Significance
Neurons and muscularis macrophages (MM) interact closely in the adult bowel and enteric neurons modulate the inflammatory response of neighboring MM. Enteric neurons also produce the essential macrophage survival factor colony stimulating factor 1. How MM–enteric neuron interactions are established during development has not been previously investigated. Surprisingly, we find that MM colonize fetal mouse bowel before enteric neurons. Furthermore, neonatal mouse and human bowel that is missing enteric neurons contains normal numbers of well-patterned, phenotypically intact MM. These data suggest that enteric neurons are not required for bowel colonization by MM and that modulatory neuro-immune circuits of the bowel mature postnatally and may be influenced by environmental factors like intestinal microbes or diet.
Keywords: muscularis macrophages, enteric nervous system, neuroimmunology, Hirschsprung disease, Ret
Abstract
The nervous system of the bowel regulates the inflammatory phenotype of tissue resident muscularis macrophages (MM), and in adult mice, enteric neurons are the main local source of colony stimulating factor 1 (CSF1), a protein required for MM survival. Surprisingly, we find that during development MM colonize the bowel before enteric neurons. This calls into question the requirement for neuron-derived CSF1 for MM colonization of the bowel. To determine if intestinal innervation is required for MM development, we analyzed MM of neonatal Ret−/− (Ret KO) mice that have no enteric nervous system in small bowel or colon. We found normal numbers of well-patterned MM in Ret KO bowel. Similarly, the abundance and distribution of MM in aganglionic human colon obtained from Hirschsprung disease patients was normal. We also identify endothelial cells and interstitial cells of Cajal as the main sources of CSF1 in the developing bowel. Additionally, MM from neonatal Ret KOs do not differ from controls in baseline activation status or cytokine-production in response to lipopolysaccharide. Unexpectedly, these data demonstrate that the enteric nervous system is dispensable for MM colonization and patterning in the bowel, and suggest that modulatory interactions between MM and the bowel nervous system are established postnatally.
Muscularis macrophages (MM) are tissue resident macrophages found within and between the circular and longitudinal muscle layers of the bowel wall. MM interact closely with enteric neurons in the myenteric plexus (1, 2) and represent a unique population of bowel macrophages that differs morphologically (3), transcriptionally (1), and likely functionally from neighboring macrophages in bowel mucosa and lamina propria.
Initial studies of MM in bowel muscle layers reported frequent tight contacts between macrophage processes and myenteric neurons in a “lock-and-key” configuration (2, 4). This suggested close communication between these cell types. However, signals exchanged between the bowel nervous system and MM are just beginning to be characterized. A recent study reported that MM produce bone morphogenetic protein 2 (BMP2), which alters enteric neuron development and influences bowel motility (5). The same study indicated that enteric neurons are the main source of the MM survival factor colony stimulating factor 1 (CSF1) in the adult bowel. Neuronal signaling within the bowel was also reported to skew MM toward an antiinflammatory, tissue protective phenotype (1, 6).
Investigation of neuronal signaling to MM has thus far been limited to adult animals. However, MM and enteric neurons exist in close contact for days within the developing bowel. Furthermore, Op/Op mice with mutant Csf1 and consequent MM depletion have defects in adult enteric nervous system (ENS) patterning (5, 7), implying that signaling during development between MM and the ENS has lasting consequences. Clarifying the developmental interplay between enteric neurons and MM may be particularly relevant to understanding what role, if any, MM have in the pathogenesis of Hirschsprung associated enterocolitis (HAEC). Hirschsprung disease (HSCR) is a birth defect where the ENS is absent from the distal bowel. HAEC is a form of severe persistent bowel inflammation that is the leading cause of death in children with HSCR (8, 9). Although several factors may contribute to HAEC (10), pathophysiologic mechanisms leading to excess inflammation are not well understood and the role of MM in HAEC has never been examined.
To determine if the ENS is important for MM development, we analyzed the bowel of Ret knockout (Ret KO) mice (11), which have no enteric neurons or enteric glia in the small bowel or colon. We find, surprisingly, that newborn Ret KO muscularis externa contains normal numbers of well-patterned MM that are phenotypically similar to control MM. Consistent with this observation, we show that the main CSF1 mRNA sources in developing bowel are nonneuronal, and include endothelial cells and interstitial cells of Cajal (ICC). Lipopolysaccharide (LPS) treatment of muscularis externa to activate Toll-like-receptor 4 (TLR4) led to similar MM responses in Ret KO vs. control tissue. These findings suggest that neuronal circuitry responsible for modulating MM activation in adults is not present or fully mature in newborn bowel and that enteric neurons are not needed to recruit or pattern MM in the bowel wall.
Results
Muscularis Externa Colonization by MM During Development Does Not Require Enteric Neural Crest-Derived Cells.
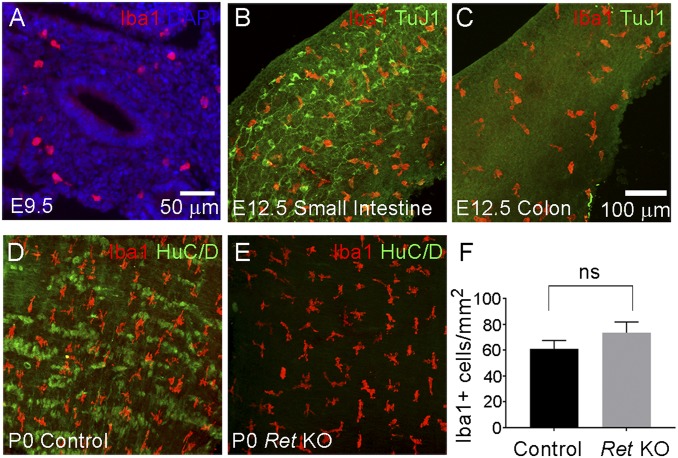
Vagal enteric neural crest-derived cells (ENCDC) that become neurons and glia of the ENS first invade the bowel foregut at embryonic day 9.5 (E9.5) in mice and then migrate through the bowel in a rostrocaudal direction (12). By E12.5, ENCDC reach midcolon and by E14.5 the bowel is fully colonized by ENS precursors. MM have been reported in the bowel as early as E15.5 (4), but whether MM are present before ENCDC colonization was not known. Since adult enteric neurons produce CSF1, which supports MM survival, we hypothesized ENCDC should colonize fetal bowel before MM to create a hospitable environment. To test this hypothesis, we used TuJ1 antibody, which binds neuron-specific β3 tubulin, to label enteric neurons in WT mice. TuJ1 identifies all bowel regions that contain ENCDC (13–15) because enteric neurons differentiate alongside migrating ENCDC. MM were identified with Iba1 antibody commonly employed for central nervous system microglia staining. We confirmed Iba1 labels all MM in the developing bowel using Cx3cr1-EGFP reporter mice that express EGFP in all bowel macrophages (Fig. S1). Iba1 also stains all CD68+ human MM (Fig. S2). Iba1+ cells were present in the outer bowel wall where muscle layers develop as early as E9.5, a time when ENCDC first invade the foregut (Fig. 1A). At E12.5, MM are present throughout the small bowel alongside enteric neurons (Fig. 1B). However, MM are also present in the distal colon, which lacks ENCDC at this age (Fig. 1C), raising the possibility that colonization of fetal bowel by MM does not require the ENS. To definitively determine if ENS is needed for MM colonization of the bowel, we analyzed muscularis externa of neonatal (postnatal day, P0) Ret KO mice that are completely missing enteric neurons and glia in the small bowel and colon. Surprisingly, the density and distribution of MM in muscularis externa of P0 Ret KO mice closely matched littermate controls, where enteric neurons were visualized with HuC/D antibody and nerve fibers can be seen with TuJ1 antibody (Fig. 1 D–F and Fig. S3A). In Ret KO mice, there are occasional extrinsic nerve fibers seen with TuJ1 antibody, but too few nerve fibers to account for MM patterning in muscularis externa (Fig. S3B).
Fig. 1.
MM colonize the bowel with or without enteric neurons. (A) Sagittal section of WT E9.5 foregut stained with Iba1 antibody (red) and with DAPI (blue). This shows Iba1+ macrophages present in the bowel at E9.5. (B) WT E12.5 whole-mount small intestine stained with Iba1 (red) and TuJ1 (green) antibodies. (C) WT E12.5 whole-mount colon stained with Iba1 (red) and TuJ1 (green) antibodies. This shows Iba1+ macrophages in E12.5 WT colon that is not yet colonized by ENCDC. (D and E) Whole-mount P0 muscularis externa from control and Ret KO distal small intestine stained with Iba1 (red) and HuC/D (green) antibodies. Ret KO bowel is devoid of HuC/D+ enteric neurons, but has well patterned Iba1+ MM present in normal abundance. (F) Quantitative analysis of Iba1+ cell density in small bowel showed no statistically significant difference between WT and Ret KO small bowel (Student’s t test, P > 0.05, n = 8 mice per genotype). NS, not significant. Error bar = SEM. (Scale bar: 100 µm.) Scale bar in C applies to B–E.
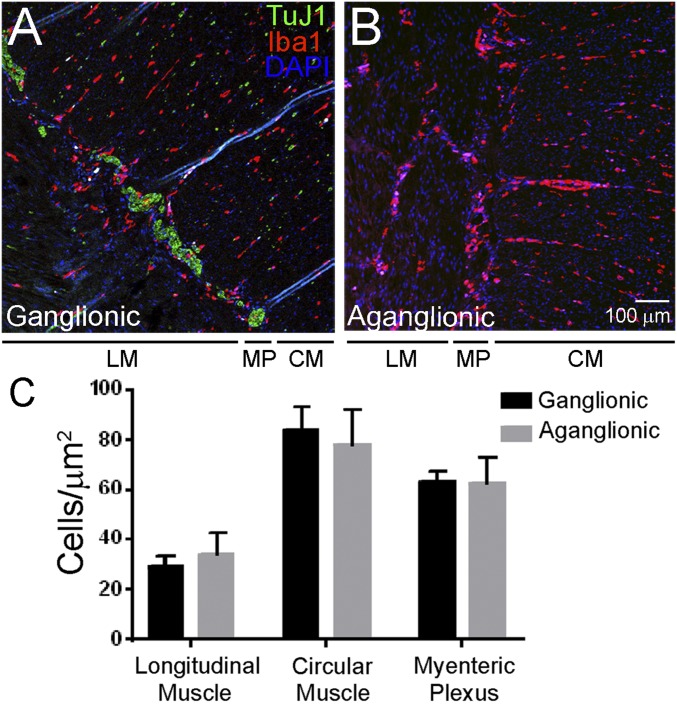
To determine if ENS is required for MM bowel colonization in humans, we analyzed enteric neuron-containing (ganglionic) and enteric neuron-deficient (aganglionic) colon segments obtained from children with HSCR at the time of pull-through surgery. Similar to findings in mice, there was no difference in Iba1+ MM number or distribution in the muscularis externa of ganglionic vs. aganglionic human colon (Fig. 2). This suggests that enteric neurons are not required for MM colonization and patterning in the bowel wall of mice or humans.
Fig. 2.
MM are present in normal abundance and distribution in human aganglionic colon from children with Hirschsprung disease. (A and B) Human colon was stained with antibodies to TuJ1 (green), Iba1 (red), and with the nuclear dye DAPI (blue). Iba1+ macrophages are present in ganglionic (A) and aganglionic (B) human muscularis externa in normal numbers and with normal distribution across muscle layers. (Scale bar: 100 µm.) (C) Quantitative analysis of MM abundance in layers of the muscularis propria. CM, circular muscle; LM, longitudinal muscle; MP, myenteric plexus. Student’s t test, P > 0.05 for all comparisons. n = 8 ganglionic and 8 aganglionic. Error bar = SEM.
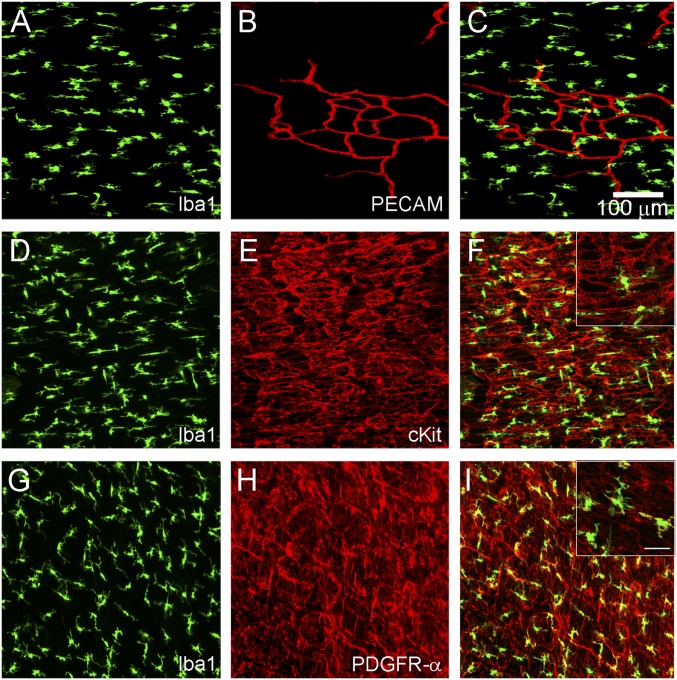
MM Do Not Distribute Along Vasculature in Muscularis Externa, but Closely Associate with ICC and PDGFRα+ Fibroblast-Like Cells.
Postnatal MM appear evenly distributed across muscularis externa with nonoverlapping processes. Because this patterning does not rely on the ENS, we determined whether MM closely associate with other muscularis cell types. Platelet endothelial cell adhesion molecule (PECAM) staining clearly shows MM are not patterned by blood vessels (Fig. 3 A–C). Some MM closely associate with cKIT+ cells of the very dense ICC network (Fig. 3 D–F), as previously reported (16, 17). MM also closely associate with PDGFRα+ fibroblast-like cells (Fig. 3 G–I). These associations are similar to those between MM and enteric neurons (Fig. 1D) (1, 2, 5). However, these occasional contacts cannot fully account for the regular patterning of MM across the muscularis externa.
Fig. 3.
MM do not distribute along endothelium in muscularis externa, but many closely associate with ICC and PDGFRα+ fibroblasts. Postnatal (P2—P4) muscularis externa whole mounts were stained with antibodies to MM marker Iba1 (A, C, D, F, G, and I), endothelial cell marker PECAM (B and C), ICC marker cKit (E and F), or fibroblast-like cell marker PDGFRα (H and I). Merged images show how MM are positioned relative to other cell types in muscle layers of the bowel wall (C, F, and I). (Scale bar for magnified Insets in F and I, 25 μm.)
Developing and Adult Bowel Contain Nonneuronal CSF1 Sources.
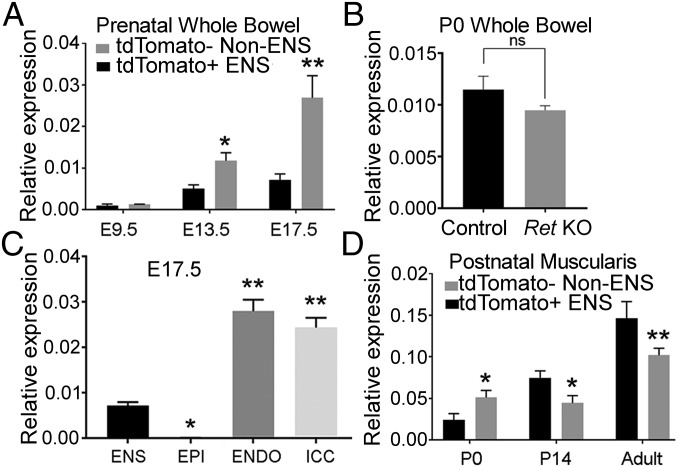
Op/Op mice that lack CSF1 are devoid of MM (7), suggesting that CSF1 is a key survival factor for these cells. It has been proposed that enteric neurons are the primary source of CSF1 in adult mice (5). However, our finding of well-patterned MM in P0 mice lacking enteric neurons strongly suggests that alternative CSF1 sources exist, at least in the developing mouse bowel. To test this hypothesis we used FACS on bowel from Wnt1Cre;tdTomato mice (1, 18–24) to isolate ENS (tdTomato+) and non-ENS (tdTomato−) cells. Csf1 expression was detectable in both ENS and non-ENS cells at E9.5 by qRT-PCR, but expression increased dramatically as development progressed (Fig. 4A). Interestingly, at E13.5 and E17.5 Csf1 mRNA levels were several-fold higher in non-ENS cells than in the ENS lineage (Fig. 4A), consistent with the observation that ENS-derived CSF1 is not needed for MM to colonize the bowel during fetal development. As further validation, we measured Csf1 mRNA levels in E13.5 bowel of a newly generated ENS reporter line called Ednrb-EGFP-L10A (Fig. S4). Similar to data in Wnt1Cre;tdTomato mice, Csf1 mRNA was much less abundant in ENS compared with non-ENS lineage cells (Fig. S4H). Additionally, whole bowel Csf1 mRNA levels did not differ significantly in P0 Ret KO mice where the ENS is missing compared with controls (Fig. 4B), further implicating non-ENS cells as CSF1 sources in the developing bowel. To identify the nonneuronal CSF1 sources in the developing bowel, we measured Csf1 mRNA in selected non-ENS cell types, including FACS-sorted epithelial cells, endothelial cells, and ICC from E17.5 bowel. We find that Csf1 is most highly expressed in endothelial cells and ICC, with significantly less expression in ENS, and virtually no expression in epithelial cells (Fig. 4C).
Fig. 4.
Csf1 is primarily expressed in non-ENS cells of fetal bowel and is only highly expressed in ENS after P14. (A) Csf1 mRNA levels in sorted tdTomato+ ENS lineage cells and tdTomato− non-ENS cells from prenatal whole bowel of Wnt1Cre;tdTomato mice. (B) Csf1 mRNA levels in P0 whole bowel from Ret KO and control mice. (C) Csf1 expression measured in E17.5 sorted tdTomato+ ENS cells, CD326+CD45−CD31− epithelial cells (EPI), CD31+ endothelial cells (ENDO), and CD117+/CD45− ICC. (D) Csf1 mRNA level in sorted tdTomato+ ENS lineage cells and tdTomato− non-ENS cells from isolated circular and longitudinal muscle layers. n = 3 biological replicates for each time point. **P < 0.01, *P < 0.05 Student’s t test (A, B, and D) or ANOVA with multiple comparisons (C). Error bar = SEM. (Scale bar, 100 µm.)
We next sought to determine when the ENS becomes the primary Csf1 source in postnatal bowel muscularis. Comparison of Csf1 levels in muscularis ENS vs. non-ENS cells at P0, P14, and 10- to 12-wk-old mice demonstrated increasing Csf1 expression in the ENS with age, with ENS Csf1 levels surpassing non-ENS cells by P14 (Fig. 4D). Interestingly, even in adult mice where ENS Csf1 expression is highest, there was significant Csf1 expression in non-ENS lineage cells of bowel muscularis (Fig. 4D).
Expression of Selected MM Genes.
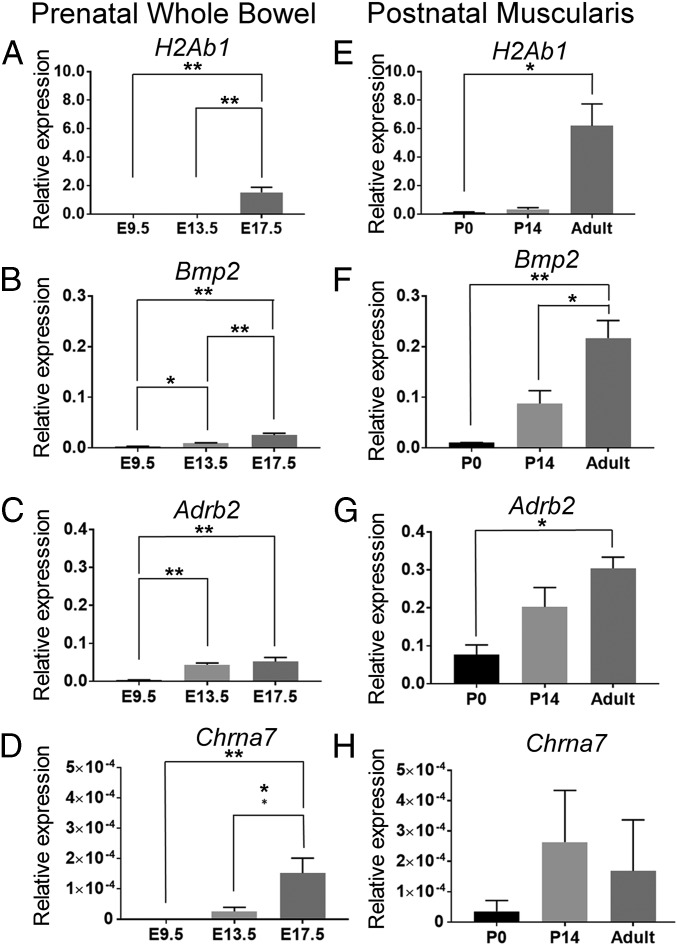
To further characterize normal MM development, we measured mRNA levels for select bowel macrophage genes over time. Selected genes included H2Ab1 (encoding MHC II), Bmp2, known to regulate enteric neuron activity in adult colon, as well as Adrb2 and Chrna7a, which relay neuronal antiinflammatory signals in MM. Prenatal data represents expression in all CD11b+/F4/80+ bowel macrophages because markers to differentiate prenatal MM from other bowel macrophages are not available and we are unable to mechanically separate prenatal muscularis from the rest of the bowel. We find that bowel macrophages express relatively low but detectable levels of H2Ab1, Bmp2, Adrb2, and Chrna7a prenatally, with all genes expressed by E17.5 (Fig. 5 A–D). Consistent with prior reports (4), H2Ab1 (MHC II) mRNA expression is negligible in MM at P0 and does not become high until after P14 (Fig. 5E), although H2Ab1 was detected in macrophages from the whole bowel at E17.5 (Fig. 5A). Postnatal Bmp2 and Adrb2 levels increase with age (Fig. 5 F and G), whereas Chrna7a remains low (note y axis scale in the figure) and relatively stable (Fig. 5H).
Fig. 5.
Gene-expression profiles for select bowel macrophage genes. (A–D) CD11b+F4/80+ macrophages were sorted from C57BL/6 whole bowel at prenatal time points (E9.5, E13.5, E17.5) and expression of select bowel macrophage genes was measured using qRT-PCR. (E–H) CD11b+F4/80+ MM were isolated from dissected muscularis layers at postnatal time points (P0, P14, adult = 10- to 12-wk old) and expression of select bowel macrophage genes was measured using qRT-PCR. n = 3 biological replicates per gene per time point. **P < 0.01, *P < 0.05 ANOVA with multiple comparisons. Error bar = SEM.
Enteric Neuron Absence Does Not Affect Baseline P0 MM Phenotype.
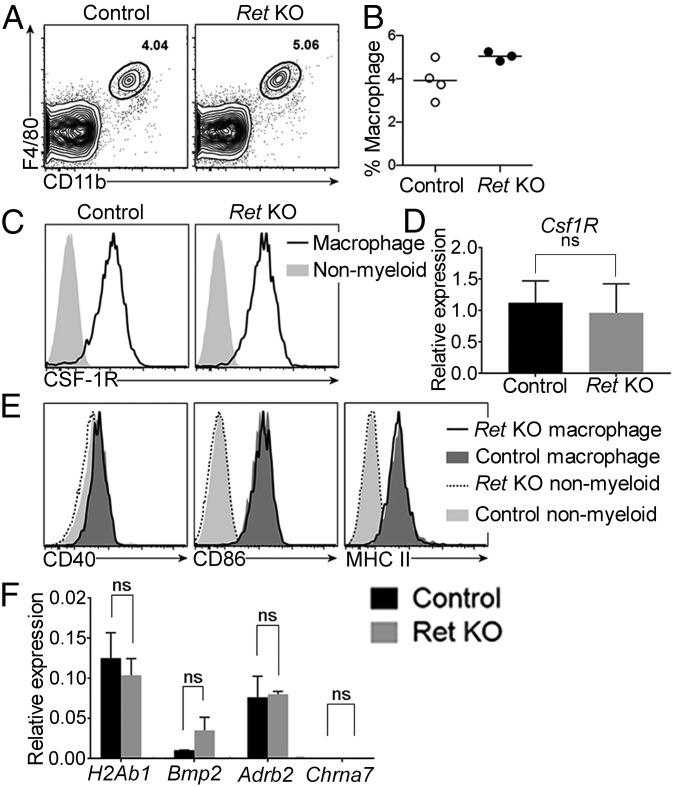
Although the ENS is not needed for MM survival or patterning, it was unclear if the ENS altered normal macrophage phenotype or function at P0. To assess the baseline activation and thus immunostimulatory potential of MM developing without enteric neurons or glia, we analyzed MM from P0 control and Ret KO mice using flow cytometry. P0 MM of control and Ret KO mice were readily detectable using the myeloid lineage marker CD11b and macrophage-specific marker F4/80 (Fig. 6A) (25). MM were present at similar frequencies in both control and Ret KO mice (Fig. 6B), consistent with our immunohistochemistry (IHC) (Fig. 1). Ret KO and control CD11b+F4/80+ cells also expressed equivalent levels of CSF1R (CSF1 receptor) (Fig. 6 C and D), suggesting that Ret KO and control MM are equally poised to respond to CSF1. Expression of the costimulatory molecules CD40 and CD86, as well as major histocompatibility complex (MHC) class II, was unaltered in mice lacking enteric neurons (Fig. 6E). Like controls, Ret KO MM expressed these activation markers at low levels, consistent with a nonactivated phenotype. We also compared the expression of MM-relevant genes previously characterized in controls and found no difference in expression of H2Ab1, Adrb2, Bmp2, or Chrna7 in Ret KO at P0 compared with controls (Fig. 6F). Thus, MM developing in the absence of enteric neurons or glia appear phenotypically normal, and enteric neurons are not needed to establish the resting phenotype of MM in neonatal mice.
Fig. 6.
Absence of enteric neurons does not affect muscularis macrophage surface phenotype, expression of MM-specific genes, or baseline activation state. P0 bowel muscle layers from control and Ret KO mice were analyzed for surface markers by flow cytometry. (A) Representative flow cytometry plots were pregated on live cells by forward and side scatter and exclusion of live/dead dye. Numbers indicate the frequency of CD11b+F4/80+ macrophages among live cells. (B) Summary data show macrophage frequencies among live cells. There was no statistically significant difference between control and Ret KO (Student’s t test). (C) Representative histograms show CSF1R expression on CD11b+F4/80+ macrophages compared with nonmyeloid (CD11b−) cells. (D) Csf1R level assessed by qRT-PCR in control and Ret KO CD11b+F4/80+ MM relative to Gapdh. (E) Representative histograms compare levels of CD40, CD86, and MHC class II in control and Ret KO macrophages. There were no statistically significant differences between control and Ret KO (Student’s t test). (F) Expression of select MM genes in P0 CD11b+F4/80+ MM from Ret KO and controls. n = 4 control and 3 Ret KO mice (B, C, and E) and 4 control, 4 Ret KO mice (D and F).
P0 MM from Ret KO Mice Respond Normally to LPS.
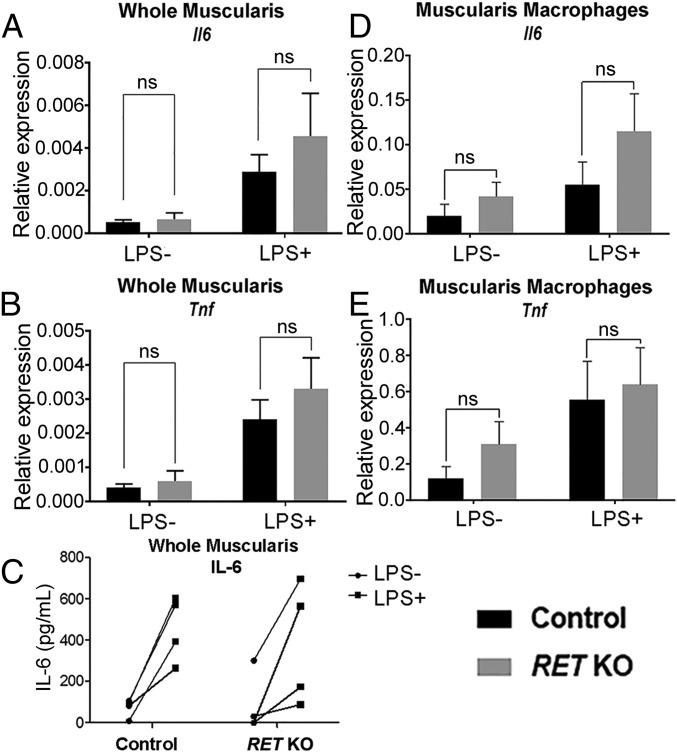
To determine if Ret KO MM have an altered LPS response, P0 Ret KO or littermate control muscularis externa was cultured 6 h with or without LPS. Following treatment, we measured tissue mRNA levels of the cytokines Il6 and Tnfα by qRT-PCR. We also measured secreted IL-6 and TNF-α protein levels by ELISA; however, TNF-α protein levels were below the limit of detection in all samples. Additionally, we measured LPS response specifically in MM by using FACS to isolate CD11b+F4/80+ MM from muscularis externa after LPS treatment. We find that LPS response was comparable for all measured parameters in control and Ret KO mice (Fig. 7). Collectively, these data suggest the ENS does not modulate early MM response to TLR activation by LPS.
Fig. 7.
MM from P0 mice lacking enteric neurons do not differ from control mice in activation by LPS stimulation. (A and B) Analysis of mRNA levels for Il6 and Tnf in whole muscularis and in isolated MM (D and E) following 6 h ex vivo culture of muscularis with and without LPS. (C) Secreted IL-6 protein levels following 6 h ex vivo culture of muscularis with and without LPS. Muscularis qRT-PCR control n = 6, Ret KO n = 4 mice. MM qRT-PCR N= 6 mice for each group. ELISA n = 4 mice for each group. Error bars = SEM. NS = P > 0.05. Data in C analyzed by two-way repeated-measures ANOVA, no significant interaction between LPS treatment and genotype (P > 0.05). All gene expression is relative to Gapdh.
Discussion
ENS Is Not Required for Mouse or Human MM to Colonize Fetal Bowel.
MM are found in mouse bowel by E15.5 (4), confirming their identity as true tissue resident macrophages. They are present along the entire bowel length within and between circular and longitudinal muscle, and often contact myenteric neurons and extrinsic neuron projections (2, 5, 6, 26). In adults, prior studies suggested that enteric neurons are a major source of the MM survival factor CSF1. Unexpectedly, we found that MM colonization of the bowel precedes colonization by enteric neurons. Iba1+ MM were observed in the region of developing muscle layers as early as E9.5. By E12.5, Iba1+ MM are present throughout the bowel, including the colon, which does not yet contain ENCDC or enteric neurons. This suggests that developing MM do not require enteric neuron-derived survival factors. Consistent with this hypothesis, Ret KO mice, which have no enteric neurons or enteric glia in the small bowel or colon, have normal numbers of well-patterned MM at birth. Normal MM numbers were also seen in human aganglionic colon, suggesting enteric neurons are not needed for initial patterning of muscularis externa by MM in mice or humans.
MM Patterning in Muscularis Externa.
MM residing in muscularis externa near enteric neurons exhibit clear patterning, with cell bodies spaced at regular intervals and generally nonoverlapping processes (1, 2, 5). Data from Ret KO mice suggest the ENS is not required to achieve this regular distribution of MM in the postnatal bowel. Furthermore, placement of MM cell bodies and processes within bowel musculature does not clearly correlate with the position of endothelial cells, ICC, or PDGFRα+ fibroblast-like cells. It is possible that MM achieve regular spacing through self-avoidance, similar to neurons and oligodendrocytes in the central nervous system (27, 28). Furthermore, although ICC and fibroblast-like cells do not appear to determine MM patterning, MM processes and cell bodies frequently associate with both cell types, suggesting functional interactions. Studies on MM-mediated ICC loss during diabetic gastroparesis (29, 30) are beginning to shed light on MM–ICC interactions, but MM interactions with fibroblast-like cells are entirely uncharacterized.
Sources of CSF1 in Prenatal and Postnatal Bowel.
Our analysis of Csf1 expression suggests that the ENS is not the major source of Csf1 in the prenatal bowel. Instead, Csf1 is highly expressed in endothelial cells and ICC, but not in epithelial cells. It is possible that other bowel cell types also make CSF1, but we did not have tools to isolate these cell populations (e.g., smooth muscle, extrinsic sympathetic and parasympathetic nerve fibers, and undifferentiated mesenchyme). Alternatively, a local source of CSF1 may not be required and circulating CSF1 may be sufficient to maintain MM. Future studies with conditional deletion of CSF1 would be required to distinguish these possibilities. It is also possible that prenatal and adult MM may differ in their dependence on CSF1 for survival. Analysis of bowel and muscularis externa from Csf1-deficient Op/Op mice at embryonic and perinatal timepoints would clarify this issue. Our findings in fetal bowel differ from results in adult mice, where CSF1 in muscularis was reported to be primarily from enteric neurons (5). Furthermore, although we confirmed that Csf1 mRNA is more abundant in the ENS than in other bowel cells at P14 and in adults, non-ENS bowel cells continue to be a prominent source of Csf1 after P14. One possible explanation for increased postnatal CSF1 production from cells of the ENS lineage is that bowel colonization by bacteria enhances neuronal CSF1 production. Consistent with this hypothesis, it has been shown that Csf1 expression in enteric neurons is regulated by LPS and that muscularis Csf1 levels and MM number decreases with antibiotic treatment (5). These observations suggest that Csf1 expression in fetal neurons remains low because bacteria have not yet colonized the bowel. Whether neuron-derived CSF1 is required for MM maintenance remains to be seen. Interestingly, germ-free mice that would be expected to have low levels of neuron-derived CSF1 do not have apparent differences in MM cell number or density (4), suggesting that nonneuronal sources of CSF1 may be sufficient to maintain the MM population.
Development of Modulatory Neuro-Immune Interactions in Muscularis Externa.
Many examples of neuronal modulation of MM have now been reported. Notably, vagus nerve stimulation of cholinergic enteric neurons in close contact with MM decreases the ATP-induced Ca2+ response in MM and reduces muscularis externa inflammation in postoperative ileus (6). This effect relies on α7nAChR cholinergic nicotinic receptors on MM and occurs independent of the spleen or adaptive immune system (6). Antiinflammatory MM phenotype is also induced by sympathetic norepinephrine signaling in response to bacterial infection (1). Signaling via β2AR β-adrenergic receptors alters gene expression in MM, leading to up-regulation of tissue-protective genes like Chi3l3 (chitinase 3-like 3) and Arg1 (arginase 1). Whether intrinsic enteric neurons mediate this extrinsic sympathetic response remains unclear.
In light of these studies, we hypothesized that MM in Ret KO mice would be hyperactivated, because Ret KO mice are missing enteric neurons and have defects in extrinsic sympathetic innervation of the bowel. However, MM sorted from neonatal Ret KO muscularis externa displayed no observable phenotypic change compared with controls. Ret KO MM also had normal cytokine production in response to LPS. The lack of a differential LPS response in ex vivo muscularis externa may reflect the requirement for intact extrinsic sympathetic and parasympathetic connections for neuronal modulation of MM, as these connections are reduced in Ret KO (31) and severed in control ex vivo preparations we used for LPS studies. Alternatively, the unaltered in vivo baseline phenotype of MM from Ret KOs may indicate that the mature neuro-immune circuitry responsible for modulating MM activation is not yet in place at P0. Ret KO mice die as neonates, so testing MM phenotypes at older ages is not possible in this model system. Postnatal maturation of ENS–MM interactions, however, seems likely because the ENS retains significant plasticity following birth that may include generation of new enteric neurons (32). In rodents the proportion of cholinergic neurons increases until P36 (33), and some enteric neurons do not obtain mature dendritic and electrophysiological properties until adulthood (34). Various environmental signals, such as intraluminal lipid and bacterial exposure, also impact postnatal enteric neuron plasticity and signaling (1, 4, 5, 35, 36) and may have important roles in shaping neuro-immune circuits in the bowel.
Clarifying which aspects of MM maturation are mediated by the ENS or extrinsic neuronal activity is an important task for future studies and may enhance our understanding of HAEC. Although MM have been implicated in pathogenesis of several gastrointestinal disorders, including diabetic gastroparesis (17, 30) and postoperative ileus (6, 26, 37), MM involvement in HAEC has not been previously explored. Our data suggest aganglionosis does not impact MM number or function at birth; however, whether MM phenotype is subsequently altered in the context of maturing neuro-immune circuits and microbial exposure remains to be seen. Of note, Ret, the major gene involved in HSCR pathogenesis, is itself expressed by a variety of immune cells in the bowel, including T and B lymphocytes, NK cells, and type 3 innate lymphoid cells (38–41). Furthermore, RET-signaling controls proinflammatory programs in peripheral blood mononuclear cells from HSCR patients and healthy donors (38). Therefore, future studies elucidating ENS–MM interactions in HAEC will need to consider non-ENS–mediated effects of Ret loss in other immune cells.
Materials and Methods
Ret-TGM mice (RRID:MGI_3623107, called Ret KO) were previously described (31). Cx3cr1-EGFP mice (B6.129P-Cx3cr1tm1Litt/J, stock #005582), tdTomato mice [Gt(ROSA)26Sortm9(CAG-tdTomato)Hze, stock #007909], and Wnt1Cre mice [Tg(Wnt1-GAL4)11Rth, stock #003829] were from The Jackson Laboratory. The use and care of mice were approved by the Children’s Hospital of Philadelphia Research Institute Institutional Animal Care and Use Committee. Ednrb-EGFP-L10a mice were made by cloning a EGFP-L10a fusion construct (42) into a vector with endothelin receptor B regulatory elements. LPS stimulation studies treated P0 bowel for 6 h in culture. Reagents, tissue processing, IHC imaging, fluorescent activated cell sorting, qRT-PCR, and statistical methods are described in more detail in SI Materials and Methods. Paraffin-embedded de-identified human colon tissue from children with Hirschsprung disease (ages ranged from 14 d to 6 mo 11 d) was obtained from the Children’s Hospital of Philadelphia Pathology Laboratory as approved by The Committees for the Protection of Human Subjects (Institutional Review Board) at The Children’s Hospital of Philadelphia.
Supplementary Material
Acknowledgments
We thank Dr. Pierre Russo for assistance with human bowel; Beth Maguire for assistance with mouse colony management; Jasbir Dalal for technical advice and assistance; Hideki Enomoto for graciously sharing his Ednrb promoter construct; Renate Lewis with the Hope Center Transgenic Constructs Core at Washington University for help with transgenic construct preparation; and the Children’s Hospital of Philadelphia Flow Cytometry Core and Pathology Core Laboratory. This work was supported by the Irma and Norman Braman Endowment (R.O.H.); the Suzi and Scott Lustgarten Center Endowment (R.O.H.); The Children’s Hospital of Philadelphia Research Institute (R.O.H.); Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital Grant MD-II-2013-269 (to R.O.H. and J.D.D.); NIH Grants R01 DK087715 (to R.O.H.) and R01 NS102272 (to J.D.D.); NIH SPARC Program OT2OD023859 (to R.O.H.); March of Dimes Grant 6-FY15-235 (to R.O.H.); and the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research Grant 1008525 (to R.O.H.). J.D.D. is a National Alliance for Research on Schizophrenia and Depression independent investigator funded by the Brain and Behavior Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802490115/-/DCSupplemental.
References
- 1.Gabanyi I, et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips RJ, Powley TL. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci. 2012;169:12–27. doi: 10.1016/j.autneu.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikkelsen HB, Larsen JO, Froh P, Nguyen TH. Quantitative assessment of macrophages in the muscularis externa of mouse intestines. Anat Rec (Hoboken) 2011;294:1557–1565. doi: 10.1002/ar.21444. [DOI] [PubMed] [Google Scholar]
- 4.Mikkelsen HB, Garbarsch C, Tranum-Jensen J, Thuneberg L. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J Mol Histol. 2004;35:377–387. doi: 10.1023/b:hijo.0000039840.86420.b7. [DOI] [PubMed] [Google Scholar]
- 5.Muller PA, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313, and erratum (2014) 158:1210. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matteoli G, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen HB, Thuneberg L. Op/op mice defective in production of functional colony-stimulating factor-1 lack macrophages in muscularis externa of the small intestine. 1999;295:485–493. doi: 10.1007/s004410051254. [DOI] [PubMed] [Google Scholar]
- 8.Gosain A. Established and emerging concepts in Hirschsprung’s-associated enterocolitis. Pediatr Surg Int. 2016;32:313–320. doi: 10.1007/s00383-016-3862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuckeroth RO. Hirschsprung disease—Integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol. 2018;15:152–167. doi: 10.1038/nrgastro.2017.149. [DOI] [PubMed] [Google Scholar]
- 10.Demehri FR, Halaweish IF, Coran AG, Teitelbaum DH. Hirschsprung-associated enterocolitis: Pathogenesis, treatment and prevention. Pediatr Surg Int. 2013;29:873–881. doi: 10.1007/s00383-013-3353-1. [DOI] [PubMed] [Google Scholar]
- 11.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 12.Burns AJ, Thapar N. Advances in ontogeny of the enteric nervous system. Neurogastroenterol Motil. 2006;18:876–887. doi: 10.1111/j.1365-2982.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 13.Bergner AJ, et al. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J Comp Neurol. 2014;522:514–527. doi: 10.1002/cne.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lake JI, Tusheva OA, Graham BL, Heuckeroth RO. Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis. J Clin Invest. 2013;123:4875–4887. doi: 10.1172/JCI69781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schill EM, et al. Ibuprofen slows migration and inhibits bowel colonization by enteric nervous system precursors in zebrafish, chick and mouse. Dev Biol. 2016;409:473–488. doi: 10.1016/j.ydbio.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikkelsen HB. Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: Morphology, distribution, spatial and possible functional interactions. J Cell Mol Med. 2010;14:818–832. doi: 10.1111/j.1582-4934.2010.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis—The missing link? Neurogastroenterol Motil. 2015;27:7–18. doi: 10.1111/nmo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H-Y. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 19.Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 20.Druckenbrod NR, Epstein ML. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005;287:125–133. doi: 10.1016/j.ydbio.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Avetisyan M, et al. Hepatocyte growth factor and MET support mouse enteric nervous system development, the peristaltic response, and intestinal epithelial proliferation in response to injury. J Neurosci. 2015;35:11543–11558. doi: 10.1523/JNEUROSCI.5267-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lake JI, Avetisyan M, Zimmermann AG, Heuckeroth RO. Neural crest requires Impdh2 for development of the enteric nervous system, great vessels, and craniofacial skeleton. Dev Biol. 2016;409:152–165. doi: 10.1016/j.ydbio.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao MM, et al. Early emergence of neural activity in the developing mouse enteric nervous system. J Neurosci. 2011;31:15352–15361. doi: 10.1523/JNEUROSCI.3053-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei J, Howard MJ. Targeted deletion of Hand2 in enteric neural precursor cells affects its functions in neurogenesis, neurotransmitter specification and gangliogenesis, causing functional aganglionosis. Development. 2011;138:4789–4800. doi: 10.1242/dev.060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koscsó B, Gowda K, Schell TD, Bogunovic M. Purification of dendritic cell and macrophage subsets from the normal mouse small intestine. J Immunol Methods. 2015;421:1–13. doi: 10.1016/j.jim.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Cailotto C, et al. Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen. PLoS One. 2014;9:e87785. doi: 10.1371/journal.pone.0087785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zipursky SL, Grueber WB. The molecular basis of self-avoidance. Annu Rev Neurosci. 2013;36:547–568. doi: 10.1146/annurev-neuro-062111-150414. [DOI] [PubMed] [Google Scholar]
- 29.Choi KM, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064, 2064.e1–2064.e2. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi KM, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–2409, 2409.e1. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enomoto H, et al. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni S, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries P, Soret R, Suply E, Heloury Y, Neunlist M. Postnatal development of myenteric neurochemical phenotype and impact on neuromuscular transmission in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G539–G547. doi: 10.1152/ajpgi.00092.2010. [DOI] [PubMed] [Google Scholar]
- 34.Foong JPP, Nguyen TV, Furness JB, Bornstein JC, Young HM. Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. J Physiol. 2012;590:2375–2390. doi: 10.1113/jphysiol.2011.225938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Quelen F, et al. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol. 2011;589:4341–4352. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heuckeroth RO, Schäfer K-H. Gene-environment interactions and the enteric nervous system: Neural plasticity and Hirschsprung disease prevention. Dev Biol. 2016;417:188–197. doi: 10.1016/j.ydbio.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008;20:81–90. doi: 10.1111/j.1365-2982.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 38.Rusmini M, et al. Induction of RET dependent and independent pro-inflammatory programs in human peripheral blood mononuclear cells from Hirschsprung patients. PLoS One. 2013;8:e59066. doi: 10.1371/journal.pone.0059066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rusmini M, et al. Expression variability and function of the RET gene in adult peripheral blood mononuclear cells. J Cell Physiol. 2014;229:2027–2037. doi: 10.1002/jcp.24660. [DOI] [PubMed] [Google Scholar]
- 40.Ibiza S, et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matera I, et al. Allele-specific expression at the RET locus in blood and gut tissue of individuals carrying risk alleles for Hirschsprung disease. Hum Mutat. 2013;34:754–762. doi: 10.1002/humu.22302. [DOI] [PubMed] [Google Scholar]
- 42.Heiman M, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]