Abstract
Objective:
To investigate scalp hair as biomarker for chronic fluoride exposure among fluoride endemic and low fluoride areas.
Methodology:
Two areas were identified in Vadodara district, Ajod, as a low fluoride area (Fluoride content of drinking water = 0.11 ppm) and Karsan, a high fluoride area (fluoride content in drinking water = 3.43 ppm). The study was performed on a total of 36 participants from the two villages, 18 from Ajod and 18 from Karsan. Thylstrup Fejerskov Index (TFI) was recorded for each of the participants and sample of hair was collected from the occipital region. Analysis of hair samples was done for assessing the fluoride content.
Results:
The study consisted of total 36 participants, ranging from the age of 34–60 years and a mean age of 46.53 years. The mean TFI score for the participants in Karsan was 3.39 (±0.979) and in Ajod was 0.83 (±0.786). The difference in this mean score between the two groups was found to be statistically significant. Furthermore, the average fluoride content in hair of the participants in Karsan was 3.40(±1.043) and that in Ajod was 0.35 (±0.063). This difference was statistically significant between the two groups. The TFI scores were found to be positively correlated with the fluoride content in hair.
Conclusion:
Hair can be used as a useful biomaterial for fluoride exposure monitoring. Having certain advantages over other biomaterials such as easy to collect, store, and transport, hair also serves as a biomarker of chronic fluoride exposure. Hair analysis should thus play a greater role in routinely measuring the chronic exposure to fluorides.
Key words: Fluoride, scalp hair, Thylstrup Fejerskov Index
INTRODUCTION
Fluorine is one of the 14 physiologically essential elements required for normal growth and development but does not exist in nature in its elemental state. The ionic form fluoride is found abundantly in a wide variety of minerals, including fluorspar and rock phosphate. Due to the universal presence of fluorides in the earth's crust, all water sources contain fluorides in varying concentrations.[1]
Fluoride has been termed as the double-edged sword. When ingested in optimum concentration, fluoride shows preventive action against dental caries. But when ingested in excess, it may lead to dental or skeletal fluorosis.
Consumption of high levels of fluorides from several sources can be detected by means of certain biomarkers. Biomarkers do not diagnose diseases but are an indicator of the disease or an altered physiological state. Plasma, bone, teeth, urine, saliva, dental plaque, plaque fluid, hair, and nails serve as exposure biomarkers for fluorosis, while genetic factors, acid-base disturbances, renal disturbances, bone growth, and nutritional state are the susceptibility biomarkers. Reduction in the activity and severity of the dental caries, dental fluorosis, and skeletal fluorosis may be considered as the biomarkers of effect.[2]
The contemporary biomarkers for fluoride have been urine, plasma, and salivary fluoride content. However, these do not measure the chronic fluoride exposure. Moreover, urinary fluoride excretions and concentrations are variable because of variations in urinary flow and pH. Hence, hair and nails are considered as the recent biomarkers and reflect the average plasma fluoride concentrations over time.[3]
In India, endemic fluorosis is considered to be a major public health problem. According to the available literature, more than 15 states in India are endemic for fluorosis, and more than 60 million people in India suffer from fluorosis, either dental or skeletal.[1]
Analyzing of fluoride intake through hair samples is not a routine practice but is gaining importance, especially when there are instances of environmental exposure to excess fluoride.[4]
Excess fluoride consumption hence shows effects on both teeth, as well as chronic deposition in hair. Thus, the present study was conducted with the aim to evaluate scalp hair as biomarker of chronic fluoride exposure among the fluoride endemic and low fluoride areas and to correlate fluoride content in hair with the levels of dental fluorosis.
METHODOLOGY
This was a cross-sectional study, which involved the collection of scalp hair samples from Ajod, a low fluoride area (fluoride content in drinking water – 0.11 ppm) and Karsan, a fluoride endemic area (fluoride content in drinking water – 3.43 ppm) from Vadodara district.[5]
Included in the study were the individuals in the age group of 35–60 years, who gave their informed written consent for the study and those who were living in the given locality since birth. Individuals living outside the study area, and those who did not agree to participate were excluded from the study.
The sample size was obtained using data from the previous similar study conducted by Parimi et al.[6] The minimum number of participants required to get probability of average hair score of 0.9 of one group higher than second at 1% risk and 90% power was 36. Hence, 18 participants were selected from each village to fulfill the minimum sample size. Systematic random sampling was carried out to enroll the participants who met the inclusion criteria.
The Modified Thylstrup Fejerskov Index (TFI)[7] [Table 1] was used to record the severity of dental fluorosis in the study participants.
Table 1.
Modified Thylstrup Fejerskov Index

The participants were asked to complete a questionnaire asking for general data: age, gender, water supply, and place of residence since birth and oral hygienic practices.
Samples of hair of minimal length 2–3 cm were taken from the occipital region using a pair of scissors and collected in a self-lock clear plastic polybag. After every use, the excess hair was brushed off the scissor blades using a small brush. The pair of scissors was then cleaned using sanitizer and cotton ball before collecting hair sample from next participant.
Following procedure was carried out for analysis of the hair samples:
Samples of hair were placed in a fritted glass filter and then rinsed with acetone, detergent, 2N sulfuric acid, and redistilled water. After drying, 100 mg aliquots were placed into test tubes, treated with concentrated sodium hydroxide solution and heated in a boiling water bath until complete solution (hair 60 min). Cooled and neutralized with 1 M HCl acid and the sample volumes were made up with water to 4 ml and diluted with equal volumes of total ionic strength adjustment buffer. Fluoride concentrations were measured by a fluoride ion specific electrode and an Ag/AgCl reference electrode with a double jacket.[6]
The collected data was entered in Microsoft (2010) spreadsheet. Descriptive statistics was used to calculate the frequency and percentage of participants suffering from different grades of dental fluorosis in the respective low fluoride and fluoride endemic areas as interpreted from the modified TFI. Mann–Whitney U-test was used to compare the mean fluoride content in the scalp hair between the two groups. Chi-square test of association was used to associate the grade of dental fluorosis with the fluoride content of scalp hair for the two groups.
RESULTS
There were a total of 36 individuals who participated in the present study. The distribution of the participants was equal in the two areas chosen for the study. There were 18 participants from Karsan, which was identified as the fluoride endemic area in Vadodara district and 18 from Ajod, identified as a low fluoride area in Vadodara district. Figures 1 and 2 show the gender-wise and age-wise distribution of the participants, respectively. The TFI scores of the participants are illustrated in Figure 3.
Figure 1.
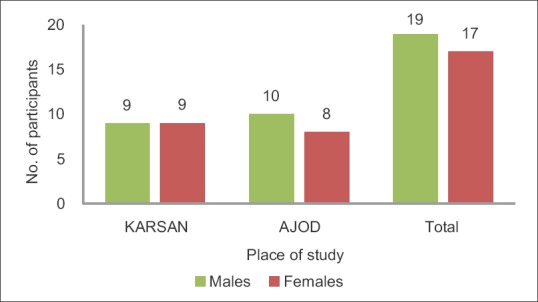
Gender-wise distribution of participants
Figure 2.
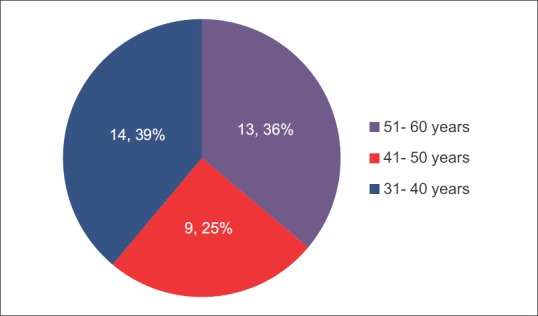
Age-wise distribution of participants
Figure 3.
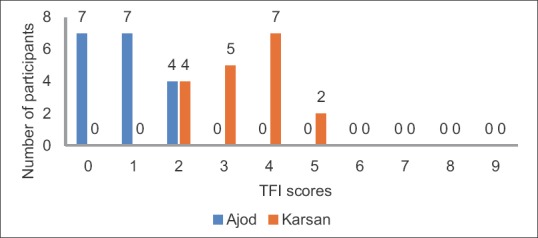
Thylstrup Fejerskov Index scores of the study participants
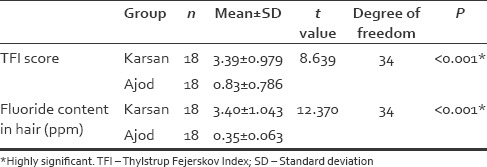
The mean fluoride content in hair samples of participants from Karsan was found to be 3.40 ppm (±1.043). Similarly, the mean fluoride content in hair samples of participants from Ajod was found to be 0.35 ppm (±0.063). The difference in means between the fluoride content in hair was also found to be statistically highly significant using the independent t-test [Table 2].
Table 2.
Comparison of TFI Score and Fluoride Content in hair between Fluoride endemic and low fluoride areas
When plotted on a graph, the TFI scores and the scalp hair fluoride content showed a positive association [Figure 4].
Figure 4.
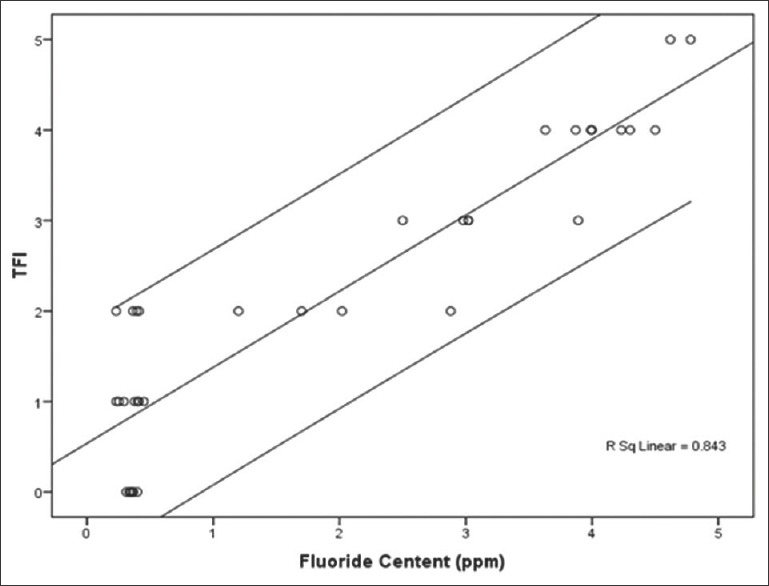
Thylstrup Fejerskov Index scores and mean hair fluoride content of study participants
DISCUSSION
Fluorine is the most abundant element in nature and is essential for the normal mineralization of bone and formation of dental enamel. Yet, fluorosis remains a very important problem in more than 24 countries in the world, including India, which lies in the geographical fluoride belt that extends from Turkey to China and Japan through Iraq, Iran and Afghanistan.
The World Health Organization (WHO) has set the upper limit of fluoride concentration in drinking water at 1.5 mg/dl. The Bureau of Indian Standards has therefore laid down Indian Standards as 1.0 mg/dl as maximum permissible limit of fluoride with further remarks as “lesser the better.” Intake of fluoride higher than the optimal level is the main reason for dental and skeletal fluorosis. The available data suggest that 15 states in India are endemic for fluorosis (fluoride level in drinking water >1.5 mg/l), and about bout 62 million people in India suffer from dental, skeletal, and nonskeletal fluorosis. Out of these, 6 million are children below the age of 14 years. Groundwater is considered as the major source of drinking water in most places on earth.[8]
Time and again, it has been proven that the levels of fluoride in water are directly proportional to the severity of dental fluorosis. High levels of fluoride in biomaterials such as urine and blood have been proposed as the most reliable indicator of the exposure to fluorides.[3]
In the present study, the fluoride content in the hair of participants from endemically fluoridated area was much higher than the participants of low fluoridated area. This finding is consistent with the results of study conducted by Parimi et al.[6] Similar results were also found by Czarnowski and Krechniak[4] and Kokot and Drzeweicki.[9]
The increase in the fluoride content of hair depends on the degree of exposure to fluorides sources, especially water. Trace elements including fluorides, which are of endogenous origin, are absorbed into the blood and subsequently incorporated into the keratin structure of hair during the growth phase. In the present study, cleaning procedure was carried out during the laboratory procedure, to eliminate surface contamination. Hence, only the fluoride of endogenous origin was measured, a major source of which being fluoride content of drinking water. The inclusion criteria for selection of the participants were that they should be residing in the said villages since birth. Hence, the accumulation of fluoride in hair in the fluoride endemic area was a cumulative process over time, which could be demonstrated by the high fluoride levels in hair of the participants from the fluoride endemic area, as compared to the low fluoride area.
Water is the major source of fluoride intake in humans. Consuming appropriate and relatively small amounts of fluoride by water is generally conceived to have a beneficial effect. However, intake of water excessively rich in fluoride results in pathological changes in teeth, leading to dental fluorosis. This has been clearly demonstrated in the present study. The TFI scores were higher for the participants chosen from Karsan, which is a high fluoride area, as compared to Ajod, which is a low fluoride area. These findings are consistent with several other studies conducted in the past. Indermitte et al.[10] studied the risk of dental fluorosis in Estonia on exposure to high fluoride drinking water and found a strong correlation between natural fluoride levels in drinking water and the prevalence of dental fluorosis. Similar positive correlation was also confirmed between fluoride content of well water and dental fluorosis level in a study conducted by Mandinic et al.,[11] who studied fluoride in drinking water and dental fluorosis in different Serbian municipalities. Firempong et al.[12] who carried out a study to investigate the relationship between fluoride ions in drinking water and the incidence of dental fluorosis in some endemic areas of Bongo district, Ghana, also found positive correlation between the two. Dean's specific index was used to assess dental fluorosis.
In the present study, a positive linear correlation was found between the fluoride levels in hair of the participants and the TFI scores. The results are similar to the results presented by Mandinic et al.[11] in their study to assess the relationship between dental fluorosis and fluoride content in hair in school children from fluorotic and nonfluorotic regions in Serbia. Statistically significant correlations were confirmed between levels of fluoride in hair and prevalence of dental fluorosis.[11]
This may be because the most common sites where the deposition of endogenous fluoride takes place during the growth phases are the hair and teeth. Thus, exposure to high contents of fluoride from drinking water up to the age of 8 years will lead to dental fluorosis. At the same time, fluoride will also get deposited in the keratin of hair, and hair being an indicator of chronic fluoride exposure shows the presence of fluoride when treated chemically.
From the present study, it is realized that the fluoride ion concentrations of Karsan village is above the WHO recommended value of 1.5 ppm for public water supplies. The human body has no mechanism to regulate excess fluoride levels. Thus, when villagers ingest the excess fluoride, it leads to dental fluorosis. This excess fluorosis also gets deposited in scalp hair, which was detected in the present study. Similar theory may be applied to other areas which fall in the fluoride endemic belt. There is therefore a need to drastically reduce the fluoride ions in drinking water for such fluoride endemic communities.
Alkaline metals are loosely bound to hair, while alkaline earth metals are bounded to a greater extent, which can be observed during removal of metals from hair in detergents. Metal analysis of hair concerning the process of incorporation of metals can also provide significant clues in the identification of metal toxicity and also the deficiency of essential metals. Since fluoride endemic belts have already been identified throughout the world, fluoride content in hair can also be used to identify geographical location and environmental conditions in forensic investigations. Ecological elements are of vital importance in forensic identification of cases, and hair has played the key evidence since the last century.[13]
Hair analysis should thus play a greater role in routinely measuring the chronic exposure to fluoride.
CONCLUSION
The current study found that there is a significant association between the fluoride content in drinking water and fluoride content of scalp hair. We can thus conclude that apart from urine and blood, hair can also contribute as biomaterials for monitoring the chronic fluoride exposure. Urine and blood serve as short term indicators of fluoride exposure. On the other hand, scalp hair is a long- term fluoride exposure indicator. Hair samples can be collected by easy, painless, and noninvasive methods, giving them further advantage over the other biomaterials. Fluoride ion-specific electrodes should be made more easily available and accessible so that the scope of hair samples as biomaterials can be further explored in different places using larger samples.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
A special acknowledgement to the Department of Chemistry, Faculty of Science, MS University, Vadodara, for helping with laboratory procedure involved in the analysis of hair samples.
REFERENCES
- 1.Khairnar MR, Dodamani AS, Jadhav HC, Naik RG, Deshmukh MA. Mitigation of fluorosis – A review. J Clin Diagn Res. 2015;9:ZE05–9. doi: 10.7860/JCDR/2015/13261.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampaio FC. Biomarkers of exposure to fluorides. J Appl Oral Sci. 2006;14:41–3. [Google Scholar]
- 3.Mehta A. Biomarkers of fluoride exposure in human body. Indian J Dent. 2013;4:207–10. [Google Scholar]
- 4.Czarnowski W, Krechniak J. Fluoride in the urine, hair, and nails of phosphate fertiliser workers. Br J Ind Med. 1990;47:349–51. doi: 10.1136/oem.47.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotecha PV, Patel SV, Bhalani KD, Shah D, Shah VS, Mehta KG, et al. Prevalence of dental fluorosis & dental caries in association with high levels of drinking water fluoride content in a district of Gujarat, India. Indian J Med Res. 2012;135:873–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Parimi N, Viswanath V, Kashyap B, Patil PU. Hair as biomarker of fluoride exposure in a fluoride endemic area and a low fluoridated area. Int J Trichology. 2013;5:148–50. doi: 10.4103/0974-7753.125613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peter S. Essentials of Public Health Dentistry. 5th ed. New Delhi: Arya Publishing House Pvt, Ltd; 2015. [Google Scholar]
- 8.Arlappa N, Qureshi A, Srinivas R. Fluorosis in India – An overview. Int J Res Dev Health. 2013;1:97–102. [Google Scholar]
- 9.Kokot Z, Drzewiecki D. Fluoride levels in hair of exposed and unexposed populations in Poland. Fluoride. 2000;33:196–204. [Google Scholar]
- 10.Indermitte E, Saava A, Karro E. Exposure to high fluoride drinking water and risk of dental fluorosis in Estonia. Int J Environ Res Public Health. 2009;6:710–21. doi: 10.3390/ijerph6020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandinic Z, Curcic M, Antonijevec E, Carevic M, Mandic J, Djukic-Cosic D, et al. Fluoride in drinking water and dental fluorosis. Sci Total Environ. 2010;408:3507–12. doi: 10.1016/j.scitotenv.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Firempong C, Nsiah K, Awunyo-Vitor D, Dongsogo J. Soluble fluoride levels in drinking water-a major risk factor of dental fluorosis among children in Bongo community of Ghana. Ghana Med J. 2013;47:16–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Dahiya MS, Yadav SK. Elemental composition of hair and its role in forensic identification. Open Access Sci Rep. 2013;2:721–6. [Google Scholar]


