SETD2 encodes a histone H3 lysine 36 (H3K36) methyltransferase affected by mutations and deletions in a wide variety of cancers. Whether SETD2 loss alone can initiate tumorigenesis has not been established previously but in the present study, Zhang et al. identify that SETD2 deficiency eventually results in outgrowth of cells with a phenotype consistent with myelodysplastic syndromes and acute myeloid leukemia.
Dynamic modifications of histones are important regulators of gene expression with H3K36me3 being tightly associated with transcriptionally active regions of chromatin and gene bodies.1 SETD2 is the sole H3K36 trimethyltransferase in eukaryotic cells and SETD2 has been ascribed to be involved in a number of biological processes mediated by diverse proteins with H3K36me3 reader domains (reviewed recently).2 This includes a role for SETD2 in DNA mismatch repair (through H3K36me3 binding of the protein hMS6), homologous recombination (mediated by LEDGF), DNA methylation (through H3K36me3 binding of DNMT3B), and nucleosome reorganization (through binding of the FACT complex to H3K36me3). Moreover, SETD2 itself physically associates with the hyperphosphorylated C-terminal domain of RNA polymerase II (RNA pol II), an association thought to link H3K36 trimethylation to sites of active transcription. The association between SETD2 and transcription has been shown to provide an indirect role for SETD2 in regulating RNA splicing, which is performed in a co-transcriptional manner and influenced by RNA pol II elongation at certain loci.
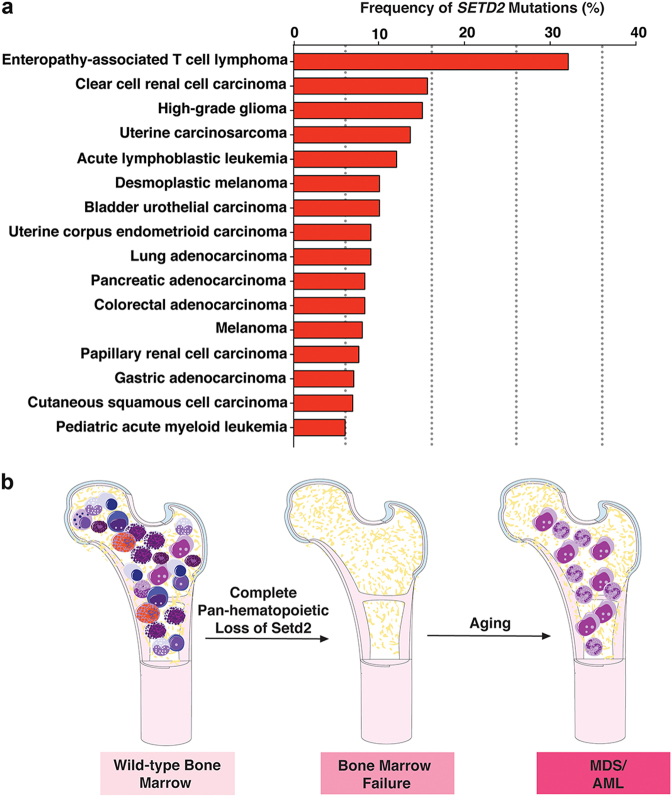
The importance of H3K36 trimethylation in cancer development is underscored by at least two striking findings from human genetic data. First, SETD2 mutations occur in many neoplasms, particularly clear cell renal cell carcinoma (ccRCC, ~15%–20%), adenocarcinoma of the lung (~5%), acute myeloid leukemia (AML, ~6%), and acute lymphoblastic leukemia (ALL, ~10%) (Fig. 1a).3,4 Second, the methyl acceptor site of H3K36 and adjacent amino acid residues in histone H3.3 are themselves mutated in specific cancers. This includes H3.3K36M mutations in chondroblastomas as well as mutations in the neighboring residue (G34) in giant-cell bone tumors. These mutations deplete H3K36me3, further implicating alterations in H3K36me3 levels in promoting tumorigenesis.
Fig. 1.
SETD2 is recurrently mutated in cancer and loss of Setd2 disrupts normal hematopoiesis. a Histogram displaying frequency of SETD2 mutations based on data from cbioportal.org, Fahey et al.2 and Moffitt et al.8 b As shown by Zhang et al.,5 complete pan-hematopoietic deletion of Setd2 in mice results in failure of hematopoiesis and normal HSC self-renewal. With time, however, a proportion of mice develop outgrowth of dysplastic Setd2-deficient cells with a clonal advantage resembling myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML)
In leukemias, SETD2 mutations were originally identified in conjunction with other alterations well established in leukemia initiation. This includes the discovery of SETD2 mutations in ~20% of MLL (also known as KMT2A)-rearranged AML.4 Consistent with this, suppression of SETD2 in combination with overexpression of MLL-AF9 fusions promoted leukemogenesis in vitro and in vivo. Despite these data supporting a role for SETD2 loss in leukemia pathogenesis, the role of SETD2 in normal hematopoiesis was not previously known. To this end, Zhang et al. generated hematopoietic-specific Setd2 knockout (KO) mice and demonstrated that Setd2 maintains a balance between hematopoietic stem cell (HSC) self-renewal and differentiation.5 Complete Setd2 loss resulted in impaired HSC self-renewal, identifying a critical role for SETD2 in normal HSC function. Based on the premise that patients with bone marrow failure syndromes eventually develop clonal transformation in the setting of ineffective hematopoiesis, with time (~8 months), abnormal cytological features suggestive of myelodysplastic syndromes (MDS) were detected in the peripheral blood of the aged Setd2 KO mice along with features of extramedullary hematopoiesis (Fig. 1b).6 Five of 8 (62%) Setd2 KO mice developed cytopenias suggestive of MDS, whereas the remaining three had a myeloproliferative phenotype (characterized by increased blood counts). Interestingly, 4 of 5 (80%) mice had bone marrow and spleen fibrosis, raising the question as to whether they developed MDS/MPN (myeloproliferative neoplasm) overlap syndrome, vs. a true MDS phenotype.
The fact that complete Setd2 loss was associated with impaired HSC self-renewal may be consistent with recent data by Mar et al. using another newly generated Setd2 conditional KO mouse model.7 In this latter model, complete Setd2 loss impaired MLL-AF9 leukemogenesis, whereas heterozygous Setd2 loss enhanced MLL-AF9 leukemogenesis. Given these findings, it will be interesting to study the potential effects of heterozygous Setd2 loss on hematopoiesis using the conditional KO models generated across both studies in the future. These issues have translational importance as ablation of residual SETD2 activity might represent an interesting therapeutic approach if complete SETD2 loss preferentially eliminates leukemia cells with heterozygous SETD2 mutations. Of note, complete ablation of Setd2 within T-cells (using Lck-Cre) promotes the development of specific T-cell populations.8 These data identify cell context-specific effects for Setd2 deletion even within the hematopoietic compartment.
Given the myriad of biological processes that SETD2’s methyltransferase activity has been linked to, deciphering which of these functions is important for tumorigenesis has been challenging. In the present study, transcriptomic analysis revealed that the gene expression profile of Setd2 KO mice was similar to that of Dnmt3a and Tet2 double KO mice. These data suggest that Setd2 and Dnmt3a/Tet2 double KO share a common DNA methylation-based mechanism contributing to leukemogenesis. Consistent with this, Setd2 KO HSCs were marked by a preponderance of hypomethylated differentially methylated regions. Despite this potential link of Setd2 loss to altered DNA methylation dynamics, Setd2 KO HSCs exhibit impaired fitness, whereas Dnmt3a/Tet2 double KO HSCs have a fitness advantage.9 However, similar to clonal selection processes in bone marrow failure syndromes, with time, Setd2 KO cells do eventually transform resulting in myeloid neoplasms.
Given these data establishing SETD2 as a bona fide regulator of HSC self-renewal and leukemia initiation, developing pharmacologic approaches to target SETD2 mutant cells could have great importance. Prior work in ccRCC has suggested that SETD2-deficient ccRCC cells exhibit preferential sensitivity to WEE1 kinase inhibition.10 Both SETD2 deficiency and WEE1 inhibition were found to result in reduced levels of RRM2, a ribonucleotide reductase. WEE1 loss in the context of SETD2 deficiency critically reduced the dNTP pool and WEE1 inhibition was thereby synthetically lethal with SETD2 loss. In the context of hematopoietic cells, reduced expression of another subunit of the complex responsible for generating dNTPs was seen in the Setd2 KO mice. Thus, further work to evaluate the sensitivity of SETD2-null and mutant leukemias to WEE1 inhibition as well as other therapies linked to increased DNA damage response and deficient mismatch repair could be therapeutically important.
References
- 1.Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat. Rev. Mol. Cell. Biol. 2012;13:115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahey CC, Davis IJ. SETting the stage for cancer development: SETD2 and the consequences of lost methylation. Cold Spring Harb. Perspect. Med. 2017;7:a026468. doi: 10.1101/cshperspect.a026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalgliesh GL, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu X, et al. Identification of functional cooperative mutations of SETD2 in human acute leukemia. Nat. Genet. 2014;46:287–293. doi: 10.1038/ng.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan-Liang, Z., et al. Cell Res.10.1038/s41422-018-0015-9 (2018).
- 6.Yoshizato T, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N. Engl. J. Med. 2015;373:35–47. doi: 10.1056/NEJMoa1414799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mar BG, et al. SETD2 alterations impair DNA damage recognition and lead to resistance to chemotherapy in leukemia. Blood. 2017;130:2631–2641. doi: 10.1182/blood-2017-03-775569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffitt AB, et al. Enteropathy-associated T cell lymphoma subtypes are characterized by loss of function of SETD2. J. Exp. Med. 2017;214:1371–1386. doi: 10.1084/jem.20160894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, et al. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat. Genet. 2016;48:1014–1023. doi: 10.1038/ng.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfister SX, et al. Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Cancer Cell. 2015;28:557–568. doi: 10.1016/j.ccell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]