Significance
The default mode network (DMN) is active both during the resting state and during internally directed cognition (e.g., remembering a past event) but is suppressed during focused attention to the external environment. However, it is currently unknown to what extent memory- and rest-related activity in the DMN originate from the same neuronal populations or exhibit similar temporal dynamics. Using direct intracranial recordings in human subjects, we observed at least three distinct, yet anatomically close, classes of sites within the posteromedial cortex, a hub of the DMN; one is active when recalling an autobiographical event and suppressed during externally directed cognition, one is active during rest, and one is active during the transition from a task to rest.
Keywords: default mode network, iEEG, posteromedial cortex, autobiographical memory, spontaneous cognition
Abstract
Neuroimaging evidence supports a role of the default mode network (DMN) in spontaneous thought and goal-driven internally oriented processes, such as recalling an autobiographical event, and has demonstrated its deactivation during focused, externally oriented attention. Recent work suggests that the DMN is not a homogeneous network but rather is composed of at least several subnetworks, which are engaged in distinct functions; however, it is still unclear if these different functions rely on the same neuronal populations. In this study, we used intracranial EEG to record from the posteromedial cortex (PMC), a core hub of the DMN, in 13 human subjects, during autobiographical memory retrieval (internally oriented), arithmetic processing (externally oriented), and cued rest (spontaneous thought), allowing us to measure activity from anatomically precise PMC sites with high temporal resolution. We observed a heterogeneous, yet spatially organized, pattern of activity across tasks. Many sites, primarily in the more ventral portion of PMC, were engaged during autobiographical recall and suppressed during arithmetic processing. Other more dorsal PMC sites were engaged during the cued-rest condition. Of these rest-active sites, some exhibited variable temporal dynamics across trials, possibly reflecting various forms of spontaneous thought, while others showed only transient activity at the beginning of cued-rest trials (i.e., after a switch from a task to cued rest), possibly involved in shifting the brain from a more focused to a more exploratory attentional state. These results suggest heterogeneity of function even within an individual node of the DMN.
The default mode network (DMN) was initially identified as a set of regions consistently suppressed during externally focused, attention-demanding tasks, relative to the “default” resting state (1, 2). However, its explicit role in ongoing behavior and cognition is not well understood. Many studies have demonstrated an increase in DMN activity during stimulus-independent thought (3) and internally directed processes, such as remembering a past event (4–7), imagining the future (4, 8), semantic/conceptual processing (9), and social cognition/mentalizing (5, 10, 11). These processes are prevalent during spontaneous cognition and must be suppressed to successfully perform many attention-demanding tasks. Other work suggests that regions within the DMN are involved in changing the locus or strength of attention (12–14), for example between stimulus-oriented and stimulus-independent thought (12), or to an overall broadening of attention (15), allowing for more exploratory thoughts and behavior. This switching or broadening of attention also occurs more at rest, when subjects are free to explore their own thoughts.
The fact that many functions are attributed to the DMN is in line with the observation that different DMN subcomponents are differentially engaged during the aforementioned processes and exhibit distinct patterns of functional connectivity at rest (16–21). Even the posteromedial cortex (PMC), a hub of the DMN and part of the “DMN-core” component (16), exhibits heterogeneous cytology (22), structural connectivity (23), and functional connectivity (17, 18, 22, 24–26). While newer fMRI studies have begun to disentangle the heterogeneity of DMN, it still remains unclear to what extent the same populations of neurons within the DMN (or its subnetworks) are engaged during distinct functions, and if so, where these distinct populations are located anatomically.
A previous study from our group (27), using intracranial recordings in the PMC of four subjects, revealed that distinct sites were activated (i.e., exhibited increased high-frequency electrical activity) during rest versus autobiographical recall. Another study by our group (28) demonstrated that memory active sites in the PMC deactivated during the math condition. These two studies demonstrated heterogeneity of function within the PMC but did not have sufficient statistical power to address whether these distinct classes of sites had consistent anatomical locations within the PMC. Moreover, they only addressed the issue of heterogeneity in the spatial, but not temporal, domain.
Examining heterogeneity across both spatial and temporal domains is crucial for understanding the DMN’s involvement across cognitive conditions. For example, during rest, one could imagine at least two general classes of activity. Mind wandering, or other types of spontaneous cognition, should exhibit variable temporal dynamics, as it is by definition not time-locked to any external events. However, there may be other neuronal activity that shifts the brain from a more focused to a broader attentional state that would occur consistently at the transition from task to rest. By measuring the temporal profile of activations at each site across trials, one can differentiate between time-locked and non-time-locked responses during rest. Intracranial EEG (iEEG) is particularly well-suited to evaluate heterogeneity of function in both the spatial and temporal domains, as it can isolate the activity of groups of neurons separated by as little as 4 mm (29) and track activity across multiple sites simultaneously with high temporal resolution (on the order of milliseconds).
In the present study, we investigate the functional heterogeneity of the human PMC in a larger cohort of subjects with denser recording sites. Our overarching prediction is that different PMC sites will exhibit different responses across task conditions. More specifically, we predict that (i) memory and rest activations will have different anatomical locations within the PMC, (ii) rest- versus memory-active sites will exhibit distinct profiles of deactivation during the math condition (i.e., externally directed cognition), and (iii) we will observe multiple temporal profiles of activity during the rest condition that reflect distinct underlying processes.
Results
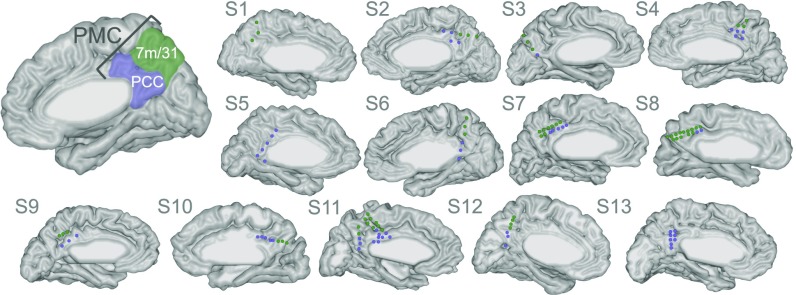
Subjects performed a task with randomly shuffled trials requiring internally directed cognition (evaluating autobiographical statements, e.g. “Yesterday I ate fruit”), externally directed cognition (evaluating arithmetic statements, e.g. “5 + 7 = 13”), or cued rest (5–10 s of undirected thought, with subjects instructed to stare at a central cross-hair). All subjects participating in this study had electrode coverage over the PMC (Fig. 1; see Table S1 for subject demographics). We measured the high-frequency broadband (HFB) activity, a signature of local cortical activation, across electrode sites (113 total) and task conditions, to identify sites that were significantly activated or deactivated during each task condition (i.e., with a significant increase or decrease in HFB power, respectively, relative to the intertrial interval). We additionally split the PMC into two anatomical subdivisions (Fig. 1), the posterior cingulate cortex (PCC; also includes the retrosplenial cortex; 56 sites) and areas 7m and 31 (7m/31; 57 sites), to determine if sites in these two subregions have differing profiles of activity across tasks.
Fig. 1.
Anatomical boundary of the PMC and two subregions of the PMC, the PCC (purple) and areas 7m and 31 (green). The electrodes falling within the PMC are marked in each individual subject’s brain and are colored according to their anatomical subregion.
Time-Locked Task-Related Neural Responses.
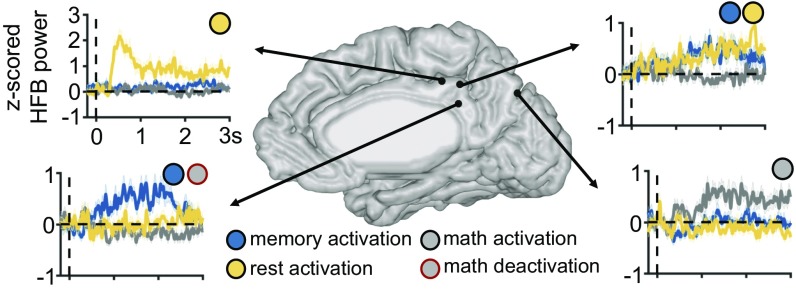
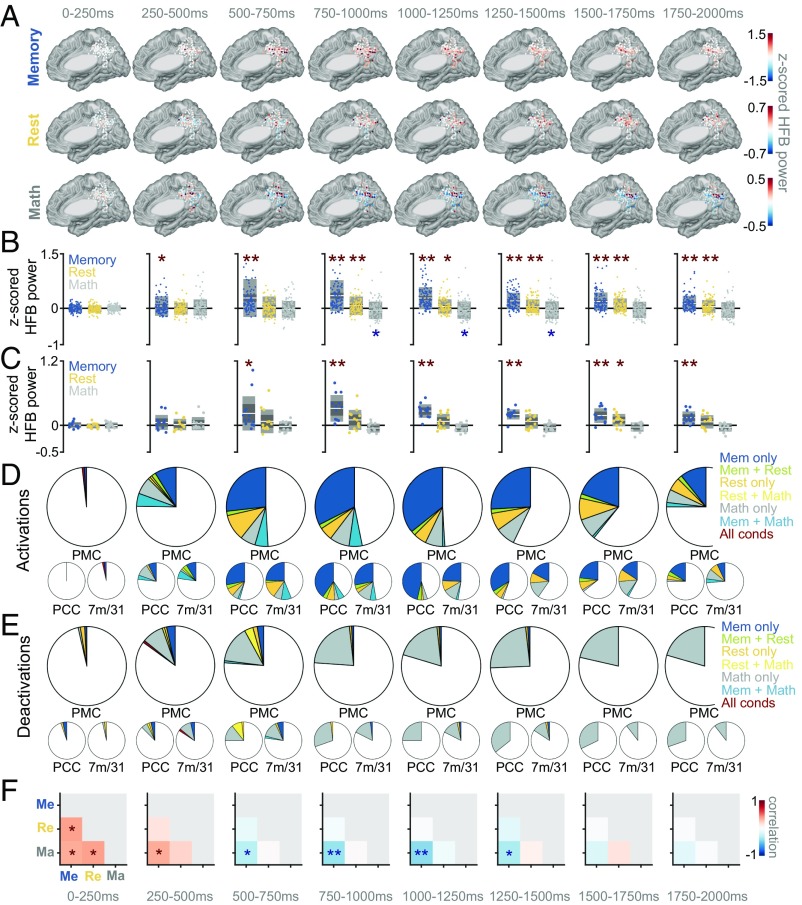
We measured changes in HFB activity in two ways. First, we computed HFB activity at each site in different time windows relative to stimulus onset, capturing solely time-locked activity. Second, we computed the total amount of time that each site was “active” (with HFB power at least two SDs above the mean of the activity during the 200-ms intertrial interval), ignoring the actual time window in which it was active (i.e., capturing both time-locked and non-time-locked activity; see SI Materials and Methods for more details). As expected, we observed several distinct profiles of activity across tasks within the PMC, a few examples of which are illustrated in Fig. 2. Many sites were activated during the memory condition (many of which were also deactivated during the math condition), a few sites were activated during the rest, and a few were activated during the math condition. Fig. 3 summarizes the time-locked HFB results across all sites in all subjects, during the three task conditions. There were several notable differences between the dynamics of response during the memory versus cued-rest conditions. First, the strength of HFB activation in the PMC was on average much larger for the memory condition than for the cued-rest condition and larger than the level of HFB deactivation during the math condition [across subjects: P < 0.05, false discovery rate (FDR) corrected by number of time windows and pairs of conditions, for every time window between 250 and 2,000 ms poststimulus, Fig. 3B; within subject: P < 0.05, FDR corrected by number of time windows and pairs of conditions, for every time window between 500 and 2,000 ms poststimulus, Fig. 3C]. Relatedly, in every time window (between 0 and 2,000 ms after stimulus onset) more sites were significantly active during the memory than during the cued-rest condition (Fig. 3D; see Table S2 for number of electrode sites active per condition/time window and number of subjects contributing to each group of active sites). Second, the time courses of activity were much different between the two conditions. HFB activity during the memory condition began to increase between 250 and 500 ms, peaked between 750 and 1,250 ms, then gradually decreased back to baseline levels. During the cued-rest condition, HFB activity on average increased slightly later, between 500 and 750 ms, but showed no clear peak in activity in time, across sites (Fig. 3 A–D). Finally, as we have previously reported (27, 28), the actual sites that were significantly active during either the memory or cued-rest condition during any individual time window were largely distinct (between 5% and 13% of memory-active sites were also active during rest, during the time windows between 500–2,000 ms after stimulus onset; Fig. 3D and Table S2). Although memory-active and rest-active sites were present in both the PCC and areas 7m/31, the majority of memory-active sites were in the PCC and the majority of rest-active sites were in areas 7m/31 (Fig. 3D and Table S2). Interestingly, we also observed several sites that were active during the math condition, which were primarily clustered in areas 7m/31 (Fig. 3 A–D and Table S2).
Fig. 2.
Example HFB time courses (z-scored relative to the 200-ms ITI) of activity across task conditions at electrode sites in a single subject (shaded region represents SEM across trials), time-locked to trial onset (vertical dotted line). Some sites were activated during the memory condition (blue circle), many of which were also and deactivated during math (gray circle, red outline), but several sites were also active during the rest condition (yellow circle), math condition (gray circle, black outline), or during multiple conditions.
Fig. 3.
(A) Progression of HFB activity in 250-ms time bins at each PMC site, separately for each condition, at all electrode sites across all subjects (transformed to common MNI space). (B) Distribution of HFB power during each time window and condition, across sites (i.e., distribution of data points from A). The center line in each box represents the mean across sites, the extents of the dark gray box represent the 95% confidence interval of the mean, and the extents of the lighter gray box represent one SD of the distribution. Asterisks indicate mean of distribution significantly different from 0 (*P < 0.05, FDR corrected; **P < 0.005, FDR corrected). (C) Average HFB power across electrodes within each subject (i.e., each dot represents a single subject) for a particular condition. Asterisks indicate mean of distribution significantly different from 0 (*P < 0.05, FDR corrected; **P < 0.005, FDR corrected). (D) Fraction of electrodes exhibiting a significant activation in each condition or combination of conditions, separately for each time window (P < 0.05, FDR corrected for number of sites, time windows, and task conditions), for the entire PMC (top), PCC (lower left), or areas 7m/31 (lower right). (E) Same as D, but showing fraction of deactivated rather than activated sites. (F) Correlation of HFB power across sites between each pair of conditions (Me, memory; Re, rest; Ma, math), separately during each time window. Asterisks indicate significant correlation, either positive or negative (*P < 0.05, FDR corrected; **P < 0.005, FDR corrected).
Another common phenomenon in the PMC besides memory-related activity was a deactivation (i.e., decrease in HFB power relative to baseline) during the math condition (Fig. 3 A–C and E and Table S2), in line with previous reports of DMN suppression during externally focused attention. Many of these math-deactivated sites were also activated during the memory condition (between 30% and 67% of math-deactivated sites, during the time windows between 500–2,000 ms after stimulus onset; Fig. S1A). Of memory-active sites, between 33% and 48% were deactivated during the math condition, in the time windows between 500–2,000 ms after stimulus onset (Fig. S1B), which were on average located more ventrally than the memory-active sites not significantly deactivated during math (Fig. S1C).
To compare the sites activated (or deactivated) across all pairs of conditions, we correlated the HFB power across sites between every pair of conditions, separately in each time window. A positive correlation between a pair of conditions suggests that the same sites are generally activated (or deactivated) during both conditions and a negative correlation indicates that sites activated during one condition tend to deactivate during the other condition, while a low (i.e., close to zero) correlation suggests that sites activated during one condition show low levels of activity in the other condition, or that the magnitudes of their activations are unrelated. In the earliest time window, HFB power was significantly positively correlated across sites between all conditions, possibly reflecting an early visual response to all stimuli at a subset of sites. However, between 500 and 1,500 ms, HFB power was significantly negatively correlated between the memory and math conditions and showed no significant correlation between the memory and rest conditions (Fig. 3F), again suggesting that the memory and rest conditions recruited largely distinct sites, at least when considering the same time window.
Non-Time-Locked Task-Related Neural Responses.
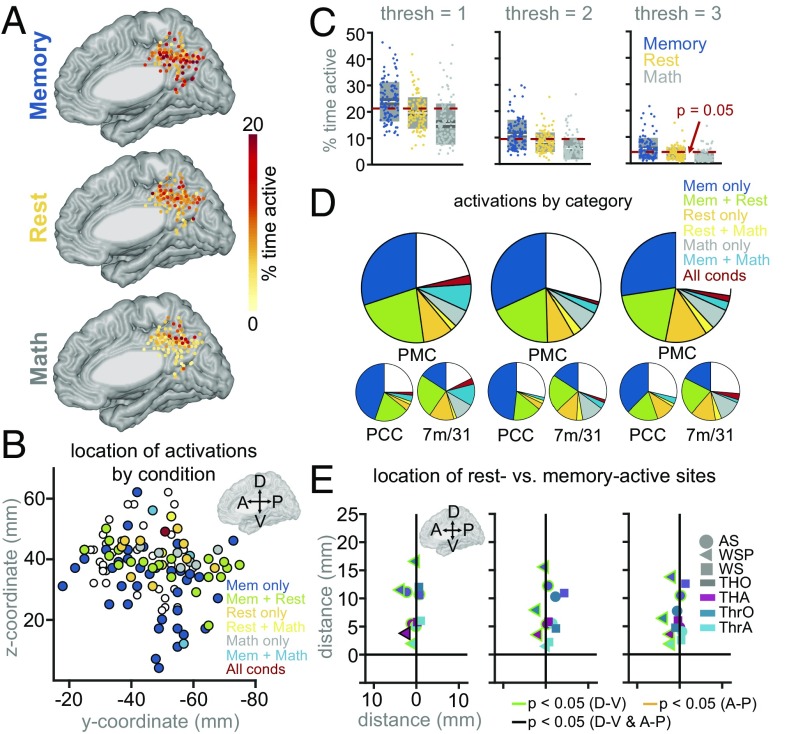
When we measured the total duration of active time in each condition, irrespective of the specific time window, we again captured sites within the PMC that were active during the memory condition. However, we also observed many sites, primarily in the more dorsal portion of PMC, that were active (many in a non-time-locked manner) during the rest condition, and many that were active during both memory and rest (Fig. 4 A, B, and D). As in the time-locked analysis, we again showed that the electrodes with the largest percentage of active time during the memory condition tended to be the least active during the math condition (r = −0.23, P = 0.01; Fig. S2A). While the correlation between activation time during the rest and memory conditions was nonsignificant (r = 0.11, P = 0.25; Fig. S2A), it was still higher than the correlations we observed between the same conditions in the time-locked analyses during every time window from 500 to 2,000 ms after stimulus onset (Fig. 3F; correlation ranged between −0.13 to −0.0017 during these windows). This suggests that more electrode sites showed activity during both memory and rest conditions when considering activations at any time. There was no significant correlation in HFB activation time between the math and rest conditions (r = 0.06, P = 0.53; Fig. S2A).
Fig. 4.
(A) Percent active time (with HFB power at least two SDs above mean of baseline) at each site, separately for each condition. (B) Anatomical location (y and z coordinates in common MNI space) of all PMC sites significantly activated during each condition or combination of conditions (i.e., those illustrated in the pie chart in D, middle column, threshold = 2; sites marked in white not significantly activated in any condition). C–E illustrate effect of changing activation threshold level. (C) Percent active time of each electrode in each condition. Dotted red line represents the P < 0.05 level, as determined by a Monte Carlo simulation (see SI Materials and Methods for more details). (D) Fraction of sites exhibiting a significant percent of activated time (i.e., above the dotted red line in C), in any condition or combination of conditions, either for the entire PMC (top), the PCC (lower left), or areas 7m/31 (lower right). (E) Difference in coordinates between rest-active and memory-active sites, computed 12 different ways (see Spatial Heterogeneity for more details). Border color indicates significance of each comparison, along the dorsal–ventral (D-V) or anterior–posterior (A-P) dimensions (green, P < 0.05 in D-V dimension, FDR corrected for 12 comparisons; yellow, P < 0.05 in A-P dimension, FDR corrected; black, P < 0.05 for both D-V and A-P dimensions, FDR corrected; otherwise, no border).
We tested several thresholds for detecting an activation (either one, two, or three SDs above the mean of the baseline HFB power), to ensure that our results were robust to changing algorithm parameters. Regardless of the threshold chosen, we found that, as in the time-locked analysis, more sites were activated during the memory condition than during the rest condition (Fig. 4 C and D and Table S3). However, relatively more sites were active during the rest-active condition in this non-time-locked analysis (Fig. 4D and Table S3) than in the time-locked analysis (Fig. 3D and Table S2), and more sites had both memory and rest-related activations when considering activations at any time (Fig. 4D and Table S3), relative to when looking within a specific time window (Fig. 3D and Table S2). For all thresholds, over half of the electrode sites active during the rest condition were also active during the memory condition (Fig. 4D and Table S3). As in the time-locked analysis, the majority of memory-active sites were located in the PCC, and the majority of rest-active sites were located in areas 7m/31 (Fig. 4D and Table S3).
Relative Anatomical Location of Rest- and Memory-Related Activity.
Next, we wanted to see if there was a consistent anatomical relationship between the rest- and memory-active sites. We measured such intersite distances in several ways. First, we defined “activated” sites either as the top half of most active sites individually within each category (thus yielding different thresholds for activation time in each category; TH for top half), or relative to the percent of activated time that would be expected by chance (determined based on a Monte Carlo simulation; see SI Materials and Methods for more details; Thr for threshold). Second, we either compared the average coordinates of all of the rest-active sites with the average coordinates of all of the memory-active sites (AS for across-subjects), in which case electrodes from the two groups may be coming from different individuals, or we only considered electrode pairs from within the same individual (WSP for within-subject pairs and WS for within-subject). The results from all of these methods are plotted in Fig. 4E (more details are included in Spatial Heterogeneity), and in all cases we found that rest-active sites were located more dorsally than memory-active sites. When only considering within-subject electrode pairs (WSP), this difference was statistically significant (P < 0.05, FDR corrected) in 11 of 12 comparisons. When looking across subjects (AS) this difference was statistically significant in 7 of 12 comparisons (P < 0.05, FDR corrected). When averaging within-subject electrode pairs within each subject separately (WS), rest-active sites were still more dorsal than memory-active sites in the majority of subjects (who had at least one rest-active and one memory-active site), but this difference was not statistically significant across subjects (perhaps because of the low statistical power with so few data points). Across methods, the actual anatomical difference between rest and memory activations in the z dimension ranged from 1.5 to 16.6 mm, with a median distance of 5.9 mm. The few math-active sites were also on average located more dorsally than the memory-active sites, and slightly dorsal and posterior to the rest-active sites (Fig. S2B). As an additional control, to ensure that the difference in trial length between rest and memory trials was not skewing our results, we reran the non-time-locked analyses after matching the average length of memory and rest trials within subject (Fig. S3) and found that rest-active sites were located more dorsally to memory-active sites.
Distinct Temporal Dynamics of Rest- and Memory-Related Neural Responses.
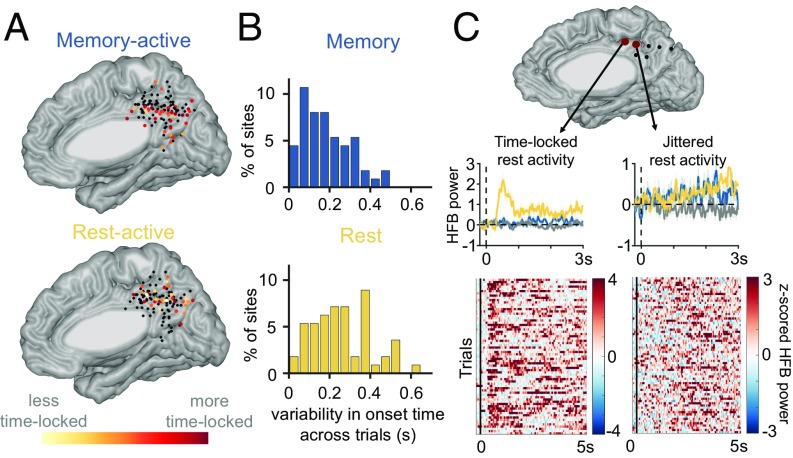
Finally, we compared the temporal dynamics during the memory versus cued-rest conditions, specifically how time-locked each site’s responses were to trial onset (Fig. 5 A and B; method illustrated in Fig. S5). We computed this difference in multiple ways, using different algorithm parameters (e.g., magnitude threshold for activation), and either across subjects (i.e., comparing all of the rest-active to all of the memory-active sites) or across within-subject pairs, to ensure that specific algorithm parameters were not driving our results. In all iterations, we found that on average the most memory-active sites exhibited more time-locked activity during the memory trials than the most rest-active sites did during rest trials (i.e., onset variability was higher for rest-active sites; Fig. S4). However, there was much variability in the temporal profiles of the rest-active sites, with some exhibiting more time-locked activity (e.g., Fig. 5C, Left) and others more jittered activity (e.g., Fig. 5C, Right).
Fig. 5.
(A) Consistency of activation time at the top 50% of memory-active sites across memory trials (Top) and at the top 50% of rest-active sites across rest trials (Bottom) are shown individually at each site (only activations within the first 2 s of each trial were included), given an activation threshold of two SDs above the mean of the baseline. (B) Distribution of across-trial activation time jitter for memory-active sites (Top) and rest-active sites (Bottom) (i.e., distribution of data points from A). (C) Temporal profiles of two exemplar cued-rest-active sites from a single subject are shown (left-hand, time-locked; right-hand, non-time-locked).
Since we predicted that spontaneous thought/mind wandering would be associated with more jittered neural activity, we reasoned that sites showing this jittered activity during rest would be more likely also to be activated during the memory condition (since much mind-wandering activity relates to remembering past events), relative to rest sites showing more time-locked activity (which we hypothesized are more related to shifting the brain’s attentional state, and thus less directly related to memory processing). We found that rest-active sites that were also active during the memory condition did show slightly less time-locked activity during the rest condition than did sites that were only active during rest (mean of 0.3-s variability versus mean of 0.28-s variability, respectively); however, this difference was not statistically significant (P = 0.79). Notably, the sites with time-locked activity following a transition to rest did not show similar activity following other state transitions, for example from rest to a task (either math or memory), or between math and memory trials.
Discussion
Summary of Findings.
In this study, we measured iEEG activity throughout the PMC across several behavioral tasks, allowing us to compare the sites engaged during distinct functions which have all been associated with the DMN. We found many sites in the PMC engaged during autobiographical recall, starting at around 250–500 ms after stimulus onset, many of which were also deactivated during the math condition (Fig. 3F and Fig. S1). When considering time-locked activity, we found few sites that responded during both the memory and rest conditions with similar timing (Fig. 3D and Table S2). However, we found greater overlap between memory- and rest-active sites when considering both time-locked and non-time-locked activations (Fig. 4D and Table S3). Of rest-active sites, some exhibited variable timing across trials, likely reflecting mind wandering or other spontaneous thought (the content of which could not be deciphered in this study). Others exhibited time-locked responses, possibly signaling a change from an attention-demanding condition to the lack thereof (Fig. 5). In general, the rest-active sites were located more dorsally than the memory-active sites within individual subjects’ brains (Fig. 4 B and E and Fig. S3C).
Distinct and Overlapping Sites Engaged During Autobiographical Recall Versus Spontaneous Thought.
When considering only time-locked activity, we found few sites that responded during both the memory and rest conditions (i.e., in the same time window; Fig. 3D and Table S2). This is perhaps unsurprising given the nature of the two task conditions. During the memory condition, subjects were cued to retrieve a memory by the written autobiographical statement; thus, memory-related activity should be time-locked to the beginning of the trial. During the rest condition, however, subjects were not given any explicit instructions on what to think about. Thus, even if they were remembering past events during a subset of these trials, such processes would unlikely occur with any consistent timing. This likely explains why we observed many more sites active during both the memory and rest conditions when considering activations occurring at any time within the trial (Fig. 4D). Our choice of baseline [the 200-ms intertrial interval (ITI)], which was most similar in nature to the rest condition, may also have hampered our ability to detect rest-related activations.
While some non-time-locked rest activations occurred at the same sites as memory activations, other did not. There are multiple possible explanations for such non-time-locked rest-selective activations. Perhaps subjects were engaged in other nonautobiographical memory-related thoughts during the cued-rest condition, such as thinking about their own condition, making plans, or inferring another’s mental state (30). Alternatively, even if subjects were at times thinking about past events during the cued-rest epochs, there may be different neural dynamics associated with focused attention toward memory-related processes to carry out a task, versus recalled memories as part of a stream of spontaneous, undirected thoughts (31). The fact that deactivations during the math condition occurred mostly at memory-active but not rest-active sites supports previous work showing competition between focused attention to externally oriented versus internally oriented processes (32–34) but suggests that spontaneous cognition, during which attention is broad and unfocused, may recruit distinct sites.
We found rest-active sites to be, on average, more dorsal than the memory-active sites within the PMC (Fig. 4 B and E and Fig. S3C). When grouping electrode sites by PMC subregion, the majority of memory-active sites were located in the PCC while the majority of rest-active sites were located in areas 7m/31. This ventral-to-dorsal memory-to-rest gradient within the PMC is in line with recent work showing fractionation within the DMN (16–18, 25). Several studies have shown that ventral PMC is more functionally connected to the hippocampus/middle temporal lobe (16, 18, 35), while the dorsal PMC is more functionally connected with sensorimotor and cognitive control networks (17, 25, 26, 35), as well as with the temporal–parietal junction (TPJ) (18), which has been associated with social cognition/theory of mind (36). Given these patterns of functional connectivity, it is not surprising that we observed memory-related activity in the more ventral portion of the PMC and rest-related activity in more dorsal portion (possibly working with the frontoparietal control networks to broaden attention during spontaneous thought, or with the TPJ to drive other nonautobiographical, self-referential processes).
Interestingly, a small cluster of PMC sites (primarily in areas 7m/31) were activated during the math condition. While we intended the math condition to elicit more externally oriented processes, subjects may also have been engaged in internally oriented processes such as retrieving an arithmetic fact from memory, or internally verbalizing the equation. It would thus be informative in future studies to compare the location of these math-related activations to those evoked by a more explicitly externally oriented task, for example visual search.
Time-Locked Cued-Rest Responses.
While neural responses that are time-locked to the beginning of math or memory trials are expected, it was surprising to find time-locked neural responses selectively at the beginning of cued-rest trials, in which individuals simply viewed a fixation cross on a dark screen. Since these time-locked responses were mostly absent during the math condition, they are unlikely related to visual processing of the rest cue (“+”) given that arithmetic statements also contained a plus sign. Moreover, these rest-locked responses had relatively late onsets (>500 ms), beyond when the initial visual processing of the stimuli should occur. One possible explanation is that this time-locked activity reflects a “switch” signal, involved in changing one’s attentional state from a more focused to a more exploratory one. Since this study only examined activity in the PMC, it remains to be determined if these switch sites also exist in other brain regions, for example in other brain regions involved in changing the locus of attention [e.g., the dorsal attention network (37) or frontoparietal control networks (35, 38)]. Also, the fact that these PMC sites were only engaged after the transition from task to rest, but not after other state transitions, suggests that different sites are engaged in different classes of “state switches,” and thus that other types of switches likely exist across the brain.
Future Directions.
In this study, we built on previous work suggesting that the DMN is composed of at least several subcomponents. We demonstrate heterogeneity of function even within the PMC, a hub of the DMN, with several distinct, but anatomically close, response profiles across tasks. It is important to note, however, that electrode sites were selected based on their anatomical location rather than their membership to the DMN as determined with resting-state functional connectivity. Gaining insight into how each distinct type of PMC site contributes, within the context of larger networks, to either autobiographical memory recall or to controlling spontaneous thought, will require future work that combines multiple recording modalities, to track the dynamics on multiple spatial and temporal scales. For instance, combining the temporal resolution of iEEG with the whole-brain coverage of fMRI, and collecting long-duration fMRI datasets within individuals to obtain spatially precise subject-specific maps (18, 39, 40), would likely be fruitful in determining the functional connectivity and network dynamics of distinct rest-active sites within the PMC.
Materials and Methods
General Methods.
We recorded iEEG responses from 13 subjects (five female) with epilepsy who were implanted with intracranial electrodes as part of their presurgical evaluation at Stanford University Medical Center, while subjects performed the behavioral task described below. All subjects had at least one electrode site placed over the PMC, but electrode locations were determined purely for clinical reasons, based on each patient’s presumed seizure focus. Demographic information is included in Table S1, and individual subject electrode coverage is displayed in Fig. 1. Each patient was monitored in the hospital for ∼6–10 d following surgery, and all subjects provided verbal and written consent before participating in any experiments. All experiments with human subjects were approved by the Stanford Institutional Review Board.
We extracted HFB (70–180 Hz) activity from each site, as a measure of local cortical activation (29, 41, 42), to identify (i) which sites were significantly activated during each condition and (ii) at what time such activations occurred.
Experimental Design: Behavioral Paradigm.
Subjects were asked to make true/false judgments on a series of visually presented statements, requiring either autobiographical memory retrieval (memory condition; e.g., “I ate fruit yesterday”) or arithmetic processing (math condition; e.g., “48 + 8 = 57”) (as described previously in refs. 27 and 28). Subjects had up to 15 s to respond (by pressing one of two keypad buttons) to each statement, but the task was self-paced and moved on to the next trial after the subject responded (usually within a few seconds; Table S1). These statements were presented in random order and were interspersed with cued-rest trials, during which subjects fixated at a center cross-hair for 5 or 10 s and were asked not to think about anything in particular. Each subject performed between 47–96 memory trials, 47–96 math trials, and 20–70 rest trials (subject specific trial numbers are given in Table S1). Arithmetic equations always consisted of one-digit number and a two-digit number, to minimize the chance that subjects were relying on rote memorization of arithmetic facts. Trials were separated by a 200-ms ITI. Stimuli were visually presented on a laptop computer (Apple MacBook or MacBook Pro) using MATLAB psychtoolbox (see Supporting Information for additional details on analysis methods).
Supplementary Material
Acknowledgments
We thank all the patients who participated in this study for volunteering their time and the Laboratory of Behavioral & Cognitive Neuroscience members for their feedback throughout this project. This work was supported by National Institute of Neurological Disorders and Stroke Grant R01NS078396, National Institute of Mental Health Grant 1R01MH109954-01, NSF Grant BCS1358907 (all to J.P.), and National Institute of Child Health and Human Development Postdoctoral Fellowship 1F32HD087028-01 (to A.L.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721714115/-/DCSupplemental.
References
- 1.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 3.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews-Hanna JR, Saxe R, Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage. 2014;91:324–335. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder JR, et al. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 10.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiers HJ, Maguire EA. Spontaneous mentalizing during an interactive real world task: An fMRI study. Neuropsychologia. 2006;44:1674–1682. doi: 10.1016/j.neuropsychologia.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. Eur J Neurosci. 2005;21:1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- 13.Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crittenden BM, Mitchell DJ, Duncan J. Recruitment of the default mode network during a demanding act of executive control. eLife. 2015;4:e06481. doi: 10.7554/eLife.06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braga RM, Buckner RL. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron. 2017;95:457–471.e5. doi: 10.1016/j.neuron.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantini D, et al. Default mode of brain function in monkeys. J Neurosci. 2011;31:12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axelrod V, Rees G, Bar M. The default network and the combination of cognitive processes that mediate self-generated thought. Nat Hum Behav. 2017;1:896–910. doi: 10.1038/s41562-017-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon ML, et al. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage. 2017;147:632–649. doi: 10.1016/j.neuroimage.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 25.Margulies DS, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Connectivity gradients between the default mode and attention control networks. Brain Connect. 2011;1:147–157. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dastjerdi M, et al. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci USA. 2011;108:3023–3028. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster BL, Dastjerdi M, Parvizi J. Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing. Proc Natl Acad Sci USA. 2012;109:15514–15519. doi: 10.1073/pnas.1206580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flinker A, Chang EF, Barbaro NM, Berger MS, Knight RT. Sub-centimeter language organization in the human temporal lobe. Brain Lang. 2011;117:103–109. doi: 10.1016/j.bandl.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 31.Christoff K, Irving Z, Fox KCR, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci. 2016;17:718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 32.Lachaux JP, et al. Silence is golden: Transient neural deactivation in the prefrontal cortex during attentive reading. Cereb Cortex. 2008;18:443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- 33.Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: Functional specialization and dynamic competition in human posterior parietal cortex. J Neurosci. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2012;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 38.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon EM, et al. Precision functional mapping of individual human brains. Neuron. 2017;95:791–807.e7. doi: 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poldrack RA, et al. Long-term neural and physiological phenotyping of a single human. Nat Commun. 2015;6:8885. doi: 10.1038/ncomms9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]