were on HFO ventilation were transferred onto conventional ventilation for the treatment. The assessors were not aware of the dosing allocations.
Adverse events
Adverse events including hypertension (defined as BP higher than 2 standard deviations [SD] above the mean for the infant’s gestational and postnatal age), [16] hyperglycemia (defined as a blood glucose > 10 μmol/L), impaired growth (defined as weight loss or head circumference decreases crossing percentile lines on the sex-specific Fenton chart), [17] sepsis, evidence of feeding intolerance, NEC or intestinal perforation, and oropharyngeal candidiasis were recorded pre- and post-treatment. The Safety Committee reviewed adverse events in a particular dosing group before providing approval for recruitment of patients into the subsequent dosing group or recommendation to stop the trial.
Primary outcome
The primary outcome was therapeutic efficacy, defined by successful extubation or reduction in FiO2 of > 75% from the baseline within the one-week study period in ≥ 6 out of 10 infants in each group set a priori. This was based on expert consensus and on previous similar studies [18, 19]. Pre- and post-treatment FiO2 was determined by calculating the mean of the FiO2 requirement over the preceding 48 h and over the final 48 h of the study period, respectively. If a patient was extubated successfully, the post-treatment FiO2 was determined by calculating the mean of the FiO2 measured over the last 48 h while intubated. A standard weaning protocol was used prior to extubation from the ventilator. Once the neonate had achieved a MAP of 8 cm H2O, ventilator rate of 10–12 breaths/minute and FiO2 < 0.30, the neonate was to be extubated, at the discretion of the clinical team. Extubation was considered successful if the infant did not require assisted ventilation in the following 48 h.
Secondary outcomes
Secondary outcomes included long-term neurodevelopmental outcome. Long-term motor and cognitive function were assessed using validated tests administered by trained personnel in the respective Neonatal Follow-up Clinics. The use of specific tests was not mandated but levels of impairment were aligned. Standardized developmental testing was performed at 18–36 months CA and the assessors were not aware of the dosing allocation. Motor impairment was defined as the presence or absence of CP and, if present, was assessed for severity by Gross Motor Functional Classification Scale- Extended and Revised (GMFCS-E&R); severe CP (non-ambulatory) was defined as GMFCS ≥3 [20]. Severe cognitive impairment was defined as a Bayley Scales of Infant Development-2nd Edition (BSID-II) Mental Developmental Index of < 70, a Bayley Scales of Infant Development 3rd Edition (BSID-III) cognitive score of < 85, [21] or scores below 2 standard deviations from the mean on the Differential Abilities Scales (DAS), a comprehensive test to assess cognitive abilities important to learning [22] or the Clinical Adaptive Test/Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS), a screening tool for children suspected of having developmental concerns [23]. Mild to moderate cognitive delay was defined as BSID-II Mental Developmental Index score 70–85 or BSID-III cognitive score of 85–100.
Statistical analysis
Continuous data were analyzed using analysis of variance while categorical data were analyzed using chi-square test. A p value of < 0.05 was considered statistically significant. The frequency of the neurodevelopmental characteristics above were calculated for each dosage group. Descriptive statistics are used to present the data for neurodevelopmental outcome given the small numbers and varying tests used. A sample size of 10 neonates per dosing group was chosen, similar to other investigational phase II clinical trials [24]. A formal sample size calculation based on a power of 80% and ɑ error threshold of 0.0125 (corrected for 4 dosing groups) for the primary outcome of extubation or reduction in FiO2 in 2/3 of the infants in each group based on previous work done by Ohlsson et al. with dexamethasone yielded a sample size of 8 per dosing group [25]. Accounting for 20% attrition, the total sample size for each group was determined to be 10. When the total sample size for each dosing level was met, data were reviewed by a Safety Committee. If no concerns were identified, approval to proceed to recruitment of the next dosing group was given. All neonates in each group were treated with the same dose of ICS.
Results
A total of 41 subjects were recruited for the study - 11, 11, 10 and 9 infants were enrolled in the 200 μg, 400 μg, 600 μg, and 800 μg bid groups, respectively. As different centers were recruiting simultaneously, 41 subjects instead of the planned 40 were recruited. In the last dosage group, once 9 patients were recruited, it was apparent that the group was not going to achieve efficacy based on the criteria defined and hence recruitment was stopped.
Table 1 shows the baseline demographic characteristics for each dosage group. No significant differences were noted for GA, BW or Apgar scores at 1 and 5 min of life. The mean GA of participating infants was 25 weeks with a mean BW of 775 g.
Table 2 shows the therapeutic efficacy and FiO2 requirements prior to and after treatment with inhaled HFA-BDP. The age of commencement of therapy varied from 10 to 28 days with a median of 22 postnatal days across dosage groups. Therapeutic efficacy was not achieved in any group. There was, however a significant reduction in post-treatment FiO2 to 0.30 in the group of neonates receiving 800 μg bid of inhaled HFA-BDP while pre-treatment FiO2 did not differ.
Table 2.
Therapeutic efficacy and FiO2 requirements at varying doses of HFA-BDPa
| Variablea | 200 μg bid (n = 11) |
400 μg bid (n = 11) |
600 μg bid (n = 10) |
800 μg bid (n = 9) |
p-value |
|---|---|---|---|---|---|
| Age at commencement of therapy (days), median (range) | 21 (12, 27) | 26 (10, 28) | 22.5 (14, 26) | 22 (12, 28) | 0.76 |
| Pre-treatment FiO2, mean (SD) | 0.40 (0.086) | 0.39 (0.039) | 0.40 (0.10) | 0.37 (0.044) | 0.78 |
| Post-treatment FiO2, mean (SD) | 0.40 (0.074) | 0.33 (0.071) | 0.32 (0.075) | 0.30 (0.064) | 0.02 |
| Therapeutic efficacy | 2/11 | 2/11 | 3/10 | 3/9 | 0.80 |
aHFA-BDP Hydrofluoralkane beclomethasone dipropionate, bid twice a day, FiO2 Fraction of inspired oxygen, μg microgram, n number, SD Standard deviation
Table 3 shows the neurodevelopmental presentations of enrolled infants. Four infants were lost to follow-up. Three infants were diagnosed with CP and all were ambulatory with GMFCS I. Cognitive function was normal in all infants except one each in the 200 μg and 400 μg groups and two infants in the 800 μg group. Additionally, 2 infants in the 400 μg and 1 infant in the 800 μg group were unable to undergo formal testing. Informal clinical assessment determined that one was normal and two had mild cognitive impairment. There were no obvious trends seen in assessment scores at escalating doses of ICS.
Table 3.
Neonatal neurodevelopmental outcomes following escalating doses HFA-BDPa
| Dose | Total Number Assessed | Cerebral palsy, n (%) | Normal or mild cognitive impairment, n (%) | Severe cognitive impairment, n (%) |
|---|---|---|---|---|
| 200 μg bid (n = 11) |
10 | 1/10 (10) | 9 (90) | 1 (10) |
| 400 μg bid (n = 11) |
10 | 1/10 (10) | 9 (90) | 1 (10) |
| 600 μg bid (n = 10) |
9 | 0/9 (0) | 9 (100) | 0 (0) |
| 800 μg bid (n = 9) |
8 | 1/8 (12.5) | 6 (75) | 2 (25) |
aHFA-BDP Hydrofluoralkane beclomethasone dipropionate, bid twice a day, μg microgram, n number, % percentage
One infant in the 800 μg group was noted to have hyperglycemia, but required no intervention. There were no other adverse events noted.
Discussion
Therapeutic efficacy as defined in the protocol was not reached. However, ICS administered to ventilator dependent preterm infants between 10 and 28 days of age at a dose of 800 μg bid resulted in a significant reduction in oxygen requirements after only 1 week of treatment. Thus, this study may provide a starting effective dose of HFA-BDP that can be utilized for future clinical trials. In comparison, Bassler et al. used inhaled budesonide at a dose of 400 μg bid using a very similar delivery system [26]. Previous pharmacokinetic studies indicate that budesonide is approximately 1.6 times more potent than beclomethasone [27]. Thus, at doses equivalent to approximately 500 μg bid of inhaled budesonide, reductions in FiO2 requirements were seen. Given that the study was not powered to detect a reduction in FiO2, these results should be interpreted with caution.
Based on limited data, even though it appears that two infants in the 800 μg group developed severe cognitive delay, no definitive conclusions can be made regarding neurodevelopmental impact. A dose-dependent trend in cognitive function was not observed and no difference in the motor function was noted.
The use of ICS for the prevention of BPD for infants born < 28 weeks GA has been studied in a number of RCTs and systematic reviews [12, 13, 26, 28]. In a recent randomized study with 441 patients randomized to budesonide and 422 patients randomized to placebo, Bassler et al. [26] investigated the use of early inhaled budesonide within the first 24 h of life. In this study, a reduction in BPD was noted but with a trend toward increased mortality. On the other hand, Nakamura et al. [28] studied 107 infants treated early with fluticasone propionate at a dose of 100 μg and 104 patients treated with placebo and demonstrated no difference in the incidence of death or oxygen dependency at discharge. However, a significant reduction in death or oxygen dependence at discharge was noted for infants between 24 and 26 weeks GA or with chorioamnionitis suggesting that ICS treatment may benefit certain high-risk subpopulations. When these two large studies were included in meta-analyses, ICS used for prevention or treatment of BPD significantly reduced the incidence of BPD and death or BPD [13, 26]. Long-term follow-up from the study by Bassler et al. is pending.
Inhaled corticosteroids used after 7 postnatal days have also been studied. A systematic review of these studies revealed no significant reduction in death or BPD, although the drug types, dosing regimens and delivery systems varied widely [29]. The sample sizes ranged from 19 to 86 infants randomized to either ICS or placebo and the total population included in the systematic review for the primary outcome of death or BPD at 36 weeks PMA was 232. There was a trend towards less use of systemic corticosteroids. In addition, a recent review found that despite small numbers in the included trials, many showed acute changes in pulmonary mechanics following administration of ICS to chronically ventilated preterm infants with BPD [14]. Two of the studies included used inhaled beclomethasone and showed higher rates of successful extubation, increased dynamic airway compliance and reduced airway resistance in oxygen-dependent preterm infants [29]. Neither study showed differences in BPD or mortality. These results are consistent with the findings in our study. However, studies using beclomethasone have been small to date.
Our delivery system included an MDI with a propellant that resulted in an aerosol with ideal particle size to enhance deposition. We also used a specifically designed spacer that employed a valve system to improve aerosol delivery. Aerosolized HFA-BDP was administered using a specifically designed neonatal aerosol delivery system (Fig. 1) which included an MDI attached to a valved aerochamber. The device was inserted between the endotracheal tube and the WYE-connector of the ventilator circuit. This device is the prototype of the Aerochamber MiniR used in the study by Bassler et al. [26]. In addition, hydrofluoralkane propellant has been found to allow generation of smaller sized particles and more uniform deposition compared to chloroflurocarbon (CFC) propellant [30]. Despite the improved characteristics of HFA-BDP, overall deposition was estimated to be only 1–2% of the administered dose based on unpublished performance studies (personal communication, M. Dolovich). Thus, therapeutic efficacy may not have been achieved due to poor deposition. On the other hand, studies have shown that MDI delivery is more efficient at ICS delivery than jet nebulizers [31, 32]. This may explain why some studies of ICS did not show significant reductions in oxygen requirements [15]. In addition, while older MDI systems had used CFC propellants, HFA propellant was used in this study and has been found to allow generation of smaller sized particles and more uniform deposition [30]. Thus, more appropriate drug delivery may explain why oxygen requirements were reduced in our study.
Fig. 1.
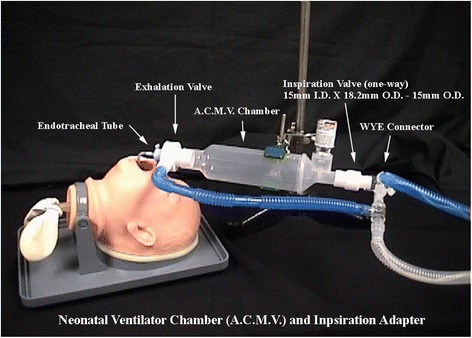
Neonatal Delivery System. Aerosolized HFA-BDP was administered using this specifically designed neonatal aerosol delivery system, including an MDI attached to a valved aerochamber (A.C.M.V). The device was inserted between the endotracheal tube and the WYE-connector of the ventilator circuit. This device is the prototype of the Aerochamber MiniR setup
Long-term outcomes of infants treated with prolonged courses of ICS are lacking. Nakamura et al. [28] did not demonstrate any significant differences in NDI or CP. In our study, it is reassuring that despite high doses of beclomethasone, there were no obvious differences in long-term neurodevelopmental outcomes. There was actually a trend toward higher rates of mild to moderate impairment in infants receiving lower doses of ICS, indicating that BPD itself may contribute more to poor neurodevelopment.
The strength of this study was its use of escalating doses to determine a pattern of response and the drug delivery system studied. The limitations include the small sample size, and lack of randomization. In addition, patients with PDA were excluded from the study, which limits in generalizability. The other limitation is the delay in publication as a result of individual and system challenges. Since the study period, delivery room practices have evolved to increasing use of early non-invasive ventilation and has resulted in decreased use of prophylactic surfactant with some evidence that these strategies may reduce exposure to invasive ventilation and eventually, chronic lung disease [33–37]. NICU practices have also changed, including less use of systemic steroids, [1] universal, early use of caffeine [38] and increased use of non-invasive modes of ventilation early. However, despite all these changes, the rates of BPD have not improved [1, 2]. As we move toward resuscitating smaller and more preterm infants, [39] BPD will likely remain a significant issue in NICU practice and safe, less invasive, effective therapies are imperative to reducing the morbidity associated with extreme prematurity. Thus, it is our view that ICS may provide a safe and reasonable alternative to systemic steroids in infants who are ventilator-dependent in their 2nd and 3rd week of life and that a larger randomized trial of late inhaled steroids for these children is warranted. In addition, this study provides evidence that the in-line MDI system is an effective way to deliver ICS and that this drug delivery system should be further developed to specifically support ventilated and non-ventilated neonates.
Conclusions
Treatment with ICS administered using a specially designed system appears to reduce the oxygen requirements in preterm neonates with early BPD with minimal impact on long-term neurodevelopment. Further research into the mechanism of action and appropriate treatment regimens is warranted. Larger studies that examine their impact on long-term neurodevelopment are also warranted.
Acknowledgements
The authors wish to acknowledge Martin Foley of Trudell Medical International for his collaboration in the development and evaluation of the valved chamber used to administer the medication.
Funding
The original study was funded by 3 M Canada™ and the aerosol delivery system was designed and provided for the study by Trudell Medical International©. The funding bodies did not participate in the design of the study, the collection of the data, its analysis or its interpretation, or in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BPD
Bronchopulmonary dysplasia
- BSID-II/III
Bayley scales of infant development mental development index
- BW
Birth weight
- CAT/CLAMS
The Clinical Adaptive Test/Clinical Linguistic and Auditory Milestone Scale
- CFC
Chloroflurocarbon
- CGA
Corrected gestational age
- CP
Cerebral palsy
- DAS
Differential Abilities Scales
- FiO2
Fraction of inspired oxygen
- GA
Gestational age
- GMFCS
Gross Motor Function Classification Scale
- HFA-BDP
Hydrofluoralkane beclomethasone dipropionate
- ICS
Inhaled corticosteroids
- MAP
Mean airway pressure
- MDI
Metered dose inhaler
- NDI
Neurodevelopmental impairment
- NEC
Necrotizing enterocolitis
- PEEP
Positive end-expiratory pressure
- RCT
Randomized controlled trial
Authors’ contributions
KR, VS, EVA, KJ and MD conceptualized the study design and wrote or edited the manuscript for publication. MR collected the majority of the data and contributed to the editing of the manuscript. EVA, EK and MV were responsible for the neurodevelopmental assessments of the children enrolled in the study and EK and MV contributed to editing the final manuscript. All authors have read and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Ethics approval and consent to participate
The study was approved by the Mount Sinai Hospital Research Ethics Board, the Sunnybrook Health Sciences Centre Research Ethics Board and the Izaak Walton Killam Research Ethics Board and Health Canada (Health Canada Clinical Trial Control Number 077174, CR File Number 9427-M2216-37C). Parents of potentially eligible neonates were identified and written consent obtained.
Competing interests
Dr. Elizabeth Asztalos is a Neonatology section editor for BMC Pediatrics, but was no involved in the review process for this article. Dr. Dunn is a co-inventor on the patent for the Neonatal Aerosol Delivery System (Trudell Medical International©) but receives no royalties. There are no other competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kamini Raghuram, Email: Kamini.raghuram@mail.utoronto.ca.
Michael Dunn, Email: Michael.dunn@sunnybrook.ca.
Krista Jangaard, Email: krista.jangaard@dal.ca.
Maureen Reilly, Email: Maureen.reilly@sunnybrook.ca.
Elizabeth Asztalos, Email: Elizabeth.asztalos@sunnybrook.ca.
Edmond Kelly, Email: Edmond.kelly@sinaihealthsystem.ca.
Michael Vincer, Email: Michael.vincer@iwk.nshealth.ca.
Vibhuti Shah, Phone: 416-586-4816, Email: vibhuti.shah@sinaihealthsystem.ca.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SK, Shah PS, Singhal N, Aziz K, Synnes A, McMillan D, et al. Association of a quality improvement program with neonatal outcomes in extremely preterm infants: a prospective cohort study. CMAJ. 2014;186(13):E485–E494. doi: 10.1503/cmaj.140399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle LW, Group VICS Respiratory function at age 8-9 years in extremely low birthweight/very preterm children born in Victoria in 1991-1992. Pediatr Pulmonol. 2006;41(6):570–576. doi: 10.1002/ppul.20412. [DOI] [PubMed] [Google Scholar]
- 4.Priante E, Moschino L, Mardegan V, Manzoni P, Salvadori S, Baraldi E. Respiratory outcome after preterm birth: a long and difficult journey. Am J Perinatol. 2016;33(11):1040–1042. doi: 10.1055/s-0036-1586172. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol. 2016;33(11):1076–1078. doi: 10.1055/s-0036-1586107. [DOI] [PubMed] [Google Scholar]
- 6.Groneck P, Götze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics. 1994;93(5):712–718. [PubMed] [Google Scholar]
- 7.Pierce MR, Bancalari E. The role of inflammation in the pathogenesis of bronchopulmonary dysplasia. Pediatr Pulmonol. 1995;19(6):371–378. doi: 10.1002/ppul.1950190611. [DOI] [PubMed] [Google Scholar]
- 8.Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014;5:CD001145. doi: 10.1002/14651858.CD001145.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014;5:CD001146. doi: 10.1002/14651858.CD001146.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development neonatal research network. N Engl J Med. 2001;344(2):95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 11.Shinwell ES, Eventov-Friedman S. Impact of perinatal corticosteroids on neuromotor development and outcome: review of the literature and new meta-analysis. Semin Fetal Neonatal Med. 2009;14(3):164–170. doi: 10.1016/j.siny.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database Syst Rev. 2012;5:CD002058. doi: 10.1002/14651858.CD002058.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Shinwell ES, Portnov I, Meerpohl JJ, Karen T, Bassler D. Inhaled corticosteroids for bronchopulmonary dysplasia: a meta-analysis. Pediatr. 2016;138(6). 10.1542/peds.2016-2511. [DOI] [PubMed]
- 14.Nelin LD, Logan JW. The use of inhaled corticosteroids in chronically ventilated preterm infants. Semin Fetal Neonatal Med. 2017;22(5):296–301. doi: 10.1016/j.siny.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Jónsson B, Eriksson M, Söder O, Broberger U, Lagercrantz H. Budesonide delivered by dosimetric jet nebulization to preterm very low birthweight infants at high risk for development of chronic lung disease. Acta Paediatr. 2000;89(12):1449–1455. doi: 10.1111/j.1651-2227.2000.tb02775.x. [DOI] [PubMed] [Google Scholar]
- 16.Dionne JM, Abitbol CL, Flynn JT. Hypertension in infancy: diagnosis, management and outcome. Pediatr Nephrol. 2012;27(1):17–32. doi: 10.1007/s00467-010-1755-z. [DOI] [PubMed] [Google Scholar]
- 17.Fenton TRA. New growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, Investigators DS. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatr. 2006;117(1):75–83. doi: 10.1542/peds.2004-2843. [DOI] [PubMed] [Google Scholar]
- 19.Kazzi NJ, Brans YW, Poland RL. Dexamethasone effects on the hospital course of infants with bronchopulmonary dysplasia who are dependent on artificial ventilation. Pediatr. 1990;86(5):722–727. [PubMed] [Google Scholar]
- 20.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised gross motor function classification system. Dev Med Child Neurol. 2008;50(10):744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharp M, DeMauro SB. Counterbalanced comparison of the BSID-II and Bayley-III at eighteen to twenty-two months corrected age. J Dev Behav Pediatr. 2017;38(5):322–329. doi: 10.1097/DBP.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 22.Elliott C. Differential ability scales ® -II (DAS-II ® ): Pearson education, Inc. 2007. [Google Scholar]
- 23.Wachtel RC, Shapiro BK, Palmer FB, Allen MC, Capute AJ. CAT/CLAMS. A tool for the pediatric evaluation of infants and young children with developmental delay. Clinical adaptive test/clinical linguistic and auditory milestone scale. Clin Pediatr (Phila) 1994;33(7):410–415. doi: 10.1177/000992289403300706. [DOI] [PubMed] [Google Scholar]
- 24.Huang J-H, Su Q-M, Yang J, Lv Y-H, He Y-C, Chen J-C, et al. Sample sizes in dosage investigational clinical trials: a systematic evaluation. Drug Des, Devel Ther. 2015;9:305–312. doi: 10.2147/DDDT.S76135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohlsson A, Calvert SA, Hosking M, Shennan AT. Randomized controlled trial of dexamethasone treatment in very-low-birth-weight infants with ventilator-dependent chronic lung disease. Acta Paediatr. 1992;81(10):751–756. doi: 10.1111/j.1651-2227.1992.tb12096.x. [DOI] [PubMed] [Google Scholar]
- 26.Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau PH, Carnielli V, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med. 2015;373(16):1497–1506. doi: 10.1056/NEJMoa1501917. [DOI] [PubMed] [Google Scholar]
- 27.Colice G. Comparing inhaled corticosteroids. Respir Care. 2000;45(7):846–853. [PubMed] [Google Scholar]
- 28.Nakamura T, Yonemoto N, Nakayama M, Hirano S, Aotani H, Kusuda S, et al. Early inhaled steroid use in extremely low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2016; 10.1136/archdischild-2015-309943. [DOI] [PubMed]
- 29.Onland W, Offringa M, van Kaam A. Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2012;4:CD002311. doi: 10.1002/14651858.CD002311.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Leach CL, Davidson PJ, Boudreau RJ. Improved airway targeting with the CFC-free HFA-beclomethasone metered-dose inhaler compared with CFC-beclomethasone. Eur Respir J. 1998;12(6):1346–1353. doi: 10.1183/09031936.98.12061346. [DOI] [PubMed] [Google Scholar]
- 31.Fok TF, Monkman S, Dolovich M, Gray S, Coates G, Paes B, et al. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1996;21(5):301–309. doi: 10.1002/(SICI)1099-0496(199605)21:5<301::AID-PPUL5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Fok TF, Lam K, Ng PC, Leung TF, So HK, Cheung KL, et al. Delivery of salbutamol to nonventilated preterm infants by metered-dose inhaler, jet nebulizer, and ultrasonic nebulizer. Eur Respir J. 1998;12(1):159–164. doi: 10.1183/09031936.98.12010159. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud RA, Roehr CC, Schmalisch G. Current methods of non-invasive ventilatory support for neonates. Paediatr Respir Rev. 2011;12(3):196–205. doi: 10.1016/j.prrv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Bhandari V. The potential of non-invasive ventilation to decrease BPD. Semin Perinatol. 2013;37(2):108–114. doi: 10.1053/j.semperi.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Patel D, Greenough A. Does nasal CPAP reduce bronchopulmonary dysplasia (BPD)? Acta Paediatr. 2008;97(10):1314–1317. doi: 10.1111/j.1651-2227.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 36.Hascoet JM, Espagne S, Hamon I. CPAP and the preterm infant: lessons from the COIN trial and other studies. Early Hum Dev. 2008;84(12):791–793. doi: 10.1016/j.earlhumdev.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Raghuram K, Mukerji A, Young J, Yee W, Seshia M, Dow K, et al. Surfactant utilization and short-term outcomes in an era of non-invasive respiratory support in Canadian neonatal intensive care units. J Perinatol. 2017;37(9):1017–1023. doi: 10.1038/jp.2017.98. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 39.Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801–1811. doi: 10.1056/NEJMoa1410689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


