Abstract
Purpose:
To determine the long-term incidence of fellow-eye surgical involvement in patients who have undergone first-eye vitreoretinal (VR) surgery for a variety of indications. This was a single-institution retrospective, consecutive series.
Methods:
Eighteen years of electronic surgical data were reviewed at our institution. All patients having surgery for the following indications were included: rhegmatogenous retinal detachment (RRD), macular hole (MH), epiretinal membrane (ERM), proliferative diabetic retinopathy (PDR), vitritis, and dropped nucleus. Primary outcome was the cumulative incidence of fellow-eye surgery at 10 years by Kaplan–Meier analysis.
Results:
Total follow-up was 29,629 patient-years. Cumulative incidence (± standard error) of fellow-eye surgery at 10 years was 7.2% ± 0.6% for RRD, 9.1% ± 1.3% for ERM, 7.5% ± 1.8% for MH, 30.6% ± 1.9% for PDR, 13.7% ± 2.9% for vitritis, and 2.8% ± 1.6% for dropped nuclei. The hazard for second-eye surgery was greatest in the early postoperative period after first-eye surgery for all indications. For RRD, the hazard was 2.7% ± 0.3% at year 1, 1.1% ± 0.2% at year 2, and 0.5% ± 0.2% at year 5. Risk factors for fellow-eye involvement for RRD were younger age (P < 0.001) and male gender (P < 0.01).
Conclusion:
We report the long-term risk of fellow-eye involvement in various VR pathologies, which is important in counseling patients regarding their risks as well as planning service provision.
Keywords: Fellow eye, hazard, retinal detachment, risk, second eye, vitreoretinal surgery
Vitreoretinal (VR) pathologies such as macular holes (MHs), epiretinal membranes (ERMs), and rhegmatogenous retinal detachments (RRDs) typically present with unilateral symptoms, but there is an increased propensity for fellow-eye involvement over time. The risk of requiring fellow-eye surgery in VR pathologies is not well established. In the case of RRD, the cumulative risk of second-eye involvement has been reported between 4% and 31% over variable follow-up periods,[1,2,3,4,5,6,7] while annual incidence has been estimated at 1%.[2] Robust, long-term data on VR patients is necessary to calculate such risks, and this data is difficult to obtain with correspondingly few reports. Yet, fellow-eye involvement remains one of the primary concerns for patients with VR pathology – and justifiably so given the potential impact on visual function. An understanding of the timing, incidence, and risk factors for second-eye surgery also has implications clinically in terms of how and when VR patients are counseled and monitored long-term.
In this retrospective study, we utilized a prospectively maintained electronic medical record (EMR) available at our institution over an 18-year period to assess the timing and incidence of second-eye surgical involvement in various VR pathologies.
Methods
A retrospective review of data was performed on a prospectively maintained EMR (Vitreor, Axsys Technologies, Glasgow, Scotland). The EMR has been used at our institution to record all VR operations since October 1998. The database captures the following compulsory fields: patient age, patient gender, laterality of affected eye, indication for surgery, and type of operation performed.
The database was reviewed for all operations meeting the following criteria:
Date of surgery: October 2001 to October 2016
-
Indication for surgery:
- RRD including any of the following: round holes, dialyses, horseshoe tear detachments, and giant retinal tears
- MH
- ERM
- proliferative diabetic retinopathy (PDR)
- Vitritis
- Dropped nucleus.
The first VR operation on the database for a particular patient (the “index” eye) was assumed to be the first-eye affected with a VR condition. By commencing study inclusion from October 2001, 3 years of database records were available for review (1998–2001) to validate index eye status at our institution.
The primary outcomes of the study were to determine for each of the six included conditions (RRD, MH, ERM, PDR, vitritis, and dropped nucleus):
The mean annual incidence of fellow-eye surgical involvement throughout follow-up
The cumulative incidence of fellow-eye surgical involvement at 5, 10, and 15 years
The hazard ratio per year of fellow-eye surgical involvement
-
The risk factors associated with fellow-eye surgical involvement among the following:
- Age at presentation
- Gender
- Right–left laterality of the first eye
- Type of first-eye surgery (vitrectomy vs buckle) for RRD cases only
- Indication of first-eye surgery (tractional retinal detachment [TRD] vs. persistent vitreous hemorrhage [VH]) for PDR cases only.
Statistics
All statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY, USA). Mean annual incidence of fellow-eye involvement was determined by dividing the total number of fellow-eyes involved by the total number of patient-years of follow-up. The follow-up for each patient was calculated as the time from first-eye surgery until fellow-eye involvement or until the eye was censored. To adjust for the absence of death data, patients who did not have fellow-eye involvement were censored when they reached the average UK life expectancy age of 79 for males and 83 for females unless the study end date arrived before.[8] If a patient was already over the UK life expectancy age at the time of their index eye operation, they were censored at 5 years from the date of surgery, unless the study end date arrived before.
The cumulative incidence of fellow-eye involvement at 5, 10, and 15 years was determined by Kaplan–Meier survival analysis. Hazard rates for each year were determined by life table analysis. Risk factors associated with fellow-eye involvement were analyzed by Cox regression. For all tests, P < 0.05 was considered statistically significant.
Results
A total of 5,749 patients had an index VR operation undertaken at our institution between October 2001 and October 2016. Total follow-up was 29,629 patient-years.
The demographics of the index eyes [Table 1] were in keeping with expected demographics for the underlying indications. There was a clear male predominance for RRD (62%) and PDR (59%), while females predominated for MH (69%) and ERM (54%). Males and females were relatively equally represented for vitritis and dropped nuclei. Mean age at index eye presentation was youngest for vitritis (53 years) and PDR (55 years) and oldest for MH (69 years) and dropped nuclei (74 years). Right eyes were the more commonly involved index eye for all indications except dropped nuclei. The mean annual incidence of fellow-eye involvement [Table 1] calculated over the entire course of follow-up showed fellow-eye involvement to be most likely with PDR (mean incidence 5.7% per year) and least common with dropped nuclei (0.3% per year).
Table 1.
Index eye characteristics and mean annual incidence of fellow-eye involvement for each surgical indication
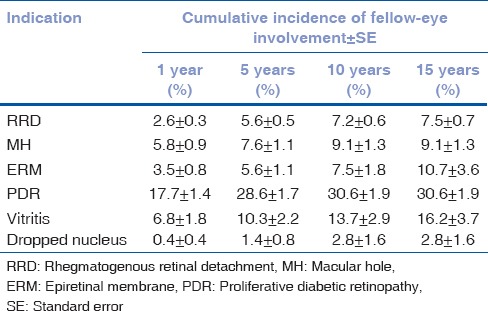
Kaplan–Meier second-eye survival curves for each indication are shown in Fig. 1. The cumulative incidence of fellow-eye involvement at 5, 10, and 15 years by Kaplan–Meier analysis for each of the indications is shown in Table 2.
Figure 1.
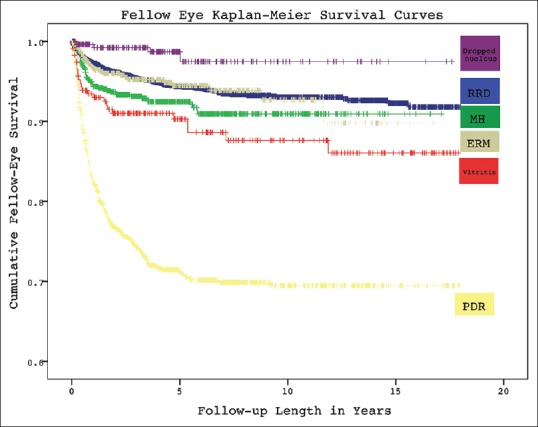
Kaplan–Meier cumulative survival curves for fellow-eye surgery after first-eye surgery for various vitreoretinal indications. RRD: Rhegmatogenous retinal detachment, MH: Macular hole, ERM: Epiretinal membrane, PDR: Proliferative diabetic retinopathy
Table 2.
Cumulative fellow-eye involvement at 1, 5, 10, and 15 years by Kaplan-Meier
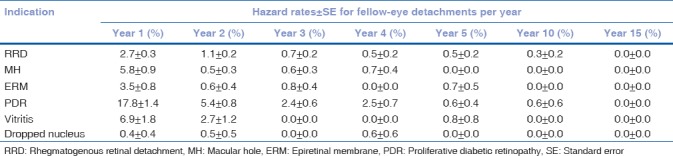
Hazard rates for fellow-eye involvement are shown in [Table 3]. They emphasize that fellow-eye involvement was heavily loaded in the early postoperative period after first-eye surgery for all indications. In the case of RRD, 49% (82/168) of fellow-eye detachments occurred in the 1st year and 77% within 3 years, while <3% occurred beyond 7 years. Hazard rates for most indications were 0 at 10 years, and for all indications were 0 at 15 years.
Table 3.
Hazard rates±standard error per year for fellow-eye detachment
Risk factors for fellow-eye rhegmatogenous retinal detachment
Cox regression found younger age at first-eye presentation was a significant risk for second-eye RRD (P < 0.001). With each additional year at presentation, the risk of second-eye RRD decreased by 1.8%. Male gender was also associated with second-eye involvement (P = 0.01). Right–left laterality of the first eye (P = 0.54) and type of first-eye surgery (vitrectomy vs buckle, P = 0.11) were not independent risk factors for second-eye involvement.
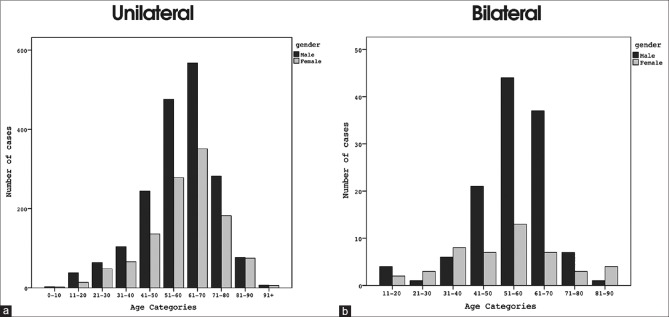
Fig. 2a shows the age and gender distribution of all RRD's, while Fig. 2b shows the age and gender distribution for bilateral cases. Fig. 2b emphasizes that bilateral retinal detachment is primarily a condition of middle-aged males.
Figure 2.
Gender and age distribution at the time of first-eye surgery for rhegmatogenous retinal detachment in cases that were ultimately (a) unilateral and (b) bilateral
In a post hoc analysis of the 434 RRD first-eyes that underwent buckling (in our institution this was a close proxy for round hole detachment or dialysis), age of first-eye surgery (P = 0.39) and male gender (P = 0.78) were no longer statistically significant risk factors for second-eye involvement. By contrast, for the 2755 first-eyes that had vitrectomies (a proxy for posterior vitreous detachment (PVD) and horseshoe breaks at our institution), younger age at presentation of first-eye RRD (P = 0.001) and male gender (P = 0.006) were both strongly associated with second-eye involvement. For cases that had a first-eye vitrectomy (implying PVD-positive RRD), the mean age of presentation was 61 years where unilateral and 56 years where bilateral (P < 0.001).
Risk factors for fellow-eye: Epiretinal membrane, macular hole, proliferative diabetic retinopathy, vitritis, dropped nuclei
For PDR, younger age at first-eye surgery was associated with second-eye surgery (P < 0.001) while indication for surgery (TRD vs. VH) and gender were not significant factors. For vitritis, older age at first-eye surgery was associated with second-eye surgery (P < 0.05). Age was not associated with second-eye involvement for ERM, MH, or dropped nuclei. Female gender was associated with increased risk of second-eye surgery for MH (P < 0.05) but gender was not associated with other indications (ERM, PDR, vitritis, or dropped nuclei). Laterality of first affected eye was not associated with second-eye involvement for any of these conditions.
Discussion
Rhegmatogenous retinal detachment and fellow-eye risks
The mean annual incidence of fellow-eye RRD in our study was 1.0%. This is consistent with findings by Hajari et al. who conducted the largest population study on the subject to date.[2] They found a mean annual incidence of 1.3% using operative records from the Danish National Health Registry covering a follow-up of 37,252 patient-years. In a smaller series, Folk and Burton reported a mean annual incidence around 1% depending on whether eyes had myopia (1.3%) or lattice (0.95%) or both (0.98%).[1] It is important to note that mean annual incidence, which is calculated across the entirety of follow-up, does not accurately reflect the variation in risk over time – and in particular the loading of fellow-eye risk in the early postoperative period after first-eye surgery. The probability (hazard rate) of fellow-eye surgery in the 1st year was 2.7% in our series, which reduced significantly to 1.1% by the 2nd year (contingent on the fellow-eye surviving to the start of the 2nd year) and 0.7% by the 3rd year. By the 5th and 10th years, the hazard rates were 0.5% and 0.3%, respectively. We are not aware of hazard rates being calculated for fellow-eye RRD before. Our results emphasize that monitoring the fellow-eye in the first few years after index eye detachment may be useful in detecting and preempting early pathology.
The cumulative incidence of fellow-eye RRD at 15 years by Kaplan–Meier analysis was 7.5% in our series. The cumulative fellow-eye risk in prior reports has been widely variable, ranging between 4% and 31% from cohorts of different sizes over variable follow-up periods, which makes useful comparison difficult.[1,3,4,5,6,7] Hajari et al. in their large, population study used a similar Kaplan–Meier analysis to our own work and found a cumulative 10-year risk of 9%.[2]
Of the risk factors examined in our study, male gender and younger age at first-eye presentation were statistically significant predictors of fellow-eye RRD involvement, which is consistent with prior reports.[2,4] Hajari et al. found pseudophakia to be an additional factor.[2] We were unable to determine the contribution of pseudophakia in our own study as it was not reliably recorded in our database. Other predisposing factors for fellow-eye involvement in prior, smaller reports include anatomical factors such as myopia, lattice, and giant retinal tears.[1,4] Again, we were unable to determine the contribution of these potential anatomical risks in our own study due to insufficient data collection on these variables. Nonetheless, the subgroup analysis of buckles versus vitrectomies in our series was instructive. At our institution, buckles are a proxy for round hole detachments or dialyses, while vitrectomy is a proxy for PVD-positive detachments with horseshoe breaks. It was interesting that gender and age were no longer statistically significant risk factors for fellow-eye involvement when the index-eye underwent a buckle, but these factors were strongly correlated to fellow-eye risk for vitrectomies. In regards to gender, these findings support a previous study by Chuo et al. which suggested a protective effect of estrogen on the integrity of vitreous architecture.[9] In regards to age, the correlation between younger presentation and fellow-eye involvement in PVD-positive detachments may be explained in two ways. First, patients who are younger when they develop a PVD (and by extension younger at first-eye presentation with a horseshoe detachment) are more likely to have inherent vitreoretinal vulnerabilities to retinal detachment such as high myopia, as confirmed by prior reports.[10] Second, patients who are younger at first-eye presentation with PVD-positive RRD are more likely to be presenting with a first-eye PVD. In other words, the fellow-eye remains at risk with an attached vitreous. On the other hand, patients presenting at a later age are more likely to be presenting with their second PVD, such that the fellow-eye has a detached vitreous and is at lower future risk. We could not test the independent contribution of PVD status to fellow-eye risk in our series due to the limitations of our data. It is conceivable that if PVD status and if intrinsic ocular factors such as myopia and lattice degeneration are taken into account, that age of first-eye presentation may no longer represent an independent risk for fellow-eye involvement.
Other vitreoretinal pathologies
The cumulative incidence of fellow-eye surgery at 15 years' follow-up Table 2 for the various presenting etiologies is instructive and provides a useful approximation for an individual's cumulative, lifetime risk. Unsurprisingly, the highest rate of fellow-eye surgical involvement occurred in diabetes (30.6%) and vitritis (16.2%). These are both conditions in which bilateral and symmetrical ocular involvement is common. There are very few studies on fellow-eye vitrectomy rates in PDR. Our incidence at 15 years (30.6%) was slightly lower than that found by Hwang et al. in a study of 358 patients from an inner-city population of predominantly uninsured, African-American patients where the 5-year fellow-eye vitrectomy rate was 36%.[11] In another study from New Zealand, on a predominantly Maori and Pacific Islander population, the fellow-eye vitrectomy rate was 38% at a mean follow-up of 4 years.[12] Of note, the population under study in New Zealand was in particularly poor health with 57% of patients dying by the time of data analysis. We are not aware of prior reports documenting cumulative fellow-eye vitrectomy rates in vitritis.
The cumulative risk of fellow-eye surgery at 15 years for ERM's was 10.7%. This appears consistent with population studies which suggest ERMs occur bilaterally (if not symmetrically) in 20%[13] to 30%[14] of patients with ERM. MH's had a cumulative 15-year risk of fellow-eye surgery of 9.1% in our series. By comparison, a prospective, observational, longitudinal study of normal fellow-eyes in patients with first-eye MH, found the risk of fellow-eye MH formation at 5 years was 15.6%.[15] The higher incidence in this report may be due to its prospective design and the fact that a proportion of patients with fellow-eye MH may not opt for surgery. Dropped nuclei had by far the lowest cumulative risk for bilaterality in our series as would be expected for an uncommon surgical complication.
In regards to risk factors, younger age at first-eye presentation was associated with fellow-eye involvement in PDR cases. This is likely reflective of younger presenters having more poorly controlled diabetes with correspondingly aggressive, bilateral disease. Hwang et al. found younger age and TRD (as opposed to nonclearing VH) to be associated with fellow-eye PDR vitrectomy.[11] TRD was not a significant risk factor in our series. A study by Song et al. assessing systemic influences on fellow-eye vitrectomy rates found renal impairment to be a significant factor.[16] For vitritis, older age was a risk factor for fellow-eye surgery in our series. This is likely to reflect the variation in presenting etiologies for vitritis in younger versus older patients.
In regards to risk over time, all 6 VR pathologies studied in our series had a significant loading of fellow-eye hazard in the early postoperative period after index eye surgery, as evidenced in [Fig. 1] and in [Table 3]. The tendency to early risk is likely multifactorial. In the case of PDR and vitritis, it likely reflects the fact that these conditions are often bilateral and relatively symmetrical. In MH's, ERM's, and RRD's, where the underlying pathophysiology centers on an aberrant PVD, fellow-eye surgery soon after the first-eye may be expected since PVD's typically occur in close succession within 6 months to 2 years of each other.[17] The loading of risk early may also be reflective of practical considerations. Patients under active review in a VR service may be more likely to plan for their second-eye surgery, especially in insidious conditions such as ERM; while for more acute pathology, patients under active review for a first-eye may be more likely to have asymptomatic pathology detected and treated in the fellow-eye.
Limitations
The main limitation of our study was its retrospective design. Moreover, like all large, retrospective studies that utilize electronic patient record (EPR) data, our results were reliant on the accuracy of data entered over many years by multiple physicians in a clinical environment. The absence of death data meant fellow-eye survival analyses relied on assumptions about patient survival, as explained in the Methods section. Given the large number of patients in our series, however, these assumptions which were based on average UK life expectancy data[8] were unlikely to introduce significant errors. The two most likely sources of error in our analyses and which would both tend to cause an underestimation of fellow-eye involvement were as follows:
The assumption that the index eye operation in our EPR database was the patient's first eye to undergo VR surgery. To validate this assumption, we recruited patients from 2001, allowing 3 years of EPR data (1998–2001) with which to cross-check index-eye status at our institution. However, if a patient had first-eye surgery performed elsewhere, or if his first-eye operation was performed at our institution before the introduction of the EPR in 1998, then his fellow-eye operation after 2001 would be considered an index eye in our study
The assumption that a patient with a first-eye VR operation at our institution would necessarily return to our institution in the event of fellow-eye involvement. In defense of this assumption, our hospital works within the UK National Health Service and the catchment area covered by our institution has remained stable during the period of follow-up of this study. However, some patients may have moved location, or chosen to have their second-eye treated in the private sector, in which case fellow-eye surgery would not be captured by our study.
For the reasons outlined above, we consider our results in respect of incidence rates and hazard rates for fellow-eye involvement, to represent a minimum estimate; with actual fellow-eye surgery rates likely to be marginally higher.
Conclusion
By utilizing EPR data available from our institution over an extended period of 18 years, our study provides valuable long-term information on the timing, incidence, and risk factors for fellow-eye surgical involvement in a variety of presenting vitreoretinal pathologies. While recognizing the limitations of retrospective data analysis, our results are useful in informing clinicians and patients about the risks of fellow-eye intervention and in providing evidence for clinicians who are seeking a rationale for when and how to screen patients for fellow-eye VR pathology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Folk JC, Burton TC. Bilateral phakic retinal detachment. Ophthalmology. 1982;89:815–20. doi: 10.1016/s0161-6420(82)34717-x. [DOI] [PubMed] [Google Scholar]
- 2.Hajari JN, Bjerrum SS, Christensen U, Kiilgaard JF, Bek T, la Cour M, et al. A nationwide study on the incidence of rhegmatogenous retinal detachment in denmark, with emphasis on the risk of the fellow eye. Retina. 2014;34:1658–65. doi: 10.1097/IAE.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 3.Delaney WV, Jr, Oates RP. Retinal detachment in the second eye. Arch Ophthalmol. 1978;96:629–34. doi: 10.1001/archopht.1978.03910050325006. [DOI] [PubMed] [Google Scholar]
- 4.Girard P, Boscher C, Merad I, Forest A. Retinal detachment in the 2d eye. Risk factors. J Fr Ophtalmol. 1983;6:975–9. [PubMed] [Google Scholar]
- 5.Gonzales CR, Gupta A, Schwartz SD, Kreiger AE. The fellow eye of patients with rhegmatogenous retinal detachment. Ophthalmology. 2004;111:518–21. doi: 10.1016/j.ophtha.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafzadeh MT, Schepens CL, Elzeneiny II, Moura R, Morse P, Kraushar MF, et al. Aphakic and phakic retinal detachment. I. Preoperative findings. Arch Ophthalmol. 1973;89:476–83. doi: 10.1001/archopht.1973.01000040478006. [DOI] [PubMed] [Google Scholar]
- 7.Gupta OP, Benson WE. The risk of fellow eyes in patients with rhegmatogenous retinal detachment. Curr Opin Ophthalmol. 2005;16:175–8. doi: 10.1097/01.icu.0000162377.55415.f3. [DOI] [PubMed] [Google Scholar]
- 8.National Life Tables. UK: Office of National Statistics; [Last accessed on 2017 Oct 10]. 2013-2015. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/20132015 . [Google Scholar]
- 9.Chuo JY, Lee TY, Hollands H, Morris AH, Reyes RC, Rossiter JD, et al. Risk factors for posterior vitreous detachment: A case-control study. Am J Ophthalmol. 2006;142:931–7. doi: 10.1016/j.ajo.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Itakura H, Kishi S, Li D, Nitta K, Akiyama H. Vitreous changes in high myopia observed by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:1447–52. doi: 10.1167/iovs.13-13496. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JC, Sharma AG, Eliott D. Fellow eye vitrectomy for proliferative diabetic retinopathy in an inner city population. Br J Ophthalmol. 2013;97:297–301. doi: 10.1136/bjophthalmol-2012-302233. [DOI] [PubMed] [Google Scholar]
- 12.Vote BJ, Gamble GD, Polkinghorne PJ. Auckland proliferative diabetic vitrectomy fellow eye study. Clin Exp Ophthalmol. 2004;32:397–403. doi: 10.1111/j.1442-9071.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 13.Duan XR, Liang YB, Friedman DS, Sun LP, Wei WB, Wang JJ, et al. Prevalence and associations of epiretinal membranes in a rural chinese adult population: The Handan eye study. Invest Ophthalmol Vis Sci. 2009;50:2018–23. doi: 10.1167/iovs.08-2624. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The blue mountains eye study, Australia. Ophthalmology. 1997;104:1033–40. doi: 10.1016/s0161-6420(97)30190-0. [DOI] [PubMed] [Google Scholar]
- 15.Ezra E, Wells JA, Gray RH, Kinsella FM, Orr GM, Grego J, et al. Incidence of idiopathic full-thickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology. 1998;105:353–9. doi: 10.1016/s0161-6420(98)93562-x. [DOI] [PubMed] [Google Scholar]
- 16.Song YS, Nagaoka T, Omae T, Yokota H, Takahashi A, Yoshida A, et al. Systemic risk factors in bilateral proliferative diabetic retinopathy requiring vitrectomy. Retina. 2016;36:1309–13. doi: 10.1097/IAE.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 17.Hikichi T, Yoshida A. Time course of development of posterior vitreous detachment in the fellow eye after development in the first eye. Ophthalmology. 2004;111:1705–7. doi: 10.1016/j.ophtha.2004.02.015. [DOI] [PubMed] [Google Scholar]