Abstract
Keratoprosthesis (Kpro) forms the last resort for bilateral end-stage corneal blindness. The Boston Type 1 and 2 Kpros, the modified osteo-odonto Kpro and the osteo-Kpro are the more frequently and commonly performed Kpros, and this review attempts to compile the current data available on these Kpros worldwide from large single-center studies and compare the indications and outcomes with Kpros in the Indian scenario. Although the indications have significantly expanded over the years and the complications have reduced with modifications in design and postoperative regimen, these are procedures that require an exclusive setup, and a commitment toward long-term follow-up and post-Kpro care. The last decade has seen a surge in the number of Kpro procedures performed worldwide as well as in India. There is a growing need in our country among ophthalmologists to be aware of the indications for Kpro to facilitate appropriate referral as well as of the procedure to enable basic evaluation during follow-ups in case the need arises, and among corneal specialists interested to pursue the field of Kpros in understanding the nuances of these surgeries and to make a judicious decision regarding patient and Kpro selection and more importantly deferral.
Keywords: Boston keratoprosthesis, keratoprosthesis, ocular surface disorders, osteoodonto keratoprosthesis
Prosthetic corneas form the last resort for corneal blindness, especially in eyes with end-stage ocular surface disorders and in those at a high risk for conventional penetrating keratoplasty.[1,2] The choice of keratoprosthesis (Kpro) depends on the underlying etiology, the anatomy of the ocular surface and the tear film status. Broadly speaking, keratoprostheses are categorized into the Type 1 and 2 Kpros based on the type of eye they cater to.
Largely, eyes with normal lids, blink and tear film without an underlying immunological etiology are considered as candidates for the Type 1 Kpro, the prototype of which is the Boston Type 1 Kpro. However, in eyes with severely dry or keratinized ocular surface with an underlying immunological disorder, associated with lid abnormalities, Type 2 Kpros are considered as the treatment option of choice. Decision-making, therefore, forms one of the most important aspects of Kpro surgery not only for choosing the appropriate patient for Kpro but also for choosing the correct type of Kpro for the patient, which would go a long way in determining a successful outcome.
Most of the series reported thus far from a single center cater to only one type of Kpro predominantly and its outcomes. The ease of availability, technique, and the lesser need for support from ancillary disciplines has allowed the Boston Type 1 to be performed easily in various centers across the globe. On the other hand, the biological Kpros are currently being performed in a few centers across the world, as is the Boston Type 2 Kpro and are usually mutually exclusive.
At the Sankara Nethralaya Ocular Surface Clinic, Kpro procedures are being performed since 2003, with the initiation of the modified osteo-odonto Kpro (MOOKP) procedure for the 1st time in India under the guidance of the father of OOKP, Professor Giancarlo Falcinelli from Italy. This was followed by initiation of the Type 1 Kpro in January 2008, followed subsequently by the Boston Type 2 Kpro in January 2013 and the osteo-Kpro in January 2014, thus in all probability making it the only center currently that actively performs and offers all types of Kpros.
This review briefly presents the results of the various types of Kpros performed at our institute, comparing the same with the outcomes from large single-center studies of different types of Kpros. Etiology-specific, complication-specific, and multicenter studies have not been included due to a possibility of an overlap with the patients from the single-center studies. Guidelines regarding choice of Kpro and surgical techniques are described. The experience with various types of Kpros has provided some insight into understanding the virtues of a particular type of Kpro as well as its shortcomings, helping apply lessons learnt from one type to another.
Types of Keratoprosthesis/Design
The design of a Kpro can be likened to some extent to that of an intraocular lens consisting of an optic and a haptic. The optic, which forms the central part of the Kpro responsible for viewing, in most types is a cylinder made of polymethyl methacrylate (PMMA) – creating an optically clear window. It is the haptic of the Kpro which determines the type of the prosthesis, and this could be divided into:
Biocompatible – usually a PMMA skirt with the corneal graft as in the Boston Type 1 and 2 Kpro
Biointegrated – as in the Dacron mesh that forms the skirt around the PMMA optic in the Pintucci Kpro
Biological – tooth or the bone that forms an autologous biological tissue that supports the optical cylinder in the osteoodonto and the osteo-Kpro, respectively.
The supporting cover tissue adds to the Kpro complex which is the bandage contact lens in the Type 1 Kpro that prevents the carrier graft desiccation. In Type 2 Kpros, the supporting cover is the skin in the Boston Type 2 and the buccal mucosa for the osteo and the osteo-odonto and Pintucci Kpros, respectively.
Indications
Kpros are performed for bilateral corneal blindness not amenable to conventional penetrating keratoplasty.
Indications for Type 1 Keratoprosthesis
With improved outcomes, the indications for Type 1 Kpro have been expanding over the past decade. However, it is best to categorize these based on prognostic hierarchy since eyes with guarded prognosis have an increased risk to develop complications.[2,3]
Good prognosis
Multiple failed grafts
Aniridia
Herpetic keratitis
Silicon oil-filled eyes.
Guarded prognosis
Pediatric corneal conditions
Chemical injuries.
Very guarded prognosis
Underlying immune conditions such as Stevens–Johnson syndrome (SJS)/ocular cicatricial pemphigoid (OCP)
Severe chemical injuries with severe forniceal shortening and lid abnormalities.
Indications for Type 2 keratoprosthesis
Based on the long-term anatomical and functional outcomes, the choice of Kpro in severe end-stage ocular surface disorders is preferably the MOOKP. In case of the patient being unsuitable for the same, the other Type 2 Kpros are chosen for the following:
SJS
OCP/mucous membrane pemphigoid
Severe chemical injuries
Severely keratinized surface.
The exclusion criteria for Kpros are tabulated in Table 1.
Table 1.
Exclusion criteria for keratoprosthesis procedures
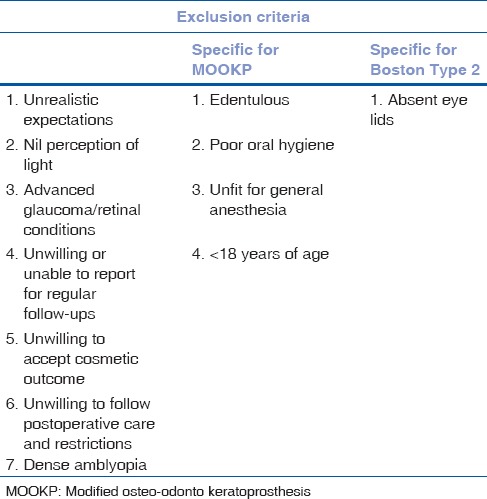
Pediatric Kpro forms a separate entity and the Type 1 Kpro is performed in pediatric population to visually rehabilitate children with congenital bilateral corneal disorders not amenable to penetrating keratoplasty. Type 2 Kpros are usually not performed in the pediatric population.
Preoperative Evaluation
A detailed history taking to determine etiology, onset (to gauge extent of amblyopia-loss of vision before 5 years of age is considered as a poor indicator for visual recovery), and previous intraocular surgeries is of paramount importance
All patients require a detailed ophthalmic evaluation including a B scan with axial length measurement
Perception of light and accurate projection of rays is assessed
Intraocular pressure is estimated by means of digital tonometry
Ultrasound biomicroscopy/anterior segment optical coherence tomography (ASOCT) helps assess the anterior segment details in eyes with scarred opaque corneas
Adequacy of blink is confirmed (Type 1)
Schirmer's I wetting is determined for adequacy of tears (Type 1)
Patency of nasolacrimal duct is confirmed by means of syringing to rule out focus of infection (for Type 1, if puncta open for Type 2)
Patients enlisted for the MOOKP should have a detailed dental and oral mucosal evaluation with a spiral computed tomography scan to evaluate the canines preoperatively along with determining fitness for general anesthesia
Counseling the patient and family with respect to realistic expectations, the need for compliance with postoperative care and follow-ups, the expected cosmetic outcome and the need to report back or to the nearest ophthalmic specialist immediately in case of unexplained drop in vision or pain, forms the most important aspect
A detailed check-list is verified before every procedure/stage to ensure a complete preoperative evaluation.
Surgical Technique and Postoperative Care
Boston Type 1 keratoprosthesis
Decide on the type of Kpro to be ordered: pseudophakic/aphakic; adult (8.5-mm backplate)/pediatric (7.0-mm backplate)
Axial length to be specified for aphakic Kpro
Kpro to be ordered for, and an extra Kpro to be ordered as a standby
Local or general anesthesia as indicated
The recipient cornea is marked with the trephine as required
Kpro to be assembled before trephining the recipient. Backplate of the Kpro measures 8.5 mm and hence the minimum donor graft size to be 8.5 mm. The donor cornea is usually oversized by 0.5 mm. The central 3 mm opening in the donor cornea is subsequently trephined
Fresh therapeutic grade donor cornea is preferred to assemble the Kpro
The optic is placed on the adhesive strip upside down. The donor graft is slid down the stem of the optic into its slot using a wrench. The back-plate is slid in place. The assembly is then locked with the titanium ring and checked for a snug fit
The recipient cornea is further trephined and removed. Any intraocular procedure as planned to be performed
The assembled Kpro is then sutured like in a penetrating keratoplasty using 16 interrupted 9-0 nylon sutures, preferably buried
A bandage contact lens is placed on the Kpro.[4]
Postoperative regimen
Fourth generation fluoroquinolone 4 times a day for a month, continued 2/day indefinitely
Topical vancomycin (14 mg/ml) 4 times a day for a month, continued 1/day indefinitely, for high-risk eyes
Topical steroids tapered to 2/day, indefinitely or discontinued after 6 months
Topical lubricants as required
BCL to be changed once in 3 months, application of 5% povidone-iodine in clinic at the time of BCL replacement
Follow-up every 3 months.[2]
Examination during each follow-up visit
Change in refraction. A hyperopic shift could indicate an early leak, a myopic shift could be indicative of raised intraocular pressure
Deposits on BCL, if any, to preferably be submitted for microbiological evaluation.
To assess for air bubbles under the optic flange as well as immobile bubbles beneath the BCL that could indicate early thinning of the carrier graft
The graft around the optic should be inspected for the presence of any infiltration
Slit-beam examination to assess for any irregularity in the carrier graft
Presence of retroprosthetic membrane (RPM), if any
Presence of loose sutures, if any, should be removed
Intraocular pressure is monitored by digital tonometry
90D lens examination to document the optic disc and posterior pole findings
Following removal of the BCL for replacement, the graft should be stained with sterile fluorescein to look for the presence of any epithelial defect or leak
Use of 5% povidone-iodine in the eye is recommended at the time of BCL replacement.
Slit-lamp photographic documentation of the eye
Humphrey visual field analysis once in 6 months
ASOCT to identify early graft thinning, periprosthetic tissue loss, retroprosthetic membrane, and angle details once in 6 months
B-scan ultrasonography once in a year.
Boston Type 2 keratoprosthesis
The procedure is largely similar to the Boston Type 1 Kpro in terms of Kpro assembly and suturing.
The differences include:
The anterior nub of the Kpro protrudes by 2 mm to accommodate the skin
The backplate is titanium and snaps onto lock the Kpro complex. There is no separate titanium ring
In the recipient, the entire conjunctival mucosa is removed from lid margin to lid margin
Sphincterotomy is done to keep the pupil mid-dilated
Following Kpro suturing, pars plana vitrectomy is performed along with Ahmed glaucoma valve implantation in all eyes
The lid margins are excised to completely be rid of hair follicles. A meticulous suturing of the lid margins in 2 layers is done around the optic.
Postoperative regimen
Systemic and topical steroids to be tapered and stopped over a month
Topical antibiotic drops – fourth generation fluoroquinolone for 2 weeks
Topical antibiotic ointment at bedtime to be continued indefinitely
Meticulous cleaning over the Kpro for the 1st postoperative week to prevent skin overgrowth
Lid sutures are removed on day 10
Follow-up once every 3 months.
Modified Osteo-Odonto Keratoprosthesis
A three-staged procedure; the MOOKP is performed largely as per the Rome-Vienna Protocol.[5] In the 1st stage, termed Stage 1 A, the eye is prepared for the procedure by removing the iris, doing a cryolens extraction and a limited anterior vitrectomy. A tectonic penetrating keratoplasty at this stage is performed only in case of any corneal thinning noted.
A month later, the Stage 1 B + C is done. This involves harvesting the chosen canine tooth, preferably maxillary and fashioning it into an osteo-odonto alveolar lamina with the optical cylinder fixed. The lamina is placed in the contralateral cheek subcutaneous pouch for it to develop its fibrovascular covering over the next 2–3 months. Simultaneously, the buccal mucosa measuring 3 cm in diameter is harvested and draped over the ocular surface securing it to the 4 recti muscles.
Three months later, the Stage 2 of the procedure is performed. The lamina is removed from the subcutaneous pouch and prepared. The mucosa over the ocular surface is reflected with an inferior hinge. The central cornea is trephined as per the posterior diameter of the optical cylinder and the lamina is placed in the eye. The oral mucosa is reflected back over the lamina and sutured and a central opening is made in the mucosa for the cylinder to protrude through.
Postoperative regimen
Systemic and topical steroids and antibiotics are administered after every stage as warranted
Topical antibiotic ointment is continued once a day indefinitely
Topical lubricants are continued indefinitely
Follow-up once every 6 months, in addition, to evaluate the health of the oral mucosa and the lamina.
Osteo-Keratoprosthesis
The procedure is very similar to the MOOKP. The bone is harvested instead of the tooth from the tibia and the same is fashioned into an osteo-lamina, in which the optical cylinder is fixed.
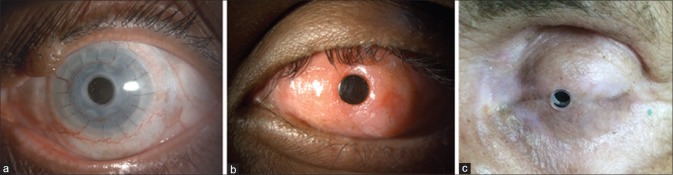
The final appearance of the eyes following each of the Kpros is illustrated in Fig. 1.
Figure 1.
Final appearance of the eye following Boston Type 1 keratoprosthesis (a), modified osteo-odonto keratoprosthesis (b) and Boston Type 2 keratoprosthesis (c) at postoperative 9, 12, and 2 years, respectively
Beyond the surgical technique and postoperative care, it is imperative to follow certain general guidelines with regard to Kpros and the same has been highlighted in Table 2.
Table 2.
General guidelines and practical pearls regarding keratoprosthesis procedures

Keratoprosthesis Setup
Setting up a Kpro unit involves considerable planning and execution. A strong sense of commitment forms the most important prerequisite. The team should constitute glaucoma, vitreoretinal, and oculoplastic colleagues along with anesthetists, and nursing staff.
For Type 2 Kpros, a more elaborate setup is required with the need for general anesthesia. Coordination with oromaxillofacial surgeons and radiologist is crucial. The appropriate instruments have to be procured for the dental or bone graft procedures.
While ordering for Type 1 Kpros, aphakic Kpros require the axial length of the eye to be provided. A second Kpro is always kept as a backup for an inadvertent loss or breakage of the Kpro during surgery.
An affiliation to an eye bank is required to procure the corneal tissue for Kpros that require a carrier graft and as a backup tissue for the others that might reveal intraoperatively, the need for a tectonic keratoplasty.
Consent forms and checklists have to be elaborate and cross-checked before every stage of surgery. Counseling by a psychologist helps the patient in a smooth transition in the perioperative period. It is essential to have a mentor and be trained in the procedure before initiating Kpro surgeries to understand better the nuances involved in the same.
Outcomes
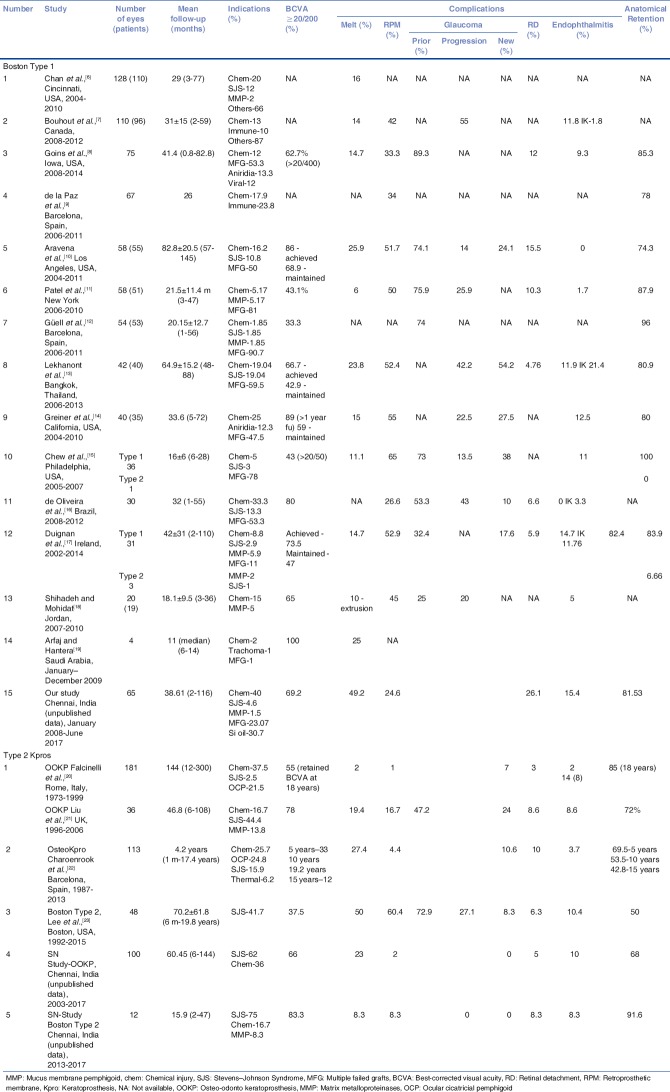
In recent times, the outcomes of especially the Type 1 Kpro have considerably improved. The visual outcome depends on the indication and is noted to be best among eyes with multiple failed grafts. A comparison of the outcomes of the different types of Kpro from single-center studies have been tabulated and compared with our outcomes (unpublished data) [Table 3].
Table 3.
A comparison of single-center indications and outcomes of Type 1 and Type 2 keratoprosthesis
The only other reported series from India include the International results of the Type 1 Kpro (59% of the 113 eyes belonging to the international arm were from 5 centers across India)[24] and our short-term outcomes of the MOOKP in 50 eyes.[25] This data has been used for comparison along with our current outcomes.
Previous donor graft failure has been the major indication for Type 1 Kpro in most series in comparison to ours that catered primarily to chemical injuries. Silicone oil-filled eyes formed the second common indication in our series for performing type 1 Kpro.[26]
The most common complications encountered are sterile melts, glaucoma, and retroprosthetic membrane and these are discussed further in detail.
Sterile melts
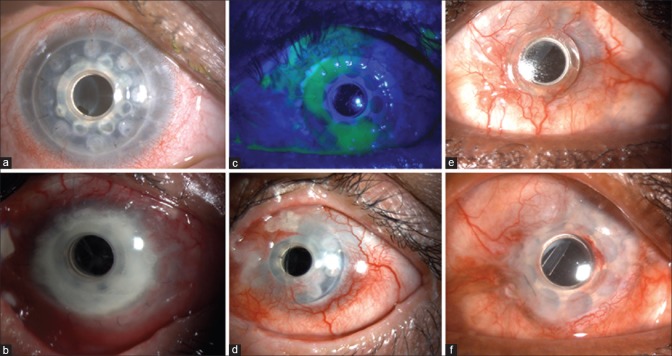
Sterile melts have been noted to occur in up to 26% of eyes in various series [Fig. 2]. It is imperative to pick up early signs described earlier.
Figure 2.
(a) Retroprosthetic membrane in a silicone oil-filled eye after Boston Type 1 keratoprosthesis, not visually significant. (b) Carrier graft infiltration in an eye with vitreous exudates and endophthalmitis 2 years following Boston Type 1 keratoprosthesis. (c) Epithelial defect noted on fluorescein staining after BCL removal, not associated with thinning. (d) Sterile carrier graft melt with edge lift of the keratoprosthesis. (e) Perioptic annular melt with no leak. Note the air bubble in the gap beneath the flange of the optic. (f) Same eye as e following an annular lamellar graft
In the presence of melt, the general dictum is to assess the extent of associated thinning. In mild cases, cyanoacrylate glue application to the area of thinning would suffice. In moderate cases involving a few or more clock hours, a crescentic or annular lamellar graft [Fig. 2] would be required to address the melt. In the presence of extensive melts, associated with aqueous leak, it would probably be best to replace the Kpro with a new one, unless the area of leak is very small and can be addressed by the above other means. In addition, medical supportive measures could include topical medroxyprogesterone and systemic doxycycline with copious lubrication and a tarsorrhaphy in cases with frequent BCL displacements leading to graft desiccation.
Sterile melts occurred in almost 50% of the cases in our series, primarily in patients with chemical injuries. This increased occurrence of melts in eyes with chemical injuries has been reported by Chan et al. with chemical injuries accounting for 35% of melts in their series. Considering that the Type 1 Kpro is performed in a relatively larger number (27% compared to 7%) of patients with chemical injuries in our country, an indication that has been seen to be associated with increased risk of sterile melts, Kpro surgeons in India should be aware of the same and attempt to pick up early signs of melt to salvage the Kpro. This can occur at any time frame following the Kpro.
Periprosthetic tissue loss has been referred to as one of the possible associations with idiopathic sterile vitritis, and the same terminology can be extended to the melts involving the haptic in other types of Kpro.[27] A similar process in the MOOKP/OKP is termed as laminar resorption[28] [Fig. 3]. A sterile inflammatory process that initiates the keratolysis could by virtue of proximity spill over into the vitreous, especially in single chamber aphakic eyes leading to a sterile vitritis. Sterile vitritis induced decrease in vision was the most common presenting feature of laminar resorption in our MOOKP series that resolved completely in most instances with systemic steroid-antibiotic management.[28]
Figure 3.
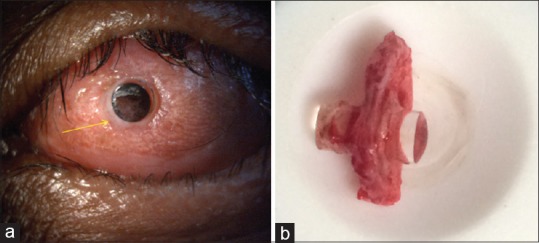
(a) Aqueous leak (indicated by yellow arrow) around the optical cylinder 8 years after modified osteo-odonto keratoprosthesis. (b) Laminar resorption seen following removal of the lamina
Retroprosthetic membrane
RPM has been reported as the most common complication in various series published so far with majority of the studies quoting an occurrence in more than 50% of the eyes. However, RPM was seen in our series in only 25% of the eyes, especially in silicone oil-filled eyes. Although RPM was noted to be the most common complication in the group performed outside of North America, it was seen in only 26.7% of the eyes at a mean follow-up of 14.2 months.[23] RPM has also been implicated as one of the causes for sterile corneal melts by virtue of preventing access of aqueous to the carrier graft.[6] A recent study has shown no benefit of titanium backplate over a PMMA backplate in the formation of RPM, with similar rates of RPM noted in both.[29] Performing a total pars plana vitrectomy appears to reduce the rate of RPM formation.[30]
Although details regarding the Kpro being aphakic or pseudophakic were not available for all the series', the international arm of the multicenter study, of which Indian centers formed an important subset had aphakic Kpros implanted in 62% of the eyes. All the Type 1 Kpros in our series were aphakic Kpros.
Whether the eye being aphakic or pseudophakic contributes toward the formation of RPM needs to be studied further. Theoretically, an aphakic eye without the posterior capsule and the iris in certain instances does not provide any scaffold for the RPM to form similar to what is seen in the MOOKP eyes where the rate of primary RPM formation is very low.
Visually insignificant RPM's can be observed and monitored [Fig. 2]. Visually significant RPM's can be addressed by means of neodymium: yttrium-aluminum-garnet laser membranotomy or a surgical membranectomy.
Glaucoma
Glaucoma continues to be the most common comorbid factor with progressive decrease in vision post-Kpro occurring most commonly secondary to continued progression of glaucoma.[2] Interestingly, there was no de novo glaucoma in the MOOKP eyes in our series. In eyes with coexistent glaucoma before Kpro placement, glaucoma needs proactive and aggressive management. It would be prudent to simultaneously place a drainage implant in eyes with the Type 1 Kpro, and the timing of placing a valve in eyes undergoing the MOOKP procedure is based on the stage of surgery.[31]
Endophthalmitis
At 15.4%, endophthalmitis in our series of Type 1 Kpros was noted to be more compared to the other single-center series' over a mean follow-up of 38 months (9% in the international study group at 14.2 months mean follow-up).[24] Endophthalmitis was noted in 10% of eyes with the MOOKP as well as the Boston Type 2 Kpro in our series. Fungal etiology was noted in almost equal number of eyes as those with bacterial endophthalmitis.
Conclusion
Considering the tropical region, in which we live with a primarily agrarian population, the risk of infection is probably bound to be more compared to the results quoted in the Western literature.[32] The indication profile in the developing countries also varies with the guarded and very guarded prognosis categories forming a major proportion of cases that undergo Type 1 Kpro. Hence, direct comparisons with outcomes and complications and applying them to different geographical zones might not be appropriate. Lekhanont et al. reported infective keratitis in 21.4% of the eyes and endophthalmitis in 11.9% at a mean follow-up of 64.9 months in a single-center series from Thailand.[13]
Among the MOOKP also, SJS forms a major indication, unlike other studies where chemical injuries predominate.[20,21] Issues specific to eyes with SJS in terms of laminar resorption and its consequences, therefore, have led to outcomes in our country that are suboptimal compared to reported outcomes in the non-SJS category.[28,33] However, the results with the MOOKP appear to be superior to the Boston Type 2 Kpro at a longer follow-up in a similar SJS population, retaining MOOKP as the procedure of choice in these eyes.
Variations and modifications from existing procedures to improvise or simplify techniques and outcomes should be done in a controlled manner, comparing outcomes with the existing gold standards, and should not be attempted by novice Kpro surgeons.
With a holistic understanding of Kpro and its implications, the need to follow strict postoperative compliance with medications, follow-ups and restrictions cannot therefore be overemphasized. Herein, decision-making and counseling plays the most crucial aspect of Kpro surgery, knowing when to operate and when not.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Professor James Chodosh, Boston Kpro Foundation.
References
- 1.Khan B, Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: An update. Curr Opin Ophthalmol. 2001;12:282–7. doi: 10.1097/00055735-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Saeed HN, Shanbhag S, Chodosh J. The Boston keratoprosthesis. Curr Opin Ophthalmol. 2017;28:390–6. doi: 10.1097/ICU.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 3.Yaghouti F, Nouri M, Abad JC, Power WJ, Doane MG, Dohlman CH, et al. Keratoprosthesis: Preoperative prognostic categories. Cornea. 2001;20:19–23. doi: 10.1097/00003226-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Dohlman CH, Mona HD, Graney J. The Boston keratoprosthesis: A new threadless design. Digit J Ophthalmol. 2007;13:3. [Google Scholar]
- 5.Hille K, Grabner G, Liu C, Colliardo P, Falcinelli G, Taloni M, et al. Standards for modified osteoodontokeratoprosthesis (OOKP) surgery according to strampelli and falcinelli: The Rome-Vienna protocol. Cornea. 2005;24:895–908. doi: 10.1097/01.ico.0000157401.81408.62. [DOI] [PubMed] [Google Scholar]
- 6.Chan CC, LoVerde L, Qiang J, Nordlund ML, Holland EJ. Incidence, risk factors, and surgical management of Boston type 1 keratoprothesis corneal melts, leaks, and extrusions. Cornea. 2016;35:1049–56. doi: 10.1097/ICO.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 7.Bouhout S, Robert MC, Deli S, Harissi-Dagher M. Corneal melt after Boston keratoprosthesis: Clinical presentation, management, outcomes and risk factor analysis. Ocul Immunol Inflamm. 2017;1:1–7. doi: 10.1080/09273948.2016.1269930. [DOI] [PubMed] [Google Scholar]
- 8.Goins KM, Kitzmann AS, Greiner MA, Kwon YH, Alward WL, Ledolter J, et al. Boston type 1 keratoprosthesis: Visual outcomes, device retention, and complications. Cornea. 2016;35:1165–74. doi: 10.1097/ICO.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 9.de la Paz MF, Stoiber J, de Rezende Couto Nascimento V, de Toledo JA, Seyeddain O, Hitzl W, et al. Anatomical survival and visual prognosis of Boston type I keratoprosthesis in challenging cases. Graefes Arch Clin Exp Ophthalmol. 2014;252:83–90. doi: 10.1007/s00417-013-2481-6. [DOI] [PubMed] [Google Scholar]
- 10.Aravena C, Yu F, Aldave AJ. Long-term visual outcomes, complications, and retention of the Boston type I keratoprosthesis. Cornea. 2018;37:3–10. doi: 10.1097/ICO.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 11.Patel AP, Wu EI, Ritterband DC, Seedor JA. Boston type 1 keratoprosthesis: The New York eye and ear experience. Eye (Lond) 2012;26:418–25. doi: 10.1038/eye.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Güell JL, Arcos E, Gris O, Aristizabal D, Pacheco M, Sanchez CL, et al. Outcomes with the Boston type 1 keratoprosthesis at instituto de microcirugía ocular IMO. Saudi J Ophthalmol. 2011;25:281–4. doi: 10.1016/j.sjopt.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekhanont K, Thaweesit P, Muntham D, Chuckpaiwong V, Vongthongsri A. Medium-term outcomes of Boston type 1 keratoprosthesis implantation in Bangkok, Thailand. Cornea. 2014;33:1312–9. doi: 10.1097/ICO.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 14.Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118:1543–50. doi: 10.1016/j.ophtha.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–96. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira LA, Pedreira Magalhães F, Hirai FE, de Sousa LB. Experience with Boston keratoprosthesis type 1 in the developing world. Can J Ophthalmol. 2014;49:351–7. doi: 10.1016/j.jcjo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Duignan ES, Ní Dhubhghaill S, Malone C, Power W. Long-term visual acuity, retention and complications observed with the type-I and type-II Boston keratoprostheses in an Irish population. Br J Ophthalmol. 2016;100:1093–7. doi: 10.1136/bjophthalmol-2015-307443. [DOI] [PubMed] [Google Scholar]
- 18.Shihadeh WA, Mohidat HM. Outcomes of the Boston keratoprosthesis in Jordan. Middle East Afr J Ophthalmol. 2012;19:97–100. doi: 10.4103/0974-9233.92123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Arfaj K, Hantera M. Short-term visual outcomes of Boston keratoprosthesis type I in Saudi Arabia. Middle East Afr J Ophthalmol. 2012;19:88–92. doi: 10.4103/0974-9233.92121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: Long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005;123:1319–29. doi: 10.1001/archopht.123.10.1319. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Okera S, Tandon R, Herold J, Hull C, Thorp S, et al. Visual rehabilitation in end-stage inflammatory ocular surface disease with the osteo-odonto-keratoprosthesis: Results from the UK. Br J Ophthalmol. 2008;92:1211–7. doi: 10.1136/bjo.2007.130567. [DOI] [PubMed] [Google Scholar]
- 22.Charoenrook V, Michael R, de la Paz MF, Ding A, Barraquer RI, Temprano J, et al. Osteokeratoprosthesis using tibial bone: Surgical technique and outcomes. Ocul Surf. 2016;14:495–506. doi: 10.1016/j.jtos.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC, et al. Long-term visual outcomes and complications of Boston keratoprosthesis type II implantation. Ophthalmology. 2017;124:27–35. doi: 10.1016/j.ophtha.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Aldave AJ, Sangwan VS, Basu S, Basak SK, Hovakimyan A, Gevorgyan O, et al. International results with the Boston type I keratoprosthesis. Ophthalmology. 2012;119:1530–8. doi: 10.1016/j.ophtha.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Iyer G, Pillai VS, Srinivasan B, Falcinelli G, Padmanabhan P, Guruswami S, et al. Modified osteo-odonto keratoprosthesis – The Indian experience – Results of the first 50 cases. Cornea. 2010;29:771–6. doi: 10.1097/ICO.0b013e3181ca31fc. [DOI] [PubMed] [Google Scholar]
- 26.Iyer G, Srinivasan B, Gupta J, Rishi P, Sen PR, Bhende P, et al. Boston keratoprosthesis for keratopathy in eyes with retained silicone oil: A new indication. Cornea. 2011;30:1083–7. doi: 10.1097/ICO.0b013e318213a8b5. [DOI] [PubMed] [Google Scholar]
- 27.Grassi CM, Cruzat A, Taniguchi EV, Crnej A, Colby KA, Dohlman CH, et al. Periprosthetic tissue loss in patients with idiopathic vitreous inflammation after the Boston keratoprosthesis. Cornea. 2015;34:1378–82. doi: 10.1097/ICO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 28.Iyer G, Srinivasan B, Agarwal S, Rachapalle SR. Laminar resorption in modified osteo-odonto-keratoprosthesis procedure: A cause for concern. Am J Ophthalmol. 2014;158:263–900. doi: 10.1016/j.ajo.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Talati RK, Hallak JA, Karas FI, de la Cruz J, Cortina MS. Retroprosthetic membrane formation in Boston keratoprosthesis: A Case-control-matched comparison of titanium versus PMMA backplate. Cornea. 2018;37:145–50. doi: 10.1097/ICO.0000000000001462. [DOI] [PubMed] [Google Scholar]
- 30.Perez VL, Leung EH, Berrocal AM, Albini TA, Parel JM, Amescua G, et al. Impact of total pars plana vitrectomy on postoperative complications in aphakic, snap-on, type 1 Boston keratoprosthesis. Ophthalmology. 2017;124:1504–9. doi: 10.1016/j.ophtha.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Iyer G, Srinivasan B, Agarwal S, Shetty R, Krishnamoorthy S, Balekudaru S, et al. Glaucoma in modified osteo-odonto-keratoprosthesis eyes: Role of additional stage 1A and Ahmed glaucoma drainage device-technique and timing. Am J Ophthalmol. 2015;159:482–900. doi: 10.1016/j.ajo.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Iyer G, Srinivasan B, Gupta N, Padmanabhan P. Outcome of Boston keratoprosthesis in a developing country-importance of patient selection, education, and perioperative care: The Indian experience. Asia Pac J Ophthalmol (Phila) 2012;1:202–7. doi: 10.1097/APO.0b013e3182607e5d. [DOI] [PubMed] [Google Scholar]
- 33.Iyer G, Srinivasan B, Agarwal S, Pillai VS, Ahuja A. Treatment modalities and clinical outcomes in ocular sequelae of Stevens-Johnson syndrome over 25 years – A paradigm shift. Cornea. 2016;35:46–50. doi: 10.1097/ICO.0000000000000680. [DOI] [PubMed] [Google Scholar]