Abstract
Purpose:
The aim of this study is to determine which parameter of Cirrus and RTVue optical coherence tomography (OCT) has the highest ability to discriminate between early, moderate, and advanced glaucoma. Simultaneously, to compare the performance of the two OCT devices in terms of their ability to differentiate the three stages of glaucoma. Further, to analyze the macular parameters of both devices and compare them with the conventional retinal nerve fiber layer (RNFL) parameters.
Methods:
One hundred and twenty eyes (30 healthy and 90 glaucomatous [30 mild, 30 moderate, and 30 advanced glaucoma]) of 65 participants (15 healthy, 50 glaucomatous [15 mild, 15 moderate, and 20 advanced glaucoma]) underwent Cirrus and RTVue OCT scanning on a single visit.
Results:
Average RNFL thickness and superior RNFL thickness of both the devices and inferior (ganglion cell complex [GCC] of RTVue device best differentiated normals from all stage glaucomatous eyes (P > 0.05). Cirrus average RNFL thickness and superior RNFL thickness performed better than other parameters (P < 0.05) in differentiating early glaucoma from moderate and advanced. In differentiating advanced from early and moderate glaucoma, RTVue average, superior, and inferior RNFL thickness and inferior GCC parameters had the highest discriminating ability (P < 0.05).
Conclusion:
Overall, average RNFL thickness had the highest ability to distinguish different stages of the disease. No significant difference was found between RTVue and Cirrus OCT device in different severity levels. No significant difference was observed between RNFL and macular parameters in different stages of glaucoma.
Keywords: Ganglion cell complex, glaucoma, optical coherence tomography, retinal nerve fiber layer
Glaucoma is a multifactorial optic neuropathy characterized by progressive structural loss of retinal ganglion cells (RGC) that may result in vision loss and irreversible blindness.[1] While the assessment of optic nerve head (ONH) and peripapillary retinal nerve fiber layer (RNFL) clinically is of paramount importance in the diagnosis of glaucoma, the recent increasing use of various imaging modalities has greatly supplemented the accuracy of diagnosing glaucoma in its different stages.[2,3]
Optical coherence tomography (OCT) is a noninvasive technology which provides cross-sectional measurements of various retinal layers. The recent introduction of spectral domain OCT has revolutionized the field of ophthalmology with a much higher axial resolution and faster scanning speed.
At present, Cirrus high-definition (HD)-OCT and RTVue 100 are the two common OCT devices widely used to quantify circumpapillary RNFL (cp-RNFL) thickness in clinical practice. Numerous studies on Cirrus OCT have revealed that RNFL thickness parameters are reproducible and have high diagnostic sensitivity and specificity in discriminating between healthy and glaucomatous eyes.[4,5] Furthermore, RNFL parameters of the RTVue-100°CT have shown high specificity for the detection of glaucoma[6]
Another extremely useful and less explored parameter of various OCT devices is the macular thickness. It has been studied that the glaucomatous damage to the macular region is common and occurs quite early in the disease.[7] It is well known that the retinal ganglion cell bodies in the inner nuclear layer of the retina are around 20-fold thicker than their axons.[8] Most of the studies have compared the discriminating ability of macular parameters of OCT devices in healthy versus early glaucoma participants and that too with variable conclusions.
In this study, we analyzed the discriminating ability of Cirrus and RTVue OCT parameters in different severity grades of glaucoma and simultaneously deduce supremacy of one OCT device over the other. In addition, we compared the retinal nerve fiber thickness and macular parameters of both the OCT devices.
Methods
Study design
This was an observational cross-sectional study. The sample size was computed using the MEDCALC-version 9.2.1.0. On keeping the power of the study, 80% (probability of type 1 (alpha) error-5%), the area under the curve (AUC) as 0.8 and the null hypothesis value of 0.5, the minimum required sample size for each group came out to be 29. Hence, we took the round figure of 30 participants for each group. Thirty early glaucomatous eyes, 30 moderate, 30 advanced glaucomatous eyes were recruited along with 30 healthy eyes.
Participants
A total of 120 eyes (30 healthy and 90 glaucomatous [30 mild, 30 moderate, and 30 advanced glaucoma]) of 65 participants (15 healthy, 50 glaucomatous [15 mild, 15 moderate, and 20 advanced glaucoma]) were recruited for this study. Both the eyes of the participants were included if they fulfilled the inclusion criteria. Five participants in the advanced glaucoma group were one-eyed, and hence, the seeing eye was recruited and the nonseeing eye excluded from the study. Another five participants with advanced glaucoma were included, and their worse eye was taken. The glaucoma participants were recruited from the Glaucoma Clinic of our hospital. The healthy participants were also recruited from the outpatient department of our hospital.
The diagnosis of primary open or closed angle glaucoma was based on gonioscopy findings, previous high intraocular pressure (IOP) (>21 mmHg) and evidence of glaucomatous optic neuropathy by any of the following signs: neuroretinal rim thinning, notching, excavation, RNFL defects or asymmetry or a vertical cup-to-disc ratio of at least 0.2 between the two eyes.
The patient group consisted of mild, moderate, and advanced primary open and closed angle glaucoma (Hodapp-Parrish-Anderson criteria[9]) [Table 1] participants with IOP controlled below 21 mmHg with antiglaucoma medication (all cases of well-established glaucoma with corroborative findings in disc and fields were taken). All patients were in the age range of 18–80 years with best-corrected visual acuity > 6/12 or better. Both the eyes of each patient were included if it fulfilled the inclusion criteria.
Table 1.
Hodapp-Parrish-Anderson classification system
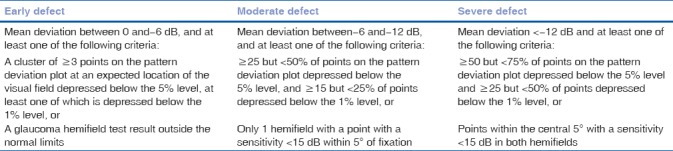
Inclusion criteria for controls or the healthy participants were IOP < 21 mm Hg, at least two reliable normal visual fields (pattern standard deviation [PSD] within 95% confidence limits and a glaucoma hemifield test result within normal limits), best-corrected visual acuity-6/9 or better, normal optic disc and macula on fundoscopic examination, vertical C/D ratio < 0.4 or a difference of < 0.2 between two eyes and age range from 18–80 years.
Exclusion criteria for all the participants were comorbid ocular conditions, for example, diabetic retinopathy, age-related macular degeneration, cataract, optic neuropathy other than glaucoma, secondary and congenital glaucoma, pathological myopia, ocular surgery within previous 6 months, media opacities precluding good quality scan, systemic diseases, and medications that could induce optic neuropathy.
Ethical clearance was obtained from the Institutional Review Board of our hospital. Informed consent was taken from all participants.
Ophthalmological examination
All participants underwent a comprehensive ophthalmologic examination: best-corrected visual acuity, a detailed slit-lamp evaluation, stereoscopic ONH photography and evaluation by a glaucoma specialist, stereoscopic evaluation of the macula, repeated White-on-White automated perimetry with the Humphrey Visual Field Analyzer 750 24-2 Sita Standard visual field testing and daytime IOP recording with Goldmann Applanation Tonometry within 1 month from the OCT imaging. Reliable visual field results are defined as ≤ 33% false positive, ≤33% false negative, a reliable factor of 15%, and pupil diameter ± 3 mm.[10] All enrolled participants were classified according to three stages of visual field damage as having early, moderate, or advanced defects using (Hodapp-Parish-Anderson criteria[9]) [Table 1].
Imaging with RTVue-100 optical coherence tomography
Each participant underwent the RTVue-100 Fourier domain-OCT and Cirrus HD-OCT scan on the same day. The standard glaucoma protocol of the RTVue-100 was used including a three-dimensional optic disc scan for the definition of the disc margin, an ONH scan, and a standard ganglion cell complex (GCC scan. The thickness of cp-RNFL was measured using ONH scan which includes nearly all the axons of the ganglion cells. The GCC scan measured the summation of three layers in the macula: the inner plexiform layer, the ganglion cell layer, and the nerve fiber layer representing ganglion cell dendrites, ganglion cell bodies, and ganglion cell axons, respectively. The parameters of the ONH scan included in our study were average, superior, and inferior cp-RNFL thickness, vertical cup/disc (C/D) ratio, and rim area. The parameters of the GCC scan analyzed in our study were average, superior, and inferior GCC thickness. Scan image with signal strength index lower than 35 was discarded.
Imaging with Cirrus high-definition optical coherence tomography
Using the Cirrus HD-OCT, each eye underwent a peripapillary scan to measure cp-RNFL thickness and a macular scan to measure the GCIPL thickness using the GCA algorithm. Different from the GCC, the GCIPL thickness measures the GCC without the cp-RNFL. Only those scans with signal strengths of 7 or more, and without motion artifacts, were considered for analysis. Similar to RTVue OCT, the parameters of the Cirrus HD OCT included in our study were average, superior, and inferior RNFL thickness in the cp-RNFL thickness map, the vertical cup-disc ratio and the rim area in ONH map and the average, superior, and inferior GCIPL thickness in the GCA map.
Statistical analysis
Baseline demographic and ocular characteristics – age/sex/C/D ratio/mean deviation/PSD were compared between the groups using one-way analysis of variance in MedCalc software- version 9.2.1.0. Mean values of different parameters of both the OCT devices were compared between all the four groups using one-way analysis of variance followed by Student–Newman–Keuls Post hoc test in MEDCALC–version 9.2.1.0 to establish the equality of means. Receiver operating characteristic (ROC) curves were used to describe the ability of each parameter to differentiate between different glaucoma stages. In this study, the AUC was classified as follows: 0.9–1 = excellent, 0.80–0.89 = good, 0.70–0.79 = fair, 0.60–0.69 = poor, and 0.50–0.59 = worthless test. Values on the ROC curves that have the best sensitivity and specificity were chosen as cutoff values that could separate between every two consecutive stages. The value of P < 0.05 was considered as statistically significant. Power of the study was 80%.
Results
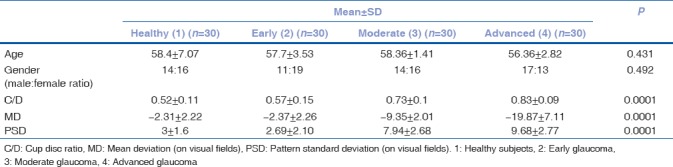
A total of 120 eyes of 65 patients were enrolled in this study. Thirty eyes were normal, 90 were glaucomatous. Glaucomatous eyes were further classified into early glaucoma (30), moderate glaucoma (30), and advanced glaucoma (30). There were 64 females and 56 males. Table 2 summarizes the baseline characteristics of the study population. There was no significant difference between normal and glaucomatous eyes with regard to gender and age. A statistically significant difference was seen in cup-disc ratio, mean deviation (on visual fields), and PSD (on visual fields) between the normal and glaucomatous eyes.
Table 2.
Clinical and ocular characteristics of the included eyes presented as mean±standard deviation, unless indicated otherwise
Receiver operating characteristic curves in differentiating stages of glaucoma
To detect the most sensitive OCT parameter which can discriminate between different stages of glaucoma, AUCs were calculated [Table 3]. Three groups were made for comparison: normal (1) participants versus all stage glaucoma participants (G1VS234); normal (1) and early (2) glaucoma participants versus moderate (3) and advanced (4) participants (G12VS34); and normal, early, and moderate glaucoma versus advanced glaucoma (G123VS4) (G stands for group).
Table 3.
Discriminating ability of different parameters between different glaucoma stages using area under receiver operating characteristic curves
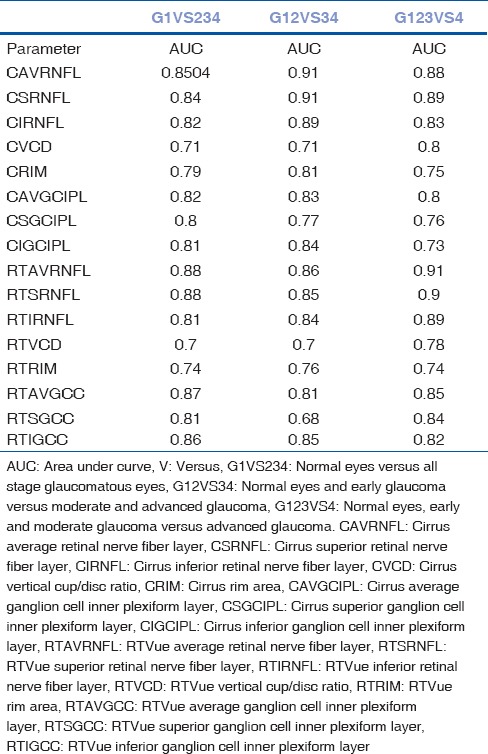
The best parameters (largest AUCs) which could differentiate normal from all stage glaucoma participants (G1VS234) were average, superior, and inferior RNFL thickness of both the OCT devices and inferior GCC of RTVue OCT device (AUC: 0.84–0.88). The best parameters which could distinguish normal and early from moderate and advanced glaucoma participants (G12VS34) were average, superior, and inferior RNFL thickness of both the devices and inferior macular parameter of RTVue device (GCC) (AUC: 0.85–0.91). Finally, the parameters which best discriminate advanced glaucoma participants from the rest (G123VS4) were again the average, superior, and inferior RNFL thickness of both the devices and inferior GCC of the RTVue device (AUC: 0.82–0.91). Overall, we can conclude that parameters average, superior, and inferior RNFL of both the devices and inferior GCC of RTVue device had the largest AUCs. The parameter which performed the poorest in all the three categories was the vertical cup-disc ratio of both the OCT devices (AUC CVCD: 0.71–0.8; AUC RTVCD: 0.7–0.78) [Table 3].
Receiver operating characteristic curves: Pairwise comparison of the receiver operating characteristic curves of the best parameters of both devices
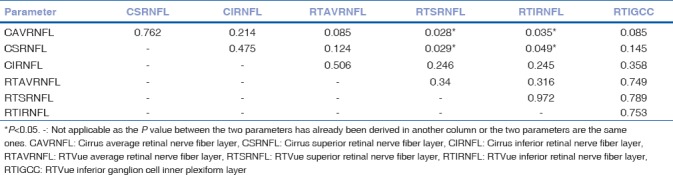
The parameters with the best performances (largest AUCs) in each category (G1VS234, G12VS34, G123VS4) [Table 4–6] were then compared among themselves. In category G1VS234, there was no statistically significant difference found between the average and superior RNFL thickness (AUC-0.0.84–0.88) of both the OCT devices and inferior macular thickness (AUC-0.86) of RTVue (P > 0.05), thus concluding that all these parameters are equally efficient and reliable in discriminating normal eyes from different severity grades of glaucomatous eyes [Table 4]. Inferior RNFL thickness was shown to have a lower AUC than average RNFL thickness in the G1VS234 category (P < 0.05). Cirrus average RNFL thickness (AUC-0.91) and superior RNFL thickness (AUC-0.91) emerged as the most reliable parameters in category G12VS34 (P < 0.05) [Table 5]. Finally, in the category G123VS4, there was no significant difference found between the average, superior, and inferior RNFL thickness of both the devices and the inferior GCC parameter of RTVue (AUC-0.89–0.91), (P > 0.05) [Table 6].
Table 4.
Comparison of receiver operating characteristic curves of best parameters of RTVue optical coherence tomography and cirrus optical coherence tomography in normal eyes versus all stage glaucomatous eyes: P values

Table 6.
Comparison of receiver operating characteristic curves of best parameters of RTVue optical coherence tomography and cirrus optical coherence tomography in normal eyes, early and moderate glaucoma versus advanced glaucoma: P values

Table 5.
Comparison of receiver operating characteristic curves of best parameters of RTVue optical coherence tomography and cirrus optical coherence tomography in normal eyes and early glaucoma versus moderate and advanced glaucoma: P values
Discussion
In the present study, our purpose was to evaluate the ability of various parameters of Cirrus and RTVue OCT devices in discriminating different stages of glaucoma. We compared normal versus all stage glaucomatous participants (G1VS234), early versus moderate and advanced (G12VS34) and advanced versus the rest (G123VS4). Overall, it was found that average RNFL thickness of both the instruments had the high discriminating ability in all the three comparisons. Our results are in agreement with Elbendary and Mohamed Helal[11] who studied the discriminating ability of RNFL thickness measured with 3DOCT 2000-Fourier Domain OCT in different stages of glaucoma and reported that average, superior and inferior RNFL thickness were the best parameters to discriminate normal from early glaucoma (AUC: 0.91–0.86), early from moderate (AUC: 0.77–0.70), and moderate form severe (AUC: 0.85–0.83) glaucoma.
In regard to the difference between two OCT devices, our results are in agreement with the study by Fenolland et al.[12] It compared the diagnostic accuracy of RTVue and Cirrus OCT and concluded that there is no significant difference between RNFL measurements and the macular measurements of the two instruments. Similarly, Leite et al.[13] compared the diagnostic accuracy of Spectralis, cirrus, and RTVue OCT devices in glaucomatous eyes and reported no statistically significant difference in the pairwise comparison among the ROC curves of all the RNFL parameters except the nasal quadrant which had significantly larger AUC in Spectralis and RTVue compared to Cirrus. The study also revealed that there is no significant difference between the two OCT devices in discriminating different severity grades of glaucoma.
We, in addition, also evaluated macular measurements in glaucomatous eyes and have found that inferior GCC of RTVue OCT device is equivalent to average and regional RNFL measurements in differentiating early moderate, and advanced glaucoma. Literature suggests that there have been variable results in deducing supremacy of macular parameter over RNFL thickness. Tan et al.[14] revealed that GCC and GCIPL thickness have a similar diagnostic power to that of cp-RNFL thickness for the diagnosis of early glaucoma. Mwanza et al.[15] also studied the diagnostic accuracy of the ganglion cell-inner plexiform layer (GCIPL) and compared it with RNFL thickness and ONH parameters in glaucomatous eyes. They also concluded that GCIPL had similar ability as peripapillary RNFL and ONH parameters in discriminating normal eyes from glaucomatous ones. On the other hand, Cennamo et al.[16] concluded that inferior GCC (AUC-0.792) and mean GCC thickness (AUC-0.742) are the best parameters to discriminate initial glaucomatous changes before the onset of visual field losses. Adding to this spectrum of variable results and conclusions, Nouri-Mahdavi et al.[17] reported that average cp-RNFL measurements are superior to average GCIPL measurements for the detection of early glaucoma. To the best of our knowledge, all these studies have compared macular and RNFL parameters in differentiating healthy participants from early glaucomatous eyes only unlike our study in which all these parameters have been studied in all three severity grades of glaucoma.
A study by Bambo et al.[18] compared the cp-RNFL and the macular GCIPL in glaucoma patients at different disease stages in a single OCT device (Cirrus HD OCT) and concluded that the inner macular parameters performed as well as cp-RNFL measurements in patients with different stages of glaucoma. However, in this study, the AUC was not calculated, and the discriminating ability of various parameters was evaluated on the basis of the difference in the mean values of the parameters in early, moderate, and advanced glaucoma. On the other hand, we have calculated the AUC for each parameter in different disease stages which is a more reliable marker to estimate the discriminating ability.
We also concluded that vertical cup-disc ratio is not a reliable parameter in discriminating different stages of glaucoma in both the OCT devices. This parameter had a poor AUC (AUC-0.7) in distinguishing normals from early glaucoma and also early glaucoma from moderate and advanced ones. This finding was consistent for both Cirrus and RTVue devices. However, this parameter has a fair discriminating ability in advanced glaucoma (AUC: 0.78–0.8).
The limitation of this study is that it is a single-center study with relatively small sample size. Further research with larger sample size is required.
Conclusion
The study is the first one in our knowledge to study the discriminating ability of both RNFL and macular parameters in two OCT devices in different severity grades of glaucoma using the area under ROC curve. Overall, average RNFL thickness was found to have the highest ability to distinguish different stages of the disease. No significant difference was found between RTVue and Cirrus OCT device in various parameters in different severity levels. We also found that macular measurements perform similarly to RNFL measurements in discriminating different severity grades of glaucoma. Finally, our findings confirm that vertical cup-disc-ratio parameter in both the instruments had the worst ability to distinguish different severity grades of glaucoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma III Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–46. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 2.Mansouri K, Leite MT, Medeiros FA, Leung CK, Weinreb RN. Assessment of rates of structural change in glaucoma using imaging technologies. Eye (Lond) 2011;25:269–77. doi: 10.1038/eye.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung KR, Wollstein G, Kim NR, Na JH, Nevins JE, Kim CY, et al. Macular assessment using optical coherence tomography for glaucoma diagnosis. Br J Ophthalmol. 2012;96:1452–5. doi: 10.1136/bjophthalmol-2012-301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwanza JC, Oakley JD, Budenz DL, Anderson DR Cirrus Optical Coherence Tomography Normative Database Study Group. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118:241–80. doi: 10.1016/j.ophtha.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung KR, Na JH, Lee Y. Glaucoma diagnostic capabilities of optic nerve head parameters as determined by cirrus HD optical coherence tomography. J Glaucoma. 2012;21:498–504. doi: 10.1097/IJG.0b013e318220dbb7. [DOI] [PubMed] [Google Scholar]
- 6.Paul C. To assess the glaucoma diagnostic ability of Fourier domain optical coherence tomography. Am J Eng Res. 2013;104 [Google Scholar]
- 7.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mwanza JC, Webel AD, Warren JL, Budenz DL. Retinal nerve fiber layer thickness floor and corresponding functional loss in glaucoma. Invest Ophthalmol Vis Sci. 2014;55:976–80. doi: 10.1136/bjophthalmol-2014-305745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodapp E, Parrish RK, Anderson DR. Clinical Decisions in Glaucoma. St Louis: Mosby; 1993. pp. 52–61. [Google Scholar]
- 10.Birt CM, Shin DH, Samudrala V, Hughes BA, Kim C, Lee D, et al. Analysis of reliability indices from humphrey visual field tests in an urban glaucoma population. Ophthalmology. 1997;104:1126–30. doi: 10.1016/s0161-6420(97)30173-0. [DOI] [PubMed] [Google Scholar]
- 11.Elbendary AM, Mohamed Helal R. Discriminating ability of spectral domain optical coherence tomography in different stages of glaucoma. Saudi J Ophthalmol. 2013;27:19–24. doi: 10.1016/j.sjopt.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenolland J, Giraud JM, El Chehab H, Francoz M, Sendon D, May F, et al. Glaucoma diagnostic accuracy: Comparison of RTVue100 and Cirrus HD. Acta Ophthalmol. 2012;90(Suppl):s249. [Google Scholar]
- 13.Leite MT, Rao HL, Zangwill LM, Weinreb RN, Medeiros FA. Comparison of the diagnostic accuracies of the spectralis, cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011;118:1334–9. doi: 10.1016/j.ophtha.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–140. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwanza JC, Durbin MK, Budenz DL, Sayyad FE, Chang RT, Neelakantan A, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: Comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012;119:1151–8. doi: 10.1016/j.ophtha.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Cennamo G, Montorio D, Romano MR, Cardone DM, Minervino C, Reibaldi M, et al. Structure-functional parameters in differentiating between patients with different degrees of glaucoma. J Glaucoma. 2016;25:e884–8. doi: 10.1097/IJG.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 17.Nouri-Mahdavi K, Nowroozizadeh S, Nassiri N, Cirineo N, Knipping S, Giaconi J, et al. Macular ganglion cell/inner plexiform layer measurements by spectral domain optical coherence tomography for detection of early glaucoma and comparison to retinal nerve fiber layer measurements. Am J Ophthalmol. 2013;156:1297–307e2. doi: 10.1016/j.ajo.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bambo MP, Güerri N, Ferrandez B, Cameo B, Fuertes I, Polo V, et al. Evaluation of the macular ganglion cell-inner plexiform layer and the circumpapillary retinal nerve fiber layer in early to severe stages of glaucoma: Correlation with central visual function and visual field indexes. Ophthalmic Res. 2017;57:216–23. doi: 10.1159/000453318. [DOI] [PubMed] [Google Scholar]


