Abstract
Aims:
The aim of this study was to observe the sequelae of fenugreek adjunctive to nonsurgical periodontal therapy and its comparison to a group treated with scaling and root planing (SRP) and metformin alone by assessing their respective effects on periodontal parameters, glycemic status, and serum lipid levels.
Materials and Methods:
The study comprised eighty patients who were further divided into two groups. Each group consisted of 40 patients. Group 1 consisted of uncontrolled noninsulin-dependent diabetes mellitus (NIDDM) patients with chronic generalized periodontitis, who received SRP and treatment with metformin. Group 2 consisted of uncontrolled NIDDM with chronic generalized periodontitis, who received SRP and metformin plus fenugreek powder. Periodontal parameters such as gingival index, plaque index, bleeding on probing, pocket depth, and clinical attachment levels were evaluated before treatment and 1 month after nonsurgical periodontal therapy. The values of low-density lipoprotein (LDL), triglycerides, total cholesterol (TC), and glycosylated hemoglobin (HbA1c) were assessed by collecting the blood samples before treatment and 1 month after treatment.
Results:
There was a statistically significant change in all the parameters seen clinically in both the groups. The glycemic status also showed a statistically significant reduction for fasting blood sugar (P < 0.001) on intragroup comparison. Intragroup comparison shows a statistically significant reduction (P < 0.001) for serum lipids, whereas intergroup comparison showed a statistically significant reduction after treatment only in TC and LDL levels (P < 0.02 and <0.012).
Conclusion:
This study shows that fenugreek powder can be used adjunctive to SRP to control the glycemic status and serum lipid levels in uncontrolled NIDDM patients.
Key words: Chronic generalized periodontitis, fenugreek, glycosylated hemoglobin, low-density lipoprotein, total cholesterol, triglycerides, type 2 diabetes mellitus
INTRODUCTION
Periodontal disease is a chronic inflammatory process activated by intense microbial activity. It is initiated by an array of complex microorganisms as well as by the susceptibility of the individual to their action. Various systemic factors, diabetes mellitus being the most common of them, help in aggravating this complex pathology.[1] It is common knowledge that there is a two-way relationship between periodontal disease and diabetes. Diabetes influences the progression of periodontitis, and it is considered as the sixth major complication of diabetes.[2] Poor glycemic control leads to worsened periodontal condition and vice versa. Nonsurgical therapy of periodontal disease has shown to improve the glycemic status of diabetic individuals.
Many herbal antidiabetic products are used for the treatment of diabetes in India. One such product which is most widely used as an oral insulin substitute for treating diabetes is fenugreek seeds (Trigonella foenum-graecum). The beneficiary effect of dietary fibers in the management of diabetes has been well recognized. Fenugreek seeds have been shown to have both antidiabetic and antilipidemic properties [3] and are commonly used as an ingredient in the food for maintaining the blood sugar level in Asia, especially in India.[4] These seeds are rich in 4-hydroxyisoleucine, saponins, trigonelline, diosgenin, and soluble fibers.[5] Fenugreek seeds possess galactomannan. These are rich in soluble fibers, and it helps in reducing blood sugar level by paring down the digestive process and inhibiting the absorption of carbohydrates. Fenugreek can be used either in powder or in the form of extract for therapeutic use. Therefore, there has been a greater source of awareness of the antidiabetic properties of fenugreek. These seeds are also rich in minerals such as iron, potassium, calcium, copper, zinc, selenium, magnesium, and manganese. Many human and animal trials have shown the antidiabetic and hypolipidemic effect of oral fenugreek powder.[6,7] This study was also done to observe the hypolipidemic effect of fenugreek powder along with metformin in comparison with metformin alone after nonsurgical periodontal therapy.
Mechanism of action
There are several reasons behind the antidiabetic effects of fenugreek. It is credited mainly to galactomannan and 4-hydroxyisoleucine (4-OH-Ile). 4-OH-Ile is a natural nonproteinogenic amino acid possessing noninsulinotropic activity, which has a direct effect on the individual islets of Langerhans causing increased glucose-induced release of insulin.[8] Studies done on human trial shows that fenugreek increased the number of insulin receptors and reduced the area under the plasma glucose curve.[9] Glucose-dependent insulin from pancreatic beta-cells is stimulated by the hypoglycemic effects of fenugreek, and also, it inhibits the activities of two intestinal enzymes involved in carbohydrate metabolism, namely, alpha-amylase and sucrase.[10] These seeds also lower serum triglycerides (TGs), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C). This effect may be due to sapogenins, which also increases biliary cholesterol excretion, ultimately leading to reduced serum cholesterol levels.[11] The impact of fenugreek on lowering the levels of serum lipids can be credited to its estrogenic constituent, by boosting the thyroid hormone T4 indirectly.[12]
MATERIALS AND METHODS
The participants were randomly selected from the outpatient clinic of the department of periodontics, Chennai. Written consent was taken from each participant. All participants completed the study. The protocol of this study was approved by the Ethical Committee of Dr. M. G. R. University and Research Institute, Maduravoyal, Chennai, India, according to the Declaration of Helsinki, which was revised in the year 2000.
This study consists of 80 participants who are divided into:
Group 1: Forty participants with chronic generalized periodontitis with uncontrolled noninsulin-dependent diabetes mellitus (NIDDM) treated with metformin alone
Group 2: Forty participants with chronic generalized periodontitis with uncontrolled NIDDM treated with metformin along with fenugreek powder as an adjuvant to scaling and root planing.
In the selected patients, detailed medical history was recorded. All the patients involved in this study had uncontrolled diabetes mellitus. The uncontrolled diabetes mellitus was defined based on HbA1c value more than 8 mg/dl. The treating physician's consent and details of the patients, regarding diabetes control, were also obtained. The history of these diabetic patients selected for the study was more than 5 years. All the clinical parameters and blood samples were obtained from these participants at baseline and after 1 month of nonsurgical periodontal therapy. The duration of this study to procure 80 patients was 2 months.
Inclusion criteria
The inclusion criteria in the study were participants who were suffering from chronic generalized periodontitis with uncontrolled type 2 diabetes mellitus
They should have at least 30% of the sites with clinical attachment level (CAL) ≥4 mm, pocket depth (PD) ≥5 mm, and bleeding on probing (BOP).
Exclusion criteria
Patients who had undergone periodontal treatment in the past 6 months
Those with a history of antibiotic administration within the past 3 months
Those with <20 remaining natural teeth
Participants who were pregnant
Participants with a history of smoking, liver diseases, and lipid-lowering and insulin therapy
Participants with tobacco and alcohol consumption.
Periodontal treatment and clinical measurements
Periodontal examination was done for all the patients in six sites per tooth except the third molar. Periodontal parameters were as follows:
Plaque index (Silness and Loe 1964)
Gingival index (Loe and Silness 1963)
BOP (Muhlemann and Son 1971).
PD and CAL were evaluated before and after 1 month of treatment. Collection of blood samples was also done for all the patients after an overnight fasting of minimum 10 h at baseline and 1 month after treatment. After documenting the periodontal status, patients were informed about the oral hygiene instructions and received full-mouth nonsurgical periodontal therapy with the administration of local anesthesia. Group 1 patients were advised to take their regular treatment protocol, that is, metformin tablets 500 mg twice a day, as per the instruction of the physician. A pilot study was done, and 25 g of fenugreek powder was standardized per day for the Group 2 patients. Group 2 patients were dispensed with fenugreek powder in a container and were advised to take 12.5 g two times daily before breakfast and lunch along with the regular metformin tablets. These patients were given a measuring cup and a chart with instructions to be followed until the next visit. Patients were asked to report once a week to check if they are consuming the fenugreek powder properly. After the periodontal treatment, patients were advised to maintain their oral hygiene with proper brushing and flossing technique. During this experimental period, patients were asked about alteration in medications concerned with diabetic treatment, use of any antibiotic or anti-inflammatory drugs, and change in lifestyle, along with exercise and diet.
Sample collection
Collection of blood samples was done in the morning after fasting for minimum 10 h the previous night. Samples were analyzed for fasting blood sugar (FBS), glycosylated hemoglobin (HbA1c), TC, LDL, and TGs. The samples were centrifuged and collected in separate test tubes to measure the levels of TC, LDL, and TGs.
Statistical analysis
Statistical analyses were done using a software program (SPSSV 16, IL). Comparison of variables within the groups was calculated by paired t-test, and intergroup comparison was done using unpaired t-test.
RESULTS
When intragroup comparison was done for all the clinical parameters, there was a statistical significance (P < 0.05) observed in both the groups [Table 1]. When intergroup comparison was done after treatment, there was statistical significance (P< 0.0031) seen only in plaque index score [Table 2]. The glycemic status was measured using FBS and HbA1c values. When intragroup comparison was done for FBS and HbA1c before and after nonsurgical periodontal treatment, there was statistically significant changes seen in both the groups for FBS (P< 0.001) [Table 3] and for HbA1c in Group 2 patients alone. Similarly, when intergroup comparison was done for FBS and HbA1c, individually, there was statistical significance observed only for FBS after treatment (P = 0.004) [Table 4]. When intragroup comparison was done in both the groups at baseline and after treatment for TC, LDL, and TGs, there was statistical significance observed in both the groups (P< 0.001) [Table 5]. When intergroup comparison was done between Group 1 and Group 2 patients for TC, LDL, and TGs, there was statistical significance seen after treatment in TC and LDL alone (P<0.02 and <0.012) [Table 6].
Table 1.
Intragroup comparison of clinical parameters at baseline and after treatment using paired t-test
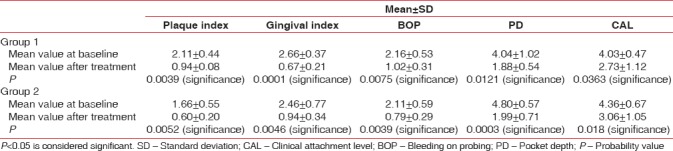
Table 2.
Intergroup comparison of mean and standard deviation of clinical parameters after treatment
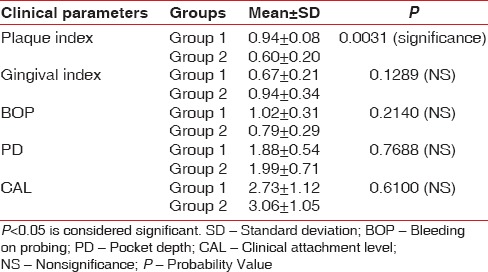
Table 3.
Intragroup comparison of fasting blood sugar and glycosylated hemoglobin at baseline and after treatment
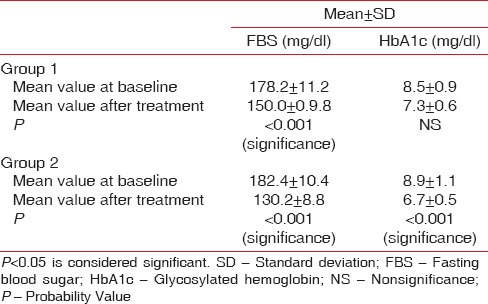
Table 4.
Intergroup comparison of fasting blood sugar and glycosylated hemoglobin at baseline and after treatment
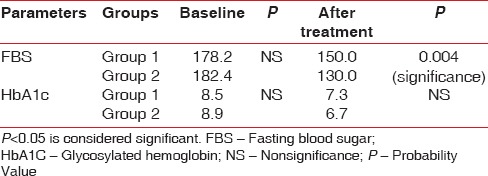
Table 5.
Intragroup comparison of serum lipids in Group 1 and Group 2 patients at baseline and after treatment using paired t-test
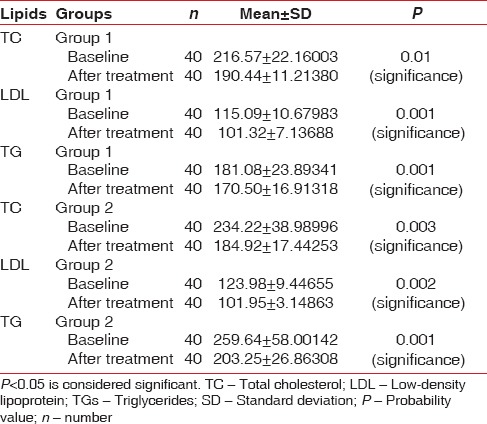
Table 6.
Intergroup comparison of serum lipids in Group 1 and Group 2 patients at baseline and after treatment using independent t-test
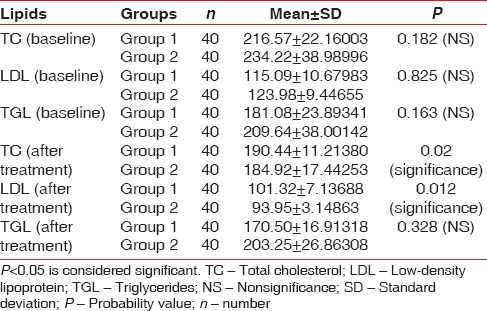
DISCUSSION
Evidences reveal that the microorganisms present in the plaque are the primary and the only extrinsic etiologic agent participating in the cause of inflammatory periodontal disease. It is a well-known fact that periodontal disease is more severe in diabetic than in nondiabetic participants. The chronic challenge of the periodontal pathogens may provide a constant source of pro-inflammatory cytokines. This may be associated with two factors: poor glycemic control and an increased tissue insulin resistance.[16] These cytokines play an important role in the diabetic pathway. This, in turn, has a direct impact on the alleviation of the disease. There are multiple abnormalities in NIDDM such as insulin resistance, lipid abnormalities, and endothelial dysfunction.
In this current study, there was a statistical significance observed in all the clinical parameters on intragroup comparison. When intergroup comparison was done, there was a statistical significance seen only in plaque index. Nonsurgical periodontal treatment helps to reduce the microbial load, thereby showing improvement in glycemic control. There is an unmistakable link between diabetes and periodontitis, both being chronic in nature. CALs in diabetic patients are significantly higher than in nondiabetic patients. Studies done by Grossi et al. have revealed that diabetics are twice more prone to clinical attachment loss than nondiabetic.[17] A study by Bridges et al. revealed the fact that the disease invades into all the periodontal parameters. Fenugreek seeds stimulate insulin secretion and produce a hypoglycemic effect. Therefore, a considerable improvement in glycemic control helps to improve the periodontal parameters significantly. The improvement in all the clinical parameters in this study was similar to a study done by Rodrigues et al.[18]
In our study, when intragroup comparison was done, there was a reduction in the FBS and HbA1c values when compared with the baseline values in both the groups. When intergroup comparison was done, there was a significant statistical reduction only for FBS. Nonsurgical periodontal therapy restores periodontal health and improves glycemic control.[19] In our study, Group 1 patients were treated with metformin and Group 2 patients were given fenugreek powder along with metformin. In many diabetic patients, blood glucose levels are not properly controlled by bonafide antidiabetic medicines, and malnourished individuals take suboptimal doses of drug to prevent hypoglycemic episodes. As fenugreek is commonly used as a condiment in India, the beneficiary effect of fenugreek in controlling blood sugar and overall cholesterol levels would have a considerable practical implication. Insulin secretion is enhanced by the fenugreek fibers due to 4-OH-Ile, which lowers the intestinal absorption of glucose, thereby regulating blood sugar.[8] Arginine and tryptophan are two other amino acids exerting an antidiabetic effect. Fenugreek seeds can also replace legumes in a diabetic patient's diet as they are excellent source of proteins. A daily intake of 50 g of fenugreek seeds can help in effectively controlling the disease.[20]
In our study, blood glucose level showed a statistically significant on intragroup comparison which was evidenced by a reduction in the FBS values for both Group 1 and Group 2 patients and HbA1c values in Group 2 patients where fenugreek seed powder was administered adjunct to nonsurgical periodontal therapy along with metformin. The galactomannan-rich soluble fiber is responsible for its antidiabetic properties.[21] The secondary mechanism for its antiglycemic effect is its high fiber content (25%).[22] A human study done by Sharma [6] revealed the positive effects of fenugreek seeds in treating both Type 1 and Type 2 DM patients. In vitro studies have revealed that the amino acid content in fenugreek possesses a direct stimulation on the β-cells. It is found to be around 0.55%. Glucose-induced insulin levels were found to be significantly higher in both humans and rats, according to studies done by Sauvaire et al.
In this current study, when intragroup comparison was done for serum lipids, there was a statistical significance seen in TC, LDL, and TG levels. Fenugreek seeds exert a distinct hypocholesterolemic effect besides lowering LDL, TG, and serum cholesterol levels. This may be due to the effect of sapogenins, which lowers serum cholesterol levels by increasing biliary cholesterol excretion.[23] When intergroup comparison was done at baseline and after treatment, there was a statistical significance observed in TC and LDL after nonsurgical periodontal treatment. The results of our study were similar to a study done by Sharma.[24] In our study, on intragroup comparison, there was significance seen in the TG level at baseline and after treatment. The TG-lowering effect may be due to the pectin component that absorbs bile acid salts. There is also an increase transformation of hepatic cholesterol to bile salts which may be caused by loss in the feces with saponins and fenugreek fiber. Fenugreek can lower lipids due to its saponin content which is converted into sapogenins in the gastrointestinal tract. Fiber galactose and mannose being the main ingredient of the gum are also present in fenugreek seeds which help in reducing the cholesterol level in hypercholesterolemic patients.[25] Cholesterol-lowering activities are also present in the fiber-rich gum portion of the seed which reduces the synthesis of cholesterol in the liver. Both these mechanisms can largely contribute to the effect of fenugreek on serum lipids.
CONCLUSION
The present study shows that fenugreek seeds decrease blood glucose and TG levels and also have a significant effect on LDL and TC levels. Since diabetic patients have an increased inflammatory cytokine which can modify the risk of diabetes, nonsurgical periodontal therapy not only helps in reducing clinically evident inflammation but also has been associated in decreasing pro-inflammatory cytokines and glycated hemoglobin levels. This is evident from our current study, which has shown statistically significant reduction in FBS and HbA1c values and also significant reduction in serum inflammatory markers when compared to baseline values.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas B, Kumari S, Ramitha K, Ashwini Kumari MB. Comparative evaluation of micronutrient status in the serum of diabetes mellitus patients and healthy individuals with periodontitis. J Indian Soc Periodontol. 2010;14:46–9. doi: 10.4103/0972-124X.65439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadkarni KM. Trigonella foenum-graecum: Indian materia medica. Prakashan LTD; 1993. pp. 1240–9. [Google Scholar]
- 4.Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: A review. J Altern Complement Med. 2004;10:369–78. doi: 10.1089/107555304323062365. [DOI] [PubMed] [Google Scholar]
- 5.Neerja A, Rajyalaxmi P. Hypoglycemic effect of processed fenugreek seeds in humans. J Food Sci Technol. 1996;33:427–30. [Google Scholar]
- 6.Sharma RD. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. Nutr Res. 1986;6:1353–64. [Google Scholar]
- 7.Valette G, Sauvaire Y, Baccou JC, Ribes G. Hypocholesterolaemic effect of fenugreek seeds in dogs. Atherosclerosis. 1984;50:105–11. doi: 10.1016/0021-9150(84)90012-1. [DOI] [PubMed] [Google Scholar]
- 8.Sauvaire Y, Petit P, Broca C, Manteghetti M, Baissac Y, Fernandez-Alvarez J, et al. 4-hydroxyisoleucine: A novel amino acid potentiator of insulin secretion. Diabetes. 1998;47:206–10. doi: 10.2337/diab.47.2.206. [DOI] [PubMed] [Google Scholar]
- 9.Raghuram TC, Sharma RD, Sivakumar B, Sahay BK. Effect of fenugreek seeds on intravenous glucose disposition in non-insulin dependent diabetic patients. Phytother Res. 1994;8:83–6. [Google Scholar]
- 10.Amin R, Abdul-Ghani AS, Suleiman MS. Effect of Trigonella feonum graecumon intestinal absorption. Diabetes. 1987;36:21–6. [Google Scholar]
- 11.Ali L, Azad Khan AK, Hassan Z, Mosihuzzaman M, Nahar N, Nasreen T, et al. Characterization of the hypoglycemic effects of Trigonella foenum-graecum seed. Planta Med. 1995;61:358–60. doi: 10.1055/s-2006-958100. [DOI] [PubMed] [Google Scholar]
- 12.Sauvaire Y, Ribes G, Baccou JC, Loubatieères-Mariani MM. Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids. 1991;26:191–7. doi: 10.1007/BF02543970. [DOI] [PubMed] [Google Scholar]
- 13.Jalali MT, Honomaror AM, Rekabi A, Latifi M. Reference ranges for serum total cholesterol, HDL-cholesterol, LDL-cholesterol, and VLDL-cholesterol and triglycerides in healthy Iranian Ahvaz population. Indian J Clin Biochem. 2013;28:277–82. doi: 10.1007/s12291-012-0268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan LA. Clinical Chemistry. St Louis, Baltimore, Philadelphia, Toronto: The CV Mosby Co.; 1989. pp. 854–6. [Google Scholar]
- 15.Koenst MH, Edstrom RD. Determination of glycosylated hemoglobin: A laboratory experiment relevant to diabetes mellitus. Biochem Educ. 1985;13:7–9. [Google Scholar]
- 16.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–84. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 17.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues DC, Taba MJ, Novaes AB, Souza SL, Grisi MF. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol. 2003;74:1361–7. doi: 10.1902/jop.2003.74.9.1361. [DOI] [PubMed] [Google Scholar]
- 19.Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135–41. doi: 10.1097/MED.0b013e3282f824b7. [DOI] [PubMed] [Google Scholar]
- 20.Senthil A, Mamatha BS, Vishwanath P, Bhat KK, Ravishankar GA. Studies on development and storage stability of instant spice adjunct mix from seaweed (Eucheuma) J Food Sci Technol. 2011;48:712–7. doi: 10.1007/s13197-010-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashmi Y, Rahul K. Study of phytochemical constituents and pharmacological actions of Trigonella foenum-graecum: A review. Int J Pharm Technol. 2011;3:1022–8. [Google Scholar]
- 22.Basch E, Ulbricht C, Kuo G, Szapary P, Smith M. Therapeutic applications of fenugreek. Altern Med Rev. 2003;8:20–7. [PubMed] [Google Scholar]
- 23.Stark A, Madar Z. The effect of an ethanol extract derived from fenugreek (Trigonella foenum-graecum) on bile acid absorption and cholesterol levels in rats. Br J Nutr. 1993;69:277–87. doi: 10.1079/bjn19930029. [DOI] [PubMed] [Google Scholar]
- 24.Sharma RD. An evaluation of hypocholeterolaemic factor of fenugreek seeds in rats. Nutr Rep Int. 1986;33:669–77. [Google Scholar]
- 25.Roberts KT. The potential of fenugreek (Trigonella foenum-graecum) as a functional food and nutraceutical and its effects on glycemia and lipidemia. J Med Food. 2011;14:1485–9. doi: 10.1089/jmf.2011.0002. [DOI] [PubMed] [Google Scholar]


