Abstract
Clinical evaluation and ultrasound examination are the first steps in the evaluation of a patient with a swelling of the parotid region. After the detection of a nodular lesion, cytological or histological confirmation is usually performed to achieve the diagnosis, while the choice of cross-sectional imaging (computed tomography scan and magnetic resonance imaging) may significantly vary from one physician to another, on the basis of the degree of confidence that both radiologist and surgeon have with this kind of imaging. This work focuses on some essential “reporting points” in cross-sectional imaging evaluation of parotid nodules, chiefly helpful to the radiologist when the ultrasonography assessment is considered incomplete and requires a further evaluation.
KEYWORDS: Computed tomography, magnetic resonance, parotid gland nodules, ultrasonography

INTRODUCTION
Salivary glands may be affected by various pathologies of inflammatory, infective, obstructive, or neoplastic origin.
In particular, the neoplastic potential is expressed by a range of benign and malignant lesions with over 45 identifiable different histotypes, which, though not frequently (<3% of head and neck tumors), has no equal in any other organ or apparatus.[1]
Salivary gland tumors are most frequently identified in the parotid gland (80% of cases), while the involvement of submandibular gland, sublingual glands, or minor salivary glands rarely occurs.[1]
The study of parotid nodular lesions relies quite often on diagnostic imaging, which is of fundamental importance for planning surgical interventions.
Ultrasonography (US), preceded by an accurate clinical evaluation, is the first essential approach in a patient presenting tumefaction of the parotid region, while performing a multidetector computed tomography (CT) or a magnetic resonance (MR) examination is a choice that may vary significantly from one physician to another. It is important to remember that the cross-sectional imaging does not allow a superior differentiation between benign and malignant parotid nodules. However, in doubtful cases, it can significantly help in further improving the evaluation of the parotid gland and the surrounding tissues, overcoming some of the main limits of the sonographic evaluation.
The aim of this work is to provide the radiologist some fundamental clues while evaluating parotid lesions on CT scan or MR imaging (MRI), starting from the anatomy of the parotid region up to the most common scenarios of the daily clinical practice, above all with regard to the surgeon's needs.
ANATOMY OF THE PAROTID SPACE
The parotid space is one of the suprahyoid neck spaces, which encompasses the homonym gland. Parotid gland occupies the craniocaudal area, from the region of the external acoustic meatus to the angle of the mandible.[2]
The parotid space is posterior to the masticator space, external to the parapharyngeal space, and anterolateral to the carotid space.[2]
It is delimited by the parotid fascia, which originates in the superficial layer of the deep cervical fascia, and surrounds the parotid gland, passing through it and forming a series of sects and subdividing the parenchyma into lobuli.
The external carotid artery and retromandibular vein pass through the parotid gland, as well as extracranial ramifications of the facial nerve, which emerges from the stylomastoid foramen.
Although it cannot be considered as a proper anatomical subdivision, a superficial lobe (the palpable portion of the gland) and a deep lobe can be identified, using the pathway of the facial nerve and its intraparotid branches as anatomical reference. In axial CT and MR images, this pathway might be identifiable by joining the stylomandibular foramen and the external margin of the retromandibular vein in a straight line [Figure 1a].[2]
Figure 1.
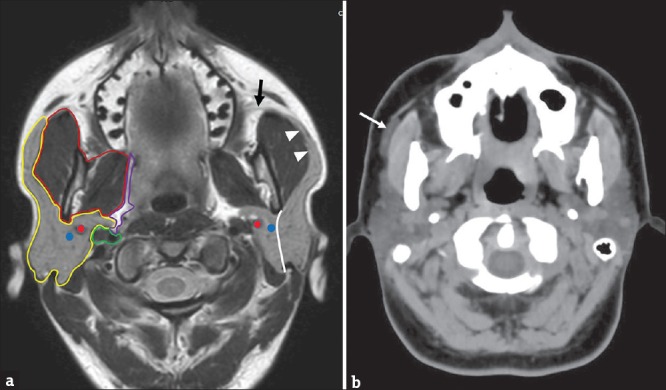
(a) T1-weighted magnetic resonance axial scan showing the anatomy of the parotid region. Yellow line: parotid space; Red line: Masticatory space; Purple line: Parapharyngeal space; Green line: Carotid space. Blue dot: Retromandibular vein; Red dot: External carotid artery. White line: Intraparotid route of the facial nerve, separating the superficial lobe from the deep lobe (black arrow), Stensen's duct; White arrowheads: anterior extension of parotid gland along the masseter muscle. (b) Axial computed tomography scan in a patient with an accessory parotid gland (arrow), lateral to the right masseter muscle along the pathway of the Stensen's duct.
Stensen's duct originates in the anterior margin of the parotid gland extending horizontally forward, along the external margin of the masseter muscle, 1–2 cm below the zygomatic arch. Beyond the masseter muscle, the parotid duct passes through the buccinator muscle, ending in the oral cavity at the level of the superior vestibule of the mouth, in the proximity of the second molar.[3]
An accessory parotid lobe may be present in 21% of participants, located about 5–6 mm in front of the main gland, along the pathway of the Stensen's duct [Figure 1b].[4,5]
In other cases, an anterior extension of the gland may be seen along the masseter muscle.
The parotid space, finally, also includes a series of periglandular and intraglandular (about 10–20) lymph nodes, located mainly on the surface of the parotid gland: diameter is normally up to 8 mm.
The anatomical subdivision into lobes is helpful, since the surgeon's aim, when removing nodular lesions in the parotid, is always to safeguard the facial nerve, performing a superficial parotidectomy in most cases, if the lesion is external to the nerve, or a total parotidectomy in the case of deep lesions.
IMAGING MODALITIES: IS ULTRASONOGRAPHY ALWAYS CONCLUSIVE?
On the basis of anatomical and anatomopathological aspects and surgical implications, radiological evaluation of the parotid nodular lesions may appear to be complex.
However, it is not so challenging taking into consideration what the real potential of diagnostic imaging is.
It is important to keep in mind that most of parotid gland tumors are benign (80%–95%):[6,7] the most common is pleomorphic adenoma (60% of all salivary gland tumors), followed by, in decreasing order of incidence, Warthin's tumor, basal cell adenoma, and oncocytoma.[8]
As for malignant tumors (10% to 25%), the most frequents are mucoepidermoid carcinoma, adenoid cystic carcinoma, and squamous carcinoma.[7]
After a clinical evaluation of a parotid swelling, even if asymptomatic, a US examination should be carried out, as the first step in the radiological framework.
Typical sonographic characteristics of benignity are well-defined margins, homogeneous echotexture, hypoechogenicity, round or ovoid shape, calcifications, and vascularization (Grade 1–2), while sonographic characteristics suggesting malignancy are irregular shape, spiculated or ill-defined margin, heterogeneous echotexture, vascularization (Grade 3–4), and the presence of large cervical lymphadenopathy.[9]
On the basis of the above-mentioned relieves, US has demonstrated a specificity that can range from 40% to 84%, a specificity of 88%–98%, and an accuracy of 57%–96%.[10]
Moreover, other US techniques, including elastography, color Doppler, and contrast-enhanced US (CEUS), can be exploited in the evaluation of parotid nodules.
Elastography is a relatively new technique, generally used in thyroid or breast imaging, whose aim is to evaluate the stiffness of the lesions.
Actually, two types of elastography are performed: strain elastography (SE) and share-wave elastography (SWE).
SE produces colored stiffness maps of the lesions as the result of the comparison of the sonographic beam before and after a compression performed by the operator.[6]
Although for breast and thyroid lesions, the stiffness is directly related to the malignant lesions, the characterization of parotid nodules is more challenging, due to their variable histoarchitecture, and overlapping features between malignant and benign can be seen.[11]
On the other hand, SWE exploits the formation of acoustic waves, perpendicular to the sonographic bean, that distribute into the tissues surrounding the lesions. Consequently, the evaluation of the stiffness is less dependent on the operator compression and more easily reproducible.[12] However, neither this technique allowed a certain differentiation between benign and malignant lesions.[6]
Color Doppler sonography evaluates macrovascularity of the lesions. Usually, a peripheral vascularization pattern is related to benignity, while an intranodular blood flow can be suspicious for malignancy.[13] Unfortunately, this kind of evaluation strictly depends on the subjective evaluation and the experience of the operator.[14]
On the other hand, CEUS, obtained after the intravenous injection of a microbubble contrast agent, allows a real-time visualization of the lesions’ perfusion.[14]
Usually, different types and subtypes of microvascularization can be distinguished, and the heterogeneous enhancement in lesions with ill-defined margins is generally associated with malignancy.[13]
Several authors suggested that a multiparametric appraisal of the parotid lesions, performed with all or some of the supra-mentioned sonographic technique and tools, could significantly improve the specificity in distinguishing benign and malignant lesions, although, up to now, precise differentiation criteria are not well established.[13,14,15]
However, the final characterization of the parotid lesions can be easily performed through cytological (fine-needle aspiration cytology [FNAC]) or histological (core needle biopsy [CNB]) procedures, to get a certain diagnosis with a high degree of accuracy.[16,17,18]
However, in some cases, the clinical picture might be still incomplete and some diagnostic questions, which can be significant to choose the most suitable surgical approach, might remain unanswered.
CROSS-SECTIONAL IMAGING MODALITIES: THE PROS AND CONS
When evaluating parotid gland lesions, the choice of the imaging modality is based on different considerations.
CT scan is a worldwide spread, rapid, and “low-cost” examination whose images are characterized by an excellent contrast resolution. CT scan leads to an accurate appraisal of parotid lesions, allowing to recognize localization, sizes, margins and density, presence of intranodular calcification, invasion of the surrounding tissues, and infiltration of bone or cartilaginous structures.
Moreover, encouraging results for what concern evaluation of conspicuity, differential diagnosis (i.e., between neoplastic and inflammatory lesions), and lymph node involvement derives from dual-energy CT, thorough its helpful tool techniques, including iodine overlay, bone removal, and virtual monoenergetic imaging.[19,20]
However, although its use is suggested especially in noncompliant patients, the major limit of CT scan remains the radiation exposure.
On the contrary, MRI is a radiation-free imaging modality that can achieve higher values of sensitivity and specificity for what concern the morphological and volumetric assessment, the lesion components, the extraglandular extension, and the perineural spread.[21]
Nevertheless, in some cases, the high costs and the prolonged scanning time may discourage its use.[22]
The accuracy of MRI can be even more increased with the complement of dynamic contrast-enhanced MRI (DCE-MRI), diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) calculation.[22,23,24]
Furthermore, functional imaging modalities failed in providing definite relieves of malignancy.
In fact, the role of DCE examination has been evaluated on both CT scan and MRI sides.
On the basis of the time-intensity curve, four types of enhancement pattern have been described for parotid gland lesion (delayed enhancement, Type A; rapid enhancement and >30% washout, Type B; rapid enhancement and 30%–10% washout, Type C; and rapid enhancement and low or no washout, Type D).[25]
However, although some patterns can be suggestive of malignancy, the collected data for both the techniques are still overlapping and not definite.[26,27]
Even DWI and ADC, in the differentiation of benign and malignant nodules, showed similar features and conflicting results among different studies.[24,28]
In fact, although previous researches had evaluated the accuracy of DWI in distinguishing pleomorphic adenomas and myoepithelial adenomas[28] and beyond the fact that ADC calculation can be useful in detecting early inflammatory or neoplastic changes in the parotid gland,[23] up to now, DWI did not show pathognomonic relieves and a univocal ADC cutoff is not yet established. Therefore, they can be considered helpful tools but not alone sufficient for a definitive characterization.[23,24,29,30]
CROSS-SECTIONAL IMAGING OF PAROTID NODULES: REFERRING KEY POINTS
Beyond the clear limitations of these techniques in the characterization of the parotid lesions, cross-sectional imaging can still be important for the surgical planning.
What?
For what concern the differentiation between benign and malignant nodules, it needs to be kept in mind that usual signs of benignity are sharp margins, round shape, homogeneous density at CT scan, and hyperintensity on T2-weighted MRI images.[22,31]
Instead, hypointensity on T2-weighted MRI images, irregular or poorly defined margins, and infiltration into surrounding tissue (parapharyngeal space, muscles, and bone) can be considered suspicious for malignancy.[25,29,32]
Where?
Due to the comprehensive evaluation of the parotid gland and the surrounding tissues, CT scan and MRI can easily distinguish the intra- or extraglandular site of a parotid lesion [Figures 2 and 3].
Figure 2.
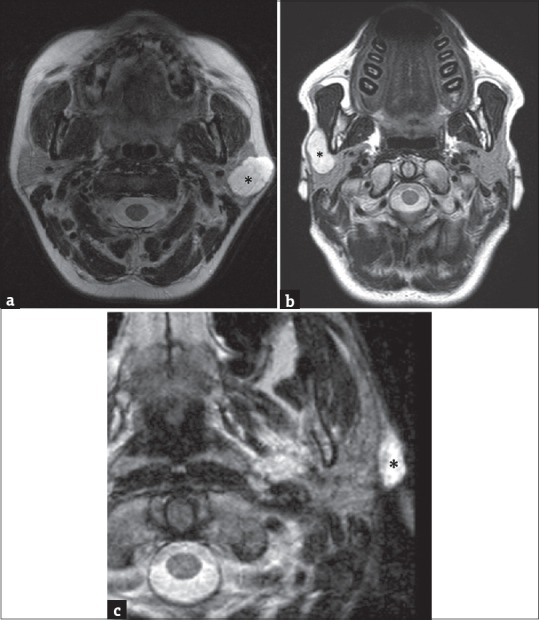
(a) A 53-year-old female axial T2-weighted magnetic resonance image showing the presence of a high-signal-intensity lesion (asterisk) in the left parotid gland. This lesion arises from the superficial lobe, presenting a partial exophytic development. (b) A 44--old male axial T2-weighted magnetic resonance scan in another patient showing a well-defined hyperintense lesion arising in the right superficial parotid lobe (asterisk); the lesion occupies the anterior extension of parotid gland superficially to the masseter muscle. In such cases, the only ultrasonography examination was not accurate enough to define the origin of lesions, while cross-sectional imaging identifies the exact location and their adjacent and distant involvement. (c) A 62-year-old male – T2-weighted axial magnetic resonance image shows an extraglandular lesion (asterisk), clearly originating from the nearby tissues.
Figure 3.
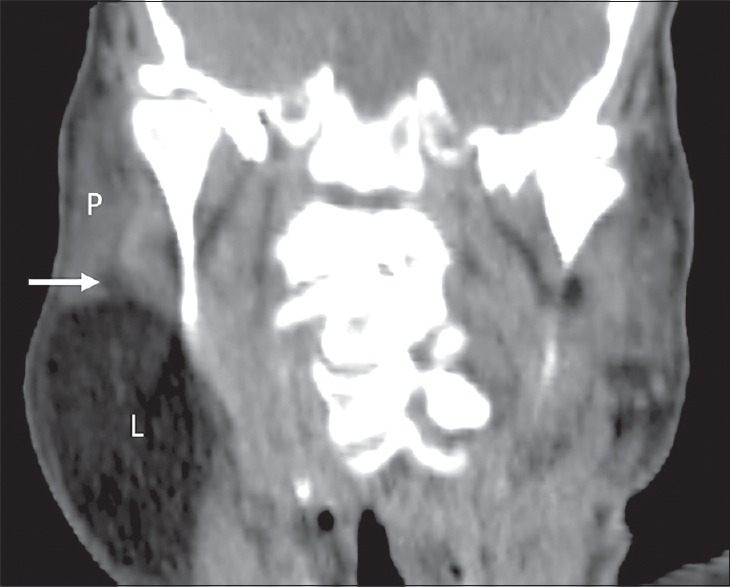
A 56-year-old male patient with a huge swelling of the right infraparotid space. Coronal unenhanced multidetector computed tomography shows a large lipoma (l) with small component (arrow) within the inferior portion of the parotid gland (p).
In nodules originating in the adjacencies of the mandibular angle, it is important to depict the exact anatomical configuration of the lower pole of the parotid gland and of the submandibular gland, to identify the exact site of origin of the lesion [Figure 4].
Figure 4.
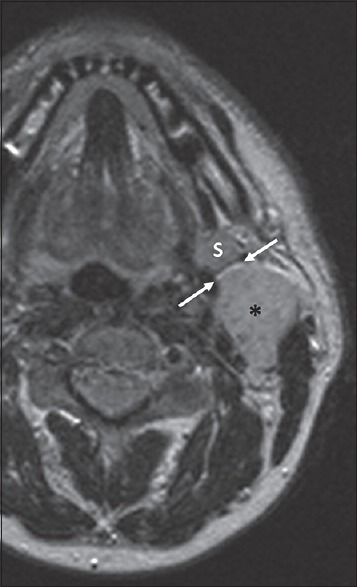
A 65-year-old female patient with swelling of the mandibular angle region, ultrasonography failed to characterize the exact origin of the lesion. Axial T2-weighted image properly demonstrates the parotid origin of the lesion (asterisk) allowing for the identification of the cleavage adipose plane (arrows) with the submandibular gland(s).
In addition, US might not permit a definite evaluation of the real depth of a parotid lesion. In fact, when a tumor has involved the deep lobe, a more drastic surgical approach is needed, requiring a total parotidectomy.
Cross-sectional imaging is also very useful in distinguishing between lesions arising from deep parotid gland and primary parapharyngeal space masses.
Larger lesions of the deep lobe, expanding toward the median line, tend to compress the adipose tissue of the parapharyngeal space, which will, instead, be obliterated in the case of expanding lesions originating from the same space [Figure 5].
Figure 5.
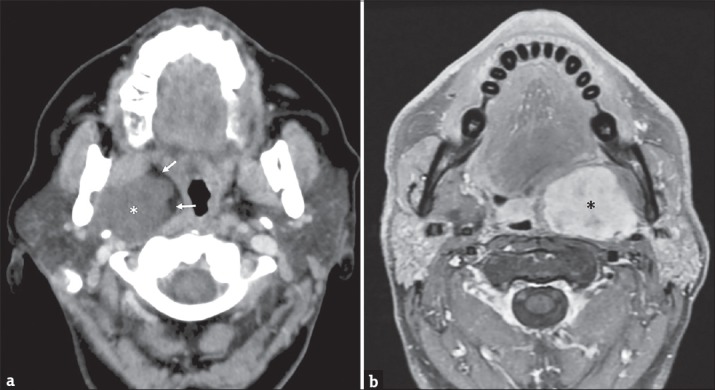
(a) A 50-year-old female axial contrast-enhanced computed tomography scan showing a large right-side parotid neoplasm (white asterisk) arising from the retromandibular part of the gland and compressing the parapharyngeal space (arrows). (b) A 64-year-old male axial T1-weighted fat-suppressed magnetic resonance image after intravenous gadolinium injection showing a voluminous mass (black asterisk) occupying the left parapharyngeal space. The mass was an adenoid cystic carcinoma of the minor salivary glands.
Moreover, the radiologist should be aware of the possible localization of the nodules into the accessory lobe, an ectopic glandular tissue separated or contiguous, usually anterior to the main gland. In fact, the typical clinical appearance of these kinds of lesions consists in a swelling of the cheek [Figures 6 and 7].
Figure 6.
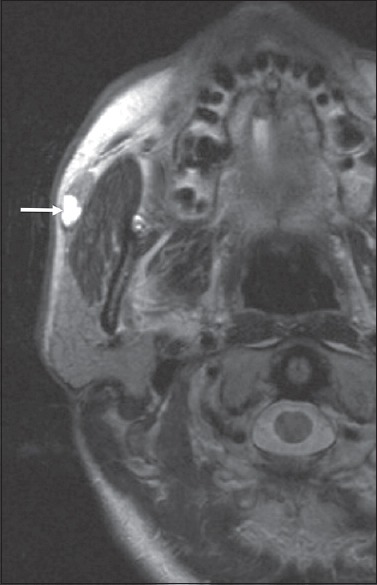
A 51-year-old female T2-weighted magnetic resonance axial image showing a lesion arising from the parotid accessory lobe (white arrow).
Figure 7.
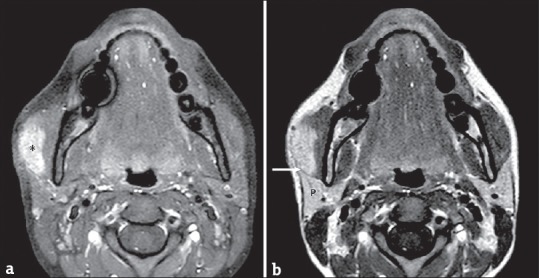
(a) A 66-year-old male axial T1-weighted fat-suppressed magnetic resonance image obtained after intravenous gadolinium administration. The images show the contrast enhancement of a lesion (asterisk) arising from the masseter muscle. (b) A 66-year-old male axial T1-weighted fat-suppressed magnetic resonance image obtained after intravenous gadolinium administration. The images show the contrast enhancement of a lesion (asterisk) arising from the masseter muscle.
How many?
With regard to the number of lesions, cross-sectional imaging helps to better evaluate glandular areas located in sites more difficult to examine at US, due to their particular anatomical position [Figures 8 and 9].[4]
Figure 8.
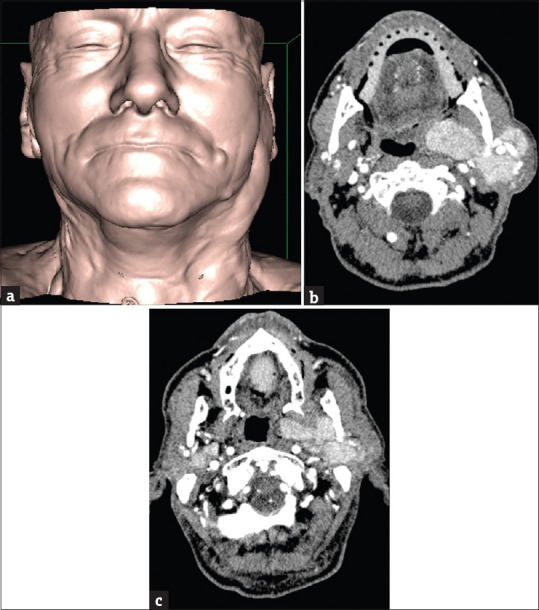
(a) A 76-year-old male three-dimensional-shaded surface display reformatted image of a patient with swelling of the left preauricular region. (b) A 76-year-old male axial contrast-enhanced computed tomography scans showing the presence of several lesions of the left parotid gland, detectable from the superficial lobe to the adjacencies of the parapharyngeal space. (c) A 76-year-old male concurrent lesions in the deep lobe of the right parotid gland can also be seen. In this patient, a previous US undervalued the number of the lesions, probably because of their deep location (i.e., deep lobe of the parotid gland or behind the shadow of the mandible).
Figure 9.
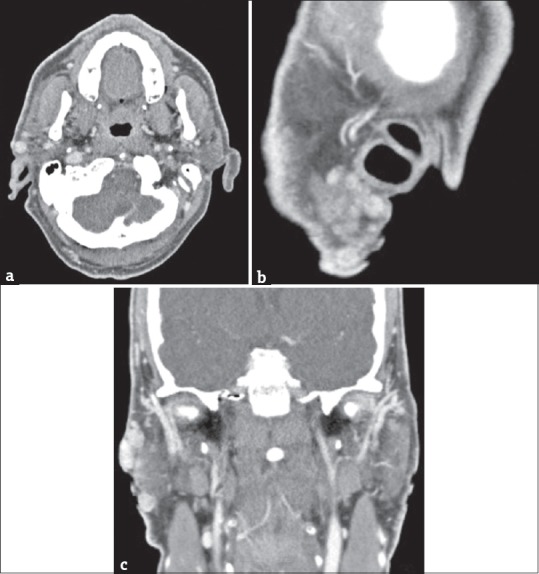
(a) A 58-year-old male postsurgical recurrences in the site of previous right parotidectomy. Axial contrast-enhanced computed tomography scan and multiplanar reformatted images, obtained along sagittal. (b) A 58-year-old male sagittal plane. (c) A 58-year-old male coronal planes show multiple parotid lesions in different locations.
True or false?
It must be pointed out how a swelling in the preauricular region, negative at US of the parotid space, may yet conceal a pathology, but located outside of the parotid gland. The so-called “pseudolesions” may include synovial chondromatosis of the temporomandibular joint, pathologies of the lower jaw, or unilateral hypertrophy of the masseter.[33] In such cases, cross-sectional techniques make it possible to observe anatomical structures bilaterally, allowing a comparative evaluation [Figure 10].[34]
Figure 10.
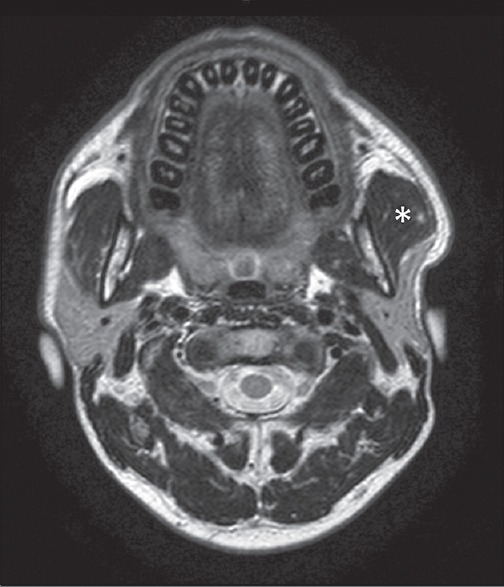
A 37-year-old female patient with swelling of the left preauricular region, negative at ultrasonography scan. Axial T2-weighted magnetic resonance scan easily demonstrates a unilateral hypertrophy of the left masseter muscle (asterisk).
CONCLUSIONS
The diagnostic evaluation of a nodular lesion of the parotid gland is based both on radiological and clinical criteria, with FNAC or CNB still representing the best and easiest available method to make the diagnosis. However, beyond the well-established role of sonography, in doubtful cases, cross-sectional imaging could be necessary to achieve a more accurate diagnosis, in terms of location, size, and topography of lesion, thus allowing to choose the most suitable surgical treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2018/8/1/14/230188.
REFERENCES
- 1.Gerwel A, Kosik K, Jurkiewicz D. US in preoperative evaluation of parotid gland neoplasms. Otolaryngol Pol. 2015;69:27–33. doi: 10.5604/00306657.1149638. [DOI] [PubMed] [Google Scholar]
- 2.Bag AK, Curé JK, Chapman PR, Pettibon KD, Gaddamanugu S. Practical imaging of the parotid gland. Curr Probl Diagn Radiol. 2015;44:167–92. doi: 10.1067/j.cpradiol.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Howlett DC, Kesse KW, Hughes DV, Sallomi DF. The role of imaging in the evaluation of parotid disease. Clin Radiol. 2002;57:692–701. doi: 10.1053/crad.2001.0865. [DOI] [PubMed] [Google Scholar]
- 4.Bialek EJ, Jakubowski W, Zajkowski P, Szopinski KT, Osmolski A. US of the major salivary glands: Anatomy and spatial relationships, pathologic conditions, and pitfalls. Radiographics. 2006;26:745–63. doi: 10.1148/rg.263055024. [DOI] [PubMed] [Google Scholar]
- 5.Frommer J. The human accessory parotid gland: Its incidence, nature, and significance. Oral Surg Oral Med Oral Pathol. 1977;43:671–6. doi: 10.1016/0030-4220(77)90049-4. [DOI] [PubMed] [Google Scholar]
- 6.Mansour N, Hofauer B, Knopf A. Ultrasound elastography in diffuse and focal parotid gland lesions. ORL J Otorhinolaryngol Relat Spec. 2017;79:54–64. doi: 10.1159/000455727. [DOI] [PubMed] [Google Scholar]
- 7.Stryjewska-Makuch G, Kolebacz B, Janik MA, Wolnik A. Increase in the incidence of parotid gland tumors in the years 2005-2014. Otolaryngol Pol. 2017;71:29–34. doi: 10.5604/01.3001.0009.8412. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi R, Dey P. Diagnostic problems of salivary gland tumors. Diagn Cytopathol. 2015;43:495–509. doi: 10.1002/dc.23255. [DOI] [PubMed] [Google Scholar]
- 9.Wu S, Liu G, Chen R, Guan Y. Role of ultrasound in the assessment of benignity and malignancy of parotid masses. Dentomaxillofac Radiol. 2012;41:131–5. doi: 10.1259/dmfr/60907848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rzepakowska A, Osuch-Wójcikiewicz E, Sobol M, Cruz R, Sielska-Badurek E, Niemczyk K, et al. The differential diagnosis of parotid gland tumors with high-resolution ultrasound in otolaryngological practice. Eur Arch Otorhinolaryngol. 2017;274:3231–40. doi: 10.1007/s00405-017-4636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heřman J, Sedláčková Z, Vachutka J, Fürst T, Salzman R, Vomáčka J, et al. Differential diagnosis of parotid gland tumors: Role of shear wave elastography. Biomed Res Int. 2017;2017:9234672. doi: 10.1155/2017/9234672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, et al. Shear-wave elastography: Basic physics and musculoskeletal applications. Radiographics. 2017;37:855–70. doi: 10.1148/rg.2017160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Li Y, Zhang S, Li X, Wang H, Yong X, et al. Evaluation of microvascularization in focal salivary gland lesions by contrast-enhanced ultrasonography (CEUS) and color Doppler sonography. Clin Hemorheol Microcirc. 2013;54:259–71. doi: 10.3233/CH-131732. [DOI] [PubMed] [Google Scholar]
- 14.Mansour N, Bas M, Stock KF, Strassen U, Hofauer B, Knopf A, et al. Multimodal ultrasonographic pathway of parotid gland lesions. Ultraschall Med. 2017;38:166–73. doi: 10.1055/s-0035-1553267. [DOI] [PubMed] [Google Scholar]
- 15.Badea AF, Bran S, Tamas-Szora A, Floareş A, Badea R, Baciut G, et al. Solid parotid tumors: An individual and integrative analysis of various ultrasonographic criteria. A prospective and observational study. Med Ultrason. 2013;15:289–98. doi: 10.11152/mu.2013.2066.154.afb2. [DOI] [PubMed] [Google Scholar]
- 16.Mantsopoulos K, Psychogios G, Agaimy A, Künzel J, Zenk J, Iro H, et al. Inflamed benign tumors of the parotid gland: Diagnostic pitfalls from a potentially misleading entity. Head Neck. 2015;37:23–9. doi: 10.1002/hed.23541. [DOI] [PubMed] [Google Scholar]
- 17.de Ru JA, van Leeuwen MS, van Benthem PP, Velthuis BK, Sie-Go DM, Hordijk GJ, et al. Do magnetic resonance imaging and ultrasound add anything to the preoperative workup of parotid gland tumors? J Oral Maxillofac Surg. 2007;65:945–52. doi: 10.1016/j.joms.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Lee YY, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol. 2008;66:419–36. doi: 10.1016/j.ejrad.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Chawla A, Srinivasan S, Lim TC, Pulickal GG, Shenoy J, Peh WC, et al. Dual-energy CT applications in salivary gland lesions. Br J Radiol. 2017;90:20160859. doi: 10.1259/bjr.20160859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roele ED, Timmer VC, Vaassen LA, van Kroonenburgh AM, Postma AA. Dual-energy CT in head and neck imaging. Curr Radiol Rep. 2017;5:19. doi: 10.1007/s40134-017-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartaglione T, Botto A, Sciandra M, Gaudino S, Danieli L, Parrilla C, et al. Differential diagnosis of parotid gland tumours: Which magnetic resonance findings should be taken in account? Acta Otorhinolaryngol Ital. 2015;35:314–20. doi: 10.14639/0392-100X-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Li J, Tan YR, Xiong P, Zhong LP. Accuracy of diagnosis of salivary gland tumors with the use of ultrasonography, computed tomography, and magnetic resonance imaging: A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:238–4500. doi: 10.1016/j.oooo.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Nada AM, Youssef AA, Basmy AA, Amin AA, Shokry AM. Diffusion-weighted imaging of the parotid gland: Can the apparent diffusion coefficient discriminate between normal and abnormal parotid gland tissues? Erciyes Med J. 2017;29:125–30. [Google Scholar]
- 24.Yuan Y, Tang W, Tao X. Parotid gland lesions: Separate and combined diagnostic value of conventional MRI, diffusion-weighted imaging and dynamic contrast-enhanced MRI. Br J Radiol. 2016;89:20150912. doi: 10.1259/bjr.20150912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M, et al. Salivary gland tumors: Diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology. 2003;226:345–54. doi: 10.1148/radiol.2262011486. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Lei GW, Wang SW, Zheng SW, Ge Y, Wei FC, et al. Diagnostic value of CT perfusion imaging for parotid neoplasms. Dentomaxillofac Radiol. 2014;43:20130237. doi: 10.1259/dmfr.20130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, et al. Parotid gland tumors: Can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology. 2008;249:909–16. doi: 10.1148/radiol.2493072045. [DOI] [PubMed] [Google Scholar]
- 28.Habermann CR, Arndt C, Graessner J, Diestel L, Petersen KU, Reitmeier F, et al. Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: Is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol. 2009;30:591–6. doi: 10.3174/ajnr.A1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H. MR imaging of parotid tumors: Typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol. 2011;32:1202–7. doi: 10.3174/ajnr.A2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yologlu Z, Aydin H, Alp NA, Aribas BK, Kizilgoz V, Arda K, et al. Diffusion weighted magnetic resonance imaging in the diagnosis of parotid masses. Preliminary results. Saudi Med J. 2016;37:1412–6. doi: 10.15537/smj.2016.12.16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joe VQ, Westesson PL. Tumors of the parotid gland: MR imaging characteristics of various histologic types. AJR Am J Roentgenol. 1994;163:433–8. doi: 10.2214/ajr.163.2.8037045. [DOI] [PubMed] [Google Scholar]
- 32.Cantisani V, David E, Sidhu PS, Sacconi B, Greco A, Pandolfi F, et al. Parotid gland lesions: Multiparametric ultrasound and MRI features. Ultraschall Med. 2016;37:454–71. doi: 10.1055/s-0042-109171. [DOI] [PubMed] [Google Scholar]
- 33.Fuller E, Bharatha A, Yeung R, Kassel EE, Aviv RI, Howard P, et al. Case of the month #166: Synovial chondromatosis of the temporal mandibular joint. Can Assoc Radiol J. 2011;62:151–3. doi: 10.1016/j.carj.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Mazziotti S, Blandino A, Gaeta M, Bottari A, Sofia C, D’Angelo T, et al. Postprocessing in maxillofacial multidetector computed tomography. Can Assoc Radiol J. 2015;66:212–22. doi: 10.1016/j.carj.2014.12.004. [DOI] [PubMed] [Google Scholar]


