Significance
The study offers a pharmacological solution to the challenging target of inducing stable chondrogenesis by human mesenchymal stromal cells (hMSCs), including protection against vascularization. Cells were reversed from the tendency to follow the default differentiation pathway, namely endochondral ossification and osteogenesis. Our findings open perspectives in articular cartilage regeneration, as well as in the establishment of hMSC-based models of cartilage development, physiology, and possibly pathology. Importantly, the results were achieved by mimicking molecular processes occurring during embryonic cartilage formation. This indicates that adult hMSCs share similarities with embryonic mesenchyme and validates the relevance to engineer developmental processes (“developmental engineering”) to control fate specification by adult stem/progenitor cell systems.
Keywords: mesenchymal stromal cells, articular cartilage, developmental engineering, microfluidics, BMPs
Abstract
It is generally accepted that adult human bone marrow-derived mesenchymal stromal cells (hMSCs) are default committed toward osteogenesis. Even when induced to chondrogenesis, hMSCs typically form hypertrophic cartilage that undergoes endochondral ossification. Because embryonic mesenchyme is obviously competent to generate phenotypically stable cartilage, it is questioned whether there is a correspondence between mesenchymal progenitor compartments during development and in adulthood. Here we tested whether forcing specific early events of articular cartilage development can program hMSC fate toward stable chondrogenesis. Inspired by recent findings that spatial restriction of bone morphogenetic protein (BMP) signaling guides embryonic progenitors toward articular cartilage formation, we hypothesized that selective inhibition of BMP drives the phenotypic stability of hMSC-derived chondrocytes. Two BMP type I receptor-biased kinase inhibitors were screened in a microfluidic platform for their time- and dose-dependent effect on hMSC chondrogenesis. The different receptor selectivity profile of tested compounds allowed demonstration that transient blockade of both ALK2 and ALK3 receptors, while permissive to hMSC cartilage formation, is necessary and sufficient to maintain a stable chondrocyte phenotype. Remarkably, even upon compound removal, hMSCs were no longer competent to undergo hypertrophy in vitro and endochondral ossification in vivo, indicating the onset of a constitutive change. Our findings demonstrate that adult hMSCs effectively share properties of embryonic mesenchyme in the formation of transient but also of stable cartilage. This opens potential pharmacological strategies to articular cartilage regeneration and more broadly indicates the relevance of developmentally inspired protocols to control the fate of adult progenitor cell systems.
Recapitulation of embryonic developmental events is receiving increasing recognition as a strategy to induce robust tissue regeneration. The paradigm of “developmental engineering” (1) indeed postulates the importance of instructing developmental processes rather than engineering replacing grafts to achieve functional tissue regeneration (2). However, it is still uncertain whether and to what extent specific adult human stem/progenitor cells conserve the capacity to follow developmental pathways. We and others previously demonstrated that the fate of human mesenchymal stromal cells (hMSCs) can be sequentially guided toward the recapitulation of the embryonic endochondral ossification program, as well as in the molecular regulation of associated processes, eventually leading to bone formation in vivo (3–7). However, the design of a reliable strategy to generate stable cartilage by adult hMSCs, as occurring in articulating surfaces, still remains an open challenge. Adult MSCs have indeed been proposed to be intrinsically committed toward terminal hypertrophic differentiation upon induction of chondrogenesis. As a consequence, hMSC-derived cartilaginous templates typically undergo remodeling into bone ossicles when transplanted in vivo (8, 9). “Re-programming” hMSCs fate by exposure to specific signals has been postulated as a potential strategy to reverse this tendency and maintain the stability of cartilage formed by hMSCs (10). In this direction, signaling factors directly mediating chondrocyte hypertrophy [i.e., parathyroid hormone-related protein (PTHrP)/Ihh regulatory loop] have been extensively considered (11–13). However, upon removal of the signal, cells typically proceed toward hypertrophy in vitro and to endochondral ossification in vivo. This suggests that the regulation of downstream signals in the hypertrophy route is not sufficient to modify the intrinsic commitment of hMSCs.
The concept of developmental engineering inspired us to operate at an earlier stage, assessing signaling pathways directly involved in embryonic articular cartilage development as potential candidates for controlling hMSC fate decision toward stable chondrogenesis. During embryonic joint development, chondroprogenitor cells lying in the interzone have been shown to contribute to either articular or transient cartilage formation (14) based on a tight regulation of bone morphogenetic protein (BMP) signaling (15). The precise spatial organization of the limb mesenchyme indeed results in a band of Noggin-expressing cells, which insulates proliferating chondrocytes in the distal part of the interzone from BMP signaling and specifies their differentiation toward articular cartilage (14). Indeed, the BMP antagonist Gremlin-1 was found among the most up-regulated genes in human adult articular compared with growth-plate cartilage, suggesting the role of BMP restriction in determining chondroprogenitor commitment (16). On the other hand, BMP signaling is involved in many phases of limb development (17, 18), being a key chondrogenic factor (19) but also a trigger for endochondral ossification (20). Recently, in vitro inhibition of the BMP pathway was exploited to force pluripotent stem cells, either embryonic (21) or induced (22), toward articular-like cartilage in vitro, although with only preliminary evidence of the in vivo phenotype stability.
In this work, we aim to demonstrate that the fate of adult hMSCs can be guided toward stable cartilage by forcing environmental conditions recapitulating specific events of articular cartilage development. We hypothesize that selective inhibition of BMP signaling during in vitro chondrogenic differentiation of adult hMSC-derived templates is sufficient to prime them toward a stable articular cartilage phenotype. We tested our hypothesis by using two BMP type I receptor-biased kinase inhibitors, differing in their kinase selectivity profile. To screen different doses and temporal stages of supplementation at a high throughput, we introduced a miniaturized microfluidic model, recently developed to allow condensation and subsequent perfusion of 3D microaggregates in each well, crucial to enabling chondrogenesis (23). We then demonstrated the long-term efficacy and the robustness of the selected developmentally inspired protocol both in vitro and in vivo using macroscale tissue models.
Results
BMP Type I Receptor Kinase Inhibitors with Different Selectivity Profiles.
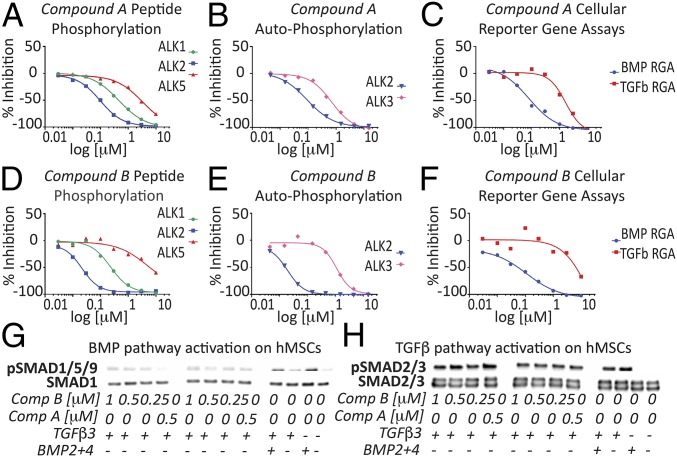
Compounds A and B are BMP type I receptor kinase inhibitors with different selectivity profiles. Their activity in biochemical kinase assays and cellular reporter gene assays for BMP and TGF-β signaling are listed in Table S1. Both compounds are biased toward ALK2 inhibition. For compound A, the selectivity for ALK2 over ALK1 is only 5-fold (Fig. 1A) and for ALK2 over ALK3 only 4.1-fold (Fig. 1B). With such small selectivity windows, efficacious compound concentrations are expected to always inhibit, at least partially, ALK1 and ALK3 in addition to ALK2. Compound B is a more potent and selective inhibitor of ALK2. Its selectivity over ALK1 is 17.2-fold (Fig. 1D) and 75.3-fold over ALK3 (Fig. 1E). Therefore, at compound concentrations that are expected to completely inhibit ALK2 (250 nM), the ALK3 receptor kinase is not inhibited (Fig. 1E).
Fig. 1.
Representative IC50 curves of both compounds for all assays. (A and D) Biochemical peptide phosphorylation assay panel. (B and E) Biochemical autophosphorylation assays. (C and F) BMP (on HuH7 cells) and TGF-β (on HEK293 cells) reporter gene assays (RGA). (G) BMP and (H) TGF-β pathway activation in adult hMSCs assessed in response to inhibitor treatment by Western blot analysis of SMAD1/5/9 and SMAD2/3 phosphorylation, respectively.
Whereas compounds A and B vary in their ability to inhibit BMP type I receptors, both compounds only poorly inhibit the TGF-β type I receptor ALK5 in biochemical kinase assays (Fig. 1 A and D and Table S1). Their activity in biochemical assays is confirmed in cellular reporter gene assays for BMP and TGF-β signaling. Both compounds potently inhibit BMP6-induced signaling in hepatocellular carcinoma cell line (HuH7) cells. Importantly, at a concentration of 500 nM, almost completely inhibiting BMP signaling, TGF-β–induced signaling in HEK293 cells is not yet compromised. Only at much higher concentrations is TGF-β signaling also inhibited in vitro, confirming the compound’s preference for BMP type I receptor inhibition (Fig. 1 C and F). The specificity in inhibiting BMP signaling was confirmed also in hMSCs (Fig. 1 G and H), whereby both compounds decreased SMAD1/5/9 phosphorylation compared with controls. Compound A exhibited the highest capacity to down-regulate BMP signaling compared with similar doses of compound B. SMAD2/3 phosphorylation was conversely not affected by the selected doses.
To understand the compound’s mechanism of action on hMSCs, the expression of ALK1, ALK2, and ALK3 receptors was evaluated. Gene-expression analysis revealed the absence of ALK1 in hMSCs, excluding binding to ALK1 as a mechanism involved in this model (Fig. S1A). ALK2 and ALK3 were instead expressed by postnatal day (P0) hMSCs, but not modulated during 14 d of treatment in 3D pellet culture (Fig. S1 B–L).
Microfluidic-Based Screening Suggested a Dose- and Timing-Dependent Effect of ALK Inhibitors on Adult hMSCs Commitment.
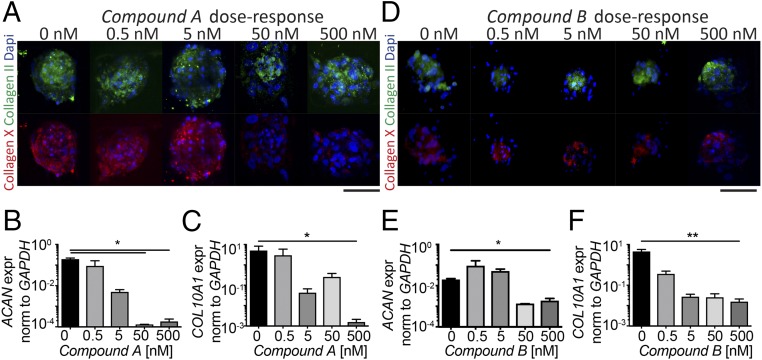
The dose-dependent effect of the BMP signaling inhibitors on adult hMSC commitment was first assessed at the microscale, by exploiting a previously developed microfluidic platform (Fig. S2A) (23). After 14 d, collagen type II deposition was not affected by any treatment at any concentration (Fig. 2 A and D). Gene-expression analysis indicates a dose-dependent reduction of ACAN, suggesting that higher doses of both compounds may delay or reduce deposition of cartilaginous matrix (Fig. 2 B and E). A concentration-dependent decrease in collagen type X deposition was detected, with increasing compound concentrations, both at the protein (Fig. 2 A and D) and mRNA (Fig. 2 C and F) levels. This effect was more pronounced upon treatment with compound A, which resulted in complete loss of staining for collagen type X at the highest concentration, correlating with a significant decrease (P < 0.05) in COL10A1 expression compared with the control. Results obtained at the microscale level were also confirmed in 3D macropellet culture (Fig. S3 A–D). Different temporal windows for compound A supplementation were then considered. A continuous treatment for 14 d in the presence of TGF-β3 resulted in the most pronounced reduction of hypertrophic matrix deposition (Fig. S2B), correlating with a consistent low expression of Msx2, a transcriptional factor downstream of the BMP pathway (Fig. S2C). Moreover, to investigate the specificity of the BMP type I receptor LMW kinase inhibitors, 3D pellets were treated with 200 ng/mL of human recombinant Noggin protein, a BMP antagonist. Such conditioning led to the generation of a cartilage matrix rich in collagen type X, thus suggesting that a generic BMP antagonist is not sufficient to avoid a commitment of hMSCs toward hypertrophy (Fig. S4). Thus, a continuous treatment with 500 nM of compound A, which targets both ALK2 and ALK3, was sufficient to maintain hMSCs in a stable cartilage phenotype in a short-term in vitro culture.
Fig. 2.
A microfluidic platform (23) for culturing hMSCs 3D micromasses was used to screen the concentration-dependent effect of (A–C) compound A and (D–F) compound B. Concentrations spanning over four orders of magnitude (0, 0.5, 5, 50, 500 nM) were assessed. (A and D) Collagen type II (green) and collagen type X (red) stainings were performed directly within the platform after 14 d. All of the images were taken at the same magnification. (Scale bars, 75 μm.) (B–F) Quantitative PCR was performed to assess the mRNA levels of ACAN and COL10A1, by extracting the RNA from the microfluidic platform. All ΔΔCt values are normalized relative to GAPDH expression and refer to basal expression in day 0 hMSCs; all fold-changes in transcript levels are shown in logarithmic scale (n = 3, *P < 0.05, **P < 0.005).
ALK2 and ALK3 Inhibition on hMSCs-Derived Constructs Is Sufficient to Maintain a Stable Cartilaginous Phenotype in Vitro.
We investigated how stable the effect of hypertrophy silencing was elicited by ALK2 and ALK3 inhibition in adult hMSC-derived constructs.
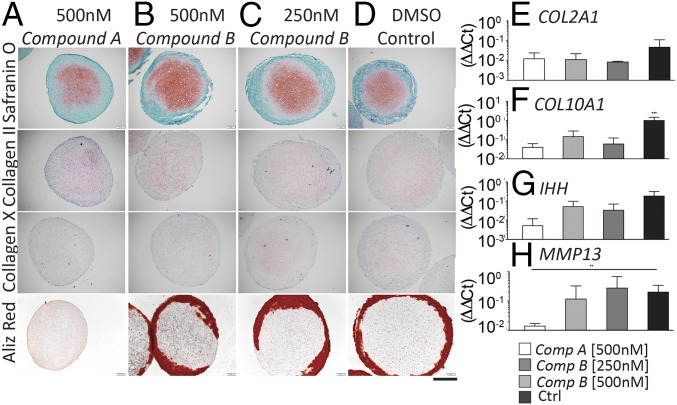
Three-dimensional hMSC-derived macropellets were incubated in standard chondrogenic medium for 28 d with either compound supplemented continuously (Fig. S3 E–H), or for the initial 14 d only, followed by incubation in standard chondrogenic medium (“recovery” phase) (Fig. S3 I–L). All constructs treated for 28 d or for 14 d + 14 d of recovery displayed a cartilaginous extracellular matrix rich in GAG and collagen type II, although chondrogenesis was less pronounced for tissues treated with compound A. Regarding early hypertrophic commitment, compound A treatment was the only condition leading to constructs negative for collagen type X after 28 d of treatment (Fig. S3E). Interestingly, no collagen type X was deposited even after removal of the compound, suggesting the stability of the established effect (Fig. S3I). To further prove the sustained effect of initial ALK2/ALK3 inhibition on favoring the generation of stable cartilage, compound A- and compound B-treated hMSC-derived macropellets were forced toward hypertrophy, following a previously established protocol (4). Hypertrophic induction after preincubation with compound A led to deposition of a cartilaginous extracellular matrix rich in GAG and collagen type II, but with negative collagen type X immunostaining (Fig. 3A). Collagen type X was instead detected in constructs treated with compound B and in the control (Fig. 3 B–D). Alizarin red staining indicated the presence of calcified areas in all conditions, but upon compound A treatment. Quantitative RT-PCR analyses indicated that compound A treatment did not change COL2A1 expression compared with compound B and control (Fig. 3E), while it significantly reduced COL10A1 and MMP13 expression compared with control (Fig. 3 F and H). IHH expression was also reduced, even if not statistically significantly (Fig. 3G). Taken together, these data indicate that inhibition of both ALK2 and ALK3 receptors (achieved by compound A treatment) is sufficient to prevent hMSCs from expressing both early and late hypertrophic markers in vitro.
Fig. 3.
(A–D) In vitro maturation and stability of 3D macropellets derived from adult hMSC cultured in chondrogenic medium, either alone or supplemented compound A or B, and forced after 14 d of treatment toward hypertrophy. Samples were histologically analyzed for GAG, collagen type II, and collagen type X deposition and Alizarin red. All images were taken at the same magnification. (Scale bar, 300 μm.) Quantitative RT-PCR analyses on (E) COL2A1, (F) COL10A, (G) IHH, and (H) MMP13 confirmed the immunohistochemistry staining. All ΔΔCt values are normalized relative to GAPDH expression and refer to basal expression in day 0 hMSCs; all fold-changes in transcript levels are shown in logarithmic scale (n = 3, *P < 0.005, ***P < 0.001). Ctrl, control.
Simultaneous Inhibition of ALK2 and ALK3 Down-Regulates the BMP Pathway and Triggers the Activation of Genes Characteristic for Articular Cartilage in Adult Human MSCs.
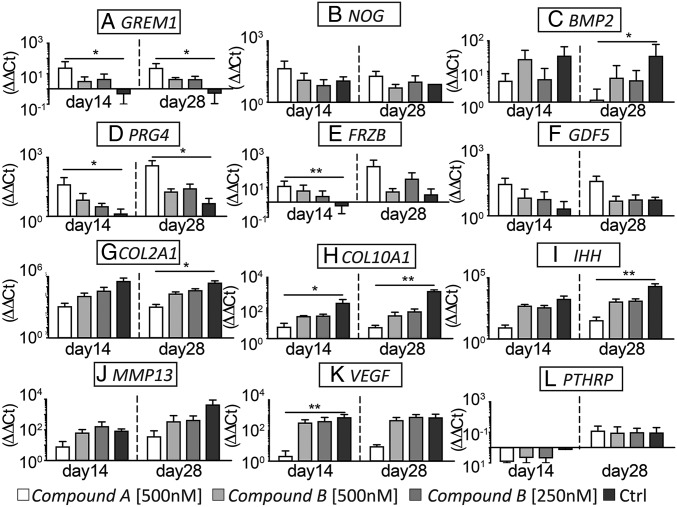
The modulation of key players of the BMP pathway was monitored upon treatment with BMP type I receptor inhibitors. Among endogenous BMP antagonists, GREM was up-regulated by both compounds, with compound A inducing the most pronounced increase. hMSCs cultured in standard chondrogenic medium instead down-regulated GREM during the whole culture period (Fig. 4A). NOG uniformly increased and no significant differences were detected among the different conditions, suggesting that Noggin is not modulated by inhibiting ALK2 and ALK3 (Fig. 4B). BMP2 was strongly up-regulated in standard chondrogenic conditions compared with treatment with compound A (Fig. 4C). This trend was confirmed by detection of secreted BMP2 protein in the culture medium using a specific ELISA, which was consistently reduced by compound A treatment (Fig. S5).
Fig. 4.
Gene-expression levels of BMP antagonists (A) GREM and (B) NOG and of (C) BMP-2. (D–G) Expression of articular cartilage related genes PRG4, FRZb, GDF5, COL2A1. (H–L) Expression of growth plate development associated genes COL10A1, IHH, MMP13, VEGF, PTHRP. All ΔΔCt values are normalized relative to GAPDH expression and refer to basal expression in day 0 hMSCs; all fold-changes in transcript levels are shown in logarithmic scale (n = 3; *P < 0.05, **P < 0.005). Ctrl, control.
To characterize the nature of the hMSC-derived cartilaginous tissues obtained by treatment with the different compounds, gene-expression changes of a panel of articular or transient cartilage signature genes were assessed by quantitative RT-PCR. Expression of Lubricin (PRG4) and Wnt antagonist Frizzled-related protein (FRZb), typical genes expressed in articular chondrocytes (16), was increased in compound A-treated cells (Fig. 4 D and E). Interestingly, FRZb expression was down-regulated in the control conditions compared with basal levels during the whole culture period. GDF5, a gene associated with joint interzone development (14), was up-regulated upon treatment with compound A (Fig. 4F). COL2A1 expression was up-regulated in all conditions compared with basal levels, with compound A-treated constructs showing the least increase of COL2A1 (Fig. 4G). Expression of genes associated with the initiation of hypertrophy and angiogenesis/vascularization—namely COL10A1, IHH, MMP13, and VEGFA—was significantly higher in hMSC-derived constructs cultured in standard chondrogenic medium, compared with compound A-treated samples (Fig. 4 H–K). PTHRP exhibited instead a variable trend (Fig. 4L), which would be challenging to interpret due to its involvement in complex feedback loops (20). Thus, inhibition of ALK2 and ALK3 contributed to the in vitro generation of hMSC-derived constructs with increased expression of genes related to articular cartilage development and function, while downregulating expression of genes associated with growth-plate development.
Preincubation with an ALK2/ALK3 Inhibitor in Vitro Favors a Stable Cartilaginous Phenotype of hMSC-Derived Constructs upon Ectopic Implantation in Vivo.
To assess whether an in vitro preincubation with BMP type I receptor inhibitors is sufficient to prevent remodeling of hMSC-derived cartilaginous constructs in vivo, compounds A and B pretreated and control samples were implanted ectopically in immunodeficient mice.
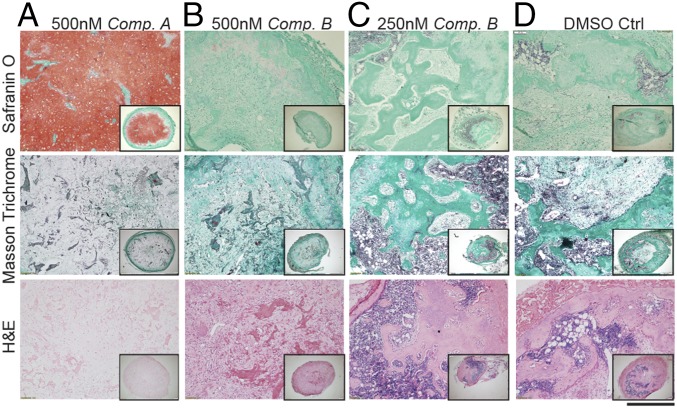
Upon retrieval (8 wk after implant), constructs displayed different morphologies. In vitro pretreatment with compound A favored the maintenance of stable cartilaginous matrices after 8 wk of implantation, characterized by high GAG content and absence of bone remodeling (Fig. 5A and Fig. S6A). Constructs treated in vitro with compound B progressed differently depending on the concentration used. The higher concentration (500 nM) resulted in a reduction of GAG accumulation over time; however, this still did not lead to complete cartilage remodeling during the observation timeframe (Fig. 5B and Fig. S6B). Constructs treated with the lower concentration (250 nM) underwent a clear remodeling, resulting after 8 wk in complete cartilage resorption and bone formation (Fig. 5C and Fig. S6C). As expected, in the control condition a complete remodeling of the tissue was observed, with the cartilage entirely replaced by bone and bone marrow after 8 wk (Fig. 5D and Fig. S6D). Taken together, these data indicate that in vitro inhibition of both ALK2 and ALK3, achieved with 500 nM of compound A, is sufficient to prevent hMSCs-derived cartilaginous constructs from remodeling in vivo. Instead, even complete inhibition of ALK2 alone with only marginal or no inhibition of ALK3, as seen with 500 and 250 nM of compound B, respectively, can only delay the remodeling process.
Fig. 5.
Development of hMSCs-derived cartilage tissues following 8 wk of ectopic implantation in vivo. Safranin O staining identifies GAG deposition; Masson Trichrome and H&E staining allow identify the presence of bone remodeling and bone marrow. (A) Compound A pretreatment was the only condition allowing the maintenance of a cartilaginous matrix still rich in GAG after 8 wk. (B–D) All of the other conditions exhibited a progression toward remodeling in bone. All images were taken at the same magnification (n = 3 donors). (Scale bar, 300 μm.)
Simultaneous ALK2 and ALK3 Inhibition Prevents hMSCs from Bone Remodeling by Activating a Protective Mechanism Against Vascularization.
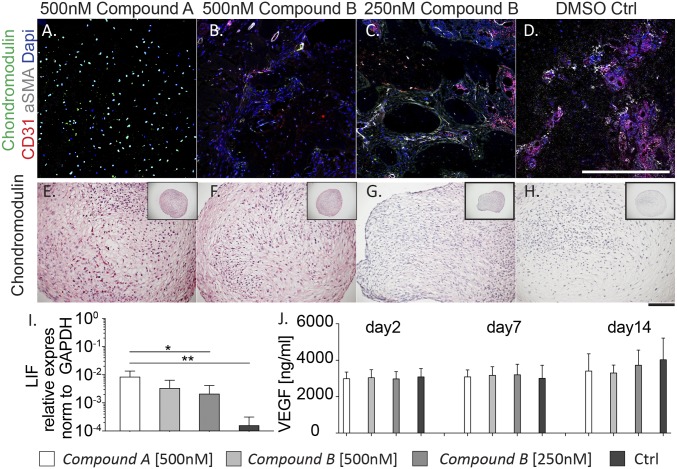
Immunofluorescence staining for vessel invasion (CD31, αSMA and DAPI) (Fig. 6 A–D) revealed different patterns of vasculature 8 wk after construct implantation. Constructs pretreated with 500-nM compound A remained avascular after 8 wk in vivo (Fig. 6A). Consistent with the observed bone remodeling (Fig. 5 C and D), both samples pretreated with ALK2-selective doses of compound B (250 nM) and control constructs resulted in efficient vascularization, exhibiting deep-vessel colonization (Fig. 6 C and D). Samples pretreated with compound B at high doses showed formation of vessels principally localized at the periphery (Fig. 6B). To further elucidate the underlying mechanism, the effect of in vitro pretreatment on regulating vascularization was assessed. Chondromodulin, an antiangiogenic factor stabilizing the chondrocyte phenotype (24), was not expressed by hMSCs cultured in standard chondrogenic medium, which committed them toward hypertrophy (Fig. 6H). In vitro pretreatment with either compound A or high doses of compound B induced Chondromodulin expression in hMSCs after 14 d (Fig. 6 E and F). Nasal chondrocytes, which were shown to readily differentiate into articular cartilage in vitro (25), were used as a positive control. In chondrogenic medium, nasal chondrocyte-derived constructs form stable cartilaginous-like matrices and high levels of Chondromodulin were confirmed (Fig. S7). Interestingly, Chondromodulin was still detectable in compound A pretreated constructs even 8 wk after implantation in vivo (Fig. 6A), while absent with all other conditions (Fig. 6 B–D). In addition, gene-expression levels of leukemia inhibitory factor (LIF), whose deficiency has been reported to enhance vascular endothelial growth factor (VEGF) levels in developing neonatal bone (26), were significantly higher in compound A treated constructs compared with both compound B treatments and control (Fig. 6I). VEGF amounts secreted by hMSCs were unaltered by any of the culture conditions, suggesting that ALK2 or ALK3 inhibition does not prevent the expression of proangiogenic factors (Fig. 6J). Taken together, these data suggest the initiation of a protective mechanism against vascularization of the cartilaginous constructs upon inhibition of ALK2 and ALK3 during the early differentiation events.
Fig. 6.
Effect of treatments with compounds A and B on vascularization in vitro and in vivo. (A–D) After 8 wk in vivo, construct vascularization was assessed by immunofluorescence staining for CD31 (red), αSMA (gray), and Chondromodulin (green). (Scale bar, 300 μm.) (E–H) Chondromodulin staining after 14 d of pretreatment in vitro. All images taken at the same magnification. (Scale bar, 300 μm.) (I) Quantitative RT-PCR was performed to assess the mRNA levels of LIF in all conditions. All ΔΔCt values are normalized relative to GAPDH expression and refer to basal expression in day 0 hMSCs; all fold-changes in transcript levels are shown in logarithmic scale (n = 3, *P < 0.05, **P < 0.005). (J) VEGF secreted by hMSCs was measured by ELISA at day 2, day 7 and day 14 of culture. Ctrl, control.
Discussion
Following the concept of developmental engineering (1), we investigated in this study the role of BMP signaling on the differentiation of adult mesenchymal progenitor cells, namely hMSCs, toward articular cartilage. The combination of (i) a microfluidic system for time- and dose-dependent screening of soluble factors on 3D microaggregates and of (ii) synthetic compounds selectively and specifically silencing the BMP pathway by targeting ALK2 and ALK3 receptors allowed us to successfully design a strategy effective in guiding hMSCs toward stable articular-like cartilage, both in vitro and in vivo. We also postulate the role of ALK2 and ALK3 inhibition in triggering a protective mechanism against vascularization, eventually favoring the maintenance of stable and avascular cartilage tissue.
Recently, the achievement of an organized cartilage template based on hMSCs was reported by recreating in vitro the native articular cartilage spatial organization. However, the only partial inhibition of hypertrophic markers achieved in this model suggests that key players are still missing to drive hMSCs toward stable cartilage (27). In this direction, few studies have recently focused on manipulating key signaling factors involved in growth-plate development. Exposure to Wnt3a (either alone or in combination with FGF2) was indeed shown to enhance MSCs undifferentiated proliferation, while priming cells toward a more efficient chondrogenic differentiation (28, 29). While providing promising results in forming stable cartilage, the expression of hypertrophic markers could not be completely inhibited by following these approaches. Sequential exposure to FGF-2, -9, or -18 was also reported as a strategy to delay, but not to avoid hypertrophic differentiation of hMSCs (30). PTHrP/Ihh regulatory loop inhibition was also proposed. However, both continuous (11) and intermittent (12, 13) supplementation of PTHrP was not sufficient to diminish exogenously induced enhancement of hypertrophy in hMSC and finally resulted in unstable in vivo cartilage formation. To overcome these issues, instead of working on downstream signals of the hypertrophy route, our approach focused on earlier stages considering signaling pathways directly involved in articular cartilage fate specification during embryonic development (14, 17). Recent evidence demonstrated the requirement of a BMP signaling restriction during mouse articular cartilage development, achieved through up-regulation of BMP inhibitors in specific spatial location (14, 15). Moreover, BMP inhibition was exploited together with manipulation of other signaling pathways to guide embryonic and induced pluripotent stem cells differentiation through mesoderm intermediates to a final chondrocyte population (21, 22). In this study, we translate this concept to an adult human population of mesenchymal progenitor cells, being in an advanced stage of commitment and typically considered intrinsically programmed on a default pathway to osteogenesis.
By using two ALK2-biased BMP type I receptor kinase inhibitors, we were able to selectively silence the BMP signaling pathway on 3D hMSC-based cultures, while minimizing unwanted off-target effects (i.e., inhibition of the TGF-β pathway). The exploitation of such synthetic low molecular-weight compounds gave us a more reproducible effect compared with the supplementation of a native BMP antagonist (e.g., Noggin), which might trigger negative feedback loops (17, 31), in turn making the system more complex and less predictable. Moreover, a microfluidic platform for generating, culturing and conditioning 3D hMSC-derived micromasses (32) allowed us to design an efficient and fine-tuned BMP inhibition strategy. Specifically, the most efficacious doses and timing of inhibitors were preselected based on their capacity to down-regulate hypertrophic markers, while maintaining high levels of cartilage-specific markers, in hMSC-derived micromass cultures. Notably, the microscale model was highly predictive of results subsequently obtained at the macroscale and in vivo. This confirms the potential of such microfluidic platforms as a powerful tool to initially set up differentiation protocols in a more efficient and medium-throughput fashion.
The different selectivity profiles of the two tested compounds allowed to unravel the contribution of ALK2- and ALK3-mediated signaling toward guiding hMSC commitment. Recent findings demonstrated how a synergistic inhibition of ALK2 and ALK3 is essential for reducing heterotopic ossification, a progressive formation of ectopic bone in soft tissue (33). In accordance, we demonstrated that inhibition of only ALK2 by using selective doses of compound B is not sufficient to sustainably silence the hypertrophic phenotype in hMSC-derived 3D constructs. Conversely, dual inhibition of ALK2 and ALK3, achieved by either nanomolar doses of compound A or by micromolar doses of compound B (Fig. S8) reduced the expression of hypertrophic markers and contributed to the maintenance of an articular-like cartilage phenotype of hMSC-derived constructs. Interestingly, a pretreatment for 14 d in vitro was sufficient for stabilizing this antihypertrophic effect, in accordance with previous reports (33). This suggests the possible induction of permanent modifications triggered within hMSCs by inhibition of ALK2 and ALK3, warranting further investigations to elucidate the responsible mechanism.
Simultaneous inhibition of ALK2 and ALK3 by compound A clearly resulted in a reduced expression of genes associated with endochondral ossification. The result could lead to the interpretation that such treatment induces a delay in chondrogenesis, rather than a specific reprogramming toward stable cartilage. However, the former possibility is not consistent with the findings that compound A resulted in (i) significantly higher expression of genes characterizing articular cartilage (e.g., Grem1, PRG4, and FRZb) (Fig. 4), and most importantly, (ii) long-term in vivo stability of the generated cartilage matrix (Fig. 5). Thus, we may conclude that cartilaginous-like constructs obtained through continuous dual inhibition of ALK2 and ALK3 in vitro contained all of the “biological instructions” necessary to prevent entering the endochondral ossification pathway. We also considered that the process of hypertrophic cartilage remodeling into bone in an ectopic in vivo environment is typically associated with invasion by new vessels. Chondromodulin is a protein expressed in the avascular zone of prehypertrophic cartilage and its expression decreases during chondrocyte hypertrophy and vascular invasion (24). Interestingly, in vitro hMSCs expressed Chondromodulin only upon a dual inhibition of ALK2 and ALK3, and maintained high-expression levels even after implantation in vivo. A previous study demonstrated the importance of silencing vascularization for generating stable cartilage templates, by genetically modifying hMSCs to continuously produce soluble VEGF receptor-2 (sFlk1) (34). We here demonstrated that an in vitro pretreatment targeting ALK2 and ALK3 is sufficient to trigger a protective mechanism against vascularization within hMSC-derived constructs, which eventually concurs in stabilizing their cartilaginous phenotype in vivo.
The implantation of hMSC-derived constructs primed toward articular cartilage in an orthotopic in vivo model will demonstrate if this system can potentially be used as a cartilage regeneration strategy. In perspective, our study may open the possibility to use hMSC-derived and primed tissues as grafts for cartilage tissue engineering. Similarly, hydrogel-based strategies could be envisaged, in which the BMP type I receptor kinase inhibitors are delivered directly at the site of the injury, potentially in combination with mobilization of progenitor cells from subchondral bone, which typically include MSC populations.
In conclusion, we demonstrate that restriction of BMP signaling can program adult hMSC to stable chondrogenesis, including protection from vessels invasion, and thus revert the postulated default commitment to osteogenesis. The strategy, which is inspired by recapitulation of critical aspects of normal articular cartilage formation during embryonic development, can be exploited to design pharmacological cartilage regeneration approaches, as well as to generate adult hMSC-based models of cartilage development, physiology, and possibly pathology. As a broader perspective, our achievements indicate that adult hMSCs share regulatory processes of embryonic mesenchyme, and strengthen the validity of developmental engineering as a paradigm to control the fate of adult progenitor cell systems.
Materials and Methods
All human samples were collected with informed consent of involved individuals, and mouse experiments were performed in accordance with Swiss law. All studies were approved by the ethics board of the canton Basel, Switzerland and by the Swiss Federal Veterinary Office. hMSCs were expanded for two passages (average of 13–15 doubling), to minimize the loss of chondrogenic potential according to previously described protocols (35). BMP type I receptor kinase inhibitors with different selectivity profiles (compounds A and B) were obtained from Novartis Pharma AG, Basel and they may be provided upon request under a material transfer agreement specifying nondisclosure conditions, and restrictions regarding the use of the materials and the results obtained therewith. The dose- and timing-dependent effects of inhibitors were tested by culturing hMSC-derived micromasses for 14 d in a previously developed microfluidic platform (23). Micromasses were analyzed by immunofluorescence directly within the platform and RNA was extracted for quantitative RT-PCR analyses. Cells were then either cultured in a 3D scaffold-free macroscale pellet model (0.25 × 106 cells per pellet) or seeded onto type I collagen meshes at a density of 7 × 106 cells/cm3 and cultured for up to 4 wk in serum-free chondrogenic medium, supplemented or not with compound A or B. After 2 wk of pretreatment in vitro, samples were implanted in the subcutaneous pouches of nude mice (four samples per mouse) and retrieved after 8 wk postimplantation. The generated tissues were analyzed histologically, immunohistochemically, and by quantitative RT-PCR; specific ELISAs were used to quantified the amounts of secreted proteins. A more complete and detailed description of the methods is included in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Schären and M. Haug for the kind provision of bone marrow aspirates and nasal cartilage biopsies. This work was partially funded by the Swiss National Science Foundation Grant NBM 1579 (to I.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720658115/-/DCSupplemental.
References
- 1.Lenas P, Moos M, Jr, Luyten FP. Developmental engineering: A new paradigm for the design and manufacturing of cell-based products. Part I: From three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev. 2009;15:381–394. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- 2.Tonnarelli B, Centola M, Barbero A, Zeller R, Martin I. Re-engineering development to instruct tissue regeneration. Curr Top Dev Biol. 2014;108:319–338. doi: 10.1016/B978-0-12-391498-9.00005-X. [DOI] [PubMed] [Google Scholar]
- 3.Scotti C, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scotti C, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheehy EJ, Vinardell T, Buckley CT, Kelly DJ. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater. 2013;9:5484–5492. doi: 10.1016/j.actbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Farrell E, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman FE, Haugh MG, McNamara LM. Investigation of the optimal timing for chondrogenic priming of MSCs to enhance osteogenic differentiation in vitro as a bone tissue engineering strategy. J Tissue Eng Regen Med. 2016;10:E250–E262. doi: 10.1002/term.1793. [DOI] [PubMed] [Google Scholar]
- 8.Pelttari K, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 9.Gaut C, Sugaya K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen Med. 2015;10:665–679. doi: 10.2217/rme.15.31. [DOI] [PubMed] [Google Scholar]
- 10.Occhetta P, Stüdle C, Barbero A, Martin I. Learn, simplify and implement: Developmental re-engineering strategies for cartilage repai. Swiss Med Wkly. 2016;146:w14346. doi: 10.4414/smw.2016.14346. [DOI] [PubMed] [Google Scholar]
- 11.Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer J, Aulmann A, Dexheimer V, Grossner T, Richter W. Intermittent PTHrP(1-34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem Cells Dev. 2014;23:2513–2523. doi: 10.1089/scd.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 14.Ray A, Singh PNP, Sohaskey ML, Harland RM, Bandyopadhyay A. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development. 2015;142:1169–1179. doi: 10.1242/dev.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 16.Leijten JCH, et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302–3312. doi: 10.1002/art.34535. [DOI] [PubMed] [Google Scholar]
- 17.Pignatti E, Zeller R, Zuniga A. To BMP or not to BMP during vertebrate limb bud development. Semin Cell Dev Biol. 2014;32:119–127. doi: 10.1016/j.semcdb.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Bénazet J-D, et al. Smad4 is required to induce digit ray primordia and to initiate the aggregation and differentiation of chondrogenic progenitors in mouse limb buds. Development. 2012;139:4250–4260. doi: 10.1242/dev.084822. [DOI] [PubMed] [Google Scholar]
- 19.Karamboulas K, Dranse HJ, Underhill TM. Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFbeta signals. J Cell Sci. 2010;123:2068–2076. doi: 10.1242/jcs.062901. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 21.Craft AM, et al. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development. 2013;140:2597–2610. doi: 10.1242/dev.087890. [DOI] [PubMed] [Google Scholar]
- 22.Craft AM, et al. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33:638–645. doi: 10.1038/nbt.3210. [DOI] [PubMed] [Google Scholar]
- 23.Occhetta P, et al. High-throughput microfluidic platform for 3D cultures of mesenchymal stem cells, towards engineering developmental processes. Sci Rep. 2015;5:10288. doi: 10.1038/srep10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiraki Y, et al. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. J Biol Chem. 1997;272:32419–32426. doi: 10.1074/jbc.272.51.32419. [DOI] [PubMed] [Google Scholar]
- 25.Pelttari K, et al. Adult human neural crest-derived cells for articular cartilage repair. Sci Transl Med. 2014;6:251ra119. doi: 10.1126/scitranslmed.3009688. [DOI] [PubMed] [Google Scholar]
- 26.Poulton IJ, McGregor NE, Pompolo S, Walker EC, Sims NA. Contrasting roles of leukemia inhibitory factor in murine bone development and remodeling involve region-specific changes in vascularization. J Bone Miner Res. 2012;27:586–595. doi: 10.1002/jbmr.1485. [DOI] [PubMed] [Google Scholar]
- 27.Ng JJ, et al. Recapitulation of physiological spatiotemporal signals promotes in vitro formation of phenotypically stable human articular cartilage. Proc Natl Acad Sci USA. 2017;114:2556–2561. doi: 10.1073/pnas.1611771114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centola M, et al. Priming 3D cultures of human mesenchymal stromal cells toward cartilage formation via developmental pathways. Stem Cells Dev. 2013;22:2849–2858. doi: 10.1089/scd.2013.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narcisi R, et al. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Reports. 2015;4:459–472. doi: 10.1016/j.stemcr.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correa D, et al. Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation. Osteoarthritis Cartilage. 2015;23:443–453. doi: 10.1016/j.joca.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman M. Feedback control of intercellular signalling in development. Nature. 2000;408:313–319. doi: 10.1038/35042500. [DOI] [PubMed] [Google Scholar]
- 32.Occhetta P, Visone R, Rasponi M. High-throughput microfluidic platform for 3D cultures of mesenchymal stem cells. In: Koledova Z, editor. 3D Cell Culture: Methods and Protocols. Springer; New York: 2017. pp. 303–323. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal S, et al. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol Ther. 2017;25:1974–1987. doi: 10.1016/j.ymthe.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsano A, et al. Spontaneous in vivo chondrogenesis of bone marrow-derived mesenchymal progenitor cells by blocking vascular endothelial growth factor signaling. Stem Cells Transl Med. 2016;5:1730–1738. doi: 10.5966/sctm.2015-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138:4456–4462. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]