Significance
PD-1–mediated inhibitory signals play a major role in T cell exhaustion during chronic infections and cancers, which makes PD-1 a valuable target of checkpoint blockade in cancer immunotherapy. However, the role of PD-1 in effector CD8 T cell differentiation during an acute viral infection is not well defined. Here, we show that PD-1 also functions as an inhibitory receptor during this early stage of T cell activation and that PD-1 blockade enhances effector function and results in faster clearance of infection.
Keywords: PD-1, viral infection, CD8 T cells, effector differentiation, memory cells
Abstract
PD-1 (programmed cell death-1) is the central inhibitory receptor regulating CD8 T cell exhaustion during chronic viral infection and cancer. Interestingly, PD-1 is also expressed transiently by activated CD8 T cells during acute viral infection, but the role of PD-1 in modulating T cell effector differentiation and function is not well defined. To address this question, we examined the expression kinetics and role of PD-1 during acute lymphocytic choriomeningitis virus (LCMV) infection of mice. PD-1 was rapidly up-regulated in vivo upon activation of naive virus-specific CD8 T cells within 24 h after LCMV infection and in less than 4 h after peptide injection, well before any cell division had occurred. This rapid PD-1 expression by CD8 T cells was driven predominantly by antigen receptor signaling since infection with a LCMV strain with a mutation in the CD8 T cell epitope did not result in the increase of PD-1 on antigen-specific CD8 T cells. Blockade of the PD-1 pathway using anti–PD-L1 or anti–PD-1 antibodies during the early phase of acute LCMV infection increased mTOR signaling and granzyme B expression in virus-specific CD8 T cells and resulted in faster clearance of the infection. These results show that PD-1 plays an inhibitory role during the naive-to-effector CD8 T cell transition and that the PD-1 pathway can also be modulated at this stage of T cell differentiation. These findings have implications for developing therapeutic vaccination strategies in combination with PD-1 blockade.
CD8 T cells play a critical role in controlling viral and also intracellular bacterial and parasitic infections (1, 2). Infection results in activation of pathogen-specific naive CD8 T cells, followed by clonal expansion and differentiation into effector CD8 T cells that kill pathogen-infected cells. After clearance of the pathogen, the majority (90–95%) of these effector CD8 T cells undergo apoptosis, but a small subset of fate-permissive effector CD8 T cells further differentiate into long-lived and functional memory cells that provide long-term protective immunity (3–6). In contrast to this generation of functional memory CD8 T cells after clearance of an acute infection, persistent antigenic stimulation as seen during chronic infections and cancer results in functional exhaustion of antigen-specific CD8 T cells (7).
The inhibitory receptor, programmed cell death-1 (PD-1), plays a major role in CD8 T cell exhaustion during chronic infections and cancer (8–10). PD-1 conveys negative signals through immunoreceptor tyrosine-based switch motif (ITSM) recruitment of SH2-domain–containing tyrosine phosphatase (SHP-2) that dephosphorylates initial stimulatory signaling molecules (11). Recently, it was reported that the PD-1–SHP-2 complex not only inhibits T cell receptor (TCR) signaling but also targets the costimulatory molecule CD28 (12, 13). Blockade of PD-1 inhibitory pathway restores function in exhausted CD8 T cells and results in reduction of viral load. This was first demonstrated using the mouse model of chronic infection with lymphocytic choriomeningitis virus (LCMV) (14–16) and then extended to chronic viral infections of nonhuman primates and humans (17–19).
Furthermore, PD-1–directed immunotherapy has shown clinical efficacy in human cancers, and several PD-1 pathway inhibitors are now licensed for the treatment of a wide variety of cancers including melanoma, lung, head and neck, renal, bladder, Merkel cell carcinoma, Hodgkin’s lymphoma, etc. (20–28).
Thus, the role of PD-1 in regulating T cell exhaustion during chronic infection and cancer is now well established (29). However, PD-1 is also expressed during the early phase of T cell activation when naive CD8 T cells differentiate into effector cells, but the physiological role of this transient PD-1 expression during acute infection is not well defined. In this study, we address this critical issue and show that the PD-1 inhibitory pathway also plays a regulatory role during naive-to-effector CD8 T cell differentiation.
Results
PD-1 Is Rapidly Expressed After Antigen Stimulation of Naive CD8 T Cells in Vivo.
We first asked how quickly naive CD8 T cells up-regulate PD-1 in vivo upon T cell activation. To examine this question, we transferred CellTrace Violet-labeled naive P14 transgenic CD8 T cells, which recognize an epitope from the LCMV glycoprotein (gp33-41), into B6 mice and infected these mice with the acute LCMV Armstrong (LCMV Arm) strain that is cleared within a week (30) (Fig. 1A). As shown in Fig. 1B, P14 cells up-regulated PD-1 at day 1 post infection even before the P14 cells had started dividing. The rapidity of PD-1 expression was dependent on the dose of the virus infection with higher doses resulting in faster PD-1 expression (Fig. 1C). PD-1 levels remained high as the LCMV-specific CD8 T cells underwent rapid cell division at days 2 and 3 post infection. Similar to PD-1, the activation markers CD25 and CD69, as well as CD44 (a marker for antigen-experienced T cells), were also rapidly up-regulated on P14 cells (Fig. 1B). CTLA-4 showed similar expression kinetics as PD-1, but other inhibitory receptors such as TIM-3, LAG-3, and 2B4 had slightly delayed kinetics compared with PD-1 expression (Fig. 2A). PD-1+ P14 cells expressed high levels of the cytotoxic molecule granzyme B and were able to produce the cytokines IFNγ and TNFα upon restimulation (Fig. 2B). Thus, these data demonstrate that PD-1 is rapidly expressed on activated CD8 T cells following viral infection in vivo.
Fig. 1.
PD-1 is rapidly expressed by antigen-specific CD8 T cells after viral infection. (A) The 1 × 106 CellTrace Violet (CTV)-labeled naive P14 TCR transgenic CD8 T cells (LCMV gp33-specific) were adoptively transferred into B6 mice. These P14 chimeric mice were infected with LCMV Arm (2 × 106 pfu/mouse i.v.). (B) Flow plots were gated on P14 cells. Expression of PD-1 and other activation markers (CD44, CD25, and CD69) was analyzed in the spleen at days 1, 2, and 3 post infection. (C) PD-1 expression on P14 cells was compared in mice infected with different doses of LCMV Arm (2 × 102, 2 × 104, or 2 × 106 pfu/mouse i.v.) at days 1, 2, and 3 post infection. Data are representative of three independent experiments with three to four mice per group.
Fig. 2.
Expression kinetics of inhibitory receptors and effector molecules on virus-specific CD8 T cells during early phase of T cell activation. CellTrace Violet-labeled P14 cells were transferred into B6 mice, followed by LCMV Arm infection (2 × 106 pfu/mouse i.v.). (A) CTLA-4, Tim-3, LAG-3, and 2B4 expression on P14 cells were examined at days 1, 2, and 3 post infection. (B) Granzyme B expression on P14 cells was observed at days 1, 2, and 3 post infection. To analyze IFNγ and TNFα production, splenocytes from P14 chimeric mice were stimulated with GP33 peptide for 5 h at days 1, 2, and 3 post infection. Data are representative of two independent experiments with three mice per group.
Next, we investigated the primary stimulus for this fast PD-1 up-regulation. Because PD-1 expression is correlated with viral titer during acute and chronic infection (9), we examined whether antigen-receptor–mediated signaling is the main regulator of PD-1 expression. We infected P14 chimeric mice with wild-type (WT) LCMV or a GP33 mutant LCMV strain that has an amino acid substitution in the GP33 epitope (V35A). This GP33 mutant LCMV strain replicates efficiently in mice but does not activate P14 CD8 T cells (31) (Fig. 3A). Comparison of WT and mutant virus infection allowed us to investigate whether environmental factors such as innate cytokines as opposed to TCR signals are involved in PD-1 expression on CD8 T cells. In the WT virus-infected mice, P14 cells highly expressed PD-1 at day 1 and maintained the expression at days 2 and 3, whereas PD-1 was minimally up-regulated on P14 cells over time in mutant virus-infected mice (Fig. 3A). These results demonstrate that the TCR signal is the principal mechanism regulating PD-1 expression and that environmental changes (cytokines) that occur during viral infection have minimal impact on PD-1 expression in virus-specific CD8 T cells in the absence of antigen stimulation. Since nuclear factor of T cell activation (NFAT), a downstream target of TCR signal, is involved in inducing PD-1 expression after T cell activation (32, 33), we also tested if inhibition of NFAT affected PD-1 expression in vivo. Following treatment with the calcineurin inhibitor FK506, PD-1 up-regulation after LCMV infection was substantially inhibited in vivo (Fig. 3B). These results strongly suggest the NFAT-dependent TCR signaling is necessary for PD-1 expression by CD8 T cells during viral infection.
Fig. 3.
TCR signal is the primary regulator of PD-1 expression. (A) P14 chimeric mice were infected with either WT LCMV or a LCMV strain with a mutation (V35A) in the GP33 epitope. PD-1 expression was examined on P14 cells in the spleen at days 1, 2, and 3 post infection. (B) After LCMV infection (2 × 106 pfu/mouse i.v.), P14 chimeric mice were treated with FK506 at day 3 post infection. PD-1 expression was examined in P14 cells at days 4 and 5 post infection. Data are representative of two to three independent experiments with three to five mice per group.
To activate T cells after viral infection, several steps are required before antigen-presenting cells (APC) can stimulate naive CD8 T cells; this involves infection of cells, expression of viral proteins, processing of these viral proteins into peptides, and then presentation by the appropriate APC. However, peptide injection allows APC to immediately present antigen to cognate T cells. Therefore, to further address the issue of how quickly PD-1 is up-regulated by naive CD8 T cells and to determine if antigen alone can do this, we injected mice with GP33 peptide and looked at the kinetics of PD-1 expression on P14 cells (Fig. 4A). We observed PD-1 expression as early as 2–4 h after peptide injection, which was much faster than what we saw after viral infection, and this further increased at 24 h (Fig. 4A). This kinetics of PD-1 expression was similar to the expression of early activation markers (CD69 and CD25) on P14 cells (Fig. 4A). Consistent with the results shown in Fig. 3B, FK506 treatment of mice immunized with GP33 peptide inhibited the expression of PD-1 on P14 cells (Fig. 4B). Taken together, the results shown in Figs. 3 and 4 show that this early PD-1 expression by CD8 T cells is primarily due to antigen-driven TCR signaling and that antigen is both necessary and sufficient for PD-1 induction.
Fig. 4.
Naive antigen-specific CD8 T cells express PD-1 within 4 h after peptide immunization. (A) GP33-41 peptide (200 μg/mouse i.v.) was injected into P14 chimeric mice, and PD-1, CD25, and CD69 expression on P14 cells was examined at 2, 4, 8, and 24 h post peptide injection. (B) Six hours after peptide injection, P14 chimeric mice were treated with FK506, and PD-1 expression was examined in P14 cells at 24 h post peptide injection. Data are representative of three independent experiments with three mice per group.
PD-1 Has an Inhibitory Role During Effector CD8 T Cell Differentiation.
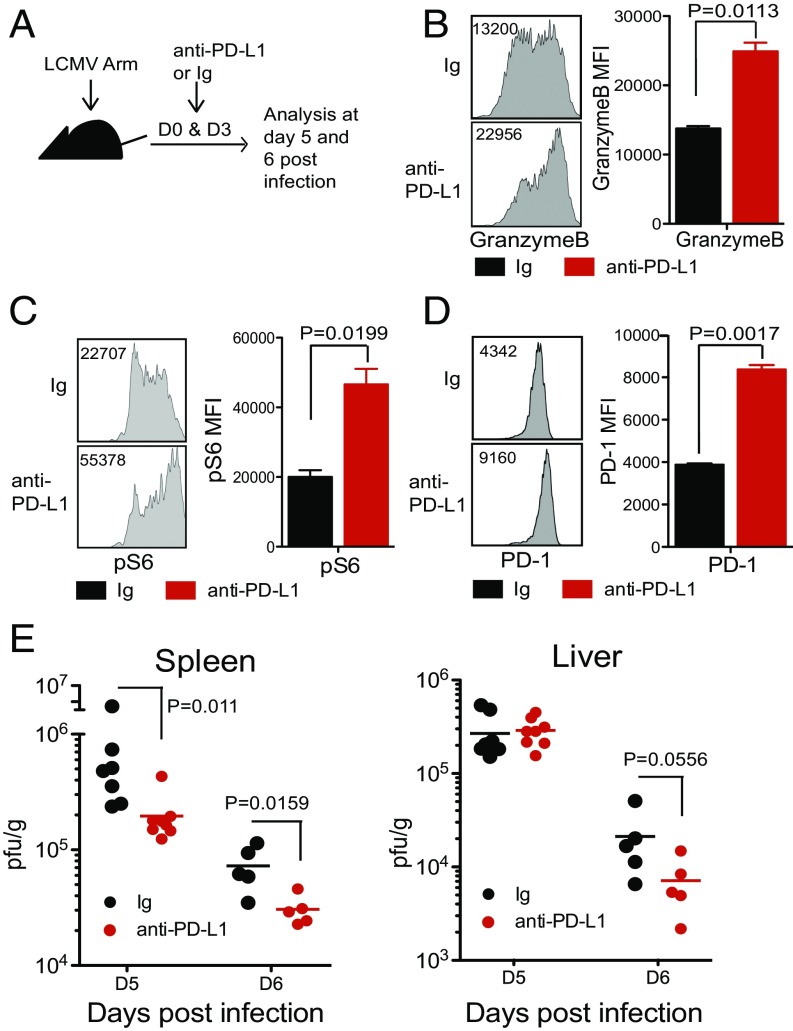
The next critical question was whether this early PD-1 expression exerted an inhibitory effect on T cells. To examine this question, LCMV Arm-infected mice were given a blocking anti–PD-L1 antibody at days 0 and 3 post infection (Fig. 5A), and the CD8 T cell response and viral titer were analyzed at days 5 and 6 post infection. Although the frequency of GP33-specific CD8 T cells and cytokine production (IFNγ and TNFα) of anti–PD-L1–treated mice were comparable to control Ig-treated mice (Fig. S1 A and B), the disruption of the PD-1 pathway during the very early phase of infection resulted in the up-regulation of granzyme B expression and increased phosphorylation of S6, a downstream target of mTOR, in antigen-specific CD8 T cells (Fig. 5 B and C). Interestingly, PD-1 expression itself was also significantly up-regulated (Fig. 5D) upon blockade of the PD-1 pathway. This is consistent with the results of Figs. 3 and 4 that show that TCR signaling regulates PD-1 expression; since PD-1 blockade enhances TCR signaling, this in turn further up-regulates PD-1 expression. Taken together, these results show that blockade of the interaction between PD-1 and PD-L1 during the early phase of acute viral infection further activates virus-specific effector CD8 T cells. Enhanced activation of virus-specific effector CD8 T cells was accompanied by significantly faster control of LCMV infection. Reduction in viral titer was evident in the spleen (P value = 0.011 at day 5 and 0.0159 at day 6) and liver (P value = 0.0556 at day 6) (Fig. 5E) of anti–PD-L1–treated mice compared with control Ig-treated mice at days 5 and 6 post infection. When we used another clone of anti–PD-L1 blocking antibody, the viral load was even more efficiently reduced (spleen P value = 0.0002 ∼ 0.0006, liver P value = 0.0002) (Fig. S2A). We also observed that anti–PD-1 antibodies were effective in reducing viral load compared with control Ig (P value = 0.0053) (Fig. S2B). Through immunopathology, we have shown that PD-L1 deficiency in mice resulted in mortality after a week of LCMV chronic infection, and another group also observed the fatal immunopathology of mice in anti–PD-1 blockade during the early phase of chronic LCMV infection (8, 34). However, in this study, we did not observe any signs of illness in anti–PD-1 or anti–PD-L1 blocking antibodies treated mice during the early phase of LCMV acute infection although there were increased CD8 T cell effector function and changes in viral load. This is most likely because the LCMV Arm stain does not cause a disseminated infection and it is cleared within a week after infection. These results show that PD-1 also acts as an inhibitory receptor during the early stage of CD8 T cell effector differentiation and that blockade of the PD-1 pathway results in more efficient resolution of the acute infection.
Fig. 5.
PD-1 pathway blockade during early phase of viral infection enhances effector function of antigen-specific CD8 T cells and reduces viral load. LCMV-infected mice were treated with anti–PD-L1 antibody (10F.9G2) or control Ig at days 0 and 3 post infection. (A) Experimental setup. (B) Granzyme B expression by DbGP33+ CD8 T cells at day 5 post infection. (C) Phosphorylation of S6 on P14 cells was examined at day 5 post infection. Representative flow plots and summary graphs on DbGP33+ CD8 T cells and P14 cells are shown. (D) PD-1 expression on DbGP33+ CD8 T cells at day 5. (E) Viral titers in spleen and liver were examined at days 5 and 6 post infection. Data are representative of two to three independent experiments with three to five mice per group. Error bars represent the SEM.
PD-1 Blockade During Acute Infection Modulates the Differentiation of Terminal Effector and Memory Precursor CD8 T Cell Subsets.
Next, we examined whether PD-1 blockade during acute infection has any effect on memory CD8 T cell formation and if this blockade modulates differentiation of the terminal effector (TE) and memory precursor (MP) CD8 T cell subsets (4). Blocking anti–PD-1 antibodies were administered from the time of viral infection until day 9 post acute LCMV infection (Fig. 6A). We did longitudinal analysis of the virus-specific CD8 T cell response and examined expression of the markers CD127 and KLRG1, which define the TE and MP effector CD8 T cell subsets, and the homing receptor L-selectin (CD62L) on LCMV-specific CD8 T cells at different time points after infection. The numbers of GP33+ CD8 T cells were comparable between mice injected with control Ig and anti–PD-1 antibodies at days 8, 15, and 30 post infection (Fig. 6B). However, the frequency of the MP population (CD127hi KLRG1low) among antigen-specific CD8 T cells (GP33+) was higher in mice that received PD-1 blockade (Fig. 6C). Likewise, GP33+ CD8 T cells in mice injected with anti–PD-1 showed better reexpression of CD62L. We also tested whether PD-1 blockade had an effect on CD8 T cell response in vaccination using recombinant adenovirus serotype 5 expressing LCMV glycoprotein (Ad5-GP) (Fig. 6D). Consistent with the results seen during acute LCMV infection, there was no significant difference in the total number of antigen-specific CD8 T cells, but frequencies of the MP CD8 T cell subset were increased in mice injected with anti–PD-1 antibody compared with control Ig during Ad5 immunization (Fig. 6 E and F). Since the amount and duration of viral antigen significantly contributes to effector and memory CD8 T cell differentiation, the increased frequency of the MP CD8 T cell subset in PD-1–treated mice is most likely due to faster viral clearance after PD-1 blockade. However, the observed 10–20% differences in the frequencies of MP vs. TE CD8 T cell subsets in the PD-1–treated mice compared with control mice were not sufficiently high to result in a detectable difference in the number of virus-specific memory CD8 T cells.
Fig. 6.
PD-1 blockade during acute viral infection enhances the frequency of the memory precursor subset of effector CD8 T cells. (A) Mice were treated with anti–PD-1(29F10) antibody or control Ig at days 0, 3, 6, and 9 post LCMV Arm. (B) The number of GP33+ CD8 T cells in PBMCs was analyzed at days 8, 15, and 30 post infection. (C) Gated on GP33+ CD8 T cells, the MP subset (CD127hi KLRG1low) and the TE subset (CD127low KLRG1hi) were analyzed at days 8, 15, and 30 post infection. (Right) Summary graph. (Bottom) CD62L expression in DbGP33+ CD8 T cells was examined at days 8, 15, and 30 post infection. (Right) Summary graph. (D) Mice were treated with anti–PD-1(29F10) antibody or control Ig at days 0, 3, 6, and 9 post Ad5-GP immunization. (E) The number of GP33+ CD8 T cells in PBMCs was analyzed at day 8, 15, and 22 post immunization. (F) Gated on GP33+ CD8 T cells, MP and TE subsets were analyzed at days 8, 15, and 22 post immunization. Summary graph is shown at Right. Data are representative of three independent experiments with five mice per group. Error bars represent the SEM.
Discussion
An interesting and unexplored aspect of PD-1 biology is that this inhibitory receptor is expressed not only on chronically stimulated exhausted CD8 T cells, but also on effector CD8 T cells during the early stage of T cell activation (9). However, it was unclear how quickly PD-1 is up-regulated and whether PD-1 on activated T cells during this early stage has an inhibitory role similar to what is seen in exhausted CD8 T cells. To address these questions, we studied the role of PD-1 during acute LCMV infection. We observed that PD-1 was rapidly up-regulated by TCR signaling upon T cell activation even before cell division. Blocking PD-1 signaling during the early phase of acute viral infection enhanced CD8 T cell effector function and viral clearance, demonstrating that PD-1 expression on early activated T cells has an inhibitory role in T cell immunity against viral infection. Thus, PD-1 acts as an inhibitory receptor not only during T cell exhaustion but also during generation of functional effector CD8 T cells.
Interestingly, we found that the PD-1 pathway blockade during the early phase of acute infection modulates the differentiation of the MP and TE subsets of virus-specific effector CD8 T cells. In our studies, there was an increase in the number of the MP CD8 T cell subset that gives rise to the pool of long-lived memory CD8 T cells. This is a beneficial thing and was most likely due to the faster clearance of infection in the anti–PD-1 antibody-treated mice. Our results are different from the finding of Odorizzi et al. (35) who showed that PD-1 deficiency results in terminal differentiation of virus-specific CD8 T cells during chronic LCMV infection. We think that the differences between the two studies are primarily due to the striking differences in both the magnitude and the duration of antigen persistence after LCMV Arm acute infection (our study) versus LCMV clone 13 chronic infection (35). Another factor is the transient PD-1 blockade that we have done using anti–PD-1 antibodies that resulted in a decrease of viral load (this is good for T cell survival and memory differentiation) compared with the PD-1 genetic deletion approach used by Odorizzi et al. (35). In this study, the small number of PD-1–deficient P14 transgenic CD8 T cells that are present in the clone 13-infected mice do not change the viral load. So, not only does the viral load remain high in these mice but also PD-1–mediated regulation is permanently lost from the transferred PD-1 KO P14 cells. Such a situation will clearly lead to more pronounced terminal differentiation and reduced cell survival of virus-specific CD8 T cells as reported by Odorizzi et al. (35).
An important question is, why is the inhibitory receptor PD-1 induced on functional CD8 T cells during the early phase of activation? As discussed above, excessive and continuous TCR stimulation can cause terminal differentiation and apoptosis of T cells. Since PD-1 levels on the early activated CD8 T cells increased in a dose-dependent manner (Fig. 1), this molecule most likely acts as a negative feedback system for T cell activation to optimize T cell effector and memory responses. Thus, an important role for this early PD-1 expression would be to function as a rheostat modulating the extent of TCR signaling in a cell-intrinsic manner (36). In addition, hyper-activated T cells can result in immunopathology, and the PD-1 pathway plays an important role in modulating excessive T cell-mediated damage. This notion is supported by previous findings in which PD-L1 deficiency in mice during the early phase (1 wk post infection) of chronic LCMV clone 13 infection resulted in fatal immunopathology (8), and PD-L1 expression on fibroblastic reticular cells in the spleen prevented tissue damage during chronic LCMV infection (37).
Consistent with our finding that blocking the PD-1 inhibitory pathway at an early stage of infection could enhance T effector function (Fig. 4), the number and function of herpes simplex virus (HSV)-specific CD8 T cells were enhanced by PD-L1 blockade during acute HSV infection in mice (38), and PD-1 blockade improved the Gag-specific CD8 T cell response following adenovirus encoding SIV-Gag immunization of rhesus macaques (39).
PD-1 directed immunotherapy is now widely used in the treatment of several different cancers (22, 25, 40). In this instance, PD-1 blockade is acting on TCF1+CD28+stem-like CD8 T cells that provide the proliferative burst and also on more exhausted CD8 T cells to increase their effector function (16, 29, 41). Our finding that PD-1 blockade can also be used to modulate naive-to-effector CD8 T cell differentiation raises some interesting possibilities about the use of therapeutic vaccination in combination with PD-1 blockade (42). Since one of the main goals of therapeutic vaccination is to induce new T cell responses, it is possible that combination therapy with PD-1 blockade will be beneficial in enhancing this naive-to-effector cell transition in cancer patients.
Materials and Methods
Mice, Infections, and Adoptive Transfer.
Six –week-old female C57BL/6 mice were purchased from The Jackson Laboratory. To generate P14 chimeric mice, we transferred 1 × 106 P14 cells (Thy1.1/1.2) into naive B6 (Thy1.2/1.2) mice. The P14 chimeric mice or B6 mice were infected with LCMV Arm (2 × 102/2 × 104/2 × 106 pfu, i.v.) or injected with gp33-41 peptide (200 μg i.v.) or Adeno5-GP [5 × 109 viral particle (v.p.), i.m.]. All mice were used in accordance with National Institutes of Health and the Emory University Institutional Animal Care and Use Committee guidelines.
PD-L1/PD-1 Blockade and Virus Titration.
Anti–PD-L1 (10F.9G2, 500 μg, i.v.) or control Ig was administered to LCMV Arm (2 × 106 pfu, i.v.)-infected B6 mice at days 0 and 3 post infection. Anti–PD-L1 (YW243.55. S70, 200 μg, i.v.) antibodies generated by Genentech (43) were administered to LCMV Arm (2 × 106 pfu, i.v.)-infected B6 mice at day 3 post infection. Anti–PD-1 (29F10, 200 μg, i.p.) or control Ig was injected into B6 mice at days 0, 3, 6, and 9 post LCMV Arm infection (2 × 106 pfu, i.v.) or Adeno5-GP immunization (5 × 109 v.p., i.m.). Titers of virus from homogenized spleen and liver were determined by plaque assay as previously described (30).
FK506 Treatment.
FK506 solution was prepared as previously described (32). FK506 (10 mg/kg) or control vehicle was s.c. administered into P14 chimeric mice at day 3 post LCMV Arm infection or 6 h after GP33 peptide injection.
Flow Cytometry and Staining.
Lymphocytes were collected from peripheral blood mononuclear cells (PBMCs) and spleen as previously described (8). To analyze cell division, we labeled P14 cells with CellTrace Violet (Invitrogen) before adoptive transfer. Live/Dead Near IR (Invitrogen) was used to remove the dead cells. To examine the expression of inhibitory receptors, cells were stained with anti–CTLA-4 (UC10-4F10-11), LAG-3 (C9B7W), Tim-3 (F38-2E2), and anti-2B4 (2B4 B6 alloantigen). To stain phospho-S6, splenocytes were fixed by Lyse/Fix buffer (BD) for 10 min at room temperature (RT). After fixation, cells were permeabilized with Perm/Wash I buffer (BD) for 10 min at RT. Then cells were stained with Alexa 488-conjugated anti-pS6 Abs (Cell Signaling) or isotype control Ig for 30 min at RT. For intracellular cytokine staining, splenocytes were incubated with gp33-41 peptide (0.4 μg/mL) for 5 h at 37 °C in the presence of brefeldin and monensin. Cells were stained with anti–IFN-γ and anti–TNF-α Abs (BD) using Cytofix/Cytoperm (BD) according to manufacturer’s recommendations. Cells were analyzed on a Canto II flow cytometer (BD Immunocytometry Systems). Data were analyzed with FlowJo v.9.9.4 (TreeStar).
Statistical Analysis.
All statistical analysis was performed using a two-tailed paired or unpaired Student’s t test.
Supplementary Material
Acknowledgments
We thank B. T. Konieczny and H. Wu for technical assistance. This work was supported by NIH Grants R01A1030048 (to R.A.) and P01AI0562999 (to R.A., G.J.F., and A.H.S.).
Footnotes
Conflict of interest statement: R.A. has patents on the PD-1 pathway and has served on the advisory board for Genentech/Roche and received research funding from Genentech and Merck. G.J.F. and A.H.S. have patents/pending royalties on the PD-1 pathway from Roche, Merck, Bristol-Myers Squibb, EMD Serono, Boehringer Ingelheim, AstraZeneca, Dako, and Novartis. G.J.F. has served on advisory boards for Roche, Bristol-Myers Squibb, Xios, and Quiet. A.H.S. has served on advisory boards for Novartis, Surface Oncology, and Elstar. A.H.S. has received research funding from Novartis, Roche, UCB, Ipsen, and Quark. B.I.A. is an employee of Five Prime Therapeutics.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718217115/-/DCSupplemental.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Obar JJ, Lefrançois L. Memory CD8+ T cell differentiation. Ann N Y Acad Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akondy RS, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552:362–367. doi: 10.1038/nature24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 5.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 6.Youngblood B, et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552:404–409. doi: 10.1038/nature25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 8.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 12.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui E, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He R, et al. Follicular CXCR5-expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 15.Utzschneider DT, et al. T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Im SJ, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fourcade J, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller MJ, et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc Natl Acad Sci USA. 2013;110:15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velu V, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garon EB, et al. KEYNOTE-001 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 23.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt LH, et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One. 2015;10:e0136023. doi: 10.1371/journal.pone.0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto M, et al. CD8 T cell exhaustion in chronic infection and cancer: Opportunities for interventions. Annu Rev Med. 2018;69:301–318. doi: 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araki K, et al. Pathogenic virus-specific T cells cause disease during treatment with the calcineurin inhibitor FK506: Implications for transplantation. J Exp Med. 2010;207:2355–2367. doi: 10.1084/jem.20100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frebel H, et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209:2485–2499. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. 2015;212:1125–1137. doi: 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 37.Mueller SN, et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Channappanavar R, Twardy BS, Suvas S. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One. 2012;7:e39757. doi: 10.1371/journal.pone.0039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finnefrock AC, et al. PD-1 blockade in rhesus macaques: Impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980–987. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- 40.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamphorst AO, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebert PJR, et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. 2016;44:609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]