Significance
As prostate cancer is the second leading cause of cancer-related death among men in the United States, an unmet medical need exists for therapies that eliminate prostate tumors while preventing toxicity in normal tissue. Recently, such tumor-targeting therapies have gained Food and Drug Administration approval in the form of antibody drug conjugates. However, a need exists for new tumor-targeting therapies that are easier to manipulate and synthesize. By selecting for RNA ligands that internalize into prostate cancer cells but not normal prostate cells, we developed another class of targeted agents. We demonstrate that the E3 RNA selectively internalizes into prostate cancer cells and that E3 highly toxic drug conjugates are potent anti-tumor agents.
Keywords: aptamer, aptamer–drug conjugate, prostate cancer, drug targeting, antidote control
Abstract
Therapies that can eliminate both local and metastatic prostate tumor lesions while sparing normal organ tissue are desperately needed. With the goal of developing an improved drug-targeting strategy, we turned to a new class of targeted anticancer therapeutics: aptamers conjugated to highly toxic chemotherapeutics. Cell selection for aptamers with prostate cancer specificity yielded the E3 aptamer, which internalizes into prostate cancer cells without targeting normal prostate cells. Chemical conjugation of E3 to the drugs monomethyl auristatin E (MMAE) and monomethyl auristatin F (MMAF) yields a potent cytotoxic agent that efficiently kills prostate cancer cells in vitro but does not affect normal prostate epithelial cells. Importantly, the E3 aptamer targets tumors in vivo and treatment with the MMAF-E3 conjugate significantly inhibits prostate cancer growth in mice, demonstrating the in vivo utility of aptamer–drug conjugates. Additionally, we report the use of antidotes to block E3 aptamer–drug conjugate cytotoxicity, providing a safety switch in the unexpected event of normal cell killing in vivo.
Prostate cancer is both the most commonly diagnosed malignancy and the second most common cause of cancer-related mortality for men in the United States (1). Therapies that eliminate both local and metastatic prostate tumor legions while sparing normal organ tissue are desperately needed. Recent exciting oncology advances have come from an old idea: antibody–drug conjugates (ADCs). While the idea was first tested 30 y ago (reviewed in ref. 2), it is only the development of new, highly toxic chemotherapy drugs coupled with improved antibody–drug linker chemistry that has led to Food and Drug Administration approval of ADCs such a brentuximab vedotin (anti-CD30–MMAE) and ado-trastuzumab emtansine (anti-HER2–DM1). Several ADCs are currently in clinical trials for prostate cancer, including both a prostate-specific membrane antigen (PSMA) antibody (3–5) and an SCL44A4 antibody (6, 7) conjugated to the highly toxic drug monomethyl auristatin E (MMAE). In all these cases, the molecular target of the antibody is a known receptor up-regulated on the target cancer cell.
Unfortunately almost all of the antigens that are recognized by antibodies and ADCs are not exclusively expressed on cancer cells, but also expressed at lower levels in normal tissue (8). Therefore, not surprisingly, toxicities have been observed with antibody-targeted therapies, resulting, at least in part, from antibody accumulation in normal tissue (9–12). For example, HER2-targeting antibodies are associated with significant cardiovascular toxicities due to HER2 expression in cardiac tissue (11), and epidermal growth factor receptor (EGFR)-targeted antibodies induce significant cutaneous toxicities (9, 10) due to cutaneous expression of EGFR. Therefore, to reduce patient morbidity and improve the therapeutic window of targeted cytotoxic agents, improved drug-targeting strategies are needed that further focus cytotoxic agents toward cancer cells and minimize drug delivery to normal cells expressing even low levels of target receptor.
While antibodies require humanization and are difficult to chemically modify with precision, aptamers, small RNA or DNA ligands, have emerged as targeting agents with antibody-like affinity (picomolar to nanomolar range) (reviewed in refs. 13–15) and the added benefit of ease of chemical synthesis and modification. Importantly, aptamers, unless formulated with polyethyleneglycol (PEG) (15), have thus far proven to be nontoxic, even when delivered to animals and humans at high doses (16–19). Traditionally aptamers are generated in the laboratory through a process known as Systematic Evolution of Ligands by EXponential enrichment (SELEX), whereby a random oligonucleotide library is repetitively bound with a protein target of interest (20, 21). In recent years, a Cell-Internalization SELEX technique, involving a cell target instead of a protein target, has been used to identify oligonucleotides that internalize into different cell types (reviewed in ref. 22). Using two independent Cell-Internalization selections against a variety of different prostate cancer cell lines, we identified an RNA aptamer, termed E3, specific for internalization into prostate cancer, but not normal prostate cells.
As a recent report suggests that aptamer–drug conjugates that utilize highly toxic drugs can kill cells in vitro (23), we sought to develop and evaluate the in vivo activity of E3–drug conjugates to determine if this approach has clinical potential. We chemically conjugated E3 to the auristatin drugs MMAE and monomethyl aruistatin F (MMAF) that have been successful in tumor targeting antibody conjugates. Both auristatin drugs are potent microtubule inhibitors that are too toxic to be used in an unconjugated state (24, 25). The MMAE-E3 and MMAF-E3 aptamer conjugates efficiently killed prostate cancer cells in culture, but not normal prostate cells, with IC50s in the nanomolar range.
We additionally aimed to exploit the unique ability of complementary antidote oligonucleotides to exquisitely and rapidly control aptamer activity (26, 27) so as to prevent unwanted aptamer targeting to normal cells if needed, as we have observed that these antidotes effectively turn off aptamer targeting and function in humans within minutes of injection (15, 28, 29). Herein, we demonstrate that E3 targeting and internalization can also be rapidly turned off via antidote addition. Treatment with antidote also neutralizes both the MMAE-E3 and MMAF conjugates and prevents cell death in vitro, providing a safety switch in the rare case that our E3 conjugates were to experience the same type of unwanted accumulation in normal tissue, as has been seen with antibody conjugates.
Most importantly, both the E3 aptamer and MMAF-E3 drug conjugate target and localize to prostate tumors in vivo, with MMAF-E3 significantly inhibiting tumor growth without engendering obvious toxicities. Thus, these animal studies indicate that conjugation of internalizing aptamers to highly toxic chemotherapeutic agents yields a class of targeted anticancer therapeutics, and the MMAF-E3 conjugate represents a targeted approach to treat patients with prostate cancer.
Results
Selection of an RNA Aptamer That Internalizes into Prostate Cancer Cells.
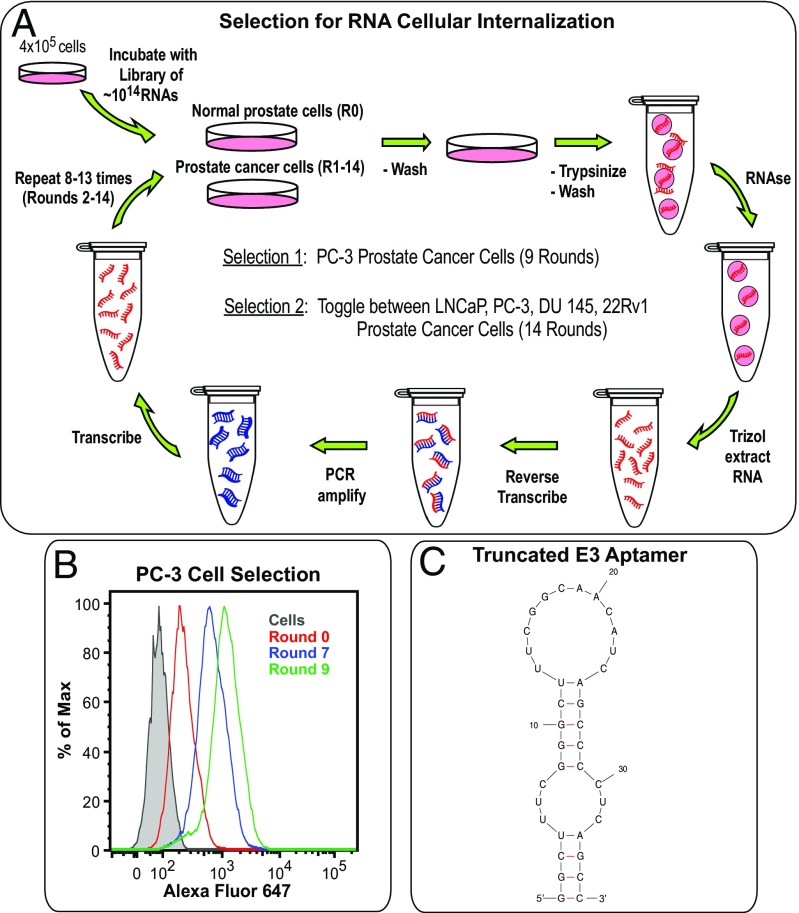
In an unbiased approach to identify RNA aptamers that internalize into prostate cancer cells at various stages of malignancy, we performed two Cell-Internalization SELEX using a 2′fluoro pyrimidine modified (30) RNA library comprising a modified, nuclease-resistant RNA pool of ∼1014 different RNAs. Two different selection strategies were employed. In the first, positive selections targeting PC-3 prostate cancer cells were combined with a strong negative selection against normal prostate epithelial cells (PrEC) to deplete RNA molecules capable of internalizing into noncancerous cells. In the second, the cell target for the positive selection was varied and included toggling between the LNCaP, PC-3, DU 145, and 22Rv1 cell lines (Fig. 1A and SI Appendix, Tables S1 and S2). These four prostate cancer cell lines represent different stages of prostate cancer progression, ranging from high sensitivity and dependence upon androgen hormones (LNCaP cells), to low androgen sensitivity (22Rv1 cells), to androgen independence (PC-3 cells), and finally to a brain metastasis derivative (DU 145). Additionally, to exclude PSMA as a target, we chose to select against both PSMA-positive (LNCaP and 22Rv1) and PSMA-negative (PC-3 and DU 145) cell lines. Here again, positive rounds of selection targeting these cells were combined with a strong negative selection against PrECs. For both selections, after incubating cells with the library of RNA variants and washing away nonbinding aptamers, cell-surface-bound RNA was degraded via treatment with a harsh RNase mixture that degrades 2′fluoro modified RNA (31), leaving only RNAs that had internalized into cells. Subsequent reverse transcription, PCR amplification, and transcription of the internalized RNA completed a round of internalization selection.
Fig. 1.
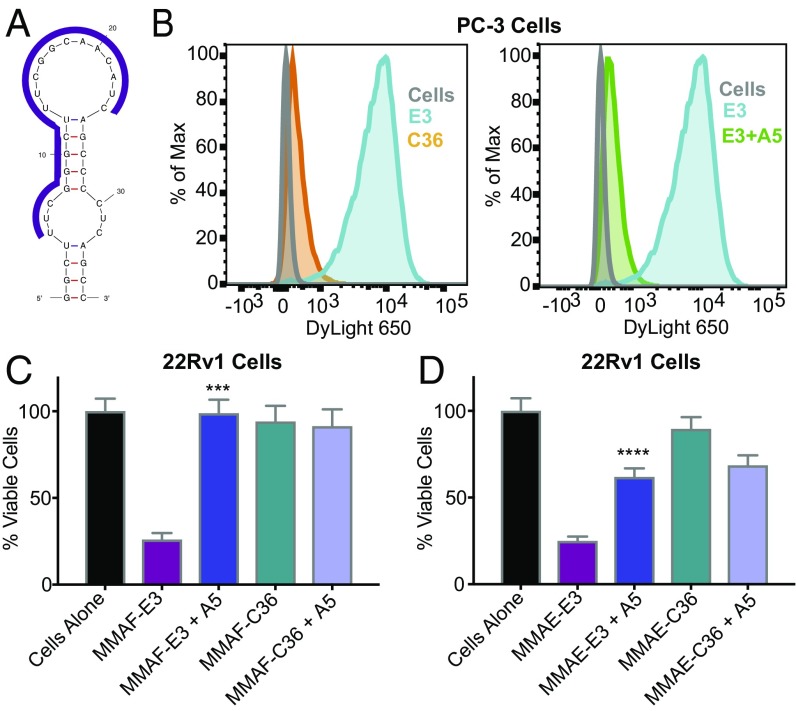
Cell-Internalization SELEX against prostate cancer cells identifies the E3 aptamer, which minimizes into an aptamer of only 36 nt. (A) Cell-Internalization SELEX scheme for selection of 2′F pyrimidine-modified RNA aptamers that internalize into prostate cancer cells. (B) Flow cytometry analysis of the internalization of different selection rounds into PC-3 prostate cancer cells. RNA pools from rounds 0, 7, and 9 of the PC-3 selection were transcribed with a 3′ 22-nt tail and annealed to an AF647-labeled reverse primer. After incubating the RNA pools with PC-3 cells for 2 h, the solutions were removed and cells washed and treated with RNase before flow analysis. (C) Predicted secondary structure of the minimized 36-nt version of the E3 aptamer, generated by mfold software.
To assess selection progress, RNA pools from different selection rounds were transcribed with a 3′ 22-nt tail that annealed to an Alexa Fluor 647-labeled reverse primer. Subsequent flow cytometry analysis clearly demonstrated an increase of internalizing aptamers as the selection progressed through additional rounds, as shown for the PC-3 cell selection (Fig. 1B). After nine rounds of selection against PC-3 cells, 12 unique RNAs were evaluated; two of them, the E3 and A3 aptamers, were found to internalize (SI Appendix, Table S3). Interestingly, after 14 rounds of toggling between LNCaP, PC-3, DU 145, and 22Rv1 cells, the E3 and A3 aptamers also emerged as well as one additional internalizing aptamer (D11, SI Appendix, Table S3). Unexpectedly, the E3, A3, and D11 aptamers all competed for cellular binding and internalization, with the E3 aptamer consistently reaching the highest levels of cellular internalization. Therefore, the E3 aptamer was chosen for further studies.
The 83-nt-long E3 aptamer folds into two predicted secondary structures (SI Appendix, Fig. S1). Truncation from the 5′ and 3′ ends of the RNA results in a 36-nt minimized E3 structure that is predicted to stably fold (G = −11.6) into two symmetric stem loops identical to the largest stem loops of the full-length aptamer (Fig. 1C). As this minimized E3 aptamer retains the cell targeting and internalization properties of the full-length aptamer, yet has a length appropriate for efficient chemical synthesis, the truncated 36-nt E3 aptamer was utilized for all subsequent studies.
In Vitro Characterization of E3 Aptamer Targeting to Prostate Cancer Cells.
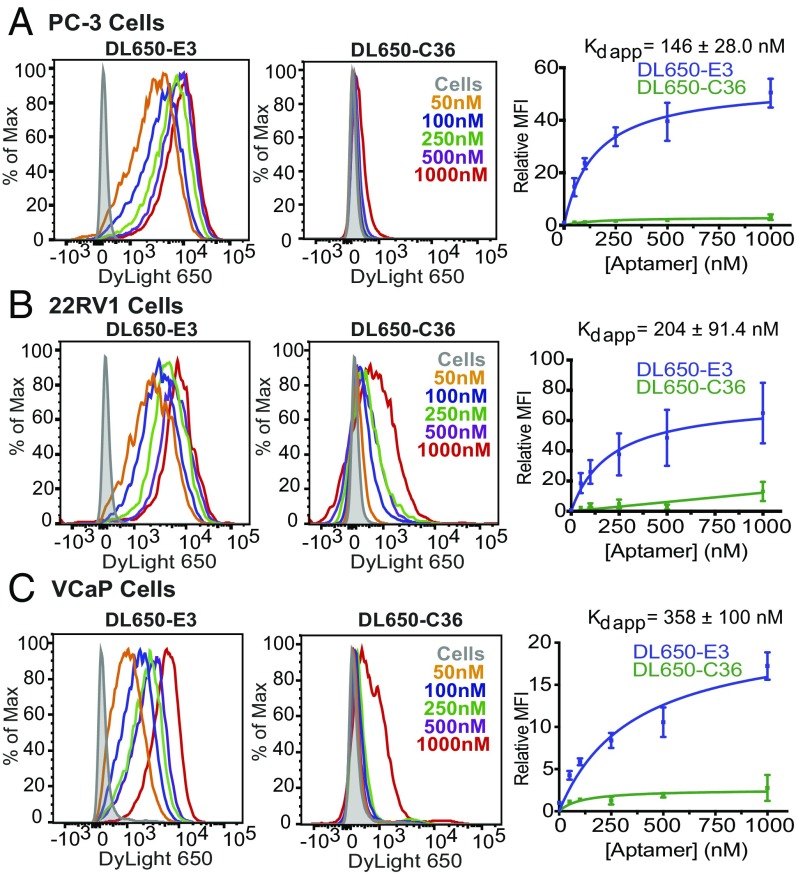
To verify that chemically synthesized E3 retains aptamer function and effectively targets a variety of prostate cancer cells, the truncated aptamer was chemically synthesized with a 5′ C6-thiol modifier and conjugated to a maleimide-DyLight 650 (DL650) fluorescent dye. The approximate binding affinity and cell specificity of DL650-E3 for five different prostate cancer cell lines was then determined by staining cells with increasing concentrations of DL650-E3. Salmon sperm DNA was included as a blocking agent to prevent nonspecific oligonucleotide binding or endocytosis and ensure that aptamer accumulation in cells was a result of specific uptake and targeting. Cells were also stained with increasing concentrations of DL650-labeled nonspecific control aptamer, C36 [cntrl.36 (32)], an unrelated RNA that serves as a size-matched control for RNA length.
As expected, for all prostate cancer cell lines tested (Fig. 2 and SI Appendix, Fig. S2), flow cytometry analysis of DL650-E3 targeting demonstrated a dose-dependent increase in aptamer association with cells as the concentration of aptamer increased. By contrast, very little cell staining was observed with the control aptamer C36, even at concentrations as high as 1 μM. Analysis of binding via nonlinear fits of the relative mean fluorescence vs. aptamer concentration gave apparent dissociation constants (Kds) for DL650-E3 ranging from 146 to 410 nM. Thus, the synthesized E3 truncate maintains nanomolar range, high-affinity targeting to prostate cancer cells. Importantly, E3 targets prostate cancer cells broadly, regardless of tumor origin or androgen sensitivity status.
Fig. 2.
E3 targets prostate cancer cells with nanomolar apparent affinity. Flow cytometry analysis of increasing concentrations of DL650-E3 aptamer or DL650-C36 control aptamer incubated with (A) PC-3, (B) 22Rv1, or (C) VCaP cells. Aptamers were incubated on cells for 1 h before washing cells and analyzing by flow cytometry. Binding curves represent data from three independent experiments, with the mean fluorescent intensity of aptamer-treated samples normalized to the mean fluorescent intensity of the cells alone signal.
Confocal Microscopy Verifies E3 Internalization into Prostate Cancer Cells.
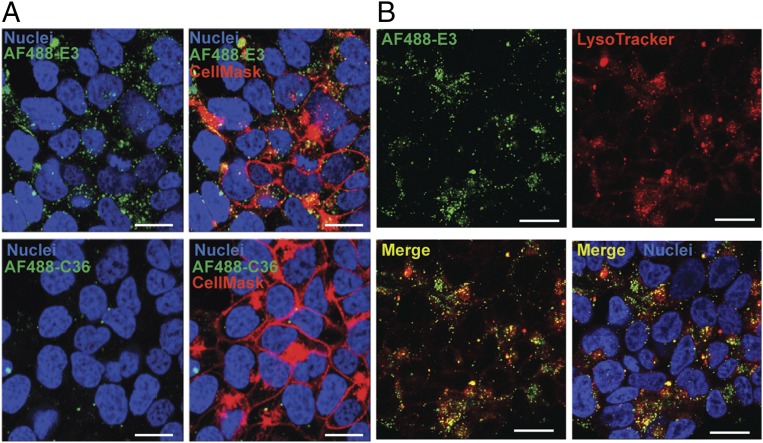
To verify internalization, and not merely cell-surface binding, of the E3 aptamer into prostate cancer cells, E3 was synthesized with a 5′ C6-thiol modifier and conjugated to the fluorescent dye maleimide-Alexa Fluor 488 (AF488). Confocal microscopy of 22Rv1 prostate cancer cells stained with the AF488-E3 conjugate shows intracellular accumulation of the aptamer, with punctate staining throughout the cytoplasm (Fig. 3A). In contrast, staining with the control AF488-C36 resulted in minimal RNA accumulation in cells. Additionally, E3 partially colocalizes with a lysosomal stain (Fig. 3B), indicating that the aptamer probably enters cells via active-targeting receptor-mediated endocytosis, resulting in endosomal to lysosomal deposition of the E3 RNA; blocking clathrin-mediated endocytosis also inhibits E3 uptake (SI Appendix, Fig. S3), further supporting this hypothesis. Thus, as expected from the internalization-biased selection for prostate cancer specific aptamers, the E3 aptamer internalizes into 22Rv1 prostate cells via an active-targeting mechanism.
Fig. 3.
Confocal microscopy visualization of E3 internalization into 22Rv1 prostate cancer cells. Cells were treated for 1 h with 1 μM of either (A) AF488-E3 or AF488-C36 ± CellMask Deep Red Plasma Membrane Stain or (B) AF488-E3 + LysoTracker Deep Red stain to label acidic vesicles. After washing, Hoechst 33342 was added to all samples to stain the nuclei. Fluorescence was observed on a Leica SP5 inverted confocal microscope. (White scale bars: 20 μm.)
E3 MMAE and MMAF Drug Conjugates Inhibit Proliferation of Prostate Cancer Cells, but Not Normal Cells.
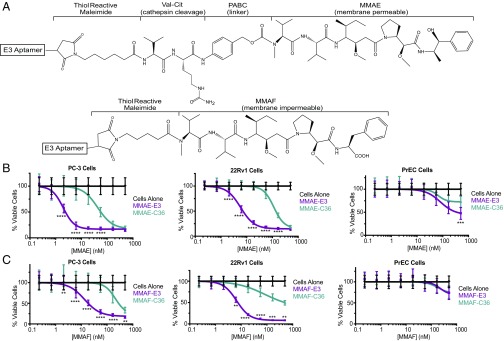
The cellular targeting and internalization abilities of E3 make it a potentially useful ligand for drug delivery. As E3 appears to enter cells via an endosomal–lyosomal pathway (Fig. 3B), we chose to conjugate E3 to two different highly toxic auristatin derivatives (Fig. 4A). The MMAE derivative is membrane permeable and able to escape from cellular compartments whereas the similar MMAF derivative is membrane impermeable. Comparing these two drugs allows us to probe the role of release from intracellular compartments in E3 drug delivery, an important consideration in MMAE and MMAF toxicity as both drugs need to reach the cytoplasm to exert their function; once in the cytoplasm, the auristatin derivatives bind to tubulin, causing apoptosis (24, 25).
Fig. 4.
E3 MMAE and MMAF drug conjugates are toxic to prostate cancer, but not normal cells. (A) Structures of E3 aptamer conjugated to maleimide-caproyl-valine-citrulline-p-aminobenzylcarbamate-MMAE or maleimidocaproyl-MMAF. (B) Viability of PC-3, 22Rv1, and VCaP prostate cancer cells or normal PrEC cells treated with MMAE-E3 and MMAE-C36 or (C) MMAF-E3 and MMAF-C36. Cells were incubated with increasing concentrations of aptamer–drug conjugates and viability determined at 144 h. Data shown are the average of three to four individual experiments. **P ≤ 0.0074, ***P ≤ 0.0003, ****P < 0.0001 versus control C36 conjugates.
We chemically conjugated the aptamer to MMAE or MMAF using the same maleimide linkers that have been used for the clinically successful antibody–MMAE and –MMAF conjugates. E3 was synthesized with a 5′ C6-thiol linker for conjugation to either maleimide-caproyl-valine-citrulline-p-aminobenzylcarbamate-MMAE or to maleimidocaproyl-MMAF, via thiol-maleimide chemistry (Fig. 4A). As free MMAE is membrane permeable, conjugation via a valine-citrulline-aminobenzylcarbamate linker inactivates the drug, preventing it from crossing cell membranes on its own, and making its cellular internalization dependent on the conjugated targeting ligand (24). Once internalized into cells, the valine-citrulline peptide portion of the linker is cleaved by the lysosomal protease cathepsin B, releasing a p-aminobenzylcarbamate intermediate of the drug that undergoes spontaneous fragmentation to release active MMAE. As MMAF is not cell permeable, its cell targeting is entirely dependent upon any attached targeting ligand and a shorter noncleavable maleimidocaproyl linker can be used for targeting ligand attachment (25).
As expected based upon the E3 aptamer’s affinity and specificity for internalizing into prostate cancer cells, the MMAE-E3 and MMAF-E3 conjugates induced cell death in all prostate cancer cells tested, with IC50s ranging from 2.3 to 152 nM (Fig. 4 B and C and SI Appendix, Fig. S4 and Table S4). Not surprisingly, the MMAE-E3 conjugates were more efficacious than the MMAF-E3 conjugates on all prostate cancer lines, likely due to the ability of MMAE to efficiently escape into the cytoplasm upon lysosomal cleavage of the MMAE-E3 linker. The MMAF-E3 conjugates exhibited a more variable effect on the different prostate cancer cell lines, with some cell lines appearing more sensitive to MMAF treatment (Fig. 4C and SI Appendix, Fig. S4B and Table S4). For example, while MMAF-E3 was only 1.6-fold less toxic to 22Rv1 cells than MMAE-E3, LNCaP cells were 26-fold less sensitive to the MMAF-E3 conjugate. These variances may be related to differential levels of MMAF endosomal or lysosomal escape, with some cells having “leakier” intracellular compartments than others.
Although MMAE-E3 was more toxic than MMAF-E3, this toxicity came at the price of reduced specificity, as the control MMAE-C36 conjugate was significantly more toxic than the MMAF-C36 conjugate on four of the five cell lines tested (Fig. 4 B and C and SI Appendix, Fig. S4 and Table S4). Even at drug concentrations of 1 μM, we failed to reach the IC50 of MMAF-C36 on the 22Rv1, DU 145, LNCaP, and VCaP cell lines, lending great specificity to the MMAF-E3 conjugate versus control. The increased toxicity of MMAE-C36 versus MMAF-C36 is not unexpected as MMAE may release from the control RNA extracellularly, allowing free MMAE to enter cells on its own. Additionally, MMAE released from the low level of internalized C36 conjugate may more efficiently escape from intracellular compartments to reach the cytoplasm.
While the MMAF-C36 conjugate was also less toxic to PC-3 cells than MMAE-C36, MMAF-C36 was only 12-fold less toxic than the targeted MMAF-E3, compared with a 25-fold difference in toxicity between MMAE-E3 and MMAE-C36. It is unclear why PC-3 cells are less resistant to the control MMAF-C36 conjugate than the other cell lines, although this may be related to a difference in cell structure and origin; the PC-3 cell line is thought to derive from the more rare form of prostatic small-cell neuroendocrine carcinoma, compared with the typical adenocarcinoma origin of most prostate cancers (33). Importantly, neither MMAE-E3 nor MMAF-E3 exhibited specific toxicity toward normal PrECs (Fig. 4 B and C and SI Appendix, Table S4). Although the slower growth of normal epithelial cells makes them inherently less susceptible to tubulin inhibitors such as MMAE and MMAF, confocal microscopy further demonstrates that E3 does not specifically target PrEC cells (SI Appendix, Fig. S5).
An Antidote to E3 Prevents Cell Targeting and Internalization and Prevents Cytotoxicity of E3–Drug Conjugates.
To explore the potential use of antidotes to control the activity of aptamer–drug conjugates, we designed a series of 18–20-nt cDNA antidotes against the E3 aptamer (SI Appendix, Fig. S6–S8). Synthesizing the best-performing DNA antidote as a 2′OMe RNA antidote resulted in complete blockage of E3-specific cell binding and internalization as monitored by flow cytometry, bringing E3 internalization down to the same background level as the nonspecific control C36 (Fig. 5 A and B), with only a 1:1 antidote:aptamer ratio required to inhibit 90% of E3 targeting (SI Appendix, Fig. S9). Most importantly, twofold excess of E3 antidote 5 neutralized both the MMAF-E3 and MMAE-E3 conjugates, preventing cell killing (Fig. 5 C and D). These results demonstrate that E3 is a tunable targeting ligand whose activity and delivery can be turned off via antidote treatment if side effects occur despite the selectivity of E3 for cancer cells.
Fig. 5.
An antidote oligonucleotide to E3 prevents cell targeting and internalization and counteracts the cytotoxicity of E3–drug conjugates. (A) Region of the E3 aptamer targeted by the antidote oligonucleotide (represented as a purple line). (B) Flow cytometry analysis of PC-3 cells incubated with 250 nM of DL650-C36 or with 250 nM of DL650-E3 ± 10-fold excess antidote 5. Antidote was incubated on cells for 1 h before incubating with aptamer solutions for 1 h. Cells were then washed and analyzed by flow cytometry. (C) Viability of 22Rv1 cells treated with 100 nM of MMAF-E3 or control conjugate MMAF-C36 ± twofold excess of antidote 5 or with (D) 100 nM of MMAE-E3 or control conjugate MMAE-C36 ± twofold excess of antidote 5. Viability was determined 144 h post aptamer addition. Data shown are the average of four individual experiments. ***P = 0.0001 versus MMAF-E3 and ****P < 0.0001 versus MMAE-E3.
The E3 Aptamer Targets Prostate Tumors in Vivo and the MMAF-E3 Drug Conjugate Inhibits Tumor Growth.
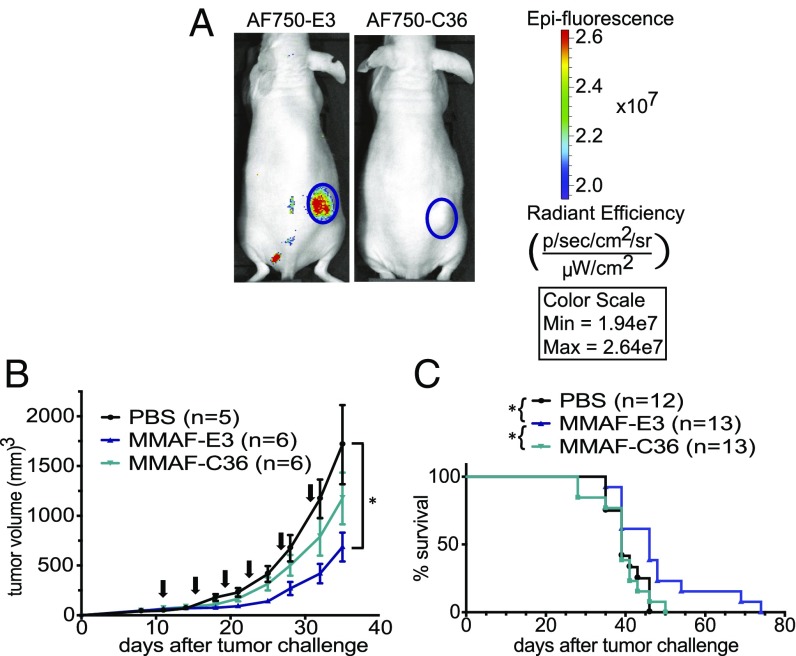
As E3 was selected in vitro, it is important to verify that the aptamer retains prostate cancer targeting in vivo. Significantly, E3 localizes to 22Rv1 prostate xenografts in vivo, as evidenced by near-infrared (NIR) in vivo imaging of mice i.v. injected with Alexa Fluor 750 (AF750)-labeled E3 (Fig. 6A). By contrast, the control AF750-C36 conjugate did not accumulate in the tumors. To investigate the ability of E3 to deliver drugs and inhibit tumor growth in vivo, we chose to test MMAF-E3 against the 22Rv1 prostate cancer model, as 22Rv1 cells were the most sensitive to MMAF-E3 in vitro while maintaining great specificity for MMAF-E3 compared with the control MMAF-C36 conjugate. Additionally, the MMAF-E3 conjugate had a half-life of 18 h in mouse serum, compared with the less-stable MMAE-E3 conjugate with a half-life of only ∼2 h (SI Appendix, Fig. S10). Mice bearing 22Rv1 xenografts were i.v. treated with PBS or with MMAF-E3 or control MMAF-C36 (1.03 mg/kg, based on drug concentration). Treatment with MMAF-E3, but not with MMAF-C36, significantly inhibited tumor growth (P = 0.0324) and increased survival (P = 0.0163) compared with buffer-treated mice. Additionally, MMAF-E3 significantly increased survival compared with mice treated with the control MMAF-C36 (P = 0.0192). Thus, the E3 aptamer maintains both its prostate cancer targeting and its therapeutic cancer cell killing effects in vivo.
Fig. 6.
The E3 aptamer targets prostate cancer xenografts in vivo, and MMAF-E3 treatment inhibits tumor growth. s.c. 22Rv1 prostate tumors were established in the right flank of nu/nu mice. (A) Tumor-bearing mice were injected via tail vein with 2 nmol of either AF750-E3 or AF750-C36 (n = 4) and imaged for NIR fluorescence. Shown are representative images from 24 h post aptamer injection. (B and C) Once tumors reached at least 50 mm3, mice were treated via tail vein with PBS or with MMAF-E3 or MMAF-C36 (1.03 mg/kg). Mice were treated q 4 d × 6, starting on day 11, as indicated by the arrows. (B) Tumor growth curves demonstrate that MMAF-E3, but not control MMAF-C36, significantly inhibits tumor growth compared with control PBS (*P = 0.0324). (C) Kaplan–Meier survival curves show a significant increase in survival for mice treated with MMAF-E3 compared with both buffer-treated and MMAF-C36–treated mice (*P = 0.0163 and *P = 0.0192, respectively). Treatment with control MMAF-C36 does not significantly change survival compared with PBS treatment.
Discussion
The need for improved cancer therapies with fewer side effects has led to the clinical development of targeted therapies such as therapeutic antibodies and ADCs. Although antibody-based cancer therapies have proven beneficial, highlighting the ability of targeted therapies to improve cancer patient outcomes, improved drug-targeting strategies are needed that further focus cytotoxic agents toward cancer cells and minimize drug delivery to normal cells that express even low levels of target receptor. Aptamers are emerging as targeting agents with antibody-like affinity coupled with the ease of chemical synthesis and modification.
With the goal of developing an aptamer-targeted therapy for prostate cancer, we used Cell-Internalization SELEX to identify the E3 aptamer, which internalizes into prostate cancer cells without targeting normal prostate cells. Truncation of the E3 aptamer into an easily synthesizable 36-nt 2′F-modified RNA allowed for subsequent conjugation to the highly toxic drugs MMAE and MMAF. Both the MMAE-E3 and MMAF-E3 conjugates are toxic to prostate cancer cells, with IC50s in the nanomolar range, but do not affect normal prostate epithelial cells above a nonspecific control. An added advantage of the E3 conjugates is their tunable toxicity: Antidote treatment abrogates the cytotoxicity of both conjugates. Importantly, MMAF-E3 maintains its efficacy in vivo, significantly inhibiting tumor growth compared with control treatment. Thus, we developed a reversible aptamer–drug conjugate for the treatment of prostate cancer.
The in vivo anti-tumor effects of MMAF-E3 are very promising for an aptamer–drug conjugate expected to have an in vivo half-life of minutes (reviewed in ref. 34). Thus, optimizing the MMAF-E3 conjugate to extend in vivo circulation time should improve its anti-tumor efficacy. Although high molecular weight PEG has previously been used to extend aptamer half-life for clinical studies (16, 19), preexisting anti-PEG antibodies caused a small percentage of patients (∼0.6%) to have severe allergic reactions (35). However, as PEG is commonly used in oncologic therapies despite its known allergic potential, it may be an acceptable way to extend aptamer half-life for the oncology setting. Alternatively, we could explore other ways to extend aptamer half-life such as conjugation to other high molecular weight carriers.
Most previously reported aptamer–drug conjugates have used the traditional chemotherapeutic doxorubicin, via either chemical conjugation or intercalation into the aptamer structure (reviewed in refs. 36 and 37). By contrast, only a few reports of aptamers conjugated to highly toxic agents have appeared (23, 38, 39), and, notably, MMAE-E3 appears ∼50-fold more potent than a recently described aptamer–MMAE conjugate (23). However, none of these highly toxic aptamer conjugates were tested in vivo. Here we report the development and in vivo activity of a class of targeted anticancer therapeutics: aptamers conjugated to highly toxic chemotherapeutics. We demonstrate that a 1:1 direct chemical conjugate of aptamer to the highly toxic drug MMAF kills prostate cancer cells in vitro and tumors in vivo. Additionally, we report the use of antidotes to block aptamer–drug conjugate toxicity, providing a safety switch in the rare event that cytotoxicity is encountered in vivo.
Materials and Methods
All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (40) and approved by the Duke University Institutional Animal Care and Use Committee (Protocols A086-14-04 & A076-17-03). Detailed methods are described in SI Appendix, Materials and Methods. RNA was synthesized (32) as previously described. Auristatins were conjugated via thiol-maleimide chemistry using the linkers shown in Fig. 4A. Cell-Internalization SELEX was performed as described in Fig. 1A, flow cytometry as in Figs. 2 and 5B, confocal microscopy as in Fig. 3, cell viability studies as in Figs. 4 B and C and 5 C and D, and animal studies as in Fig. 6.
Supplementary Material
Acknowledgments
We thank Keith E. Maier for his assistance with oligonucleotide synthesis. This work was supported by Department of Defense (DoD) Prostate Cancer Research Program (PCRP) Postdoctoral Training Award PC131874 (to B.P.G.) and Synergistic Idea Award PC111812P2/W81XWH-12-1-0262 (to B.A.S.); Stand Up to Cancer Innovative Research Grant SU2C-AACR-IRG-0809 (to M.L.); and National Cancer Institute (NCI) Grants R21CA182330 (to M.L.) and R21CA157366-03 (to M.L.). Additionally, this work was supported by Duke Cancer Institute (DCI) NCI Grant P30-CA014236 funding for the Duke Optical Molecular Imaging and Analysis Facility (in vivo imaging), DCI Flow Cytometry Shared Resource (flow cytometry), and Duke Light Microscopy Core Facility (confocal microscopy).
Footnotes
Conflict of interest statement: b3 bio, inc. (D.P.A. and A.P.B.) and Duke University (B.P.G. and B.A.S.) have submitted patent applications.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717705115/-/DCSupplemental.
References
- 1.American Cancer Society . Cancer Facts & Figures 2016. American Cancer Society; Atlanta: 2016. [Google Scholar]
- 2.Chari RVJ. Targeted delivery of chemotherapeutics: Tumor-activated prodrug therapy. Adv Drug Deliv Rev. 1998;31:89–104. doi: 10.1016/s0169-409x(97)00095-1. [DOI] [PubMed] [Google Scholar]
- 3. Progenics Pharmaceuticals, Inc. (2015) Progenics Pharmaceuticals presents positive phase 2 data for PSMA ADC in metastatic castration-resistant prostate cancer. Available at https://globenewswire.com/news-release/2015/02/26/710376/10122204/en/Progenics-Pharmaceuticals-Presents-Positive-Phase-2-Data-for-PSMA-ADC-in-Metastatic-Castration-Resistant-Prostate-Cancer.html. Accessed May 18, 2017.
- 4.Wang X, Ma D, Olson WC, Heston WD. In vitro and in vivo responses of advanced prostate tumors to PSMA ADC, an auristatin-conjugated antibody to prostate-specific membrane antigen. Mol Cancer Ther. 2011;10:1728–1739. doi: 10.1158/1535-7163.MCT-11-0191. [DOI] [PubMed] [Google Scholar]
- 5. Progenics Pharmaceuticals, Inc. (2013) Progenics pharmaceuticals presents updated data from phase 1 study of PSMA ADC. Available at www.globenewswire.com/news-release/2013/02/14/523727/10021886/en/Progenics-Pharmaceuticals-Presents-Updated-Data-From-Phase-1-Study-of-PSMA-ADC.html?print=1. Accessed May 18, 2017.
- 6.Coveler AL, et al. A phase 1 clinical trial of ASG-5ME, a novel drug-antibody conjugate targeting SLC44A4, in patients with advanced pancreatic and gastric cancers. Invest New Drugs. 2016;34:319–328. doi: 10.1007/s10637-016-0343-x. [DOI] [PubMed] [Google Scholar]
- 7.Mattie M, et al. The discovery and preclinical development of ASG-5ME, an antibody-drug conjugate targeting SLC44A4-positive epithelial tumors including pancreatic and prostate cancer. Mol Cancer Ther. 2016;15:2679–2687. doi: 10.1158/1535-7163.MCT-16-0225. [DOI] [PubMed] [Google Scholar]
- 8.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 9.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203–218, quiz 219–220. doi: 10.1016/j.jaad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Onitilo AA, Engel JM, Stankowski RV. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: Prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf. 2014;5:154–166. doi: 10.1177/2042098614529603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Kurzrock R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat Rev. 2014;40:883–891. doi: 10.1016/j.ctrv.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Conrad RC, Giver L, Tian Y, Ellington AD. In vitro selection of nucleic acid aptamers that bind proteins. Methods Enzymol. 1996;267:336–367. doi: 10.1016/s0076-6879(96)67022-0. [DOI] [PubMed] [Google Scholar]
- 14.Osborne SE, Ellington AD. Nucleic acid selection and the challenge of combinatorial chemistry. Chem Rev. 1997;97:349–370. doi: 10.1021/cr960009c. [DOI] [PubMed] [Google Scholar]
- 15.Nimjee SM, White RR, Becker RC, Sullenger BA. Aptamers as therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:61–79. doi: 10.1146/annurev-pharmtox-010716-104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan MY, et al. Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation. 2008;117:2865–2874. doi: 10.1161/CIRCULATIONAHA.107.745687. [DOI] [PubMed] [Google Scholar]
- 17.DeAnda A, Jr, et al. Pilot study of the efficacy of a thrombin inhibitor for use during cardiopulmonary bypass. Ann Thorac Surg. 1994;58:344–350. doi: 10.1016/0003-4975(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 18.Eyetech Study Group Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: Phase II study results. Ophthalmology. 2003;110:979–986. doi: 10.1016/S0161-6420(03)00085-X. [DOI] [PubMed] [Google Scholar]
- 19.Chan MY, et al. A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J Thromb Haemost. 2008;6:789–796. doi: 10.1111/j.1538-7836.2008.02932.x. [DOI] [PubMed] [Google Scholar]
- 20.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 21.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 22.Yan A, Levy M. Cell internalization SELEX: In vitro selection for molecules that internalize into cells. Methods Mol Biol. 2014;1103:241–265. doi: 10.1007/978-1-62703-730-3_18. [DOI] [PubMed] [Google Scholar]
- 23.Yoon S, et al. Aptamer-drug conjugates of active metabolites of nucleoside analogs and cytotoxic agents inhibit pancreatic tumor cell growth. Mol Ther Nucleic Acids. 2017;6:80–88. doi: 10.1016/j.omtn.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doronina SO, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 25.Doronina SO, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: Effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17:114–124. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- 26.Rusconi CP, et al. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- 27.Nimjee SM, et al. A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Mol Ther. 2006;14:408–415. doi: 10.1016/j.ymthe.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Dyke CK, et al. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: A phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation. 2006;114:2490–2497. doi: 10.1161/CIRCULATIONAHA.106.668434. [DOI] [PubMed] [Google Scholar]
- 29.Sullenger BA, Nair S. From the RNA world to the clinic. Science. 2016;352:1417–1420. doi: 10.1126/science.aad8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pieken WA, Olsen DB, Benseler F, Aurup H, Eckstein F. Kinetic characterization of ribonuclease-resistant 2′-modified hammerhead ribozymes. Science. 1991;253:314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- 31.Magalhães ML, et al. A general RNA motif for cellular transfection. Mol Ther. 2012;20:616–624. doi: 10.1038/mt.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilner SE, et al. An RNA alternative to human transferrin: A new tool for targeting human cells. Mol Ther Nucleic Acids. 2012;1:e21. doi: 10.1038/mtna.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai S, et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71:1668–1679. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maier KE, Levy M. From selection hits to clinical leads: Progress in aptamer discovery. Mol Ther Methods Clin Dev. 2016;5:16014–16024. doi: 10.1038/mtm.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganson NJ, et al. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol. 2016;137:1610–1613.e7. doi: 10.1016/j.jaci.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K, et al. Advances in the development of aptamer drug conjugates for targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1438–e1453. doi: 10.1002/wnan.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruno JG. A review of therapeutic aptamer conjugates with emphasis on new approaches. Pharmaceuticals (Basel) 2013;6:340–357. doi: 10.3390/ph6030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly L, Kratschmer C, Maier KE, Yan AC, Levy M. Improved synthesis and in vitro evaluation of an aptamer ribosomal toxin conjugate. Nucleic Acid Ther. 2016;26:156–165. doi: 10.1089/nat.2015.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu TC, et al. Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res. 2006;66:5989–5992. doi: 10.1158/0008-5472.CAN-05-4583. [DOI] [PubMed] [Google Scholar]
- 40.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]