Significance
In vector-borne disease systems, there is mounting evidence that vertebrate hosts become more attractive to disease vectors during infection, yet in human malaria, the underlying mechanism has not been studied. We identified compounds, including aldehydes, that are produced in relatively greater amounts in the skin odor of individuals with malaria, thus demonstrating a basis for this phenomenon in the cues used during mosquito host location. By establishing the attractiveness of these compounds to malaria mosquito vectors in laboratory bioassays, we characterize a process by which Plasmodium infection of humans could lead to increased mosquito biting. These compounds may serve as biomarkers of malaria or be used to enhance the efficacy of chemical lures used to trap mosquitoes.
Keywords: malaria transmission, host attractiveness, parasite–vector–host interactions, aldehydes, disease biomarkers
Abstract
Malaria parasites (Plasmodium) can change the attractiveness of their vertebrate hosts to Anopheles vectors, leading to a greater number of vector–host contacts and increased transmission. Indeed, naturally Plasmodium-infected children have been shown to attract more mosquitoes than parasite-free children. Here, we demonstrate Plasmodium-induced increases in the attractiveness of skin odor in Kenyan children and reveal quantitative differences in the production of specific odor components in infected vs. parasite-free individuals. We found the aldehydes heptanal, octanal, and nonanal to be produced in greater amounts by infected individuals and detected by mosquito antennae. In behavioral experiments, we demonstrated that these, and other, Plasmodium-induced aldehydes enhanced the attractiveness of a synthetic odor blend mimicking “healthy” human odor. Heptanal alone increased the attractiveness of “parasite-free” natural human odor. Should the increased production of these aldehydes by Plasmodium-infected humans lead to increased mosquito biting in a natural setting, this would likely affect the transmission of malaria.
Parasite transmission often constitutes a population bottleneck: Of the many parasites within one host, only a few are successfully transmitted to the next (1). Hence, parasites often evolve to exert influence over the transmission events. The malaria parasite Plasmodium would benefit from increasing its infected vertebrate host’s attractiveness to susceptible Anopheles mosquito vectors, if this resulted in increased contact rates between the two hosts. Such changes in attractiveness have been demonstrated in both animal (2–6) and human (7–9) malaria systems, as well as in other vector-borne disease systems (10–13). While manipulation of the “attractiveness” phenotype by the parasite has been suggested (5–9), it is difficult to disentangle this from some by-product of infection that fortuitously leads to increased host attractiveness and subsequently transmission. Body odor, comprising the volatile compounds emitted from the skin of vertebrates, is the most important cue used by Anopheles for host location (14). It has been shown that differences in the composition of skin odor are responsible for the variation in attractiveness to biting insects known to exist between people (15, 16), and these differences may be influenced by body weight and/or surface area, hormones, or genetic factors (17–19). Human body odor can also be influenced by disease, including metabolic disorders, genetic disorders, and infections (20). A study of Plasmodium infection in mice found such changes in body odor to be associated with changes in attractiveness to mosquitoes (6), and another found compositional changes in skin odor during controlled human malaria infection (CHMI), with a variable effect on attractiveness (21). While increased attractiveness of Plasmodium-infected individuals has been demonstrated in a malaria-endemic setting (9), remarkably, no study has yet investigated the skin chemistry underlying this phenomenon. Given the crucial importance of body odor to mosquito host location, and the proposition that body odor can be altered during disease, here, we hypothesize that infection with Plasmodium parasites changes the odor of humans and that this influences attractiveness of humans to mosquitoes. To test this hypothesis, we first confirmed that asymptomatic children in Western Kenya were more attractive to mosquitoes when harboring Plasmodium parasites, before comparing skin odor composition between Plasmodium-infected and parasite-free children from the same population. Using analytical chemistry, and the antennal and behavioral responses of Anopheles mosquitoes, we identified and established the role of Plasmodium infection-associated compounds (IACs) in human body odor.
Results
Attractiveness and Plasmodium Infection.
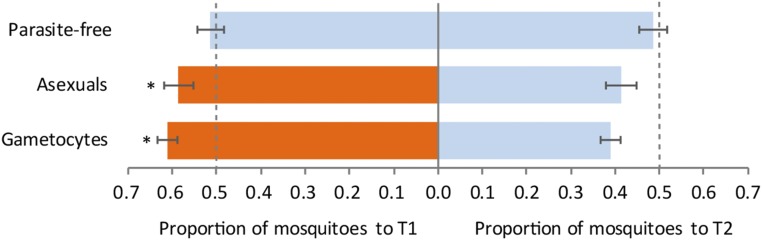
We measured the behavioral response of Anopheles gambiae sensu stricto (s.s.) to the foot odor of 5- to 12-y-old schoolchildren at two sampling time points, to assess whether Plasmodium infection changes the attractiveness of human hosts to mosquitoes. At time point one (T1; Fig. 1), foot odor of asymptomatic Plasmodium falciparum-infected and uninfected children was collected on socks for 20 h. For infected individuals, this occurred immediately after administration of the first dose of treatment with the antimalarial artemether-lumefantrine (AL), which is known to allow residual parasitemia during this time period (22). Odor samples were collected in the same manner from the same children 21 d later, following confirmed parasite clearance (T2; Fig. 1). Odor samples from participants with malaria parasites were categorized by those who harbored transmissible gametocyte stages [microscopy positive and/or >50 gametocytes per microliter by the molecular diagnostic quantitative nucleic acid sequence-based amplification (QT-NASBA), which detects female gametocyte Pfs25 mRNA, n = 23] or those with asexual stage parasites (microscopy negative for gametocytes and/or QT-NASBA gametocytes <50 per microliter, n = 10). Samples were considered parasite-free (n = 12) when no parasites were detected by microscopy and 18S quantitative PCR (qPCR) (23). An. gambiae s.s. mosquitoes were offered the choice of either T1 or T2 odor samples from the same child, in a dual-choice cage assay (Fig. S1). The proportion of mosquitoes choosing the odors collected from children at T1 was significantly affected by parasitological status [generalized linear model (GLM), F test, P < 0.001]. Mosquitoes were more attracted to odors collected at T1 from children harboring asexual or gametocyte-stage parasites relative to T2 odor samples [GLM, 95% confidence intervals (95 CI) 0.55–0.62 and 0.59–0.63, respectively; Fig. 1]. Across both groups, the ratio of attraction to “infected” (asexual or gametocyte carriers) vs. “parasite-free” odor was 0.6 to 0.4. Mosquitoes did not differentiate between T1 and T2 odor samples from parasite-free children, indicating that the difference observed between T1 and T2 odor was not an effect of sampling time point (GLM, 95 CI: 0.48–0.54; Fig. 1). This effect was independent of age, sex, tympanic (in-ear) temperature, or hemoglobin level at T1. These results indicate that infection with microscopically observable densities of either asexual-stage parasites [median, 1,340 (interquartile range; IQR: 480–2,720) parasites per microliter (p/µL)] or gametocytes [median, 80 (IQR: 40–680) p/µL] is associated with changes in odor profile that increase attraction to mosquitoes. This finding supports previous studies that demonstrate the heightened attractiveness of infected hosts, although here, by offering foot odor alone, we preclude the influence of other factors including breath. We did not observe the gametocyte-specific effect that was described (7–9), although we cannot rule out the possibility that low densities of gametocytes in some “asexual” participants contributed to their increased attractiveness. To determine which chemicals in body odor are responsible for the observed differences in attractiveness, we repeat-sampled 56 Plasmodium-infected and parasite-free children from the same locality, using air entrainment to collect foot odor samples onto polymeric filters for further analysis.
Fig. 1.
Effect of parasitological status on An. gambiae s.s. preference for body odor sampled at two time points, one during Plasmodium infection (T1) and the other following parasite clearance (T2). Blue bars represent attraction to odor from parasite-free samples, and orange bars represent attraction to odor samples from individuals with parasites. Groups of 10 mosquitoes were given a choice between socks worn by each participant at both T1 and T2, in a dual-choice cage assay, with the number of mosquitoes that chose the T1 or T2 odor sample being summed over six replicates per participant. Participants were grouped into those with gametocytes by microscopy or QT-NASBA (at >50 gametocytes per microliter) (n = 23), those with asexual stages only by microscopy (n = 10), or parasite-free (n = 12). Of those with asexual parasites, three had submicroscopic gametocytes (1–34.9 gametocytes per microliter of blood), and three were not tested. Predicted mean proportions from the GLM are plotted with 95 CI. *P < 0.05. (GLM included infection status only as predictor of proportion of mosquitoes attracted to T1 odor samples.)
Antennal Response to Malaria Odor.
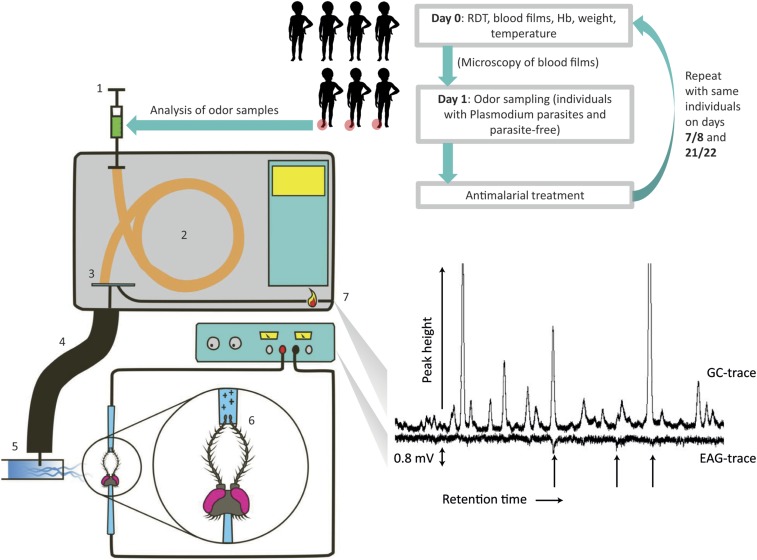
We analyzed air entrainment odor extracts using coupled gas chromatography-electroantennography (GC-EAG) (15). A change in the electric potential across the antenna resulting from stimulated neuropsychological activity (i.e., the EAG response) is caused during olfactory nerve cell response. This allows detection of compounds to which the mosquitoes are potentially behaviorally active (Fig. 2). Point-of-care malaria diagnostics [rapid diagnostic test (RDT) and microscopy], used to inform odor sampling from asymptomatic individuals, were retrospectively confirmed by using molecular diagnostics. Infected children were treated after odor sampling, and repeat sampling of all individuals was attempted 1 and 3 wk later alongside repeat parasitological diagnoses (Fig. 2). Odor samples from individuals harboring similar Plasmodium parasite stages or densities were extracted into solvent and mixed to create blends of “average” odor with the following infection profiles: (i) Plasmodium infection, no gametocytes; (ii) Plasmodium infection, high-density gametocytes; and (iii) parasite-free individuals (Table S1). A further group, (iv) Plasmodium infection, submicroscopic gametocytes, was included due to the frequency of submicroscopic gametocytemia in endemic infections. P. falciparum gametocyte densities were determined by Pfs25 mRNA QT-NASBA, while 18S qPCR and duplex qPCR were used to determine P. falciparum and Plasmodium densities, respectively. Twenty-two analytes (Table S2) were found to elicit antennal response in Anopheles coluzzii (formerly the M-form of An. gambiae s.s. Giles), including the aldehydes heptanal, octanal, and nonanal. No EAG-active analytes were specific to any of the infection profiles (i–iv), indicating that any Plasmodium-induced change in the compounds used by host-seeking Anopheles must occur by variation in the relative amounts of compounds that are present in parasite-free individuals.
Fig. 2.
Schematic of protocol (Upper) for odor sampling by air entrainment from Plasmodium-infected individuals, for use in GC-EAG analysis (Lower; here with An. coluzzii) and direct GC analysis of entire odor profile. Children were recruited for odor sampling in groups of three to represent parasite-free, asexual parasite carriers, and gametocyte carriers, if parasite prevalence allowed. Following malaria diagnosis by point-of-care methods and odor sampling, malarious individuals were treated, and the same cohort was resampled on days 8 and 22. Whole blood samples were also taken for retrospective molecular analysis. During GC-EAG, odor samples are injected by syringe at the inlet directly into the column (1), where they are vaporized, and carried through the column by the carrier gas (here, hydrogen) (2). During passage through the (50 m) HP1 column, constituents of the sample are separated by GC, and analytes are split as they elute from the column (3). A proportion is directed, via a heated transfer line (4), into a humidified, purified, airflow (5), which is then directed over the insect antennae (6), simultaneously to the proportion that is detected by a flame ionization detector on the GC (7). GC analytes are represented by peaks (GC trace), while antennal response by nerve cell depolarization causes a perturbation in the electroantennographic detection (EAG trace), indicating entomologically significant analytes. Image courtesy of Iain Robinson (Iain-robinson.com).
Plasmodium IACs.
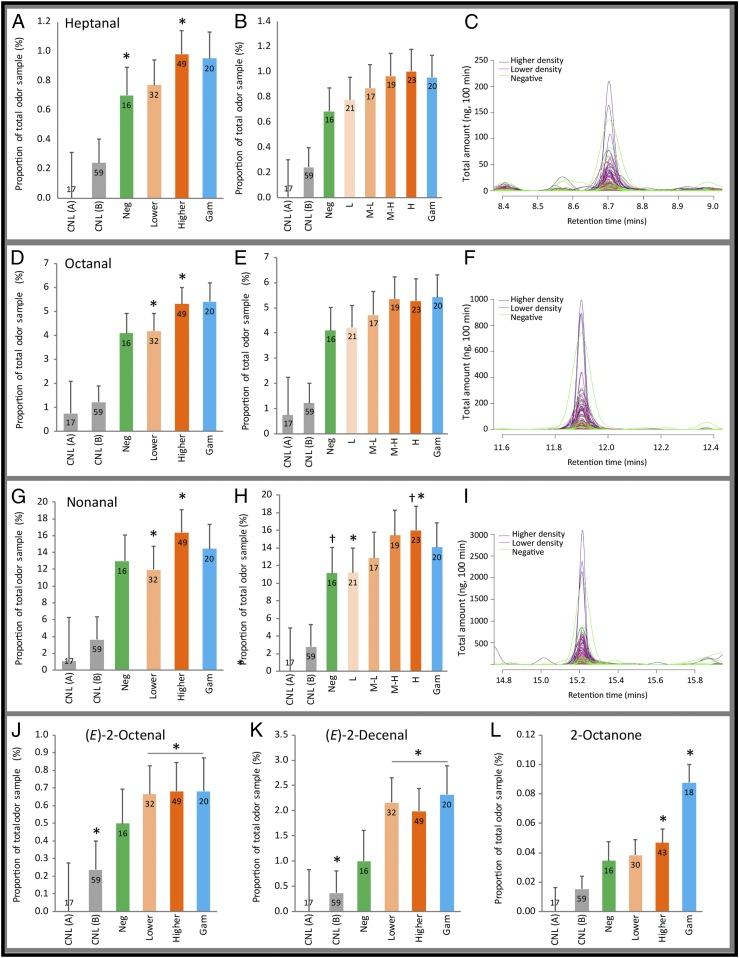
To investigate whether Plasmodium infection indeed results in quantitative changes in the production of volatile compounds, we compared the profiles of 117 foot and 59 control (empty entrainment bag) odor samples. A total of 56 individuals participated in air entrainment odor sampling (Fig. 2); however, not all individuals were available at follow-up time points. Foot odor samples from Plasmodium-infected individuals were categorized by infection status: those from individuals with “higher” (>50 p/µL, which approximates the microscopy limit of detection) and “lower” (<50 p/µL) density infections and those from individuals harboring microscopic gametocytes (“total density” categorization). As the prevalence of non-P. falciparum infections was low [5.05% (n = 5) and 3.96% (n = 4) for P. malariae and P. ovale spp., respectively, at day 0, and eight of nine had concurrent P. falciparum parasites], we did not separate samples from individuals with non-falciparum infections. Our analysis revealed increases in the production of the aldehydes heptanal, octanal, nonanal, (E)-2-octenal, and (E)-2-decenal by infected individuals. Increases were broadly associated with infections of high parasite density, relative to either low density or production by parasite-free individuals. High-density infections were also correlated with the presence of gametocytes in this dataset (Fig. S2C). Heptanal was produced in significantly greater amounts by individuals with higher parasite densities (>50 p/µL) relative to parasite-free individuals [residual maximum likelihood (REML), least significant difference (LSD), 5%; Fig. 3 A and C]. Octanal and nonanal were produced in significantly greater amounts by individuals with higher-density, relative to those with lower-density (<50 p/µL), infections (REML, LSD, 5%; Fig. 3 D, F, G, and I). To investigate further this seemingly density-dependent effect, we divided the higher- and lower-density individuals into quartiles, representing “low,” “medium-low,” “medium-high,” and “high” density. We observed a clear correlation between increased production of heptanal, octanal, and nonanal and increased parasite density (Fig. 3 B, E, and H). The difference in production of nonanal between low, or negative, and high individuals was significant (REML, LSD, 5%; Fig. 3H and Table S3). Relative to parasite-free individuals, there was a trend for all Plasmodium parasite-positive individuals to produce more of the unsaturated aldehydes (E)-2-octenal and (E)-2-decenal (Fig. 3 J and K), and for the latter, this difference was significant if individuals were categorized simply as Plasmodium-positive or parasite-free (REML, “positive vs. negative” categories, LSD 5%; Table S3). It is well-established that aldehydes are among the many volatiles that constitute human skin odor (24–26), where they are frequently cited as being predominant (27). Additionally, the ketone 2-octanone was found to be associated with the presence of microscopic gametocytes (REML, LSD, 5%; Fig. 3L). Again, ketones are known volatiles of human skin. For all IACs, we found a quantitative relationship: The majority of individuals produced these compounds, but the quantity produced increased with Plasmodium infection. An average of 177 (SE 5.23) analytes were captured per sample, and the IACs were disproportionately abundantly produced (Fig. 4), comprising on average 22.92% of the total odor profile across all 117 samples. When production was ranked relative to all other compounds sampled, nonanal had a median rank of one, octanal two, and heptanal five. While specific IACs were produced in greater amounts by individuals harboring parasites, an overall increase in volatile emissions from infected persons was not observed (REML, LSD, 5%; Fig. S3), contrary to findings in the mouse or CHMI system (6, 21). Among the IACs, the antennal response, observed by GC-EAG to heptanal, octanal, and nonanal, suggests that changes in the production of these compounds could affect mosquito behavior.
Fig. 3.
Amount of IACs produced by individuals of differing parasitological status. (A–C) Heptanal, (D–F) octanal, (G–I) nonanal, (J) (E)-2-octenal, (K) (E)-2-decenal, (L) 2-octanone production (relative to all compounds in odor sample) per group (100-min odor profile sampling). Predicted means (+SE) are given by linear mixed modeling (REML). See Table S3 for details of the models and Table S4 for SE of the difference (SED) values for comparison of predicted means. Sample size is in bar ends. *,†P < 0.05 (significant pairwise difference in mean amount between two groups indicated, tested by LSD). A, D, G, J, K, and L, total density categorization: Gam, microscopic gametocytes; Neg, negative; lower and higher refer to parasite densities of lesser or greater than 50 p/μL. (B, E, and H) Quartile categorization. Neg and Gam are defined as before. L, low, mean/median parasite density 0.38/0.3, n = 21; M-L, medium-low, mean/median parasite density 16.77/8.3, n = 17; M-H, medium-high, mean/median parasite density 296.60/214.18, n = 19; H, high, mean/median parasite density 102,669.46/13,304.54, n = 23. For bar charts CNL(A), solvent control; CNL(B), empty bag control. C–I show raw GC output for heptanal, octanal, and nonanal. Individual traces represent odor samples, colored according to the parasitological status of the individual from whom the odor sample was taken, Higher-density, lower-density, and negative definitions are as described above. Gametocyte carriers are excluded for clarity, as compound production spanned higher and lower parasite density groups.
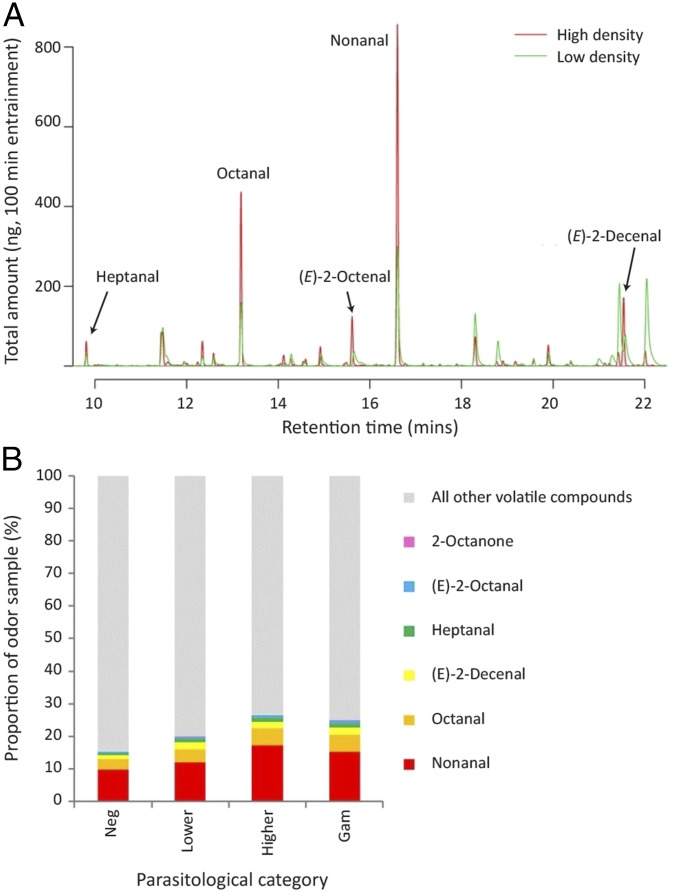
Fig. 4.
Comparison of odor profiles from parasite-free individuals vs. those harboring bloodstream parasites. (A) Representative GC traces from an individual with a high-density infection (>50 p/μL blood) and low-density infection (<50 p/μL blood). Compounds found to be associated with infection (other than 2-octanone; not visible due to very small amounts) are annotated. (B) The proportion (percent) that IAC contributed toward the entire odor profile, grouped by parasitological category (total density categories). The average number of non-IAC per group (i.e., all other volatile compounds; gray bar) was 171.27 (SE = 5.23) across all groups.
Mosquito Response to IAC.
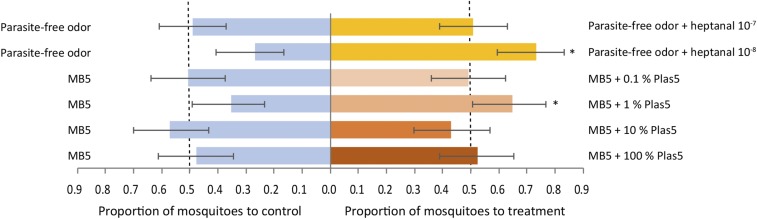
To determine whether the IACs were attractive to mosquitoes, and therefore likely to be responsible for the increased attractiveness observed in infected individuals, we tested all six IACs [heptanal, octanal, nonanal, (E)-2-decenal, (E)-2-octenal, and 2-octanone] in behavioral bioassays with An. coluzzii. We tested for a behavioral response toward the latter three compounds because of their positive association with the presence of parasites in the bloodstream (Fig. 3), despite a lack of antennal response in An. coluzzii (Table S2). First, the odor of parasite-free children (worn socks) was supplemented with the IAC individually and tested at a minimum of two concentrations each. Of these, adding 10 μL of heptanal at a concentration of 10−8 g/mL to parasite-free odor significantly increased attractiveness, relative to parasite-free odor alone (GLM, 95 CI: 0.60–0.84; Fig. 5), while heptanal at a concentration of 10−7 g/mL had no effect. The attractive concentration is ∼1/10th of the additional heptanal isolated in odor samples from individuals with higher-density Plasmodium infections, relative to negative individuals, over the corresponding time period. This suggests that elevated emission of heptanal, at specific concentrations, by parasitemic children could contribute to their increased attractiveness to mosquitoes. Supplementing with octanal, nonanal, (E)-2-decenal, (E)-2-octenal, or 2-octanone alone did not induce altered behavioral responses, despite the EAG activity observed in response to octanal and nonanal (Fig. S4). We then tested whether the addition of heptanal to a current best-practice synthetic mosquito lure, MB5 [comprising ammonia, l-(+)-lactic acid, tetradecanoic acid, 3-methyl-1-butanol, and butan-1-amine (28)], might further increase attractiveness to mosquitoes. However, MB5 supplemented with heptanal was equally attractive as control MB5, at three concentrations (Fig. S4). This suggests that the attractiveness of heptanal observed with parasite-free odor was dependent on synergism with other volatile compounds naturally present, but absent from the synthetic MB5 blend. Because odor detection and response are highly contextual, this was not an unexpected outcome. To investigate further the behavioral role of IACs, but allowing for such synergistic effects between these compounds, we tested two blends with MB5: Plas 5 contained the aldehydes found to be associated with increased total parasite density [heptanal, octanal, nonanal, (E)-2-octenal, and (E)-2-decenal], and Plas 6 additionally contained the ketone 2-octanone that was associated specifically with gametocytes. Each was tested at four concentrations. The Plas 5 blend enhanced attractiveness of MB5 (1% concentration, GLM, 95 CI: 0.51–0.77; Fig. 5). However, the Plas 6 blend was not found to increase attractiveness of MB5 at any concentration (Fig. S4), which suggests that the gametocyte-associated 2-octanone moderated the attractiveness of the Plas 5 aldehydes. Given the presence of small amounts of 2-octanone in parasite-free odor, however (Fig. 3I), which increases in attractiveness on addition of heptanal (Fig. 5), it appeared that this repellency of 2-octanone is not observed in the context of natural human odor. In previous studies describing the increased attraction of gametocyte carriers, the odor tested included both body and breath (7, 9), leaving open the possibility that the gametocyte-specific attraction may have originated in the breath. Indeed, “malaria-associated” volatile compounds have been identified in the breath, whose production varied cyclically according to P. falciparum parasitemia, although no investigation of the mosquito response to those compounds was undertaken (29). Our behavioral tests showed that supplementing parasite-free odor with heptanal increased attractiveness to mosquitoes. However, heptanal alone did not increase the attractiveness of a basic synthetic lure, while a blend of infection-associated aldehydes including heptanal (Plas 5) was attractive. Therefore, in both instances, the increased attraction was dependent on additive effects among the infection-associated aldehydes, which are naturally present in parasite-free odor at lower concentrations (Fig. 3).
Fig. 5.
An. coluzzii responses in a dual-port olfactometer to heptanal and a blend of five infection-associated aldehydes, Plas 5. Heptanal (10 µL) at two concentrations (g/mL) was presented with (yellow bars) and tested against (blue bars) odor (socks) from parasite-free study participants (5- to 12-y-old Kenyan children) over eight replicates. Plas 5 [heptanal, octanal, nonanal, (E)-2-octenal, and (E)-2-decenal] at four concentrations (10 µL of 100% approximating the amounts found in the foot odor samples) was presented with (orange bars) and tested against (blue bars) the synthetic lure MB5 [ammonia, l-(+)-lactic acid, tetradecanoic acid, 3-methyl-1-butanol, and butan-1-amine] over 10/11 replicates. Each replicate tested 30 mosquitoes. Predicted mean proportions and 95 CI are presented, from two separate GLMs (for heptanal and Plas 5 assays), assuming a binomial distribution and using a logit link function. *P < 0.05. (See Table S6 for details of the GLMs.)
Discussion
Aldehydes are found in the skin odor of various mammalian species (30) and have been determined to be among the chemicals used by hematophagous insects for host location (31). These oxygenated compounds can be synthesized when reactive oxygen species attack lipid-dense membrane structures (32), i.e., lipid peroxidation, caused by oxidative stress. Oxidative stress is known to characterize malaria infection (33), occurring in the erythrocytes and liver. Alternatively, or additionally, the aldehydes found here may have been produced directly by Plasmodium parasites: A recent publication found the aldehydes octanal, nonanal, and decanal to be among volatile compounds emitted by red blood cell (RBC) cultures that had been supplemented by (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) (34). HMBPP is a precursor in the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway, apparently used by Plasmodium for isoprenoid production, and it was suggested that HMBPP triggered enhanced release of these compounds from infected RBCs, with a subsequent impact on mosquito attraction. Additionally, terpenes were isolated from HMBPP RBCs, and another study also isolated terpenes above Plasmodium-infected RBC cultures (35). Although the MEP pathway is a possible source of terpenes via isoprenoid production in infected RBCs (iRBCs) (35), the source of terpenes in HMBPP RBCs remains unknown (34). We did not find an association between Plasmodium infection and the emission of terpenes from the skin, corroborating earlier findings in Plasmodium-infected mice (6). It should be emphasized that laboratory-based studies of the volatile compounds isolated above iRBC cultures do not characterize the human body odor used by mosquitoes during host location. As such, they do not fully capture the complex biological and biochemical host–parasite interactions that occur in natural Plasmodium infections. In our study, the production of aldehydes was increased in individuals with Plasmodium infection. The extent to which parasite-specific release of aldehydes from iRBCs would contribute to a profound and systemic increase in aldehyde production, caused by malaria-induced oxidative stress, remains unexplored. Finally, it is important to note that while the lipid peroxidation pathway for aldehyde production is well-established, the skin microbiota is also known to produce aldehydes. This is particularly relevant to our study, as odor samples were taken from the feet. Feet harbor skin microflora that produce volatiles that are attractive to mosquitoes (36), and differences in microflora have been associated with differences in attractiveness (16).
We demonstrated that elevated production of specific aldehydes in skin odor is associated with increased attractiveness to mosquitoes in Plasmodium-infected people. Our findings are in accordance with the “deceptive signaling” hypothesis, whereby host cues already used by host-seeking insects are exaggerated, increasing the attractiveness of that vertebrate to biting insects, but when the blood-meal is in fact unfavorable to the insect (37). If the disadvantages [e.g., reductions in fecundity (38–40) or shortened lifespan (41–43)] of taking an infected blood-meal outweigh any advantages [e.g., reduced host defenses (2) or faster engorgement (44)], the evolution of an infected-host avoidance phenotype might be expected. Anopheles may less easily select against an infected-host phenotype comprising “normal” stimuli. It is possible that the observed changes to skin odor are specific to P. falciparum, which constituted the majority of infections in the cohorts that we studied. In similar studies of mosquito attraction, increases are often associated with the chronic phase of malaria and/or increased density of bloodstream gametocytes (2, 5–9). We found that odor from all P. falciparum-infected individuals was more attractive than that of parasite-free individuals, and the increased production of IACs was correlated with total parasite density. Although it is possible that gametocytes at densities below the detection limit of our assay (45) contributed to the attractiveness of individuals with asexual malaria parasites, the greatest production of IACs did not correlate with gametocyte density. The association between P. falciparum asexual parasite biomass and gametocyte density is generally positive (46–48), and we also observed this in our study. Thus, our findings are in broad agreement with studies that observed an increase in attractiveness related to the presence of gametocytes (2, 5–9) or general malaria infection (4).
The compounds we observed to be associated with infection could be derived from malaria-induced oxidative stress. Although there is in vitro evidence that suggests both octanal and nonanal could be produced by RBCs via interactions with components of the isoprenoid production pathway (34), found in Plasmodium parasites, both this and the oxidative stress mechanism would result in the observed correlation between increased parasite density and increased compound production. More generally, this correlation would be observed if IACs were the by-product of any sequelae of Plasmodium infection, with the resulting influence on mosquito behavior a coincidental benefit to the parasite. This raises the question of whether the IACs found in our study are specific to Plasmodium infection or could be a general “scent of infection.” Indeed, increased aldehydes have been found to signify the presence of other diseases (32, 49, 50). However, our data do indicate that the observed increase in these IACs is specific to Plasmodium infection, because a general scent of infection might have been expected in malaria parasite-free children who harbored other infectious organisms, and as such there would have been no observed difference in IAC production between the uninfected and infected children in this study. For example, Schistosoma mansoni was recently found in 51% of 9- to 12-y-old children in the neighboring Asembo District (51) and was likely present in individuals in our malaria parasite-free cohort. To date, only one study on the effects of Plasmodium infection on volatile emission in humans included a control group of bacteria-infected participants, and they found that increased levels of thioethers in human breath were indeed malaria-specific (29). Nevertheless, an important follow-up to our study would be to examine skin odor profiles, and behavioral responses of mosquitoes toward these, of individuals affected by other diseases thought to be characterized by similar emissions but not vectored by an insect.
Although it is not possible to infer from this study whether the increased production of IACs is under the control of malaria parasites, it could be argued that if the parasites indirectly stimulated compound production (e.g., via triggering oxidative stress), the parasite genes underlying this stimulation would be selected for via enhanced transmission. However, a mutation benefitting transmission may be costly to the parasite and would therefore only be selected if the trade-off resulted in a net increase in transmission. Further studies that explore the relative costs and benefits of manipulation to the parasite, for example, through modeling (52), are merited. The implications of identifying IACs, with their demonstrated impact on mosquito behavior, are far-reaching: We better understand parasite–vector–host transmission events and their overdispersed nature in human populations. These compounds may permit further improvement of already highly functional lures for trapping malaria mosquitoes or even serve as biomarkers for malaria, providing a basis for novel and noninvasive diagnostic tools.
Materials and Methods
Ethics.
Study participants were 5- to 12-y-old children local to the Thomas Odhiambo Campus of icipe in Western Kenya (0°25′48.1″S, 34°12′24.5″E), including Rusinga Island, in Suba District, Homa Bay County. Participants were recruited after obtaining signed consent. The study protocol (NON SSC 389) was approved by the Scientific and Ethical Review Committee of the Kenya Medical Research Institute (KEMRI/RES/7/3/1). Subsequent analyses were conducted at the London School of Hygiene and Tropical Medicine (LSHTM) (ethics reference 8510).
Attractiveness of “Infected Odor” (Socks) by Cage Assays.
A cohort of Plasmodium-infected, asymptomatic (tympanic temperature <37.5 °C) individuals that participated in an olfactometer study (9) was studied for the attractiveness of their skin odor to An. gambiae s.s. Forty-five children were included, of which there were 23 with microscopic gametocytes or an estimated gametocyte density of >50 gametocytes per microliter of blood by QT-NASBA, 10 positive for asexual parasites but not gametocytes by microscopy, and 12 that tested Plasmodium-parasite free by 18S-qPCR (23). Samples were collected at two time points: within 24 h of antimalarial treatment but while children still harbored parasites (22) (T1 samples) and 21 d later (T2 samples). Antimalarial treatment with AL was administered to Plasmodium-positive individuals according to manufacturer’s instructions (20 mg of artemether/120 mg of lumefantrine per tablet; Coartem; Novartis), and socks were put on within 1 h of treatment. Age, hemoglobin (Hb), weight, and temperature were measured as covariates (9). At day 21 (“after”), both parasitological testing and participant covariate measurements were repeated.
Procedures for collection of body odor.
Body odors were collected for 20 h on nylon socks (97% polyamide, 3% elastane, 20 denier; Hema), which were washed by using 70% ethanol and dried at 70 °C for 2 h before use. Surgical gloves were worn throughout collection procedures. Children were assisted in putting on and removing the socks. These were stored in clean glass jars at −20 °C until use in cage assay experiments. Children were asked not to bathe during this time, but had no other behavioral restrictions.
Behavioral assays for attractiveness of odor samples.
A dual-choice cage assay was modified (53) to determine the relative attractiveness of odor samples from T1 and T2. Three World Health Organization bioassay tubes (12.5 cm long, 5 cm wide) (54) were connected with sliding units between the inner and outer tubes (Fig. S1). Mosquito cages (15 × 15 × 15 cm) were wrapped with transparent kitchen cling-film (Chandaria Industries Ltd.) to prevent movement of volatiles between different assays running in parallel. The outer tubes were inserted 6 cm into the cages. Per individual, T1 and T2 samples (sock pairs) were placed in opposing cages, with the feet cut off to remove environmental soiling.
Six-to 8-d-old, non-blood-fed, female An. gambiae s.s. mosquitoes [Mbita strain, with published rearing methods (55)] were collected before the experiment and allowed 8 h acclimatization. Ten mosquitoes were released into the central tube per bioassay, and the gates of the tubes were opened for 15 min to allow mosquitoes to make a choice of odor source. Experiments were conducted between 18:30 and 22:30 under ambient conditions, in a red fluorescent-lit room (average temperature, 24.1 °C) with the dual cage covered by black cotton cloth. After 15 min, mosquito choice was recorded. All sock pairs were tested simultaneously on the same nights, and in total, each pair (child) was tested six times, replicating over experimental nights, dual cage set-ups, and between cages. All disposable equipment was changed, and cages were cleaned (70% ethanol), between experiments/replicates.
Statistical analysis.
Per child, the number of mosquitoes that chose the T1 or T2 odor sample was summed over six replicates, and the relative attractiveness of samples was determined as the proportion of mosquitoes that selected a sample over the total number of mosquitoes that made a choice. A GLM (binomial distribution, logit link function, and dispersion estimated) was used to test the effect of parasitological status (parasite-free, asexual, or gametocytes) on the relative attractiveness. The number of mosquitoes caught in the cage with the T1 sample was used as the response variable and all mosquitoes caught in both cages as the binomial total. Covariates associated with participants (age, sex, Hb, and tympanic temperature measured at T1) were tested, but removed from the model because they were not significant (P > 0.05, F tests). Per parasitological group, we used the 95 CI of the predicted proportion of mosquitoes choosing T1 odor samples, derived from the GLM, to assess whether mosquito choice differed significantly from a 50:50 distribution over the two odor samples. SPSS (2016; Version 24; IBM) was used for the analyses.
Collection of Volatile Odor Samples.
Sampling procedure.
In the same locality, a separate cohort of schoolchildren, of varying Plasmodium infection status, were sampled for foot odor (Fig. 2, Upper). On day 0, 20 children for whom the parent or guardian had given full consent were tested for their malaria status by RDT and microscopy. Tympanic temperature, age, weight, and Hb levels were recorded. Symptomatic children and/or those with a temperature >37.5 °C with RDT positivity were treated with AL (as described above) and excluded from the study. Overnight, microscopy was conducted, and three children were selected for odor sampling, with the intention to sample one child with asexual parasites, one with gametocyte stages, and one with no parasites. On day 1, odor sampling was conducted by air entrainment, after which all malarious children were treated. Days 0 and 1 constituted round one (R1), and the same procedures were conducted at days 7 and 8 (R2) and days 21 and 22 (R3), with the intention to repeat-sample the same children at two points posttreatment (Fig. 2). R1–R3 were repeated for 6 mo between January and June 2014. In this way, 56 children were repeat-sampled, but a total of 117 odor samples and 59 accompanying empty bag control samples was achieved, due to loss-to-follow-up.
Air entrainment.
For each child, one foot was placed in a prepared bag (Fresh and Eazy oven bags, 45 × 50 cm; Meda-Pak), clipped shut around the calf. At each sampling round (R1–R3), a control (empty) bag was tightly closed and sampled in the same manner. Bags were fitted with Swagelok fittings at opposing corners, allowing connection to polytetrafluoroethylene (PTFE) tubing for airflow. Air (charcoal-filtered) was pumped into the top of the bag and vacuumed from the bottom (both at 500 mL/min), with a 30-min purge before fitting the polymer filters, to ensure system cleanliness. Porapak filters were connected (Porapak Q, mesh size 50/80; Supelco Analytical) and sampled for 100 min, then stored in stoppered glass vials in a cool box before sealing under filtered nitrogen on the same day. Ampoules were stored at −20 °C until shipping to LSHTM. Before use, all PTFE tubing, Swagelok fittings, and glassware were cleaned with 70% ethanol, then baked in an oven at 150 °C for 2 h. Sampling bags and charcoal filters were baked in the same manner. Cotton gloves were worn by the investigators throughout.
Infection status.
Odor sampling was informed by RDT [One Step malaria HRPII and pLDH antigen rapid test (SD BIOLINE, catalog no. 05FK60)], performed as per manufacturer’s guidelines, and thick and thin blood films were made by using peripheral blood from a finger prick. Whole blood (50 μL) was stored in RNAprotect (250 μL; QIAGEN). Retrospectively, DNA/RNA extraction was performed by using a Total Nucleic Acid Isolation Kit [with methods as published (56)], and P. falciparum parasite density and stage V gametocyte density were determined by 18S qPCR (23) and QT-NASBA (57). Additionally, dried blood stored on both Whatman No. 3 filter paper [Whatman (wDBS)] and used RDTs [air-dried and stored in sealed plastic bags containing the desiccant silica gel (uRDT)] was used as a DNA template. DNA was extracted from circles (3 mm) punched from the wDBS, and sections (3 × 2 mm) were cut from the central section of the nitrocellulose strips in the uRDTs (58). Extraction was performed in a deep well plate by using an automated extraction system (QIAsymphony), with the QIAsymphony DSP DNA mini kit (QIAGEN) according to the manufacturer’s instructions, and a Plasmodium tRNA methionine-based duplex qPCR was used to measure Plasmodium density (22). Good correlation in parasite density was obtained between duplex qPCR by using wDBS or uRDT whole blood template. Where available, the same DNA extracts were used for species-specific (P. falciparum, P. ovale spp., and P. malariae) nested PCR (59), with some P. ovale spp. identifications confirmed by the P. ovale spp. tryptophan-rich antigen (PoTRA) assay (60).
GC-EAG of Pooled Odor Samples.
GC-EAG odor sample blends.
Porapak filters were eluted by using redistilled diethyl ether (750 μL), and, to approximate an “average” odor per category, extracts were pooled according to the individual’s parasitological status: (i) Plasmodium infection, no gametocytes; (ii) high-density P. falciparum gametocytes; (iii) parasite-free individuals; or (iv) Plasmodium infection, submicroscopic P. falciparum gametocytes (Table S1). Aliquots (400 μL) of extracts were mixed, then concentrated (to 60 µL) under a stream of nitrogen (charcoal-filtered). Glassware, charcoal filters, and PTFE tubing were cleaned as before.
Experimental set-up.
GC-EAG was conducted during the scotophase, using 4- to 8-d-old, unfed female An. coluzzii [N’gousso strain (61)]. Adults were maintained at 70% relative humidity (RH), with a 12-h light/dark cycle (scotophase 09:00–21:00) and access to 50% glucose solution. The order of testing blends was determined by a 5 × 5 Latin square (including control blend). The mosquito head was dissected, and the palps, proboscis, and half of the terminal (13th) antennal flagellomere were cut off. The indifferent electrode was inserted into the back of the head, and the antennal tips were guided into the recording electrode to complete the circuit (Fig. 2). Electrodes were hand-pulled glass tips inserted over silver wire (diameter 0.37 mm; Harvard Apparatus) and filled with Ringers’ solution (15). GC was performed on a 7890A machine (Agilent Technologies) with the following program: oven temperature maintained at 40 °C for 0.5 min, increased by 10 °C per min to 230 °C, then held for 20 min. Blends were injected at 4 μL, and the eluate was split to the flame ionization detector (FID) and EAG interface at a ratio of 1:1. At the EAG interface, the eluate passed from the heated splitter column to a stream of charcoal-filtered, humidified air (flow rate 400 mL/min). This airflow was directed over the antenna at a distance of 5 mm. The signal was amplified 10,000× by the Intelligent Data Acquisition Controller-4, and signals were analyzed by using EAD 2000 software (both Syntech). Responses were signified by a depolarization of sufficient amplitude. Peaks that elicited responses in more than three of the six/seven total repetitions were considered to be EAG-active.
Analysis of Odor Profiles by GC.
Instruments used for GC analysis were 7890A, 6890N, and HP6890 (Agilent Technologies). Each was fitted with a cool-on-column injector, FID, and used hydrogen carrier gas, and 1-μL injections were performed. All were fitted with an HP1 column, 50 m × 0.32 mm, film thickness 0.52 μm, and the following program was used: oven temperature maintained at 40 °C for 0.5 min, increased by 5 °C per min to 150 °C, held for 0.1 min, raised by 10 °C per min to 230 °C, and held for 40 min. Traces were analyzed by using the R package MALDIquant (62) (R Version 3.3.0, 2016, The R Foundation for Statistical Computing). In brief, raw x, y coordinates for GC traces were exported from Agilent ChemStation (C.01.04), and the y value (height, for 1 µL) was multiplied by total extract to represent actual amount per sample (nanograms). Following baseline removal, traces were visually inspected for consistent differences between parasitological groupings. Compounds of interest (COIs) were then compared quantitatively, by integrating peaks in ChemStation, and calculating retention index and amount relative to a standard series of n-alkanes (C7-C25), using Eq. 1.
Retention index (RI) calculation
| [1] |
where RtX is the retention time for the COI, Rtn is the retention time for alkane before the COI, Rtn + 1 is retention time for alkane after the COI, and n is the number of carbons in alkane before the COI.
Compound identification.
Following statistical analysis (below), IACs were tentatively identified by GC-MS, using either a Micromass Autospec Ultima [a magnetic sector mass spectrometer equipped with a Programmed Temperature Vaporizing inlet (GL Sciences B.V.)] and Agilent 6890N GC or a Mass Selective Detector (quad GC-MS). Peaks were compared with MS databases (National Institute of Standards and Technology). For confirmation of identification, authentic standards were injected onto two GC columns (HP1 and DB wax) simultaneously with samples containing those compounds. Standards were heptanal (Sigma-Aldrich), octanal (Sigma-Aldrich), nonanal (Sigma-Aldrich), (E)-2-octenal (Acros Organics), (E)-2-decenal (SAFC), and 2-octanone (Sigma-Aldrich). Identifications were considered certain when the resultant peak increased in height without increasing in width. Coinjections were conducted for all IACs.
Statistical analysis.
Any sample that had detectable parasite DNA at amounts greater than published limits of detection (LOD) for the assays [0.02 p/µL for 18S (23) and 5 p/µL for duplex qPCR (22)] was considered positive, and those with DNA amounts beneath these thresholds were excluded. Only samples that were negative by all measures, including at least one molecular diagnostic measure, were taken to be negative, other than RDTs for which positivity was acceptable (on an assumption of positivity due to circulating HRP-2 protein) (63). Individuals with Plasmodium parasitemia, but without microscopic gametocytes, were divided into higher and lower parasite density categories: higher density with >50 p/µL and lower density with between the LOD and 50 p/µL (Fig. S2A). Categorization was informed by using 18s qPCR, then duplex qPCR (wDBS > uRDT), then microscopy, according to assay result availability. Instances suggesting no parasites by 18S qPCR but with a robust parasite signal from one or more other measures were allocated to the appropriate positive category. For “quartile” categories, higher- and lower-density samples (n = 81) were then subdivided into quartiles according to density (Fig. S2B). Again, samples were allocated according to a hierarchy of procedures, in the order 18S qPCR > duplex qPCR > microscopy. Where 18S and/or duplex qPCR result was zero or missing but microscopy was positive, the film was reread and that value assumed. Two samples with low parasite density by 18S but high and corresponding density by duplex qPCR and microscopy were allocated according to the two corresponding outcomes, and one further lower-density sample was excluded from quartile analysis due to imprecise parasite density. Gametocyte densities per group, “total density” categories, are given in Fig. S2C (measured QT-NASBA, where available), and the correlation between 18S qPCR and duplex qPCR by two templates is in Fig. S2D.
The association between the production of COI (variate: percentage of total entrainment) and parasitological category was assessed by linear mixed models fitted using the method of REML. This modeling allowed for unequal sample sizes (per parasitological category) and repeated measures on the same individuals. We tested (F tests) for the main effects of covariates (age, Hb, day of the year, and weight) before the treatment (parasitological status) term and for factors (sex and round) after the treatment term, in a forward selection, parallel-lines, regression analysis approach (Table S3). Pairwise comparisons between groups of most biological interest were made by using the LSD at the 5% level (Table S4), and COI demonstrating significant (REML, LSD, 5%) differences between groups were termed IACs. Data analysis was conducted by using Genstat (2013, 16th ed.; VSN International).
Behavioral Testing of Candidate Compounds.
Testing IACs individually.
Six IACs [heptanal, octanal, nonanal, (E)-2-octenal, (E)-2-decenal, and 2-octanone] were tested in a background of odor from the worn nylon socks of 12 parasite-free children (18S qPCR confirmed). Each sock pair was cut into 12 strips after removing the foot part, and then 12 bundles were made, each containing a strip from each individual. Bundles were stored at −20 °C until, and between, experiments. IACs were positioned downwind and separated from sock bundles by a metal grid, ensuring no contact. Parasite-free odor (bundles) was tested with or without individual IAC (in 10 µL of hexane on filter paper) and against the same but with hexane alone. For each IAC, a decimal dilution series was made (in hexane) and two/three concentrations chosen, to bracket the differential amount between significantly different groups (LSD 5%, REML), adjusted to represent 15 min of compound release (test duration).
Improving a mosquito lure.
Next, we verified whether the IAC could improve a mosquito lure for monitoring or mass trapping of Anopheles. Heptanal, the most promising candidate from the above experiment, was tested as well as two blends: Plas 5 contained the IAC that were associated with parasitological positivity [nonanal, heptanal, octanal, (E)-2-decenal, and (E)-2-octenal], and Plas 6 additionally contained the gametocyte-associated 2-octanone (Fig. 3). Ratios were derived, and amounts of compounds were taken (Table S5) from predictions for compounds for parasitological groups with significantly increased quantity (LSD 5%, REML). Plas 5 and 6 were tested with the synthetic lure MB5 (28) at four concentrations, each decreasing by a factor of 10 from the 100% concentration (Table S5).
Assay.
A triple-chamber dual-port olfactometer (64) was used to test the preference of 30 5- to 8-d-old female, non-blood-fed An. coluzzii [Suokoko strain, rearing procedures as published (21)] for parasite-free odor or MB5, supplemented with IAC or IAC blends, against background odor alone (parasite-free odor or MB5). Mosquitoes were maintained in a release cage before testing (for 24 h). Experiments took place during the last 4 h of the scotophase under near-dark conditions (<1 lx). Mosquitoes were allowed to fly for 15 min, and then those that had entered the traps with test/control odors were counted. Each IAC/concentration combination was tested eight or nine times on different days, and each Plas concentration 10 or 11 times on different days, rotating treatments between the left and right port of the olfactometer. Climatic data (RH, temperature, and air pressure) were recorded in the flight chambers and in the surrounding room.
Statistical analyses.
GLMs were used to test the effect of odors (individual IAC/heptanal/Plas blends) on relative attractiveness (the proportion of mosquitoes selecting the test odor). GLMs were run as described above [attractiveness of infected odor (socks) by cage assays], testing parameters associated with the set-up as additional factors or covariates in the model, and retaining when significant (P < 0.05, F test). Sets of compounds were run in separate models (Table S6). SPSS was used for the analyses.
Supplementary Material
Acknowledgments
We thank all the participants in this study, and their families, for consenting participation. We are indebted to the field assistants, David John Odoyo and Geoffrey Omondi Olweru, for all of their help and hard work. Patrick Sawa (St Jude’s Clinic, icipe), and the Minister for Education, Mbita district, were most helpful while the study was being conducted. The Sutherland group at LSHTM was kind to allow use of laboratory space, consumables, and parasitological advice; and Mary Oguike assisted with PoTRA assays to confirm species-specific PCR where necessary. Mojca Kristan and Mary Oguike allowed access to and use of An. coluzzii for EAG experiments at LSHTM, and we thank the staff of the insectaries at icipe for providing An. gambiae for the cage assay experiments and to Wouter van Veen and colleagues of the Experimental Zoology Group at Wageningen University for help with rearing An. coluzzii for IAC behavioral experiments. Angela Hunt-Cooke reread the malaria films. We also thank Tom Walker and Tom Ant for reading the manuscript. We thank Iain Robinson for his contribution to the GC-EAG graphics. P.W. received center support from the Medical Research Council and Department for International Development and research grant support from the Bill & Melinda Gates Foundation. This work was supported by the Netherlands Organization for Health Research and Development (ZonMW TOP Grant 91211038, to W.T and R.W.S.). T.B. is further supported by a grant from The Netherlands Organization for Scientific Research (Vidi fellowship; NWO Project 016.158.306). Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
Footnotes
Conflict of interest statement: A.R., J.G.d.B., J.G.L., and W.T. are inventors on a patent application filed with the UK Intellectual Property Office (application no. 1805023.7).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721610115/-/DCSupplemental.
References
- 1.Poulin R. Parasite manipulation of host behavior: An update and frequently asked questions. Adv Study Behav. 2010;41:151–186. [Google Scholar]
- 2.Day JF, Edman JD. Malaria renders mice susceptible to mosquito feeding when gametocytes are most infective. J Parasitol. 1983;69:163–170. [PubMed] [Google Scholar]
- 3.Coleman RE, Edman JD, Semprevivo LH. Interactions between malaria (Plasmodium yoelii) and leishmaniasis (Leishmania mexicana amazonensis): Effect of concomitant infection on host activity, host body temperature, and vector engorgement success. J Med Entomol. 1988;25:467–471. doi: 10.1093/jmedent/25.6.467. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson HM, Rivero A, Read AF. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology. 2003;127:9–19. doi: 10.1017/s0031182003003287. [DOI] [PubMed] [Google Scholar]
- 5.Cornet S, Nicot A, Rivero A, Gandon S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett. 2013;16:323–329. doi: 10.1111/ele.12041. [DOI] [PubMed] [Google Scholar]
- 6.De Moraes CM, et al. Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci USA. 2014;111:11079–11084. doi: 10.1073/pnas.1405617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:e298. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista EP, Costa EF, Silva AA. Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasit Vectors. 2014;7:251. doi: 10.1186/1756-3305-7-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busula AO, et al. Gametocytemia and attractiveness of Plasmodium falciparum-infected Kenyan children to Anopheles gambiae mosquitoes. J Infect Dis. 2017;216:291–295. doi: 10.1093/infdis/jix214. [DOI] [PubMed] [Google Scholar]
- 10.Turell MJ, Bailey CL, Rossi AA. Increased mosquito feeding on rift valley fever virus-infected lambs. Am J Trop Med Hyg. 1984;33:1232–1238. doi: 10.4269/ajtmh.1984.33.1232. [DOI] [PubMed] [Google Scholar]
- 11.Coleman RE, Edman JD. Feeding-site selection of Lutzomyia longipalpis (Diptera: Psychodidae) on mice infected with Leishmania mexicana amazonensis. J Med Entomol. 1988;25:229–233. doi: 10.1093/jmedent/25.4.229. [DOI] [PubMed] [Google Scholar]
- 12.Baylis M, Nambiro CO. The effect of cattle infection by Trypanosoma congolense on the attraction, and feeding success, of the tsetse fly Glossina pallidipes. Parasitology. 1993;106:357–361. doi: 10.1017/s0031182000067093. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea B, et al. Enhanced sandfly attraction to Leishmania-infected hosts. Trans R Soc Trop Med Hyg. 2002;96:117–118. doi: 10.1016/s0035-9203(02)90273-7. [DOI] [PubMed] [Google Scholar]
- 14.Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 15.Logan JG, et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol. 2008;34:308–322. doi: 10.1007/s10886-008-9436-0. [DOI] [PubMed] [Google Scholar]
- 16.Verhulst NO, et al. Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS One. 2011;6:e28991. doi: 10.1371/journal.pone.0028991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muirhead-Thomson RC. The distribution of anopheline mosquito bites among different age groups; a new factor in malaria epidemiology. BMJ. 1951;1:1114–1117. doi: 10.1136/bmj.1.4715.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert IH, Gouck HK, Smith N. Attractiveness of men and women to Aedes aegypti and relative protection time obtained with Deet. Fla Entomol. 1966;49:53. [Google Scholar]
- 19.Fernández-Grandon GM, Gezan SA, Armour JA, Pickett JA, Logan JG. Heritability of attractiveness to mosquitoes. PLoS One. 2015;10:e0122716. doi: 10.1371/journal.pone.0122716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prugnolle F, et al. Infection and body odours: Evolutionary and medical perspectives. Infect Genet Evol. 2009;9:1006–1009. doi: 10.1016/j.meegid.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 21.de Boer JG, et al. Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci Rep. 2017;7:9283. doi: 10.1038/s41598-017-08978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beshir KB, et al. Measuring the efficacy of anti-malarial drugs in vivo: Quantitative PCR measurement of parasite clearance. Malar J. 2010;9:312. doi: 10.1186/1475-2875-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermsen CC, et al. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol. 2001;118:247–251. doi: 10.1016/s0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 24.Bernier UR, Booth MM, Yost RA. Analysis of human skin emanations by gas chromatography/mass spectrometry. 1. Thermal desorption of attractants for the yellow fever mosquito (Aedes aegypti) from handled glass beads. Anal Chem. 1999;71:1–7. doi: 10.1021/ac980990v. [DOI] [PubMed] [Google Scholar]
- 25.Curran AM, Rabin SI, Prada PA, Furton KG. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J Chem Ecol. 2005;31:1607–1619. doi: 10.1007/s10886-005-5801-4. [DOI] [PubMed] [Google Scholar]
- 26.Penn DJ, et al. Individual and gender fingerprints in human body odour. J R Soc Interface. 2007;4:331–340. doi: 10.1098/rsif.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dormont L, Bessière J-M, Cohuet A. Human skin volatiles: A review. J Chem Ecol. 2013;39:569–578. doi: 10.1007/s10886-013-0286-z. [DOI] [PubMed] [Google Scholar]
- 28.Menger DJ, Van Loon JJA, Takken W. Assessing the efficacy of candidate mosquito repellents against the background of an attractive source that mimics a human host. Med Vet Entomol. 2014;28:407–413. doi: 10.1111/mve.12061. [DOI] [PubMed] [Google Scholar]
- 29.Berna AZ, et al. Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J Infect Dis. 2015;212:1120–1128. doi: 10.1093/infdis/jiv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaleta KT, Hill SR, Birgersson G, Tekie H, Ignell R. Chicken volatiles repel host-seeking malaria mosquitoes. Malar J. 2016;15:354. doi: 10.1186/s12936-016-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puri SN, et al. Electroantennogram and behavioral responses of Culex quinquefasciatus (Diptera: Culicidae) females to chemicals found in human skin emanations. J Med Entomol. 2006;43:207–213. doi: 10.1603/0022-2585(2006)043[0207:eabroc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. Int J Cancer. 2010;126:2663–2670. doi: 10.1002/ijc.24970. [DOI] [PubMed] [Google Scholar]
- 33.Becker K, et al. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int J Parasitol. 2004;34:163–189. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Emami SN, et al. A key malaria metabolite modulates vector blood seeking, feeding, and susceptibility to infection. Science. 2017;4563:1–9. doi: 10.1126/science.aah4563. [DOI] [PubMed] [Google Scholar]
- 35.Kelly M, et al. Malaria parasites produce volatile mosquito attractants. MBio. 2015;6:e00235-15. doi: 10.1128/mBio.00235-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhulst NO, et al. Cultured skin microbiota attracts malaria mosquitoes. Malar J. 2009;8:302. doi: 10.1186/1475-2875-8-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauck KE, De Moraes CM, Mescher MC. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc Natl Acad Sci USA. 2010;107:3600–3605. doi: 10.1073/pnas.0907191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hacker CS. The differential effect of Plasmodium gallinaceum on the fecundity of several strains of Aedes aegypti. J Invertebr Pathol. 1971;18:373–377. doi: 10.1016/0022-2011(71)90040-1. [DOI] [PubMed] [Google Scholar]
- 39.Freier JE, Friedman S. Effect of host infection with Plasmodium gallinaceum on the reproductive capacity of Aedes aegypti. J Invertebr Pathol. 1976;28:161–166. doi: 10.1016/0022-2011(76)90117-8. [DOI] [PubMed] [Google Scholar]
- 40.Vézilier J, Nicot A, Gandon S, Rivero A. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc Biol Sci. 2012;279:4033–4041. doi: 10.1098/rspb.2012.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson RA, Knols BG, Koella JC. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology. 2000;120:329–333. doi: 10.1017/s0031182099005570. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson HM, Read AF. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18:256–261. doi: 10.1016/s1471-4922(02)02281-x. [DOI] [PubMed] [Google Scholar]
- 43.Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basáñez MG. Anopheles mortality is both age- and Plasmodium-density dependent: Implications for malaria transmission. Malar J. 2009;8:228. doi: 10.1186/1475-2875-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossignol PA, et al. Enhanced mosquito blood-finding success on parasitemic hosts: Evidence for vector-parasite mutualism. Proc Natl Acad Sci USA. 1985;82:7725–7727. doi: 10.1073/pnas.82.22.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stone W, et al. A molecular assay to quantify male and female Plasmodium falciparum gametocytes: Results from 2 randomized controlled trials using primaquine for gametocyte clearance. J Infect Dis. 2017;216:457–467. doi: 10.1093/infdis/jix237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koepfli C, et al. Blood-stage parasitaemia and age determine Plasmodium falciparum and P. vivax gametocytaemia in Papua New Guinea. PLoS One. 2015;10:e0126747. doi: 10.1371/journal.pone.0126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slater HC, et al. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature. 2015;528:S94–S101. doi: 10.1038/nature16040. [DOI] [PubMed] [Google Scholar]
- 49.Phillips M, et al. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis (Edinb) 2007;87:44–52. doi: 10.1016/j.tube.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, et al. A study of the volatile organic compounds exhaled by lung cancer cells in vitro for breath diagnosis. Cancer. 2007;110:835–844. doi: 10.1002/cncr.22844. [DOI] [PubMed] [Google Scholar]
- 51.Butler SE, et al. Mechanism of anemia in Schistosoma mansoni-infected school children in Western Kenya. Am J Trop Med Hyg. 2012;87:862–867. doi: 10.4269/ajtmh.2012.12-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vickery WL, Poulin R. The evolution of host manipulation by parasites: A game theory analysis. Evol Ecol. 2010;24:773–788. [Google Scholar]
- 53.Okal MN, Francis B, Herrera-Varela M, Fillinger U, Lindsay SW. Water vapour is a pre-oviposition attractant for the malaria vector Anopheles gambiae sensu stricto. Malar J. 2013;12:365. doi: 10.1186/1475-2875-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization 2006. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets (World Health Organization, Geneva)
- 55.Busula AO, et al. Mosquito host preferences affect their response to synthetic and natural odour blends. Malar J. 2015;14:133. doi: 10.1186/s12936-015-0635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dicko A, et al. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: A single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016;16:674–684. doi: 10.1016/S1473-3099(15)00479-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider P, et al. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol. 2004;137:35–41. doi: 10.1016/j.molbiopara.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Cnops L, Boderie M, Gillet P, Van Esbroeck M, Jacobs J. Rapid diagnostic tests as a source of DNA for Plasmodium species-specific real-time PCR. Malar J. 2011;10:67. doi: 10.1186/1475-2875-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snounou G, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 60.Oguike MC, et al. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol. 2011;41:677–683. doi: 10.1016/j.ijpara.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Habtewold T, Duchateau L, Christophides GK. Flow cytometry analysis of the microbiota associated with the midguts of vector mosquitoes. Parasit Vectors. 2016;9:167. doi: 10.1186/s13071-016-1438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibb S, Strimmer K. MALDIquant: A versatile R package for the analysis of mass spectrometry data. Bioinformatics. 2012;28:2270–2271. doi: 10.1093/bioinformatics/bts447. [DOI] [PubMed] [Google Scholar]
- 63.Abba K, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011:CD008122. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verhulst NO, Weldegergis BT, Menger D, Takken W. Attractiveness of volatiles from different body parts to the malaria mosquito Anopheles coluzzii is affected by deodorant compounds. Sci Rep. 2016;6:27141. doi: 10.1038/srep27141. [DOI] [PMC free article] [PubMed] [Google Scholar]