Significance
Urbanization reduces exposure risk to many wildlife parasites and in general, improves overall health. However, our study importantly shows the complicated relationship between the diffusion of zoonotic pathogens and urbanization. Here, we reveal an unexpected relationship between hemorrhagic fever with renal syndrome incidence caused by a severe rodent-borne zoonotic pathogen worldwide and the process of urbanization in developing China. Our findings show that the number of urban immigrants is highly correlated with human incidence over time and also explain how the endemic turning points are associated with economic growth during the urbanization process. Our study shows that urbanizing regions of the developing world should focus their attention on zoonotic diseases.
Keywords: urbanization, immigration, hantavirus, endemic turning point, China
Abstract
Urbanization and rural–urban migration are two factors driving global patterns of disease and mortality. There is significant concern about their potential impact on disease burden and the effectiveness of current control approaches. Few attempts have been made to increase our understanding of the relationship between urbanization and disease dynamics, although it is generally believed that urban living has contributed to reductions in communicable disease burden in industrialized countries. To investigate this relationship, we carried out spatiotemporal analyses using a 48-year-long dataset of hemorrhagic fever with renal syndrome incidence (HFRS; mainly caused by two serotypes of hantavirus in China: Hantaan virus and Seoul virus) and population movements in an important endemic area of south China during the period 1963–2010. Our findings indicate that epidemics coincide with urbanization, geographic expansion, and migrant movement over time. We found a biphasic inverted U-shaped relationship between HFRS incidence and urbanization, with various endemic turning points associated with economic growth rates in cities. Our results revealed the interrelatedness of urbanization, migration, and hantavirus epidemiology, potentially explaining why urbanizing cities with high economic growth exhibit extended epidemics. Our results also highlight contrasting effects of urbanization on zoonotic disease outbreaks during periods of economic development in China.
Urbanization is a complex phenomenon that has been associated with rapid environmental changes and population movements, factors implicated as drivers of the transmission dynamics of communicable disease (1). On the one hand, urbanization has been shown to alter the composition of wildlife communities and increase the number of species that thrive in urban areas (2), which has the potential to pose short-term and long-term health problems (3, 4) and facilitate infectious disease emergence (5). On the other hand, urbanization often improves infrastructure and environmental health and leads to increases in health care provision (6, 7). It is, therefore, generally recognized that urban living has contributed to an overall improvement of health (8). However, the relationship between the urbanization process and health seems to be complex. The process of urbanization has also been shown to promote the emergence of infectious diseases (9, 10), a public health problem that has particularly affected large- and medium-sized cities (11). Many cities in 19th century Europe, for instance, suffered an “urban penalty” from infectious disease, such that deaths exceeded births, and urban growth was only sustained by immigration subsidies from the countryside (12). Our current understanding of the relationship between urbanization/urban living and the health threats posed by infectious diseases is, therefore, incomplete (12).
Hantaviruses (family Hantaviridae) are known to cause two serious human diseases: hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome (HFRS) (13–15). Case fatality rates range from 0.5 to 40% depending on the viral strain (15, 16). Hantaviruses are primarily transmitted from infected animals to humans via inhalation of aerosols contaminated by virus shed in excreta, saliva, and urine (15, 17). Epidemiological evidence indicates that areas with the greatest risk for human infection include those that experience more migrants and crowded working and living conditions, especially poor housing conditions, where close contact with the excreta of infected rodents is frequent (18, 19).
China has experienced an urbanization process at an unprecedented rate and scale over the past half-century (20), with the proportion of the population living in urban areas increasing from 18% in 1978 to 51% in 2011, exceeding the population living in rural areas for the first time (21). Furthermore, HFRS is still an important public health problem in China, with over 1.4 million cases in humans (including 45,000 deaths) reported between 1950 and 2010 (22). Of the major endemic countries for HFRS, China accounts for 90% of total HFRS cases worldwide (23, 24), mainly caused by two strains of hantavirus: Hantaan virus (HTNV; carried by Apodemus agrarius) and Seoul virus (SEOV; carried by Rattus norvegicus) (25, 26). More than 1.2 billion people live in provinces of China where HFRS has been reported (27).
An increasing number of studies point to links between the urbanization process and the spread of zoonotic diseases (28–31). Previous studies have shown that HFRS incidence is linked to land use change (32–34), especially along the urban–rural gradient (35, 36). However, studies that address the dynamics of zoonotic spillover, with particular focus on the role of urbanization over long timescales, are lacking. We set out to disentangle the complex relationship between urbanization and infectious disease dynamics at the human–wildlife interface using an unprecedented amount of data covering 48 y (1963–2010). These data date back to the first HFRS case reported in Hunan Province, China.
Results
Dynamics and Spatial Clustering.
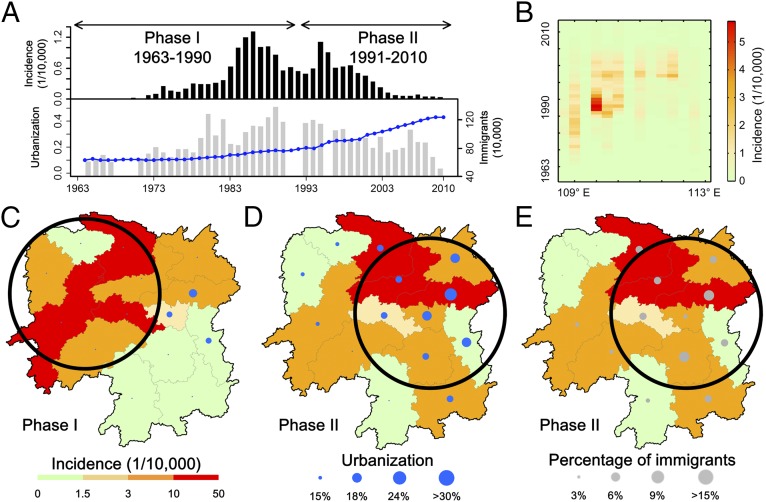
More than 110,000 HFRS cases were reported in Hunan Province from 1963 to 2010 in a province with more than 60 million people, covering an area of 211,800 km2 in southern China. The incidence curve over the 48-y period is reasonably characterized by two phases: phase I spanning from 1963 to 1990 and phase II covering the period between 1991 and 2010 (Fig. 1A). The time series of incidence highlights the geographic pattern of the epidemic, which spread from western cities to eastern ones throughout the 1980s and 1990s (Fig. 1B and SI Appendix, Fig. S1). During phase I, the main endemic areas of HFRS were located in western Hunan Province (Fig. 1C), while in phase II, newly established endemic areas emerged in the east (Fig. 1D). It is worth noting that the spatial and temporal patterns of HFRS incidence coincide with trends in urbanization and immigration patterns across Hunan (Fig. 1 D and E) rather than native population growth or the balance between immigration and emigration (SI Appendix, Figs. S2 and S3). A second notable feature is the synchronized pattern of the urbanization process and immigration across the cities.
Fig. 1.
Time series dynamics of HFRS outbreaks in Hunan Province. (A) Temporal distribution patterns of HFRS incidence (black bars), urbanization (blue line), and number of immigrants (gray bars) in Hunan Province, 1963–2010. The number of immigrants in Hunan is not available for the years 1967–1969, 1990–1991, and 1995. (B) HFRS incidence in the 14 cities of Hunan sorted by longitudes. (C) The spatial distribution of HFRS incidence and urbanization in each city for phase I (1963–1990). (D) Urbanization (blue circles) and (E) percentage of immigrants (gray circles) in each city for phase II (1991–2010). In C and D, the background maps with color gradient present the incidence of HFRS for phase II (1991–2010). Urbanization of cities in our study is defined as the percentage of the nonagricultural population (which is recorded in China’s Hukou household registration system) to the total population.
Urbanization and Immigration.
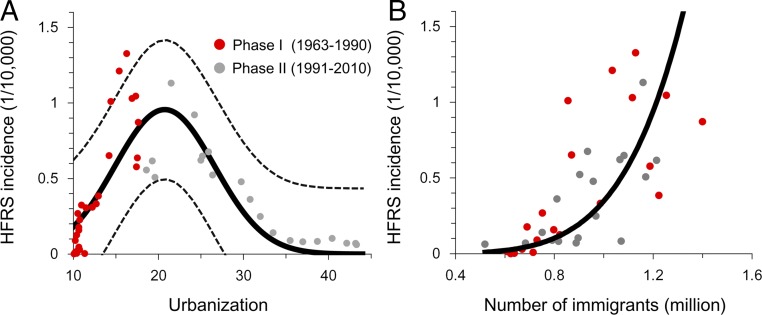
We compared patterns of urbanization and number of immigrants at both provincial and city scales with those of HFRS incidence from 1963 to 2010. HFRS incidence and urbanization followed an inverted U-shaped relationship at the provincial scale (Fig. 2A). In phase I, the primary stage of urbanization, HFRS incidence and urbanization were strongly positively correlated, whereas in phase II, they were negatively correlated. We propose the existence of an endemic turning point (ETP) between urbanization and zoonotic trends as a result of interventions reducing incidence during phase II (Fig. 2A). The HFRS incidence and number of immigrants are strongly positively correlated for both phases (1963–2010) (Fig. 2B). This result indicates that the effect of urbanization on HFRS epidemics changed, while the effect of immigration remained constant (SI Appendix, Fig. S4).
Fig. 2.
Urbanization, immigration, and HFRS outbreak. (A) Urbanization and HFRS incidence in Hunan Province. The thick black line shows the fit of urbanization and HFRS cases; 95% prediction ranges of regression are shown as dashed lines. (B) Scatterplot of the number of immigrants (in millions) and HFRS incidence. The thick black line shows the regression of the annual number of immigrants and HFRS incidence.
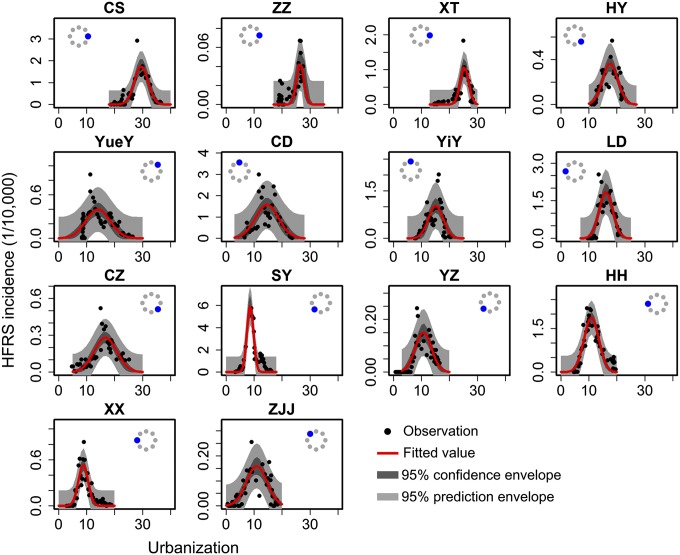
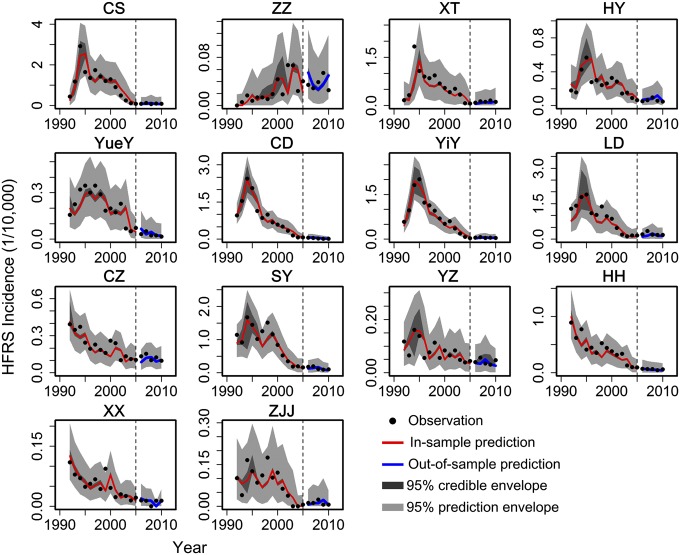
We used the city level to parameterize the spatiotemporal model. Fig. 3 shows the incidence of HFRS and pattern of urbanization for each city. The patterns are conspicuously different between locations—most, although not all, eastern cities experienced more prolonged epidemic buildup than western cities. This difference may be associated with specific socioeconomic profiles, a point to which we return later. To account for uncertainty in the ETPs, we used a Bayesian approach (Materials and Methods). Posterior medians and 95% credible intervals for the model parameters are provided in SI Appendix, Table S3. Our final results focus on the posterior median values. HFRS incidence exhibits a first-order autoregressive signature, indicating that the occurrence of HFRS in a given year is related to the numbers in the preceding year. In addition, we found that cities with a large immigrant population have a higher incidence of HFRS than cities with a small immigrant population. The predicted incidence from the spatiotemporal model fit well with the observations over the 1992–2010 period, including peak values (Fig. 4) (the average R2 train and R2 test of cross-validation are 0.89 and 0.71, respectively). Trace plots (SI Appendix, Fig. S5) and the Gelman and Rubin diagnostic indicate convergence of the Markov chain Monte Carlo chains [all scale reduction factors were estimated as one (37)], and all posterior distributions were approximately normal (SI Appendix, Fig. S6). In diagnosing the residuals of the model, a random pattern was observed, with no autocorrelation (SI Appendix, Fig. S7).
Fig. 3.
Incidence of HFRS and urbanization process for each city in Hunan Province. Observations are shown as black points, and red lines represent fitted curves. The dark-gray areas indicate the 95% confidence envelopes, and the light-gray areas indicate the 95% prediction envelopes. The city locations, relative to the center of Hunan Province, are signified by the blue compass indicator. CD, Chang De; CS, Chang Sha; CZ, Chen Zhou; HH, Huai Hua; HY, Heng Yang; LD, Lou Di; SY, Shao Yang; XT, Xiang Tan; XX, Xiang Xi; YiY, Yi Yang; YueY, Yue Yang; YZ, Yong Zhou; ZJJ, Zhang Jia Jie; ZZ, Zhu Zhou.
Fig. 4.
The estimated HFRS disease temporal profiles of cities in Hunan Province. The solid lines correspond to the estimated HFRS incidence, and the dark-gray areas correspond to pointwise 95% credible envelopes. The light-gray areas indicate the 95% prediction envelopes for new observations, which account for the estimated observation error. The observed data are plotted as black points, and the red lines represent the in-sample prediction of HFRS incidence. The blue lines represent the out-of-sample prediction of HFRS incidence separated from the simulated HFRS incidence by dashed vertical lines. CD, Chang De; CS, Chang Sha; CZ, Chen Zhou; HH, Huai Hua; HY, Heng Yang; LD, Lou Di; SY, Shao Yang; XT, Xiang Tan; XX, Xiang Xi; YiY, Yi Yang; YueY, Yue Yang; YZ, Yong Zhou; ZJJ, Zhang Jia Jie; ZZ, Zhu Zhou.
ETP.
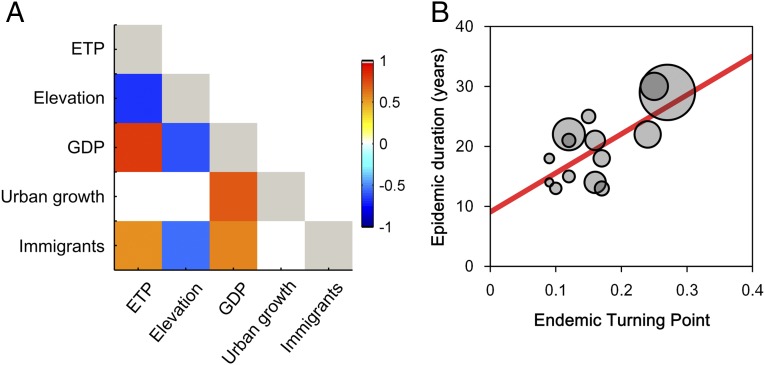
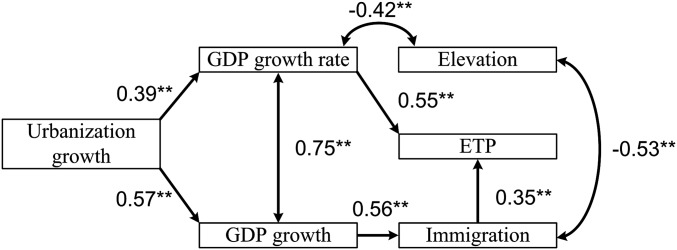
We studied the variation in ETPs among cities by examining associations with potential socioeconomic covariates. The results reveal strong correlations between the ETPs, economic growth, and immigration (Fig. 5A). It is important to note that ETP is positively associated with per capita gross domestic product growth (r = 0.80, P < 0.01). Urbanization growth correlated with per capita gross domestic product growth as well (r = 0.69, P < 0.01). Note that all of these quantities are significantly negatively correlated with elevation of city (unit: meters). Overall, the duration of HFRS epidemics (represented by the time to peak of HFRS incidence since the end point of the epidemic, which may be influenced by vaccination campaigns that started after 2010; note that the epidemic is still ongoing) was prolonged in cities with higher ETPs (r = 0.70, P < 0.01) (Fig. 5B) and relatively high levels of urbanization. These results suggest that cities with faster economic growth during the urbanization process may reach their ETPs later (i.e., experienced more prolonged epidemics) due to the large number of immigrants prolonging the epidemic duration, while economic growth contributes toward improvements in the living conditions and general health of a population. These contrasting effects may influence the dynamics of the epidemics (e.g., epidemic duration and ETP). A structural equation model analysis consistently supported our hypothesis and previous analysis and indicated the following pathways (Fig. 6). Urbanization growth correlated with per capita gross domestic product growth followed by a direct positive effect of per capita gross domestic product growth on immigration. This result is consistent with previous studies, which found evidence of long-run causality from per capita economic growth to immigration but not vice versa (38). Additionally, a long-term balanced relationship exists between urbanization and economic growth in China (39). The urbanization process seems to have a positive indirect effect on the ETP via gross domestic product growth rate, and immigration has a positive direct effect on the ETP.
Fig. 5.
Interrelatedness of the urbanization process, immigration, ETPs, and epidemic duration between cities. (A) Correlation matrix for the estimated ETPs, median elevation, per capita gross domestic product growth, growth of urbanization, and number of immigrants among cities. Only significant relationships are shown. The growth of urbanization is not linearly correlated with ETP but rather, influences it indirectly through its effect on economic growth. The relationship between urbanization rate and immigration over time between cities is provided in SI Appendix, Fig. S8. (B) Duration of HFRS epidemics as a function of ETPs (urbanization). Epidemic duration is presented by the duration between the origin and the peak of HFRS incidence of the corresponding curve. Circle size is proportionate to the per capita gross domestic product growth. There are significant correlations between epidemic duration and gross domestic product (r = 0.67, P < 0.01) and between epidemic duration and ETPs (r = 0.70, P < 0.01). GDP, gross domestic product.
Fig. 6.
Structure and results from our structural equation models for the city-level data: χ2/df = 10.17/5, comparative fit index = 0.90. There is no statistical difference between the predicted and observed covariance matrices (P > 0.05). Double-headed arrows indicate correlations. Straight lines indicate direct relationships. Numbers are standardized path coefficients (55) Gross domestic product growth (purchasing power parity) indicates per capita gross domestic product growth. Gross domestic product growth rate (percentage) indicates per capita gross domestic product growth rate. Immigration indicates number of immigrants. The coefficients of determination for equations of endogenous variables are 0.62 for ETP, 0.32 for immigration, 0.27 for elevation, 0.16 for gross domestic product growth rate, and 0.34 for gross domestic product growth. The structural equation model was implemented by using the R package lavaan with maximum likelihood estimation procedures. GDP, gross domestic product. **P < 0.05.
Discussion
The transition of populations from rural to urban environments has resulted in changing global patterns of disease and mortality (40, 41). Our study of hantavirus spillover raises a number of issues. We clearly show the important roles of urbanization and population migration in epidemic spread using historical datasets of time series comprising half a century of records. In particular, the long-term data from Hunan Province allow us a unique opportunity to study the relationship between urbanization and zoonotic diseases. In our study area, HFRS was first reported in 1963; a relatively low prevalence of hantavirus infections among both rodent and human cases would be expected to be seen in the early years (accompanying a relatively low level of urbanization). Then, during the period of eastward spread, the virus spread among rodents from endemic to nonendemic areas. This was followed by an increase in incidence of HFRS until urbanization finally resulted in an ETP.
In industrialized nations, urban living has generally contributed to an overall improvement of health (8). During the last 50 y, we find a biphasic inverted U-shaped relationship between urbanization and HFRS rates in Chinese cities. We use the ETP to quantify the difference in peak between cities. Our results show that cities with a higher economic growth rate experienced more rapid urbanization and prolonged HFRS epidemics. We infer that the process of urbanization and associated economic growth may delay zoonotic disease decline, possibly due to a higher volume of immigrants and the specific living conditions of recent immigrants (SI Appendix, Fig. S8). The statistical relationship between HFRS, urbanization, and immigration plausibly results from a chain effect from economic growth, which drives the urbanization process and regulates/encourages immigrant movement, thereby influencing overall epidemic situations. The number of immigrants is also affected by many factors, such as urbanization, economic growth, increased demand for labor, and other socioeconomic factors. Therefore, the relationships between immigration and zoonotic spillover and between immigration and urban growth may be complex and may not be linear for every case (SI Appendix, Figs. S4 and S8). In addition to the urbanization process, there is evidence that elevation is also an important factor with regards to immigration. Higher-elevation areas tend to have lower economic growth rates and in turn, attract fewer immigrants (Fig. 5A and SI Appendix, Fig. S9). Perhaps this is not a surprising result: lower average elevation contributes positively to economic growth in China (42). Thus, the complexity of zoonotic spillover is likely to be a result of a combination of environmental, biological, and anthropogenic dimensions to the interactions at the human–wildlife interface (7, 16). The detailed interrelatedness of urbanization rates, immigration, and economic growth is an important area for future work.
Urban environments have proven to be favorable for rat (Rattus spp.) population growth and associated zoonoses (43), with a notable increase in SEOV-related HFRS in cities of China due to the increased contact between rats and humans (44). The early stage of urbanization is characterized by mass rural–urban immigration alongside rapid urban expansion with poorly developed infrastructure (45). New immigrants usually enter urban regions with poor housing and health care conditions, which are factors contributing toward a high risk of human infection. Moreover, owing to farmland conversion, deforestation, and other land use changes induced by urban expansion, habitat loss and fragmentation could drive rodent movement and thereby, increase human exposure to rodents (46, 47).
Finally, we show that epidemic levels of HFRS generally decline when the urbanization process (SI Appendix, Figs. S10 and S11 and Table S1) results in an ETP. Urbanized settings are driven by economic growth, and economic growth contributes to improvements in the general health of a population. Increased development generally improves living conditions, thereby reducing rat populations. The key to HFRS control is to both reduce peridomestic rodent abundance and enhance vaccination coverage. Vaccination programs need to take into account the particular challenge of human migration from rural to urban settings. In particular, our analysis shows that immigration influences spatiotemporal patterns of HFRS incidence during the urbanization process, with resulting health implications. On the one hand, immigrants traveling from nonendemic rural areas may be susceptible to pathogens to which they have not previously been exposed. On the other hand, immigrants originating from high endemic areas can be expected to have cross-protection. Therefore, the implementation of rigorous vaccination campaigns is crucial to controlling HFRS in urbanized China.
Limitations of this study should also be noted. First, HFRS in China may be caused by HTNV harbored by A. agrarius or by SEOV harbored by the genus Rattus (48, 49). As these two rodent reservoirs have a different ecology and live in different habitats, this may influence the epidemiologic characteristics of HFRS. However, in our analysis, we did not differentiate between sero-/genotypes of hantavirus due to limited data. Thus, the relative importance of SEOV vs. HTNV in fueling HFRS epidemics is not elucidated by our study. Second, the number of HFRS cases was likely underreported at the beginning of the study period. Although strict criteria exist to ensure proper clinical diagnosis and reporting is required by law, an increase in the number of hospitals and trained doctors and nurses over the past 50 y will have skewed the number of disease cases reported, with an increase in cases reported in more recent years. In addition, the increased use of telephones and cellular telephones will likewise have increased the number of disease cases reported in more recent years. Given that HFRS cases are to be reported within 24 h by law, regardless of the urban or rural residence, and the severe clinical course of HFRS, it could be expected that differences in detection rate between urban and rural communities have been greatly diminished. Third, socioeconomic statistics were not available for some cities before 1992, and therefore, these statistics could not be taken into account for those cases. Better data on rodent population density and virus prevalence in rodents will be important to fully understand the relationship between urbanization and HFRS risk.
On a global scale, urbanization is increasing, with the world’s urban population forecasted to reach 6.3 billion in 2050, potentially leading to significant changes in infectious disease risks and disease burden. Our results should motivate the development of practical predictive frameworks for HFRS epidemics during the urbanization process in developing countries and in hantavirus-endemic regions of the world.
Materials and Methods
Data.
We used official reports and associated socioeconomic data for Hunan Province. Records of HFRS cases from 1963 to 2010 were obtained from the Hunan Provincial Center for Disease Control and Prevention. As HFRS is a severe viral disease in China, strict criteria are applied to both clinical diagnosis and reporting procedures, and the surveillance strategies for HFRS in Hunan remained constant during the study period. Since the 1980s, cases were also confirmed by detecting antibodies against hantavirus in patients’ serum samples. We also conducted the analysis using the dataset from 1980 to 2010. The results consistently supported our analysis (SI Appendix, Figs. S14 and S15). In 2008, the HFRS-targeted Expanded Program on Immunization was implemented to reduce the incidence of HFRS, and a vaccination campaign was conducted in our study area since 2009 (50). Therefore, the incidence of HFRS after 2010 was not included in our analysis.
Demographic data, including the total population size and number of immigrants (from 1963 to 2010), were obtained from Hunan Province’s statistical yearbook, China’s statistical yearbook of cities, and the Hunan Public Security Bureau. Here, immigration is defined as the movement of people into a destination city to settle or to take up employment as migrant workers. In our study, the urbanization at the provincial level is defined as the percentage of the total population living in urban areas (Fig. 1), while the urbanization at the city level is defined as the percentage of the nonagricultural population (which is recorded in China’s Hukou household registration system) to the total population. Due to changes in the official statistical category of “urban population” at the city level, these data span a shorter period. The data were grouped by city according to the administrative boundaries for 14 cities. There is high concordance between the urban population (SI Appendix, Fig. S12) and the nonagricultural population at both the provincial and city levels (SI Appendix, Fig. S13). Remotely sensed data were acquired using Landsat Thematic Mapper and Enhanced Thematic Mapper Plus as the base images used to extract coverage of urban areas and to quantify urban area expansion.
Urbanization and HFRS Dynamics.
The relationship between urbanization and HFRS incidence is explored as a function of the urbanization and the number of immigrants. Early urbanization information was incomplete for some cities; these were, therefore, replaced through linear extrapolation. We note that it is difficult to accurately reconstruct the missing number of annual immigrants for the early study period (details are in SI Appendix, Table S2).
Estimating the ETP.
At some time point during the course of the urbanization process, all cities exhibit an ETP defined as the inflection point along the epidemic curve at which incidence changes from increasing to decreasing. This point is likely a complex function of improved sanitation, health care access, and shifts in peridomestic abundance of the rodent reservoir host. To investigate HFRS dynamics across cities, we used a flexible Bayesian estimation method, which links observed HFRS incidence in city s and year t, Y(s,t), to immigration (N), urbanization (U), and the city-specific empirical ETPs. The spatiotemporal Poisson regression model considered is given by
| [1] |
| [2] |
| [3] |
| [4] |
where μ denotes the fitted model, Eu is the overall urbanization effect, and Ei is the overall immigration effect on HFRS epidemics, with the coefficients β, γ, and φ representing regression parameters. Temporal random effects were modeled by a first-order autoregressive process: g(s,t) = θg(s, t − 1) + ε. The parameter θ is the temporal correlation between consecutive years. The spatial random effect used a spatial process η(s), which was assumed to have a conditionally autoregressive (CAR) structure (51). The CAR structure incorporates spatial dependence by specifying the distribution of the random effect for a city as being dependent on the collection of random effects for all geographically adjacent cities: η(s) ∼ N(‾η(s), τ/n(s)); n(s) is the number of neighbors corresponding to city s, ‾η denotes the mean of neighboring random effects for city s, and τ is a scaling variance parameter. Hence, cities that border many other cities have a smaller variance.
We fitted the epidemic model (Eqs. 1–4) to the reported incidence for each city using a subset of data from 1992 to 2010 (due to the missing covariate information) (SI Appendix, Table S2) using hierarchical Bayesian modeling with sampling-based methods. To assess the accuracy of the model, we used the dataset (1992–2005) for training runs (in-sample prediction) and used the remaining data (2006–2010) to test the model (out-of-sample prediction). Metropolis–Hastings Markov Chain Monte Carlo was used for sampling the posterior distributions (52). Model fitting and model convergence were conducted using MATLAB (vR2009b) tool box Delayed Rejection Adaptive Metropolis (53). Three chains with different initial conditions were used to check for convergence of posterior distribution estimates. Priors for all parameters were defined as Gaussian distributions, with a mean of 0 and a variance of 100. Each chain was run for 5 million iterations, with a burn-in of 500,000 iterations.
Structural Equation Model.
Urbanization has a complex effect on the incidence of zoonotic disease; hence, we used structural equation models as an integrated approach to estimate the structural correlation between variables by using the R package lavaan (54) with maximum likelihood estimation procedures.
Supplementary Material
Acknowledgments
We thank Sari C. Cunningham for her valuable comments and help. We thank the hundreds of Hunan Provincial Center for Disease Control and Prevention staff and local health workers in Hunan Province who collected successive data from 1963 to 2010. We also thank the Hunan Public Security Bureau for providing valuable assistance in the data collection. Funding has been provided by National Natural Science Foundation of China Grant 81673234; National Key Research and Development Program of China Grant 2016YFA0600104; Chinese Health Industry’s Special Research Funds for Public Welfare Projects Grant 201502020; Science and Technology Planning Project of Hunan Province, China Grant 2015JC3063; Shaanxi Provincial Projects for Serious Disease Prevention and Control Grant 0617-15240415; and the Fundamental Research Funds for the Central Universities.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712767115/-/DCSupplemental.
References
- 1.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol. 2006;21:186–191. doi: 10.1016/j.tree.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 2.McKinney ML. Urbanization, biodiversity, and conservation. Bioscience. 2002;52:883–890. [Google Scholar]
- 3.Hu X, Cook S, Salazar MA. Internal migration and health in China. Lancet. 2008;372:1717–1719. doi: 10.1016/S0140-6736(08)61360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong P, et al. Urbanisation and health in China. Lancet. 2012;379:843–852. doi: 10.1016/S0140-6736(11)61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife–Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Rao K, Wu J, Gakidou E. China’s health system performance. Lancet. 2008;372:1914–1923. doi: 10.1016/S0140-6736(08)61362-8. [DOI] [PubMed] [Google Scholar]
- 7.Tian H, et al. Anthropogenically driven environmental changes shift the ecological dynamics of hemorrhagic fever with renal syndrome. PLoS Pathog. 2017;13:e1006198. doi: 10.1371/journal.ppat.1006198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis. 2011;11:131–141. doi: 10.1016/S1473-3099(10)70223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YZ, et al. Seoul virus and hantavirus disease, Shenyang, People’s Republic of China. Emerg Infect Dis. 2009;15:200–206. doi: 10.3201/eid1502.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo SQ, et al. Seoul virus in patients and rodents from Beijing, China. Am J Trop Med Hyg. 2008;78:833–837. [PubMed] [Google Scholar]
- 11.Zhang YZ, Zou Y, Fu ZF, Plyusnin A. Hantavirus infections in humans and animals, China. Emerg Infect Dis. 2010;16:1195–1203. doi: 10.3201/eid1608.090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dye C. Health and urban living. Science. 2008;319:766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- 13.Glass GE, et al. Using remotely sensed data to identify areas at risk for hantavirus pulmonary syndrome. Emerg Infect Dis. 2000;6:238–247. doi: 10.3201/eid0603.000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmaljohn C, Hjelle B. Hantaviruses: A global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson CB, Figueiredo LTM, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23:412–441. doi: 10.1128/CMR.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian H, et al. Interannual cycles of Hantaan virus outbreaks at the human-animal interface in Central China are controlled by temperature and rainfall. Proc Natl Acad Sci USA. 2017;114:8041–8046. doi: 10.1073/pnas.1701777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallio ER, et al. Prolonged survival of Puumala hantavirus outside the host: Evidence for indirect transmission via the environment. J Gen Virol. 2006;87:2127–2134. doi: 10.1099/vir.0.81643-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, et al. Epidemic characteristics of hemorrhagic fever with renal syndrome in China, 2006-2012. BMC Infect Dis. 2014;14:384. doi: 10.1186/1471-2334-14-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C, et al. Analysis of an outbreak of hemorrhagic fever with renal syndrome in college students in Xi’an, China. Viruses. 2014;6:507–515. doi: 10.3390/v6020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu YG, Ioannidis JP, Li H, Jones KC, Martin FL. Understanding and harnessing the health effects of rapid urbanization in China. Environ Sci Technol. 2011;45:5099–5104. doi: 10.1021/es2004254. [DOI] [PubMed] [Google Scholar]
- 21.Roger CK, Shimou Y. Urbanization and sustainable metropolitan development in China: Patterns, problems and prospects. GeoJournal. 1999;49:269–277. [Google Scholar]
- 22.Liu QY, Meng FX, Fan JC. Vector surveillance and control in emergencies in China: Proceedings and perspectives. Zhongguo Meijie Shengwuxue Ji Kongzhi Zazhi. 2011;22:1–4. [Google Scholar]
- 23.Huang X, Yin H, Yan L, Wang X, Wang S. Epidemiologic characteristics of haemorrhagic fever with renal syndrome in Mainland China from 2006 to 2010. Western Pac Surveill Response J. 2012;3:12–18. doi: 10.5365/WPSAR.2011.2.2.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang WY, et al. Climate variability and hemorrhagic fever with renal syndrome transmission in Northeastern China. Environ Health Perspect. 2010;118:915–920. doi: 10.1289/ehp.0901504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao H, et al. Environmental variability and the transmission of haemorrhagic fever with renal syndrome in Changsha, People’s Republic of China. Epidemiol Infect. 2013;141:1867–1875. doi: 10.1017/S0950268812002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang LQ, et al. Spatiotemporal trends and climatic factors of hemorrhagic fever with renal syndrome epidemic in Shandong Province, China. PLoS Negl Trop Dis. 2010;4:e789. doi: 10.1371/journal.pntd.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao H, et al. Atmospheric moisture variability and transmission of hemorrhagic fever with renal syndrome in Changsha City, Mainland China, 1991-2010. PLoS Negl Trop Dis. 2013;7:e2260. doi: 10.1371/journal.pntd.0002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao H, et al. Animal reservoir, natural and socioeconomic variations and the transmission of hemorrhagic fever with renal syndrome in Chenzhou, China, 2006-2010. PLoS Negl Trop Dis. 2014;8:e2615. doi: 10.1371/journal.pntd.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patz JA, et al. Working Group on Land Use Change and Disease Emergence Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver SC. Urbanization and geographic expansion of zoonotic arboviral diseases: Mechanisms and potential strategies for prevention. Trends Microbiol. 2013;21:360–363. doi: 10.1016/j.tim.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamer SA, Lehrer E, Magle SB. Wild birds as sentinels for multiple zoonotic pathogens along an urban to rural gradient in greater Chicago, Illinois. Zoonoses Public Health. 2012;59:355–364. doi: 10.1111/j.1863-2378.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 32.Yan L, et al. Landscape elements and Hantaan virus-related hemorrhagic fever with renal syndrome, People’s Republic of China. Emerg Infect Dis. 2007;13:1301–1306. doi: 10.3201/eid1309.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang WY, et al. Predicting the risk of hantavirus infection in Beijing, People’s Republic of China. Am J Trop Med Hyg. 2009;80:678–683. [PubMed] [Google Scholar]
- 34.Liu HN, et al. Time-specific ecologic niche models forecast the risk of hemorrhagic fever with renal syndrome in Dongting Lake district, China, 2005-2010. PLoS One. 2014;9:e106839. doi: 10.1371/journal.pone.0106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang LQ, et al. Spatiotemporal dynamics of hemorrhagic fever with renal syndrome, Beijing, People’s Republic of China. Emerg Infect Dis. 2009;15:2043–2045. doi: 10.3201/eid1512.081078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang LQ. 2009. Study on spatiotemporal distribution and environmental risk factors of hemorraghic fever with renal syndrome. PhD thesis (Academy of Military Medical Sciences, Beijing)
- 37.Laneri K, et al. Forcing versus feedback: Epidemic malaria and monsoon rains in northwest India. PLoS Comput Biol. 2010;6:e1000898. doi: 10.1371/journal.pcbi.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morley B. Causality between economic growth and immigration: An ARDL bounds testing approach. Econ Lett. 2006;90:72–76. [Google Scholar]
- 39.Zhu K, Li J, Yue F. Empirical analysis on the relationship between China urbanization and economic growth. Statistical Research. 2011;28:80–87. [Google Scholar]
- 40.Harpham T. Urbanisation and health in transition. Lancet. 1997;349:11–13. [Google Scholar]
- 41.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng L, Cai Z, Zhang L. Geography-included regional economic growing model and case study. Resources & Industries. 2012;14:182–188. [Google Scholar]
- 43.Himsworth CG, Parsons KL, Jardine C, Patrick DM. Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis. 2013;13:349–359. doi: 10.1089/vbz.2012.1195. [DOI] [PubMed] [Google Scholar]
- 44.Xiao H, et al. Ecology and geography of hemorrhagic fever with renal syndrome in Changsha, China. BMC Infect Dis. 2013;13:305. doi: 10.1186/1471-2334-13-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Normile D. China’s living laboratory in urbanization. Science. 2008;319:740–743. doi: 10.1126/science.319.5864.740. [DOI] [PubMed] [Google Scholar]
- 46.Desselberger U. Emerging and re-emerging infectious diseases. J Infect. 2000;40:3–15. doi: 10.1053/jinf.1999.0624. [DOI] [PubMed] [Google Scholar]
- 47.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song G. Epidemiological progresses of hemorrhagic fever with renal syndrome in China. Chin Med J (Engl) 1999;112:472–477. [PubMed] [Google Scholar]
- 49.Tian HY, et al. Changes in rodent abundance and weather conditions potentially drive hemorrhagic fever with renal syndrome outbreaks in Xi’an, China, 2005-2012. PLoS Negl Trop Dis. 2015;9:e0003530. doi: 10.1371/journal.pntd.0003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He X, Wang S, Huang X, Wang X. Changes in age distribution of hemorrhagic fever with renal syndrome: An implication of China’s expanded program of immunization. BMC Public Health. 2013;13:394. doi: 10.1186/1471-2458-13-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Besag J, Kooperberg C. On conditional and intrinsic autoregression. Biometrika. 1995;82:733–746. [Google Scholar]
- 52.Chib S, Greenberg E. Understanding the metropolis-hastings algorithm. Am Stat. 1995;49:327–335. [Google Scholar]
- 53.Haario H, Laine M, Mira A, Saksman E. DRAM: Efficient adaptive MCMC. Stat Comput. 2006;16:339–354. [Google Scholar]
- 54.Rosseel Y. lavaan: An R package for structural equation modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 55.Petraitis P, Dunham A, Niewiarowski P. Inferring multiple causality: The limitations of path analysis. Funct Ecol. 1996;10:421–431. [Google Scholar]