Significance
Eliminating malaria from islands should, in theory, be easier than eliminating malaria from countries in mainland Africa because of restricted movement of insects and people between treated and untreated areas. The example of Bioko Island, where the entomological inoculation rate in 2004 was among the highest in Africa, demonstrates how difficult this can be. Vector control has eliminated two of the four vector species, and malaria has been dramatically reduced. This study demonstrates rapid evolution of resistance following reintroduction of pyrethroid-based control interventions, with the selection of a P450-based mechanism (CYP9K1). Urban malaria, movement of infected people from the mainland, and selection of this pyrethroid-resistance mechanism in addition to knockdown resistance has impeded progress and forced a change to nonpyrethroid indoor residual spraying.
Keywords: cytochrome P450, resistance, pyrethroid, malaria, control
Abstract
Since 2004, indoor residual spraying (IRS) and long-lasting insecticide-impregnated bednets (LLINs) have reduced the malaria parasite prevalence in children on Bioko Island, Equatorial Guinea, from 45% to 12%. After target site-based (knockdown resistance; kdr) pyrethroid resistance was detected in 2004 in Anopheles coluzzii (formerly known as the M form of the Anopheles gambiae complex), the carbamate bendiocarb was introduced. Subsequent analysis showed that kdr alone was not operationally significant, so pyrethroid-based IRS was successfully reintroduced in 2012. In 2007 and 2014–2015, mass distribution of new pyrethroid LLINs was undertaken to increase the net coverage levels. The combined selection pressure of IRS and LLINs resulted in an increase in the frequency of pyrethroid resistance in 2015. In addition to a significant increase in kdr frequency, an additional metabolic pyrethroid resistance mechanism had been selected. Increased metabolism of the pyrethroid deltamethrin was linked with up-regulation of the cytochrome P450 CYP9K1. The increase in resistance prompted a reversion to bendiocarb IRS in 2016 to avoid a resurgence of malaria, in line with the national Malaria Control Program plan.
Malaria prevalence in Africa has been halved since 2000, with 80% of the reduction being the result of the use of vector control (1). This reduction is primarily attributable to pyrethroid-treated bed nets and indoor residual spraying (IRS). Pyrethroid resistance in mosquito populations is at a critical tipping point in Africa, threatening the progress made in reducing malaria transmission (2, 3).
The most prevalent pyrethroid resistance mechanisms in Anopheles affect the voltage-gated sodium channel target site of the insecticide or its metabolic degradation pathway. Additional resistance mechanisms include epicuticular changes, which slow the uptake of pyrethroids (4). Knockdown resistance (kdr) mutations at the target site typically alter the insecticide binding affinity, and the most common in Anopheles are mutations L1014F and L1014S in the voltage-gated sodium channel (5, 6). In addition, the mutation N1575Y combined with L1014F strengthens resistance to pyrethroids and dichlorodiphenyltrichloroethane (DDT) (7, 8), which used to be one of the most widely used insecticides for malaria control and is still being used in several parts of the world. Kdr resistance alone has not been correlated with operational control failure in Anopheles gambiae. The accumulation of kdr and metabolic resistance mechanisms, however, have resulted in much higher pyrethroid resistance levels, which may have an operational impact (9–11).
Overexpression of detoxification enzymes such as cytochrome P450 monooxygenases (henceforth “P450s”) is frequently found in association with metabolically based insecticide resistance in An. gambiae (12). The P450 genes Cyp6M2 and Cyp6P3 are often overexpressed in resistant An. gambiae (13), and both metabolize permethrin and deltamethrin (14, 15). Cyp6Z2 is also sometimes overexpressed, but binds/sequesters rather than metabolizes the pyrethroids permethrin and cypermethrin (16).
Analysis of metabolic-resistance mechanisms typically involves transcriptomic comparisons between resistant laboratory strains or field-caught populations and laboratory susceptible strains (17), with occasional examples of spatial or temporal comparisons made between field mosquitoes that differ in resistance phenotype (18). In Burkina Faso, significant changes in expression of P450s were linked to an increase in pyrethroid resistance (10), and, in Benin, expression of Cyp6M2 and Cyp6P3 increased in An. gambiae after mass distribution of long-lasting insecticide-treated nets (LLINs) (19). Kdr frequencies were unchanged in both cases. An association between pyrethroid use and an increase in the relative frequency of kdr 1014F and a nearly fixed X-linked haplotype containing Cyp9K1 was demonstrated in a genome-wide study of mosquitoes from Mali (20), but the role of this P450 gene in resistance has not been established.
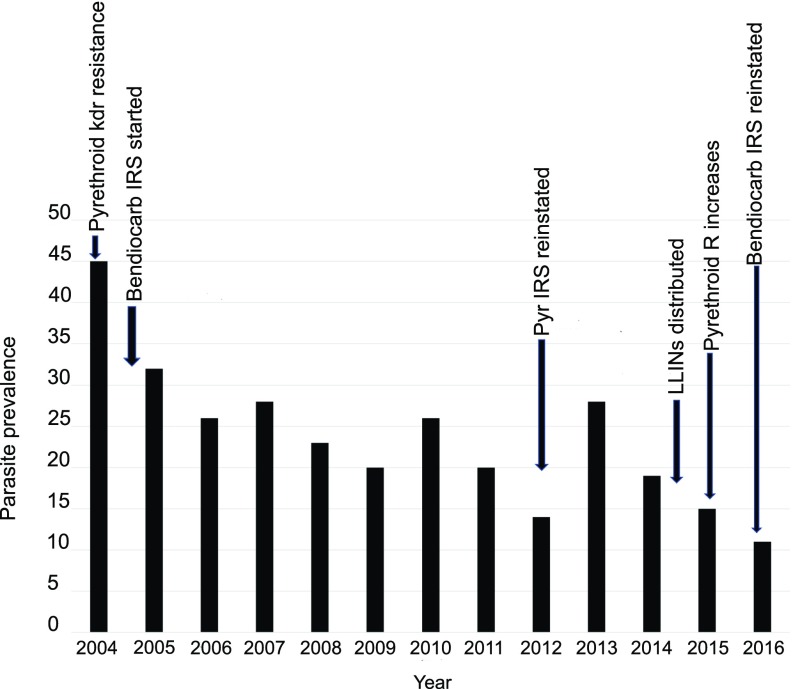
Before the Bioko Island Malaria Control Project (BIMCP) and the Ministry of Health and Social Welfare established the malaria control program in 2004, Bioko had one of the highest entomological inoculation rates recorded in Africa, at ∼1,200 infectious bites per person per year (21). In 2004, there were three major mosquito vector species on the island: Anopheles funestus and the M and S forms of An. gambiae, now elevated to species as An. gambiae (S) and Anopheles coluzzii (M) (22). Malaria prevention efforts used pyrethroid-impregnated LLINs and IRS, with the carbamate bendiocarb or the pyrethroid deltamethrin (see Fig. 1 for timeline). A country-level operational implementation of the World Health Organization (WHO)-recommended Global Plan for Insecticide Resistance Management (GPIRM) was established in 2012 (23), with insecticide resistance monitoring providing robust data to support evidence-based insecticide resistance management strategies.
Fig. 1.
Timelines for vector-control events and Plasmodium falciparum parasite prevalence data in 2–14-y-old children in 2004–2016. Arrows indicate major vector control events over time in Bioko. Pyr, pyrethroids; R, resistance.
Pyrethroid-based IRS was used when the program was initiated, but resistance was quickly detected. Bendiocarb-based IRS was instigated in the second spray round, but this switch increased cost and reduced the effective longevity of the IRS. A comprehensive study of the resistance mechanisms present in An. gambiae from Bioko was conducted in 2011; kdr 1014F genotypes were at high frequencies (28.5% FF, 57.14% LF), with no significant metabolic resistance (23). With kdr alone expected to have no operational impact on pyrethroid-based IRS efficacy, the BIMCP reintroduced pyrethroids in 2012.
In 2015, bioassays indicated that pyrethroid resistance frequency and level were increasing rapidly, which triggered detailed analysis of the local vector population composition and insecticide-resistance mechanisms to determine the appropriate vector control interventions for 2016.
Results
Bioassays and Genotypes.
A timeline for introduction of different vector control activities in Bioko, overlaid on the annual malaria prevalence figures for children 2–14 y old, is given in Fig. 1. There were some issues with the pyrethroid IRS formulation in 2013, which were corrected in 2014, but there were no indications from bioassay data that resistance increased from 2013 to 2014.
The levels of insecticide resistance increased in 2015 compared with earlier years, with mosquitoes from two field sites (hospital and industrial) having <30% mortality after exposure to the WHO discriminating dosage of deltamethrin but remaining susceptible to bendiocarb (mortality rate > 99%; Table S1).
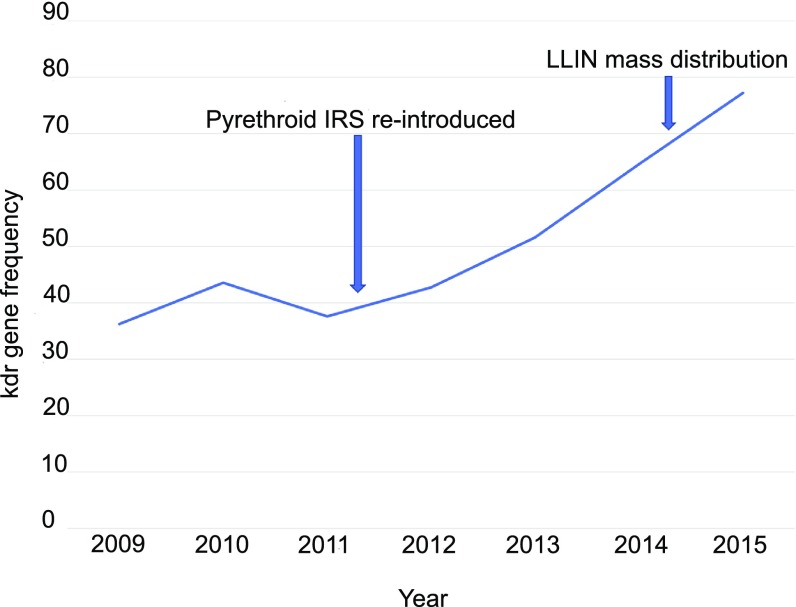
From the two sites where resistance was analyzed in detail, all mosquitoes were identified as An. coluzzii (Table 1). All samples collected in 2015 were homozygous for the L1014F kdr mutation, but the L1014S and N1575Y and AChE G119S mutations were not detected. This finding represents a significant increase in kdr from 2011 (28.5% 1014F/F; χ22 = 46.5, P = 8 × 10−11; Table 1). Annual island-wide surveys of kdr confirmed the increase in frequency (Fig. 2) from 2011 to 2015.
Table 1.
Molecular form composition and frequencies of target-site resistance mutations in field-caught An. gambiae s.l. populations collected in Bioko
| Population | Genotypes | Target-site allele frequency, %* | |||||
| No. of specimens | Molecular form, % | No. of specimens | 1014F/F | 1014F/L | 10114L/L | ||
| M/M | S/S | ||||||
| Malabo 2004† | — | 36 | 64 | 4,500 | 14 | 41 | 45 |
| Malabo 2011 | 75 | 100 | 0 | 75 | 28.5 | 57.14 | 14.3 |
| Malabo hospital 2015 | 16 | 100 | 0 | 16 | 100 | 0 | 0 |
| Malabo industrial 2015 | 16 | 100 | 0 | 16 | 100 | 0 | 0 |
M, An. coluzzii (M form); S, An. gambiae s.s. (S form).
Data from Hemingway et al., 2013 (23).
Mutations L1014S and N1575Y on the voltage-gated sodium channel, as well as mutation G119S on acetylcholinesterase, were absent in all alleles (n > 200) genotyped in both 2011 and 2015. F/F, individuals homozygous for mutation L1014F on the voltage-gated sodium channel; F/L, heterozygotes; L/L, homozygous individuals for the WT (i.e., susceptible) allele.
Fig. 2.
Frequency of the L1014F kdr mutation in A. coluzzii on Bioko Island, 2009–2015. Data generated from combined routine island-wide annual entomological surveillance data and the more detailed studies undertaken in 2011 and 2014 at two sites in Malabo. Arrows indicate time points at which new pyrethroid-based operational IRS and LLIN control interventions occurred.
Gene Expression.
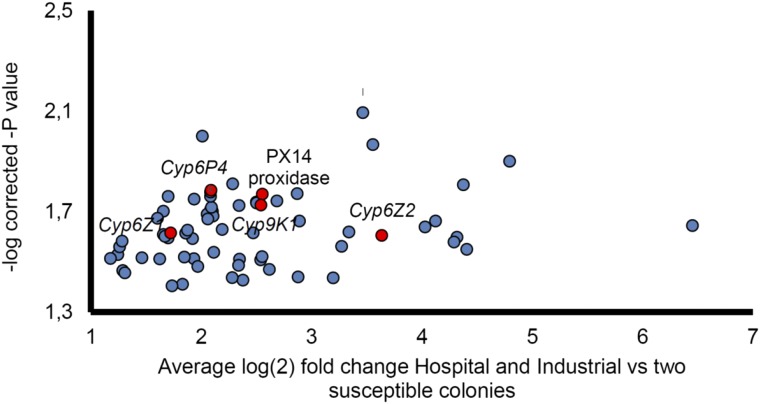
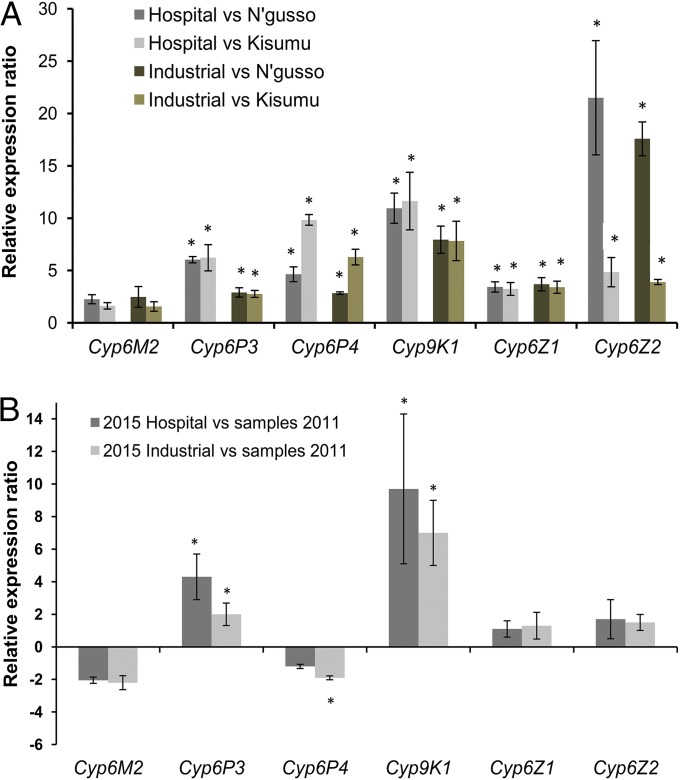
Several genes were overexpressed when the Bioko field-collected populations were compared with the insecticide-susceptible laboratory colonies. Of the 48 genes that were significantly overexpressed in all comparisons performed, four were P450s: Cyp6P4, Cyp9K1, Cyp6Z1, and Cyp6Z2 (Fig. 3 and Dataset S1). The up-regulation of each gene was assessed by quantitative RT-PCR (qPCR). We also included Cyp6P3 in the analysis as a strong pyrethroid metabolizer that narrowly missed statistical significance in one of the four comparisons, in addition to the other known strong metabolizer, Cyp6M2, even though this was not consistently differentially expressed. The qPCR results confirmed the statistically significant up-regulation of these P450 genes in most comparisons, with the exception of Cyp6M2 (Fig. 4A).
Fig. 3.
Significantly up-regulated transcripts found in field-caught resistant populations. Transcripts were found to be significantly up-regulated in the hospital 2015 and industrial 2015 populations in all comparisons performed with the two susceptible laboratory colonies N’gusso and Kisumu. The average log(2) FC between resistance and susceptible replicates is shown. Detoxification genes are highlighted in red.
Fig. 4.
Expression analysis of selected P450s by qPCR. (A) qPCR comparisons of selected P450 transcripts between hospital 2015 and industrial 2015 An. gambiae field populations from Bioko vs. the two susceptible laboratory colonies (N’gusso, Kisumu). Error bars represent the SE based on four biological replicates. Asterisk indicates a statistically significant difference in expression levels between the tested samples (Mann–Whitney U test, P < 0.05). (B) Comparison of P450 transcript expression levels between field-caught populations in 2015 (hospital and industrial) vs. samples collected in 2011. Error bars represent the SE based on four biological replicates. Asterisk indicates a statistically significant difference in expression levels between the tested samples (Mann–Whitney U test, P < 0.05).
We then compared expression levels of the same P450 genes between the populations collected in 2011 and 2015 (Fig. 4B). There was a significant and substantial elevation of expression of Cyp9K1 and, to a lesser extent, of Cyp6P3 in 2015 samples compared with 2011. Cyp6P4 showed a significant but slight decrease in one site. The expression of the other P450s was unchanged.
Functional Expression and Characterization of CYP9K1.
As the role of the Cyp9K1 gene in insecticide resistance had not been evaluated previously to our awareness, CYP9K1 and An. gambiae cytochrome P450 reductase (CPR) were functionally coexpressed in Escherichia coli and directed to the inner bacterial membrane by using the leading sequences ompA and pelB, respectively. The reduced carbon monoxide-difference spectrum obtained indicated that CYP9K1 was expressed predominately in its active P450 form, characteristic of a well-folded and functional enzyme (Fig. S1A). The isolated bacterial membranes contained 1.5 µM P450, whereas the measured P450 reductase activity (AgCPR) was 136.5 μmol cytochrome c reduced/min/g protein. CYP9K1 showed high activity for the fluorescent substrate 7-ethoxycoumarin (Table 2).
Table 2.
Activity of recombinant CYP9K1 against model substrates and insecticides
| Substrate | Specific activity/depletion of parental compound, % | |
| −NADPH | +NADPH | |
| 7-Ethocycoumarin | No activity | 10.6 ± 1.1 |
| Deltamethrin | No depletion | 32.0 ± 3.24 |
| Bendiocarb | No depletion | No depletion |
| Pyriproxyfen | No depletion | 38.0 ± 1.3 |
Activities are given in picomoles of 7-hydroxycoumarin per minute per picomole of P450 for the substrate 7-ethocycoumarin and as percentage depletion of the parental insecticides under assay conditions.
CYP9K1 was able to metabolize 32% of the deltamethrin applied following a 2-h incubation (Table 2). Deltamethrin metabolism followed Michaelis–Menten kinetics (Km = 28.2 μΜ and Vmax = 0.57 pmol deltamethrin/min/pmol P450; Fig. S1B). CYP9K1 likewise metabolized pyriproxyfen, as observed by the NADPH-dependent depletion of this insecticide (Table 2) and the formation of two metabolites, which eluted at 6.2 min and 7.4 min, respectively (24). In contrast, bendiocarb was not metabolized by CYP9K1, with no substrate depletion after a 20-min incubation, after which spontaneous, non–P450-specific, degradation occurred.
Discussion
Bioko Island is implementing a country-level operational implementation of the GPIRM with evidence-based malaria vector control. A robust program for insecticide resistance monitoring has been in place since 2004 (23). In 2011, pyrethroids were still operationally functional, and the BIMCP rotated back to pyrethroid IRS in 2012 after 7 y of bendiocarb use to maintain levels of disease control, reduce cost, and reduce the selection pressure on bendiocarb. Three years later, after bioassays signaled a change in pyrethroid-resistance status of the local vector (deltamethrin mortality rate of 25.7% in 2015 vs. 40% in 2011) but detected no difference in the susceptibility to bendiocarb (Table S1), a detailed insecticide-resistance analysis was undertaken. The frequency of the target site pyrethroid resistance mutation L1014F was dramatically higher in 2015 (100% in 2015 and 57% in 2011 at the same collection sites), but the Anopheles pyrethroid kdr-based resistance enhancing mutation N1575Y and the AChE resistance mutation (G119S) were not found. The P450s Cyp6P4, Cyp9K1, Cyp6Z1, Cyp6Z2, and Cyp6P3 were overexpressed in An. coluzzii from Bioko compared with the susceptible colonies (N’gusso and Kisumu), indicating a possible link to the increased pyrethroid resistance. Specific P450 genes are repeatedly implicated in pyrethroid resistance in An. gambiae and An. coluzzii populations (13), and, in some cases, single P450s have been demonstrated to confer resistance via heterologous expression in Drosophila (9). In resistant mosquito field populations, however, multiple P450s are typically overexpressed to moderate levels, in contrast with Drosophila and agricultural pest examples, in which resistance is often attributed to dramatic elevation of single P450s (25, 26).
In the “hospital 2015” and “industrial 2015” An. coluzzii populations, there was a 6–10-fold increase in expression of Cyp9K1, and lower, but significant, overexpression of Cyp6P3 compared with mosquitoes sampled from the same regions in 2011. Selection by pyrethroid-based IRS and more recently LLINs in Bioko may have given rise to this metabolic resistance coupled with an increase in kdr frequency. Resistance is likely to have arisen de novo in An. coluzzii on the island, as there is no evidence over several years of An. funestus or An. gambiae s.s. reinvading from the mainland after local elimination, coupled with previous evidence of a de novo local origin of kdr 1014F (27). The situation on Bioko is one of very few examples of documented changes in metabolic gene expression linked to control interventions.
Relatively few candidate genes in An. gambiae spp. have been subjected to functional validation, without which a definitive causal link with insecticide resistance cannot be made. CYP9K1 was able to metabolize deltamethrin (Kcat, 0.57 min−1; Km, 28.2 μM), albeit with lower in vitro catalytic efficiency compared with other major P450s linked with resistance, CYP6P3 (Kcat, 1.8 min−1; Km, 5.9 µM) (14) and CYP6M2 (Kcat, 1.1 min−1; Km, 2 µM) (15). However, the actual in vivo contribution of each P450 to the resistance phenotype is a more complex issue, as factors such as tissue localization of each enzyme may affect the resistance phenotype. Moreover, combined metabolic activity of more than one P450 on pyrethroid substrates and their metabolites has been reported (28, 29), which might also be the case here, perhaps mediated by synergistic interaction with the other overexpressed enzyme, CYP6P3. An interesting consideration for alternative interventions is that CYP9K1 was also able to metabolize the juvenile hormone analog pyriproxyfen, in line with other major cytochrome P450 metabolic enzymes (30). This result is a concern because pyriproxyfen is being combined with pyrethroids in some next-generation LLINs. CYP9K1 did not metabolize bendiocarb and CYP6P3 has very low catalytic efficiency for bendiocarb (9), consistent with the absence of phenotypic resistance.
It has been hypothesized that the coevolution of P450s and target-site resistance mutations can lead to high levels of resistance that challenge operational efficacy of pyrethroids (20, 31). When metabolic pyrethroid resistance was first selected in Africa, Cyp9K1 did not appear to be a strong indicator of metabolic resistance in An. gambiae, a pattern that may now be changing. In addition to the present data from Bioko, Cyp9K1 was found to be up-regulated in DDT-resistant An. gambiae and An. coluzzii populations from Cameroon possessing kdr 1014F (32) and also in deltamethrin-resistant but kdr-free Anopheles parensis from Uganda (33). A section of the X-chromosome that included Cyp9K1 was detected as being under positive selection, together with the L1014F kdr mutation, in An. coluzzii populations from Mali after an increase in insecticide pressure by the widespread use of insecticide-treated nets with pyrethroids (20). This growing body of evidence suggesting a role for CYP9K1 in resistance is now further supported by demonstration of functional expression and metabolic activity.
In 2004, the majority of malaria mosquito vectors collected on Bioko were An. gambiae s.s. In 2015, An. coluzzii and Anopheles melas were the only vector species collected. Hence, the vector-control activities may now have eliminated An. funestus and An. gambiae s.s. from the island. In Mali, Cyp9k1 was selected as an An. coluzzii haplotype from standing variation, rather than via introgression from An. gambiae (20). On Bioko, the simultaneous detection of increased Cyp9k1 expression, coupled with an increase in kdr 1014F frequency and higher levels of pyrethroid resistance, suggests that the two mechanisms may act synergistically.
Conclusion
The change in the resistance profile of An. coluzzii observed over a period of 3 y after pyrethroid IRS was reinstated is mediated by target site and metabolic resistance mechanisms involving a newly validated cytochrome P450 pyrethroid metabolizer, CYP9K1. In line with the national evidence-based resistance management plan, a recommendation that a nonpyrethroid IRS formulation should be used in 2016 was made. The susceptibility of An. coluzzii and An. melas vectors to organophosphates and carbamates indicates that bendiocarb or the longer-lasting formulation of Actellic 300CS (34) are the best alternatives.
Materials and Methods
Malaria Prevalence Surveys.
Annual malaria prevalence surveys in children 2–14 y of age were undertaken as described by Bradley et al. (35). Informed consent was obtained from all participants. Annual parasite prevalence surveys are covered by ongoing IRB clearance through the Ministry of Health and Marathon Oil Malaria elimination programme in Equatorial Guinea, which has been in place since 2003.
Mosquito Collections and Bioassays.
Island-wide routine entomological monitoring was undertaken annually from 2009 to 2015 to assess species composition and the frequency of the kdr-pyrethroid resistance mechanism.
More intensive analysis of resistance was undertaken in 2004, 2011, and 2014 at more limited sites in response to changes in resistance levels detected in routine surveys. For the intensive surveys, immature Anopheles were collected from the same sites each year: the grounds of the general hospital in the Ela Nguema district of Malabo (03°45.583 N, 008°47.364 E; referred to as “hospital”) and a former industrial site along the harbor (03°45.325 N, 008°46.003 E; “industrial”). Both sites are in the center of Malabo, close to housing. Field-collected larvae were reared to adulthood, and 3-d-old non–blood-fed females were bioassayed by using the WHO susceptibility tests (36). Briefly, mosquitoes were exposed to insecticide-impregnated papers (0.05% deltamethrin or 0.1% bendiocarb; WHO recommended doses) for 1 h, and mortality rates were recorded 24 h later.
Molecular Genotyping for Species Identification and Target Site Resistance Mutations.
An. gambiae s.l. were identified by using a PCR-based diagnostic assay (37) with An. gambiae s.s. and An. coluzzii, two members of this complex subsequently separated by using the short interspersed element (SINE) PCR method (38). SINE PCR is needed because the two species are morphologically indistinguishable. TaqMan assays with probes detecting the mutant resistant allele and the WT susceptible allele were used to screen for the presence of three kdr mutations (L1014F, L1014S, N1575Y) and the G119S AChE mutation conferring resistance to pyrethroids and DDT and to organophosphates, respectively (7, 39).
Analysis of Gene Expression (Metabolic Resistance).
Three-day-old field-caught, non–blood-fed female mosquitoes were compared with adult females of the same age from the N’gusso (An. coluzzii) and Kisumu (An. gambiae s.s.) insecticide-susceptible laboratory colonies. Total RNA was extracted from four replicate batches of 10 mosquitoes from each group (N’gusso, Kisumu, hospital, and industrial) by using a PicoPure RNA isolation kit (Thermo Fisher Scientific) and treated with DNase using the Qiagen RNase-free DNase kit according to the manufacturer’s instructions. RNA quantity and quality were evaluated with a NanoDrop spectrophotometer (Thermo Fisher Scientific) and a model 2100 Bioanalyzer (Agilent Technologies). RNA pools from each group were amplified and labeled by using the Low Input Quick Amp Labeling Kit (Agilent Technologies). The Agilent Agam15k array (40) was used for dual-color hybridizations (hospital vs. N’gusso, hospital vs. Kisumu, industrial vs. N’gusso, and industrial vs. Kisumu). Each mosquito population comparison was repeated four times.
Agilent Feature Extraction Software v12 and GeneSpring v13 were used for the analysis of the microarray data. Significance was determined by a one-sample t test (against the null hypothesis, which was a ratio of field/colony sample expression of 1) with the P value threshold set at P < 0.05 following correction for multiple testing (Benjamini–Hochberg method). A fold change (FC) criterion of FC > 2 or FC < −2 (for underexpressed probes) was used.
RNA was reverse-transcribed by using SuperScript III (Invitrogen) and oligo(dT)20 primers to produce cDNA. Expression of candidate genes from the microarray analysis was validated by qPCR (primers listed in Table S2) and normalized using the Ribosomal S7 (AGAP010592) and Elongation Factor (AGAP005128) reference genes (10). Mosquito samples from 2011, before the reintroduction of deltamethrin in 2012, were included in this analysis to allow direct comparisons between temporally separated field-caught samples. Experiments were performed by using four biological replicates from each treatment group and two technical replicates for each reaction. Relative expression analysis was performed according to ref. 41.
Functional Expression of CYP9K1.
cDNA from the adult hospital population was used as a template to amplify the full-length Cyp9K1 by using a proof reading polymerase (Kappa Taq DNA polymerase, long range) and primers listed in Table S2. The hospital strain was used because it had the highest levels of CYP activity. Conditions of amplification were 94 °C for 2 min, followed by 30 cycles of denaturation at 94 °C for 25 s, annealing at 54 °C for 15 s, and extension at 72 °C for 2 min, followed by a final extension step for 5 min. Primers used for the amplification introduced a EcoRI restriction site before the start ATG codon and an XbaI site after the stop codon. The purified PCR product (NucleoSpin PCR and gel extraction purification; Macherey–Nagel) was ligated into the pCWompA2 expression plasmid (16) and sequenced. For protein expression, the strategy described in ref. 42 was followed by using E. coli BL21STAR cells cotransformed with the pCWompA2-Cyp9K1 P450 and the pACYC-AgCPR (expression plasmid for the An. gambiae CPR). Membrane preparations were tested for total protein concentration (43), P450 concentration (44), and CPR activity (45).
Cytochrome P450 Activity and Insecticide Metabolism Measurements.
Activity of recombinant CYP9K1 against the fluorogenic P450 substrate 7-ethoxycoumarin and eight different luciferin-conjugated substrates (P450 Glo proluciferin substrates Luciferin-H, Luciferin-ME, Luciferin-CEE, Luciferin-H EGE, Luciferin-PFBE, Luciferin-PPXE, Luciferin-ME EGE, and Luciferin-IPA; Promega) was measured (42).
Metabolism of deltamethrin, bendiocarb, and the insect growth regulator pyriproxyfen by CYP9K1 was measured. Deltamethrin (10 µM Fluka; Pestanal), bendiocarb (10 µM Fluka; Pestanal), and pyriproxyfen (50 µM Fluka; Pestanal) were incubated with 25 pmol recombinant CYP9K1 and 200 pmol b5 (AgCytb5; GB AY183376) in 100 µL Tris⋅HCl buffer (0.2 M, pH 7.4), containing 0.25 mM MgCl2. The incubation was performed in the presence and absence of an NADPH generating system: 1 mM glucose-6-phosphate (Sigma-Aldrich), 0.1 mM NADP+ (Sigma-Aldrich), and 1 U/mL glucose-6-phosphate dehydrogenase (G6PDH; Sigma-Aldrich). Reactions were incubated at 30 °C, agitated at 9,600 × g oscillation, and stopped at different time points by using 100 µL acetonitrile. The quenched reactions were centrifuged at 9,600 × g (MicroCL 17R centrifuge; Thermo Scientific) for 10 min, and 100 µL of the supernatant was assessed by HPLC by using a UniverSil HS C18 (250 mm 5 µm) reverse-phase analytical column (Fortis). Reactions with deltamethrin were run with an isocratic mobile phase of 10% H2O and 90% acetonitrile with a flow rate of 1 mL/min for 20 min. Deltamethrin elution was monitored at 225-nm absorbance at a retention time of 9.8 min. Reactions with bendiocarb were run with an isocratic mobile phase of 65% H2O and 35% acetonitrile with a flow rate of 1 mL/min for 20 min. Bendiocarb elution was monitored at 205-nm absorbance at a retention time of 17.2 min. Reactions with pyriproxyfen were run with an isocratic mobile phase of 30% H2O and 70% acetonitrile with a flow rate of 1.5 mL/min for 25 min. Pyriproxyfen elution was monitored at 232-nm absorbance and at a retention time of 18.1 min. For kinetics of deltamethrin, varying concentrations of substrate were used (0.5–150 μM). Rates of deltamethrin turnover were plotted vs. deltamethrin substrate concentrations, and the kinetic parameters Km and Vmax were determined by using SigmaPlot 12.0 (Systat Software) by fitting to the Michaelis–Menten equation.
Supplementary Material
Acknowledgments
We thank Patricia Pignatelli (Liverpool School of Tropical Medicine) for her help with the microarray work and Dr. Mark Paine (Liverpool School of Tropical Medicine) who provided the pCWompA2 expression plasmid. This work was supported by the donors to the BIMCP, Marathon Oil Corporation, Noble Energy, AMPCO, SonaGas, GEPetrol, and the Government of Equatorial Guinea (Ultimate Funder, Medical Care Development International Grants RBPS108052 and RBPS107175).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719663115/-/DCSupplemental.
References
- 1.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Hemingway J. Malaria: Fifteen years of interventions. Nature. 2015;526:198–199. doi: 10.1038/526198a. [DOI] [PubMed] [Google Scholar]
- 4.Balabanidou V, et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc Natl Acad Sci USA. 2016;113:9268–9273. doi: 10.1073/pnas.1608295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranson H, et al. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Torres D, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones CM, et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci USA. 2012;109:6614–6619. doi: 10.1073/pnas.1201475109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, et al. A mutation in the intracellular loop III/IV of mosquito sodium channel synergizes the effect of mutations in helix IIS6 on pyrethroid resistance. Mol Pharmacol. 2015;87:421–429. doi: 10.1124/mol.114.094730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edi CV, et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10:e1004236. doi: 10.1371/journal.pgen.1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:146. doi: 10.1186/s12864-015-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toé KH, et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis. 2014;20:1691–1696. doi: 10.3201/eid2010.140619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–559. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly MJ, Isaacs AT, Weetman D. Identification, validation, and application of molecular diagnostics for insecticide resistance in malaria vectors. Trends Parasitol. 2016;32:197–206. doi: 10.1016/j.pt.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller P, et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008;4:e1000286. doi: 10.1371/journal.pgen.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson BJ, et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem Mol Biol. 2011;41:492–502. doi: 10.1016/j.ibmb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin LA, et al. Characterization of inhibitors and substrates of Anopheles gambiae CYP6Z2. Insect Mol Biol. 2008;17:125–135. doi: 10.1111/j.1365-2583.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 17.Ingham VA, et al. Dissecting the organ specificity of insecticide resistance candidate genes in Anopheles gambiae: Known and novel candidate genes. BMC Genomics. 2014;15:1018. doi: 10.1186/1471-2164-15-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller P, et al. Pyrethroid tolerance is associated with elevated expression of antioxidants and agricultural practice in Anopheles arabiensis sampled from an area of cotton fields in Northern Cameroon. Mol Ecol. 2008;17:1145–1155. doi: 10.1111/j.1365-294X.2007.03617.x. [DOI] [PubMed] [Google Scholar]
- 19.Yahouédo GA, et al. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large scale implementation of vector control interventions. Parasit Vectors. 2016;9:385. doi: 10.1186/s13071-016-1661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Main BJ, et al. Complex genome evolution in Anopheles coluzzii associated with increased insecticide usage in Mali. Mol Ecol. 2015;24:5145–5157. doi: 10.1111/mec.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overgaard HJ, et al. Malaria transmission after five years of vector control on Bioko Island, Equatorial Guinea. Parasit Vectors. 2012;5:253. doi: 10.1186/1756-3305-5-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.della Torre A, et al. Speciation within Anopheles gambiae–The glass is half full. Science. 2002;298:115–117. doi: 10.1126/science.1078170. [DOI] [PubMed] [Google Scholar]
- 23.Hemingway J, et al. Country-level operational implementation of the Global Plan for Insecticide Resistance Management. Proc Natl Acad Sci USA. 2013;110:9397–9402. doi: 10.1073/pnas.1307656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauen R, et al. Development of a lateral flow test to detect metabolic resistance in Bemisia tabaci mediated by CYP6CM1, a cytochrome P450 with broad spectrum catalytic efficiency. Pestic Biochem Physiol. 2015;121:3–11. doi: 10.1016/j.pestbp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Daborn PJ, et al. A single p450 allele associated with insecticide resistance in Drosophila. Science. 2002;297:2253–2256. doi: 10.1126/science.1074170. [DOI] [PubMed] [Google Scholar]
- 26.Karunker I, et al. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae) Insect Biochem Mol Biol. 2008;38:634–644. doi: 10.1016/j.ibmb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Reimer LJ, et al. An unusual distribution of the kdr gene among populations of Anopheles gambiae on the island of Bioko, Equatorial Guinea. Insect Mol Biol. 2005;14:683–688. doi: 10.1111/j.1365-2583.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 28.David JP, Ismail HM, Chandor-Proust A, Paine MJ. Role of cytochrome P450s in insecticide resistance: Impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120429. doi: 10.1098/rstb.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandor-Proust A, et al. The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling. Biochem J. 2013;455:75–85. doi: 10.1042/BJ20130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yunta C, et al. Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance in An. gambiae. Insect Biochem Mol Biol. 2016;78:50–57. doi: 10.1016/j.ibmb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardstone MC, et al. Cytochrome P450 monooxygenase-mediated permethrin resistance confers limited and larval specific cross-resistance in the southern house mosquito, Culex pipiens quinquefasciatus (vol 89, pg 175, 2007) Pestic Biochem Physiol. 2008;91:191. [Google Scholar]
- 32.Fossog Tene B, et al. Resistance to DDT in an urban setting: Common mechanisms implicated in both M and S forms of Anopheles gambiae in the city of Yaoundé Cameroon. PLoS One. 2013;8:e61408. doi: 10.1371/journal.pone.0061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulamba C, et al. Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: A potential challenge for malaria vector control in Uganda. Parasit Vectors. 2014;7:71. doi: 10.1186/1756-3305-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxborough RM, et al. Long-lasting control of Anopheles arabiensis by a single spray application of micro-encapsulated pirimiphos-methyl (Actellic® 300 CS) Malar J. 2014;13:37. doi: 10.1186/1475-2875-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley J, et al. Infection importation: A key challenge to malaria elimination on Bioko Island, Equatorial Guinea. Malar J. 2015;14:46. doi: 10.1186/s12936-015-0579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization . Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. WHO; Geneva: 2013. [Google Scholar]
- 37.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 38.Santolamazza F, et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bass C, et al. The Vector Population Monitoring Tool (VPMT): High-throughput DNA-based diagnostics for the monitoring of mosquito vector populations. Malar Res Treat. 2010;2010:190434. doi: 10.4061/2010/190434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell SN, et al. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc Natl Acad Sci USA. 2012;109:6147–6152. doi: 10.1073/pnas.1203452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riga M, et al. Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochem Mol Biol. 2014;46:43–53. doi: 10.1016/j.ibmb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 44.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J Biol Chem. 1964;239:2379–2385. [PubMed] [Google Scholar]
- 45.Strobel HW, Dignam JD. Purification and properties of NADPH-cytochrome P-450 reductase. Methods Enzymol. 1978;52:89–96. doi: 10.1016/s0076-6879(78)52009-0. [DOI] [PubMed] [Google Scholar]