Terminal transition metal-oxo (oxo = O2−) intermediates enjoy storied status in inorganic chemistry. The elucidation of the vanadyl (VO2+) electronic structure by Ballhausen and Gray (1) helped usher in the era of molecular orbital depictions of bonding in transition metal chemistry. Since then, metal-oxo species have been implicated in vital roles throughout biological catalysis, with key examples including the ferryl-porphyrin radical intermediate, compound I (2) used by cytochrome P450 enzymes to hydroxylate C–H bonds and high-valent Mn-oxo species postulated to participate in O–O bond formation by the oxygen-evolving complex of photosystem II (3, 4). These natural examples have inspired considerable synthetic efforts: The past few decades have witnessed the syntheses of numerous examples of terminal metal-oxo complexes capable of activating moderately strong bonds (5–7). The ubiquity of reactive terminal metal-oxos has also reached the field of heterogeneous catalysis. Recently, Solomon and coworkers (8) characterized a potent zeolite-supported ferryl (FeO2+) species capable of hydroxylating methane at room temperature. Now, in PNAS, Synder et al. (9) extend characterization of this species, using synchrotron-based spectroscopies to access valuable bonding parameters essential to defining the electronic structure underpinning the reactivity of this ferryl.
The capacity for Fe-doped zeolites to effect methane hydroxylation under mild conditions has been known for decades (10). However, identifying the “active ingredient” in materials with low (ca. 0.3 wt%) Fe loading required adopting a “bioinorganic approach” of site-selective spectroscopies (8). Variable-temperature/variable-field magnetic circular dichroism (VTVH-MCD) allowed direct focus on the paramagnetic FeII active site (α-Fe) and its methane-hydroxylating oxygenated derivative (α-O). VTVH-MCD intensifies transitions between d-d (ligand-field) excited states as a function of temperature and applied magnetic field, and the resulting magnetization curves may be fit to yield two parameters: geff (effective g value) and a δ (rhombic zero field splitting) (11). Extracted parameters indicated that both α-Fe and α-O are mononuclear high-spin (S = 2) species; α-Fe is an oxygen-coordinated, square-planar FeII, and α-O is a tetragonal ferryl. These findings distinguished α-O from binuclear “intermediate Q,” which is the methane-hydroxylating active species formed by the enzyme-soluble methane monooxygenase (12, 13). Although the methane-oxidizing intermediate had a face at this point, mystery persisted. High-spin ferryls have been reported and characterized previously (14, 15), but without such oxidative alacrity as exhibited by α-O.
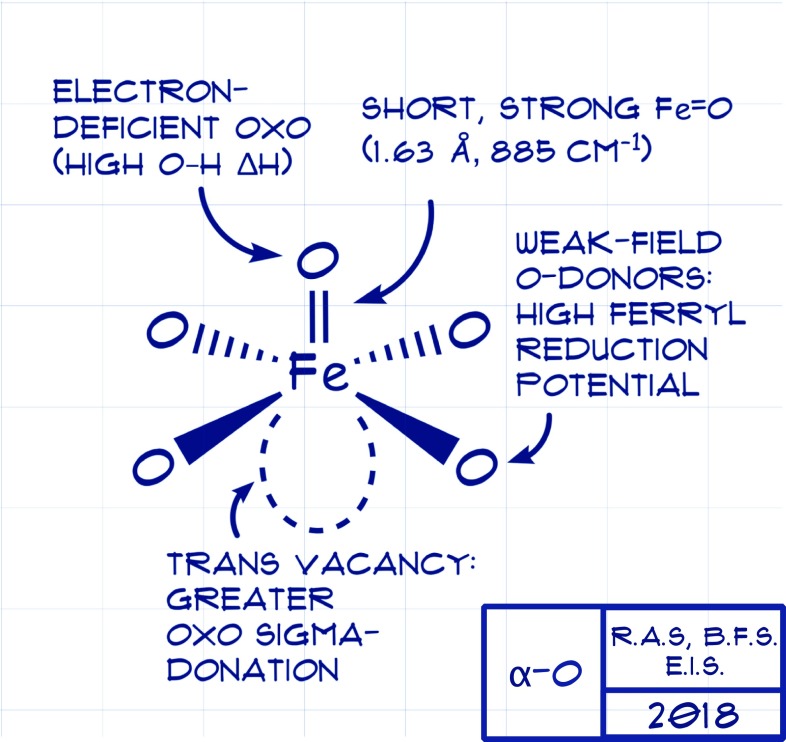
The structural characterization of α-Fe and α-O reported in PNAS (9) offers a rationale for the oxidative potency. Using Fe K-edge X-ray absorption spectroscopy and extended X-ray absorption fine structure (EXAFS) data obtained at the Stanford Synchrotron Radiation Lightsource, Synder et al. (9) established the inner-sphere metrical parameters of α-Fe: four inner-sphere zeolite O ligands at 2.02 ± 0.03 Å. For α-O, a 1.63 ± 0.03 Å Fe=O scatterer completes the inner coordination sphere. Further support for a five-coordinate ferryl came from the particularly intense 1s → 3d pre-edge excitation reflective of a high degree of 3d/4p mixing due to diminished site centrosymmetry. However, ambiguities intrinsic to EXAFS analysis, particularly the inability to distinguish neighboring elements on the periodic table at similar distances (16), necessitated additional recourse to completing the local structural description, including disposition of Si and Al about the Fe active site.
For this, the investigators used 57Fe nuclear resonance vibrational spectroscopy (NRVS). This technique measures vibrational fine structure proximal to the 57Fe nuclear excitation that reports directly on vibrational modes involving Fe (17). These modes are sensitive not only to the nature of the inner coordination sphere but also to the placement of Al and Si in the β-type six-membered ring (β-6MR) housing the active Fe. Using normal mode analysis, Synder et al. (9) could exclude all but a β-6MR, where Al centers bridge Fe-coordinating zeolite O-donors.
The final, key experimental data furnished by this work concern the nature of the Fe=O bond. The 1.63 ± 0.03 Å Fe=O bond in α-O is typical for both S = 1 and S = 2 ferryls, and thus affords no clues concerning reactivity (18). However, NRVS directly afforded a stretching frequency, 885 cm−1, for νFe=O, where ν is vibration. This stretching frequency, which is over 50 cm−1 greater than the next reported value for a nonheme ferryl (19), reveals α-O to have a considerably stronger Fe=O interaction. Density functional theory calculations were carried out that effectively reproduce the experimental spectra features of α-O. Further examination of these experimentally validated electronic structure calculations reveal that the Fe=O interaction is highly covalent, manifesting an electron-deficient oxo primed for facile H-atom abstraction.
All told, the structural and spectroscopic characterization of α-O affords a blueprint for a “hot” ferryl capable of activating E–H bonds as recalcitrant as the 104 kcal⋅mol−1 C–H bond of methane (Fig. 1). The active ingredients contributing to the reactivity of α-O manifest from the well-defined, constrained environment afforded by its host zeolite lattice. Not unlike how a protein defines the inner- and outer-sphere coordination about a metal to imbue reactivity properties, the present study shows that the “entatic state” effect (20–22) is alive and well in a heterogeneous catalyst. These crucial, synergistic features of α-O include (i) low coordination number, (ii) a set of weak inner-sphere donors, and (iii) a highly covalent Fe=O interaction. Together, these attributes impart high driving force for and a low activation barrier to E–H bond activation. The weak field imparted by the four inner-sphere zeolite O-donors favors the high-spin configuration, a feature implicated in bolstering in the reactivity of metal oxos (23). What makes α-O truly special is the high Fe=O covalency, which manifests in an electron-deficient oxo with a large driving force for O–H bond formation. Metal-ligand covalency, recognized early on as essential to properly describing bonding in coordination compounds (24), is once again seen as a vital contributor to reactivity, and thus should influence the design of future transition metal catalysts and reagents (25).
Fig. 1.
Blueprints afforded by Synder et al. (9) for a potent methane hydroxylation intermediate.
Opportunity abounds to push the limits of ferryl reactivity: Judicious ligand design or material choice could afford a three-coordinate Fe that, upon receipt of an O-atom, imposes even heavier electronic demands on the oxo ligand. Thus, the race is on to leverage the insights afforded in this article by Synder et al. (9) toward the design of new catalysts, be they homogeneous or heterogeneous, for hydroxylation of strong bonds. Such systems would find welcome use in pharmaceutical synthesis, hydrocarbon valorization, and other applications.
Acknowledgments
My related work is supported by the National Science Foundation (CAREER Award CHE-1454455), as well by a research fellowship from the Alfred P. Sloan Foundation.
Footnotes
The author declares no conflict of interest.
See companion article on page 4565.
References
- 1.Ballhausen CJ, Gray HB. The electronic structure of the vanadyl ion. Inorg Chem. 1962;1:111–122. [Google Scholar]
- 2.Rittle J, Green MT. Cytochrome P450 compound I: Capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, et al. High-spin Mn-oxo complexes and their relevance to the oxygen-evolving complex within photosystem II. Proc Natl Acad Sci USA. 2015;112:5319–5324. doi: 10.1073/pnas.1422800112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEvoy JP, Brudvig GW. Water-splitting chemistry of photosystem II. Chem Rev. 2006;106:4455–4483. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]
- 5.Borovik AS. Role of metal-oxo complexes in the cleavage of C-H bonds. Chem Soc Rev. 2011;40:1870–1874. doi: 10.1039/c0cs00165a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunay A, Theopold KH. C-H bond activations by metal oxo compounds. Chem Rev. 2010;110:1060–1081. doi: 10.1021/cr900269x. [DOI] [PubMed] [Google Scholar]
- 7.McDonald AR, Que L., Jr High-valent nonheme iron-oxo complexes: Synthesis, structure, and spectroscopy. Coord Chem Rev. 2013;257:414–428. [Google Scholar]
- 8.Snyder BE, et al. The active site of low-temperature methane hydroxylation in iron-containing zeolites. Nature. 2016;536:317–321. doi: 10.1038/nature19059. [DOI] [PubMed] [Google Scholar]
- 9.Snyder BER, et al. Structural characterization of a non-heme iron active site in zeolites that hydroxylates methane. Proc Natl Acad Sci USA. 2018;115:4565–4570. doi: 10.1073/pnas.1721717115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smeets PJ, Woertink JS, Sels BF, Solomon EI, Schoonheydt RA. Transition-metal ions in zeolites: Coordination and activation of oxygen. Inorg Chem. 2010;49:3573–3583. doi: 10.1021/ic901814f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neese F, Solomon EI. MCD C-term signs, saturation behavior, and determination of band polarizations in randomly oriented systems with spin S ≥ 1/2. Applications to S = 1/2 and S = 5/2. Inorg Chem. 1999;38:1847–1865. doi: 10.1021/ic981264d. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee R, Proshlyakov Y, Lipscomb JD, Proshlyakov DA. Structure of the key species in the enzymatic oxidation of methane to methanol. Nature. 2015;518:431–434. doi: 10.1038/nature14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo RG, et al. High-energy-resolution fluorescence-detected X-ray absorption of the Q intermediate of soluble methane monooxygenase. J Am Chem Soc. 2017;139:18024–18033. doi: 10.1021/jacs.7b09560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.England J, et al. A synthetic high-spin oxoiron(IV) complex: Generation, spectroscopic characterization, and reactivity. Angew Chem Int Ed Engl. 2009;48:3622–3626. doi: 10.1002/anie.200900863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigi JP, et al. A high-spin iron(IV)-oxo complex supported by a trigonal nonheme pyrrolide platform. J Am Chem Soc. 2012;134:1536–1542. doi: 10.1021/ja207048h. [DOI] [PubMed] [Google Scholar]
- 16.Riggs-Gelasco PJ, Stemmler TL, Penner-Hahn JE. XAFS of dinuclear metal sites in proteins and model compounds. Coord Chem Rev. 1995;144:245–286. [Google Scholar]
- 17.Scheidt WR, Durbin SM, Sage JT. Nuclear resonance vibrational spectroscopy–NRVS. J Inorg Biochem. 2005;99:60–71. doi: 10.1016/j.jinorgbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.England J, et al. The crystal structure of a high-spin oxoiron(IV) complex and characterization of its self-decay pathway. J Am Chem Soc. 2010;132:8635–8644. doi: 10.1021/ja100366c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell CB, 3rd, et al. A combined NRVS and DFT study of Fe(IV)=O model complexes: A diagnostic method for the elucidation of non-heme iron enzyme intermediates. Angew Chem Int Ed Engl. 2008;47:9071–9074. doi: 10.1002/anie.200803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallee BL, Williams RJ. Metalloenzymes: The entatic nature of their active sites. Proc Natl Acad Sci USA. 1968;59:498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray HB, Malmström BG, Williams RJ. Copper coordination in blue proteins. J Biol Inorg Chem. 2000;5:551–559. doi: 10.1007/s007750000146. [DOI] [PubMed] [Google Scholar]
- 22.Mara MW, et al. Metalloprotein entatic control of ligand-metal bonds quantified by ultrafast X-ray spectroscopy. Science. 2017;356:1276–1280. doi: 10.1126/science.aam6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook SA, Lacy DC, Borovik AS. Terminal metal–oxo species with unusual spin states. In: Swart M, Costas M, editors. Spin States in Biochemistry and Inorganic Chemistry: Influence on Structure and Reactivity. Wiley; Chichester, UK: 2015. pp. 203–228. [Google Scholar]
- 24.Griffiths J, Owen J. Complex hyperfine structures in microwave spectra of covalent iridium compounds. Proc R Soc Lond A Math Phys Sci. 1954;226:96–111. [Google Scholar]
- 25.Walroth RC, et al. Electronic structural analysis of copper(II)-TEMPO/ABNO complexes provides evidence for copper(I)-oxoammonium character. J Am Chem Soc. 2017;139:13507–13517. doi: 10.1021/jacs.7b07186. [DOI] [PubMed] [Google Scholar]