Abstract
Early language delay has often been associated with atypical language/literacy development. Neuroimaging studies further indicate functional disruptions during language and print processing in school-age children with a retrospective report of early language delay. Behavioral data of 114 5-year-olds with a retrospective report of early language delay in infancy (N = 34) and those without (N = 80) and with a familial risk for dyslexia and those without are presented. Behaviorally, children with a retrospective report of early language delay exhibited reduced performance in language/reading-related measures. A voxel-based morphometry analysis in a subset (N = 46) demonstrated an association between reduced gray matter volume and early language delay in left-hemispheric middle temporal, occipital, and frontal regions. Alterations in middle temporal cortex in children with a retrospective report of early language delay were observed regardless of familial risk for dyslexia. Additionally, while children with isolated familial risk for dyslexia showed gray matter reductions in temporoparietal and occipitotemporal regions, these effects were most profound in children with both risk factors. An interaction effect of early language delay and familial risk was revealed in temporoparietal, occipital, and frontal cortex. Our findings support a cumulative effect of early behavioral and genetic risk factors on brain development and may ultimately inform diagnosis/treatment.
Keywords: developmental disorder, developmental dyslexia, familial risk, language delay, voxel-based morphometry
Introduction
The beginning of a child's acquisition and command of language is marked by milestones such as producing first words (∼12 months) and first sentences (∼2 years; Zubrick et al. 2007). However, up to 19% of all children arrive at these milestones later than expected (Horwitz et al. 2003; Zubrick et al. 2007). These children are identified as language delayed and often referred to as late talkers (Lyytinen et al. 2005; Zubrick et al. 2007; Fernald and Marchman 2012). Previous research has shown that language abilities in early development predict later language and literacy skills (Rescorla 2000, 2002, 2009; Lyytinen et al. 2005; Puolakanaho et al. 2008). Furthermore, early language delay has been identified as a precursor of clinically significant language-based learning disabilities, such as developmental dyslexia and specific language impairment (SLI) (Scarborough 1990; Pennington and Bishop 2009; Hayiou-Thomas et al. 2010; Torppa et al. 2010; McBride-Chang et al. 2011).
Expressive and receptive language skills, as well as phonological processing and rapid automatized naming, are some of the strongest predictors of later reading and language abilities (Gallagher et al. 2000; Pennington and Lefly 2001; Snowling et al. 2003; Puolakanaho et al. 2008; Flax et al. 2009). Similarly, longitudinal studies have linked delays in speech production and perception in infancy (i.e., receptive or expressive language) to deficits in receptive language, verbal memory, phonological skills, rapid naming, and letter knowledge (Guttorm et al. 2005; Guttorm et al. 2010). For example, an infant's delay in language production, as measured by vocabulary at 18 months of age, predicts vocabulary growth by 30 months (Fernald and Marchman 2012). Infants identified with language delay (e.g., measured by onset of talking) continue to show performance deficits on various language and literacy assessments as they grow older (Zubrick et al. 2007; Rice et al. 2008; Skibbe et al. 2008). Such differences are detectable from early childhood until adolescence (Rescorla 2000, 2002, 2005, 2009). These variations in behavioral measures are independent of nonverbal cognitive abilities or socioeconomic status (Gallagher et al. 2000; Zubrick et al. 2007).
Even though most language impairments have a known biological basis (e.g., Newbury and Monaco 2010; Reilly et al. 2010), there is limited evidence on the neuronal underpinnings of early language delay. Preston et al. investigated the effects of both late talking and early speech sound errors on language and literacy outcomes in elementary school. Their research linked these early dysfunctions with functional (Preston et al. 2010, 2012) and structural brain alterations (Preston et al. 2014) in school-aged children. In particular, neuronal alterations were identified in regions including bilateral thalamus, putamen, left insula, and middle/superior temporal and occipitotemporal gyrus during tasks of language perception and reading (Preston et al. 2010, 2012). This finding was corroborated by structural alterations in similar regions, including increases in gray (bilateral superior temporal gyrus) and white (corpus callosum) matter volume indices (Preston et al. 2014). Evidence of the neural basis of early language delay is also based on studies in SLI (Bishop and Snowling 2004; Sheng and McGregor 2010) or the “KE” family (a family with high rates of speech and language disorders in combination with a mutation in the FOXP2 gene [Watkins et al. 1999; Lai et al. 2003]). Voxel-based morphometry (VBM) has, for example, revealed bilateral structural brain volume reductions in SLIs in various regions within the anterior (Plante et al. 1991; Clark and Plante 1998) and posterior (Plante et al. 1991; Preis et al. 1998; Vargha-Khadem et al. 1998; Belton et al. 2003; Herbert et al. 2005; Leonard et al. 2006) components of the left-hemispheric perisylvian language areas.
In summary, research on early language delay shows that toddlers' language abilities predict subsequent language and literacy skills and that early language delay has been linked to differential brain functions as observed in elementary school children who previously experienced early language delay (e.g., Preston et al. 2010, 2012). To the best of our knowledge, unlike functional findings, no reports about the effects of early language delay on brain structure in preschoolers or kindergarteners exist. Therefore, our first aim is to replicate previous behavioral findings through in-depth behavioral characterization of cognitive and language skills, as well as home literacy and socioeconomic variables in preschoolers/kindergarteners with a retrospective report of early language delay and those without. Second, we aim to examine structural brain characteristics in preschool children with and without a retrospective report of early language delay. Based on behavioral literature (Rescorla 2000, 2002, 2009; Rescorla et al. 2000a; Lyytinen et al. 2005; Puolakanaho et al. 2008), we expect that a retrospective report of early language delay as observed in infancy can still differentiate children on measurements of expressive and receptive language abilities in preschool/kindergarten. Based on the study of functional alterations in children with early language delay (Preston et al. 2010) and early speech sound errors (Preston et al. 2012), we further expect that children with a retrospective report of early language delay exhibit structural brain alterations, particularly in areas known to be integral to expressive language skills (e.g., temporal lobe).
Research also indicates that children with a familial risk for reading and language impairments commonly show early language delay (McBride-Chang et al. 2011; Nash et al. 2013). For example, compared with typically developing controls, children at familial risk for dyslexia have a heightened risk for early language delay. As early as 17 months of age, there are significant reductions in vocabulary size and syntactic complexity in infants at familial risk for dyslexia (Koster et al. 2005). In preschool-age children, familial risk for dyslexia was found to be predictive of reading outcome (Thompson et al. 2015) and at-risk status was shown to predict later reading abilities (Duff et al. 2015). Therefore, as a third aim, we intend to disentangle common and distinct effects of a retrospective report of early language delay and familial risk for dyslexia on brain structure in preschoolers/kindergarteners. Alterations in brain structure and function have previously been reported in preschoolers with a familial risk for dyslexia compared with those without a familial risk (Maurer et al. 2003; Blau et al. 2009; Maurer et al. 2011; Raschle et al. 2011; Raschle, Zuk, Gaab 2012; Raschle et al. 2014; Im et al. 2015). Furthermore, previous studies have suggested that individual differences in language skills arise from various causal factors (Rescorla and Achenbach 2002; Oliver et al. 2004), where each single factor may have a small impact; however, progressive accumulation of genetic, behavioral, and environmental risk factors may increase the risk for language difficulties exponentially (Henrichs et al. 2011). Therefore, we hypothesize that behavioral (retrospective report of early language delay) as well as genetic (familial risk for dyslexia) risk will lead to shared and distinct variations in brain structure and behavioral skills, with the strongest/most widespread alterations in those children who have a concurrent risk for dyslexia and early behavioral risk factors (e.g., a retrospective report of early language delay). Our results may elucidate alterations associated with later, persistent deficits and thereby contribute to efforts to improve early identification and intervention practices.
Materials and Methods
Subjects
All participants included in the current analysis are part of a longitudinal ongoing study at Boston Children's Hospital investigating early differences in children with a familial risk for dyslexia compared with children without a familial risk for dyslexia (Boston Longitudinal Study of Dyslexia, BOLD). Potential participants were recruited by means of public notices, the Boston Children's Hospital Research Participant Registry, local public and private schools, as well as learning disability clinics. Participating families are invited for 2 visits per year, one behavioral standardized testing session and one neuroimaging session. As part of our longitudinal study protocol, we explicitly recruited children with and without a familial risk for dyslexia. Familial risk for dyslexia, however, is known to be associated with higher reports of early language delay (Zubrick 2007; Reilly et al. 2010).
There is wide variation in the use of the term “language delay” across different studies. The most common practices are to either use self-report or objective measures (in longitudinal studies) in order to determine past risk status. In the current report, we use the term “retrospective report of early language delay” to refer to a delay in language development as retrospectively indicated by parents (“To the best of your knowledge, did your child experience any delays in language development?” Multiple Choice Answer Format [yes/no]). Because we relied on a categorical retrospective report (early language delay: yes/no) for determining group classification, converging evidence from the parent questionnaire was sought to validate the groups. Consequently, a retrospective report of early language delay was linked to 2 additional items on the developmental questionnaire: age when the child produced his/her first words and age when the child produced his/her first sentences (significant Pearson correlation of P < 0.001; see Supplementary Tables I1/I2). All 3 variables have individually been used to quantify early language delay in past research (e.g., Rescorla et al. 2000b; Rescorla and Alley 2001; Rice et al. 2006; Zubrick et al. 2007; Preston et al. 2010; Pecini et al. 2011). Furthermore, all 3 variables are known to predict later language and literacy performance (Scarborough 1990; Lyytinen et al 2001; Lyytinen et al. 2005; Torppa et al. 2010; McBride-Chang et al. 2011). However, no linear developmental trajectory of early behavioral characteristics and later language abilities exists (Scarborough 1990; Storch and Whitehurst 2002; McGuiness 2005; Flax et al. 2009; Torppa et al. 2010; Rescorla 2011).
Overall, parents of 117 children reported on their children's early language development by means of a questionnaire. Those children who scored below average during tests of nonverbal IQ (standard score of <85; Kaufman Brief Intelligence Test, 2nd edition [KBIT-2]; Kaufman and Kaufman 1997) or did not complete IQ assessments (N = 3) were removed from further statistics. In order to examine the influence of familial risk factors for reading and language disabilities, we further grouped all children according to familial risk for dyslexia. Children with at least 1 first-degree relative with a report of a clinical diagnosis of developmental dyslexia were labeled as children at familial risk for dyslexia; children with no first-degree relative with developmental dyslexia or reading disability were labeled as no-risk. The familial risk variable was assessed in a prescreening interview. All procedures were approved by the local Institutional Review Board, and informed written consent and verbal assent were given by parents and children, respectively.
Behavioral Group Characteristics and Demographics
In the current publication, we present data on 2 groups of subjects. Data from 114 children were included in a behavioral analysis (“BEH group”). A smaller subset of 46 children was included in our voxel-based morphometry analysis (“VBM group”). Group characteristics and matching procedures are described in the following sections.
BEH Group
A total of 114 (59 boys/55 girls) 65.6-month-old, healthy, native English-speaking children with a retrospective report of early language delay (N = 34; boys = 24/girls = 10; average age = 65.5 months; 22 with a familial risk for dyslexia and 12 without a risk) and those without (N = 80; boys = 35/girls = 45; average age = 66.0 months; 26 with a familial risk for dyslexia and 54 without a risk) completed standardized behavioral testing. Behavioral assessments included a wide range of standardized prereading and language skills, such as expressive and receptive vocabulary (Clinical Evaluation of Language Fundamentals, Fourth Edition [CELF-4]; Semel et al. 2004), phonological processing (Comprehensive Test of Phonological Processing [CTOPP]; Wagner et al. 1999), rapid automatized naming (Rapid Automatized Naming [RAN]; Wolf and Denckla 2005), and word and letter identification (Woodcock Reading Mastery Tests-Revised [WRMT-R]; Woodcock 1998). Additionally, all participating families were given a socioeconomic background questionnaire (questions adapted from the MacArthur Research Network: http://www.macses.ucsf.edu/) and answered questions concerning the home literacy environment (as cited in Katzir 2009). All of the children were monolingual. Fifteen families reported some exposure to a second language in a daycare, nanny or preschool setting; however, we assured that the amount and onset of exposure did not qualify these children as simultaneous bilinguals (having learned 2 languages from birth [Costa and Sebastian-Galles 2014]). None of the families reported any history of neurological or psychological disorder, head injury, or poor vision or hearing.
VBM Group
The majority of all 114 children further completed a pediatric neuroimaging session, including several functional MRI tasks as well as structural image acquisition. However, not every structural or functional task was available for every child due to compliance issues or data quality. Of the 34 children with a reported early language delay, 23 successfully completed structural image acquisition (13 with a familial risk for dyslexia and 10 without a risk; average age = 67.08 months; boys = 16/girls = 7). For consequent VBM analysis, a control group of children with no retrospective report of early language delay (N = 23; 13 with a familial risk for dyslexia and 10 without a risk; average age = 66.45 months; boys = 16/girls = 7) was selected to best match the experimental group with a retrospective report of early language delay, considering distribution of gender, age, familial risk for dyslexia, and general cognitive ability. The 2 groups did not differ significantly in gender, age, familial risk for dyslexia, or general cognitive ability (P>0.05). One child in each group was exposed to some Spanish within a school or daycare setting (both children with a familial risk for dyslexia) but did not fulfill criteria for simultaneous bilingualism (Costa and Sebastian-Galles 2014). Neuroimaging and behavioral testing were conducted during separate visits. Both sessions were performed on average within 6.57 weeks of each other (6.71 weeks for the group with a retrospective report of early language delay and 6.42 weeks for the group without a report of early language delay).
Imaging Procedure
Prior to neuroimaging, all children underwent an extensive preparation session within a mock-scanner environment (see also Raschle et al. 2009; Raschle, Zuk, Ortiz-Mantilla et al. 2012). Whole-brain structural T1-weighted MPRAGE MRI sequences were acquired on a Siemens 3T whole-body scanner with the following specifications: 128 slices, TR 2000 ms; TE 3.39 ms; TI = 900 ms; flip angle 9°; field of view 256 mm; voxel size 1.0 × 1.0 × 1.3 mm.
VBM Analysis and Statistics
We utilized the voxel-based morphometry toolbox (VBM8; http://dbm.neuro.uni-jena.de/vbm) as implemented in SPM8 http://www.fil.ion.ucl.ac.uk/spm/) and executed in MATLAB (Mathworks). All images were bias-corrected, normalized using the high-dimensional DARTEL approach and segmented into gray matter, white matter, and cerebrospinal fluid. Quality control was performed on all images through a visual check, as well as by displaying the sample homogeneity using standard deviations through the VBM toolbox. Volumes with an overall covariance below 2 standard deviations were visually inspected (high covariance values indicate data similarity; for VBM manual and details, see http://dbm.neuro.uni-jena.de/vbm8/VBM8-Manual.pdf). One T1 image from a control participant did not pass quality control, and the subject was replaced (since the number of children without early language delay exceeded the number of children in the experimental group, we were able to select the best matching control group from a pool of participants). Finally, the modulated (nonlinear only) dartel-warped segmented gray matter images were smoothed with an 8-mm full-width at half maximum isotropic Gaussian kernel.
The modulated smoothed gray matter volumes were analyzed using a flexible full-factorial 2 × 2 × 2-design, with factors early language delay (early language delay/no early language delay; 2 levels) and familial risk for dyslexia (familial risk for dyslexia/no familial risk for dyslexia; 2 levels). We performed F-tests (P < 0.005, uncorrected) to investigate main effects of a retrospective report of early language delay, familial risk for dyslexia and the interaction effects between early language delay and familial risk for dyslexia. Follow-up two-sample T-tests were employed to investigate group effects further. The degrees of freedom were 42 for all comparisons.
Results
Behavioral Results
Behavioral group differences between children with a retrospective report of early language delay compared with those without a retrospective report of early language delay included in the behavioral and neuroimaging analysis are listed in Table 1 (“BEH group”) and Table 2 (“VBM group”). Overall, children with a retrospective report of early language delay compared with those without and children with a familial risk for dyslexia compared with those without do not differ significantly in assessments targeting socioeconomic status (e.g., total family income or parental education) or home literacy environment (e.g., total number of adult books at home, time a child is read to, help with schoolwork; all P > 0.05). However, there is one question that differs in both “BEH” and “VBM” groups: The total number of children's books at home is higher for the group of children with a retrospective report of early language delay. Since 1) this is the only home literacy environment variable that exhibited a differences between the groups and 2) the effect is in favor of the group with a retrospective reported language delay, it is very unlikely that it has influenced our final results (for a complete overview of socioeconomic status and home literacy environment questions, see Supplementary Tables I3/I4 and I5/I6). All participants were tested prior to or during the first year of kindergarten and the majority of the children tested were still pre- or beginning readers. Most children only recognized a few or no isolated sight words during the time of neuroimaging (average number of sight words recognized according to the Word Identification subtest of the Woodcock Reading Mastery Tests-Revised = 3.14 words, range = 0–43; average numbers of sight words in children without an early language delay: 3.55, range = 0–43; average numbers of sight words in children with an early language delay: 2.18, range = 0–24). According to the retrospective parent reports, we identified 24 boys and 10 girls as early language delayed (for the “BEH group”, 16 boys and 7 girls were described in retrospective parent report as having early language delay within the “VBM group”). Our groups are in line with studies that indicate a higher prevalence of early language delay in boys compared with girls (Rescorla and Achenbach 2002; Horwitz et al. 2003; Zubrick et al. 2007). Developmentally, it is further notable that our grouping variable (reported early language delay) was strongly associated with categorical reports on the children's first words and first sentences produced but also linked to motoric development (significant Pearson correlation between the variables language delay, first word/sentence spoken [P ≤ 0.001] and age the child started walking [P = 0.016]; see Supplementary Tables I1/I2).
Table 1.
Effect of retrospective report of early language delay on language measures (MANCOVA) of all children included in the behavioral analysis (“BEH group”)
| ELD+ | ELD− | Multivariate tests |
Main effect of group |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD [N] | Mean ± SD [N] | F-value | P | F-value | P | |||
| Behavioral measures | ||||||||
| CELF-4 | ||||||||
| Core language | 102.50 ± 11.32 [32] | 112.92 ± 13.54 [75] | F4,101 = 4.04 | 0.004** | 0.138 | F1,105 = 10.81 | 0.001*** | 0.094 |
| Receptive language | 101.25 ± 12.85 [32] | 110.43 ± 12.08 [75] | F1,105 = 8.60 | 0.004** | 0.076 | |||
| Expressive language | 102.06 ± 11.79 [32] | 113.63 ± 14.09 [75] | F1,105 = 13.02 | <0.001*** | 0.111 | |||
| Language structure | 101.91 ± 12.36 [32] | 113.19 ± 13.68 [75] | F1,105 = 12.68 | 0.001*** | 0.109 | |||
| CTOPP | ||||||||
| Elision | 9.19 ± 1.66 [31] | 10.33 ± 2.18 [79] | F3,105 = 1.32 | 0.274 | 0.036 | F1,108 = 3.35 | 0.070 | 0.030 |
| Blending | 10.32 ± 1.60 [31] | 11.01 ± 1.71 [79] | F1,108 = 1.95 | 0.165 | 0.018 | |||
| NW repetition | 8.77 ± 2.17 [31] | 9.41 ± 2.17 [79] | F1,108 = 0.59 | 0.446 | 0.005 | |||
| RAN | ||||||||
| Objects | 94.68 ± 15.30 [31] | 102.50 ± 12.95 [74] | F2,101 = 2.88 | 0.061 | 0.054 | F1,103 = 5.82 | 0.018* | 0.054 |
| Colors | 92.68 ± 13.85 [31] | 99.68 ± 16.10 [74] | F1,103 = 2.94 | 0.090 | 0.028 | |||
| WRMT-R | ||||||||
| Letter ID | 98.88 ± 10.99 [34] | 102.68 ± 9.80 [78] | F2,108 = 1.50 | 0.227 | 0.027 | F1,110 = 1.93 | 0.168 | 0.017 |
| Word ID | 93.53 ± 18.70 [34] | 95.77 ± 21.67 [78] | F1,110 = 0.03 | 0.872 | <0.001 | |||
Note: Measures' standard scores are reported.
ELD+, with a retrospective report of early language delay; ELD−, without a retrospective report of early language delay; NW, nonword; ID, identification, = partial eta squared; MANCOVA = multivariate analysis of covariance (KBIT-2 as a covariate).
*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Table 2.
Effect of retrospective report of early language delay on language measures (MANCOVA) of all children included in the VBM analysis (“VBM group”)
| ELD+ | ELD− | Multivariate tests |
Main effect of group |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD [N] | Mean ± SD | F-value | P | F-value | P | |||
| Behavioral measures | ||||||||
| CELF-4 | ||||||||
| Core language | 103.36 ± 10.69 [N = 22] | 110.55 ± 11.34 [N = 22] | F4,38 = 1.73 | 0.164 | 0.154 | F1,42 = 4.34 | 0.044* | 0.096 |
| Receptive language | 101.64 ± 12.40 [N = 22] | 108.18 ± 7.98 [N = 22] | F1,42 = 3.83 | 0.057 | 0.086 | |||
| Expressive language | 102.95 ± 10.80 [N = 22] | 110.82 ± 13.98 [N = 22] | F1,42 = 4.08 | 0.050* | 0.090 | |||
| Language structure | 102.95 ± 12.70 [N = 22] | 110.18 ± 13.47 [N = 22] | F1,42 = 2.99 | 0.091 | 0.068 | |||
| CTOPP | ||||||||
| Elision | 9.36 ± 1.81 [N = 22] | 9.82 ± 1.79 [N = 22] | F3,39 = 0.56 | 0.647 | 0.041 | F1,42 = 0.42 | 0.522 | 0.010 |
| Blending | 10.36 ± 1.71 [N = 22] | 11.05 ± 1.76 [N = 22] | F1,42 = 1.51 | 0.226 | 0.036 | |||
| NW repetition | 8.95 ± 2.15 [N = 22] | 9.41 ± 2.11 [N = 22] | F1,42 = 0.49 | 0.490 | 0.012 | |||
| RAN | ||||||||
| Objects | 96.90 ± 16.19 [N = 21] | 97.41 ± 13.28 [N = 22] | F2,39 = 0.66 | 0.524 | 0.033 | F1,41 = 0.01 | 0.922 | 0.000 |
| Colors | 94.86 ± 13.85 [N = 21] | 91.64 ± 15.14 [N = 22] | F1,41 = 0.57 | 0.455 | 0.014 | |||
| WRMT-R | ||||||||
| Letter ID | 100.57 ± 10.53 [N = 23] | 101.61 ± 11.35 [N = 23] | F2,42 = 0.04 | 0.965 | 0.002 | F1,44 = 0.06 | 0.809 | 0.001 |
| Word ID | 94.74 ± 17.94 [N = 23] | 95.78 ± 20.50 [N = 23] | F1,44 = 0.00 | 0.982 | <0.001 | |||
Note: measures' standard scores are reported.
ELD+, with a retrospective report of early language delay; ELD−, without a retrospective report of early language delay; NW, nonword; ID, identification, = partial eta squared; MANCOVA = Multivariate analysis of covariance (KBIT-2 as a covariate).
*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Multivariate Testing
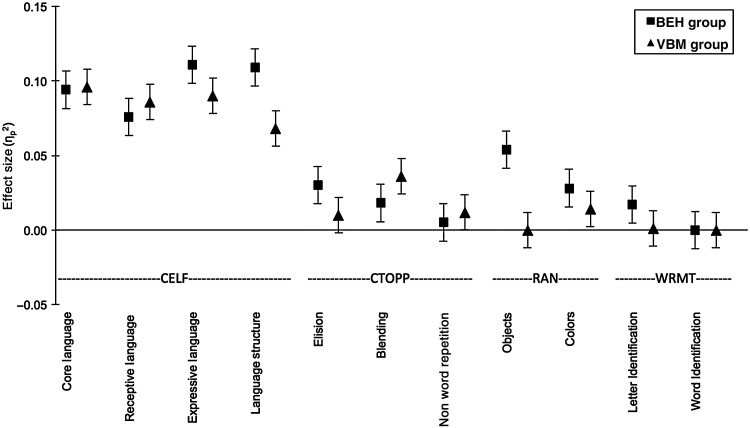
Various previous studies have reported differences in nonverbal IQ in children with early language delay. Furthermore, children with early language delay in combination with concurrent cognitive delay have the poorest prognosis for later language and literacy outcomes (Stothard et al. 1998; Young et al. 2002; Preston et al. 2010). Therefore, we decided to conduct multivariate analysis of covariance (MANCOVAs) on different language and reading measures (including CELF-4, CTOPP, RAN, and WRMT-R) using nonverbal IQ (KBIT-2) as a covariate. The MANCOVA model testing the effect of a retrospective report of early language delay on expressive and receptive language skills (CELF-4 performance; all subscales) was significant (F4,101 = 4.04, P = 0.004). Moreover, the follow-up ANCOVAs within the MANCOVA model revealed that children with a retrospective report of early language delay performed significantly lower than children with no delay on all CELF-4 indices (including core language [F1,105 = 10.81, P = 0.001], receptive [F1,105 = 8.60, P = 0.004], and expressive language [F1,105 = 13.02, P ≤ 0.001], as well as language structure [F1,105 = 12.68, P = 0.001]). The main model (MANCOVA) for CTOPP, RAN, and WRMT-R failed to reach significance (see Table 1). The same analysis for the 46 children included in our “VBM Group” did not reach significance (P > 0.05 for all) (Table 2). The effect sizes (partial eta squared) for group differences between children with a retrospective report of early language delay compared with those without a delay are displayed in Figure 1.
Figure 1.
Effect sizes (partial eta squared, ) with standard error bars using a 95% confidence interval based on multivariate analyses of covariance (MANCOVAs) using nonverbal IQ as a covariate for group differences between children with a retrospective report of early language delay compared with those without for the subscales of each test. Effect sizes are shown for the “BEH” (squares) and “VBM” (triangles) group separately. CELF-4, Clinical Evaluation of Language Fundamentals, Fourth Edition; CTOPP, Comprehensive Test of Phonological Processing; RAN, Rapid Automatized Naming; WRMT-R, Woodcock Reading Mastery Tests-Revised.
Using a 2×2 MANCOVA, we further examined performance including both retrospective report of early language delay and familial risk for dyslexia as grouping variables (see Table 3). The MANCOVA revealed a main group effect for the group with a retrospective report of early language delay on CELF-4 performance (F4,99 = 2.61, P = 0.040), and a main group effect of familial risk for dyslexia on WRMT-R performance (F2,106 = 12.07, P < 0.001). No interaction effect reached significance. Follow-up ANCOVAs on the main effect of the early language delay group disclosed that children with a retrospective report of early language delay performed significantly lower on all CELF-4 indices (including core language [F1,103 = 7.47, P = 0.007], receptive [F1,103 = 5.52, P = 0.021] and expressive language skills [F1,103 = 8.79, P = 0.004], and language structure [F1,103 = 8.42, P = 0.005]). Moreover, the follow-up ANCOVAs on the main effect of familial risk for dyslexia revealed that children with a familial risk performed significantly lower on the letter identification subscale of the WRMT-R (F1,108 = 22.93, P < 0.001). None of the other MANCOVAs on group effects and none of the MANCOVAs on interaction effects reached significance. To summarize, the present study demonstrates that children with a retrospective report of early language delay in infancy display reduced behavioral performance on several language and reading-related measures, including expressive and receptive language skills.
Table 3.
Effects of retrospective report of early language delay and familial risk for dyslexia (2 × 2 MANCOVA) on language measures of all children included in the behavioral analysis (“BEH group”)
| ELD+ FHD+ | ELD+ FHD− | ELD− FHD+ | ELD− FHD− | Main effect of group (ELD) |
Main effect of group (FHD) |
Interaction effect (ELD × FHD) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD [N] | Mean ± SD [N] | Mean ± SD [N] | Mean ± SD [N] | F-value | P | F-value | P | F-value | P | |||
| Behavioral measures | ||||||||||||
| CELF-4 | ||||||||||||
| Core language | 100.15 ± 10.86 [20] | 106.42 ± 11.44 [12] | 110.22 ± 15.87 [23] | 114.12 ± 12.35 [52] | F1,103 = 7.47 | 0.007** | 0.068 | F1,103 = 2.63 | 0.108 | F1,103 = 0.88 | 0.350 | 0.009 |
| Receptive language | 100.05 ± 13.02 [20] | 103.25 ± 12.88 [12] | 105.57 ± 11.93 [23] | 112.58 ± 11.62 [52] | F1,103 = 5.52 | 0.021* | 0.051 | F1,103 = 3.01 | 0.086 | F1,103 = 0.02 | 0.884 | <0.001 |
| Expressive language | 98.55 ± 11.25 [20] | 107.92 ± 10.66 [12] | 111.22 ± 17.04 [23] | 114.69 ± 12.60 [52] | F1,103 = 8.79 | 0.004** | 0.079 | F1,103 = 4.18 | 0.044* | F1,103 = 2.03 | 0.157 | 0.020 |
| Language structure | 99.00 ± 11.27 [20] | 106.75 ± 13.06 [12] | 109.83 ± 16.99 [23] | 114.67 ± 11.82 [52] | F1,103 = 8.42 | 0.005** | 0.076 | F1,103 = 4.13 | 0.045* | F1,103 = 0.77 | 0.381 | 0.008 |
| CTOPP | ||||||||||||
| Elision | 9.15 ± 1.60 [20] | 9.27 ± 1.85 [11] | 9.52 ± 1.78 [25] | 10.70 ± 2.25 [54] | F1,106 = 2.08 | 0.152 | 0.019 | F1,106 = 1.30 | 0.257 | F1,106 = 0.17 | 0.679 | 0.002 |
| Blending | 10.05 ± 1.64 [20] | 10.82 ± 1.47 [11] | 11.08 ± 1.53 [25] | 10.98 ± 1.81 [54] | F1,106 = 1.27 | 0.262 | 0.012 | F1,106 = 0.39 | 0.536 | F1,106 = 3.09 | 0.082 | 0.029 |
| NW repetition | 8.85 ± 2.48 [20] | 8.64 ± 1.57 [11] | 9.40 ± 2.16 [25] | 9.41 ± 2.19 [54] | F1,106 = 0.75 | 0.389 | 0.007 | F1,106 = 0.28 | 0.598 | F1,106 = 0.167 | 0.683 | 0.002 |
| RAN | ||||||||||||
| Objects | 94.29 ± 15.36 [21] | 95.50 ± 15.96 [10] | 96.05 ± 12.75 [23] | 105.23 ± 12.15 [52] | F1,101 = 3.23 | 0.076 | 0.031 | F1,101 = 2.66 | 0.106 | F1,101 = 1.41 | 0.238 | 0.014 |
| Colors | 92.48 ± 13.93 [21] | 93.10 ± 14.40 [11] | 94.91 ± 19.08 [22] | 101.69 ± 14.39 [52] | F1,101 = 1.78 | 0.186 | 0.017 | F1,101 = 0.72 | 0.400 | F1,101 = 0.27 | 0.607 | 0.003 |
| WRMT-R | ||||||||||||
| Letter ID | 96.00 ± 11.6 [22] | 104.17 ± 7.65 [12] | 95.31 ± 7.97 [26] | 106.37 ± 8.5 [52] | F1,108 = 0.03 | 0.854 | <0.001 | F1,108 = 22.93 | 0.000*** | F1,108 = 0.18 | 0.669 | 0.002 |
| Word ID | 92.86 ± 19.11 [22] | 94.75 ± 18.69 [12] | 86.96 ± 12.50 [26] | 100.17 ± 23.94 [52] | F1,108 = 0.28 | 0.600 | 0.003 | F1,108 = 2.04 | 0.156 | F1,108 = 0.37 | 0.544 | 0.003 |
Note: measures' standard scores are reported.
ELD+, with a retrospective report of early language delay; ELD−, without a retrospective report of early language delay; FHD+, with a familial risk of dyslexia; FHD−, without a familial risk of dyslexia; NW, nonword; ID, identification, = partial eta squared; MANCOVA = multivariate analysis of covariance (KBIT-2 as a covariate).
*P ≤ 0.05; **P ≤0 .01; ***P ≤ 0.001.
Neuroimaging Results: VBM
Voxel-based morphometry (VBM8) revealed alterations in gray matter volume indices when comparing children with a retrospective report of early language delay to those without a report of early language delay. Our results indicate shared but also distinct influences on brain structure through early language delay and familial risk, respectively. A detailed overview of the results is given below. Overall, there are no differences in total gray matter volume between children with a retrospective report of early language delay compared with those without (P = 0.773).
Main Effect of Early Language Delay
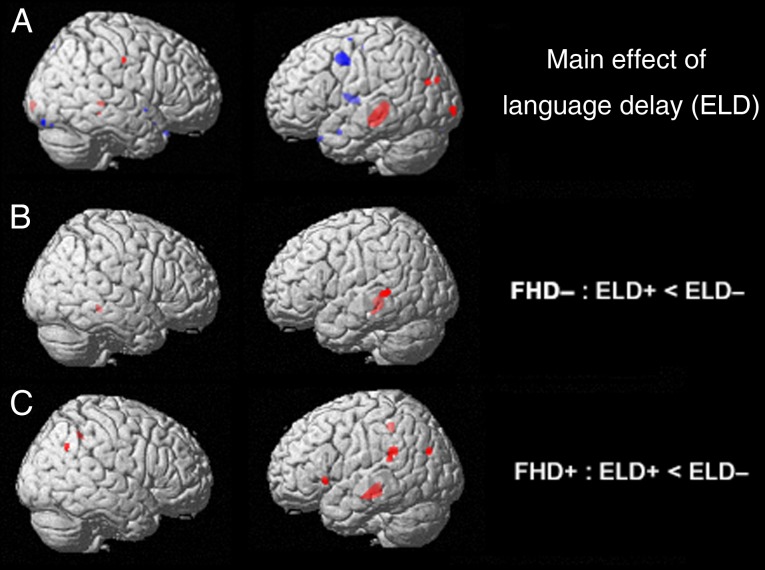
Main effects of a retrospective report of early language delay were identified in left-hemispheric temporal (anterior fusiform/parahippocampal gyrus), occipital (middle occipital gyrus/cuneus), and frontal (middle frontal/precentral gyrus) brain regions, as well as left putamen and caudate (Fig. 2A and Table 4). Children with a retrospective report of early language delay showed a significant reduction in gray matter volume in middle temporal and middle occipital gyrus (Fig. 2A). Comparing the effects of early language delay in children with familial risk for dyslexia to those without a familial risk resulted in reduced gray matter volume in overlapping, but also distinct regions of the brain. In particular, T-tests revealed reduced gray matter volume mainly in left-hemispheric middle temporal brain regions for children without a familial risk for dyslexia, but with early language delay compared with those without (Fig. 2B). Children at familial risk for dyslexia with a retrospective report of early language delay compared with those without early language delay demonstrated reduced gray matter volume in the same temporal brain areas but exhibited additional reductions in regions including temporoparietal and occipitotemporal brain areas (Fig. 2C).
Figure 2.
(A) Main effect of a retrospective report of early language delay with gray matter volume decreases (in red: middle temporal and middle occipital gyrus) and increases (blue: frontal brain regions); (B) effect of early language delay within typically developing children without a risk for dyslexia in middle temporal gyrus; (C) effect of early language delay within children at familial risk for dyslexia in areas including middle temporal, temporoparietal, and occipitotemporal brain regions (P = 0.005). ELD, early language delay; FHD, familial risk of dyslexia; + = with; − = without.
Table 4.
Peak coordinates representing cortical areas with a significant main effect of language delay
| Region | k | (Zo) | x | y | z |
|---|---|---|---|---|---|
| Main effect of language delay | |||||
| GMVI reductions | |||||
| Temporal/limbic lobe | |||||
| Anterior fusiform/parahippocampal gyrus/caudate [L] | 436 | 3.69 | −33 | −30 | −14 |
| Occipital lobe | |||||
| Middle occipital gyrus/cuneus [L] | 29 | 2.87 | −26 | −99 | −3 |
| GMVI increases | |||||
| Frontal lobe | |||||
| Middle frontal/precentral gyrus [L] | 145 | 3.14 | −32 | 2 | 43 |
| Other | |||||
| Putamen [L] | 76 | 4.15 | −26 | −9 | 9 |
Note: GMVI, gray matter volume indices; L, left.
Main Effect of Familial Risk of Dyslexia
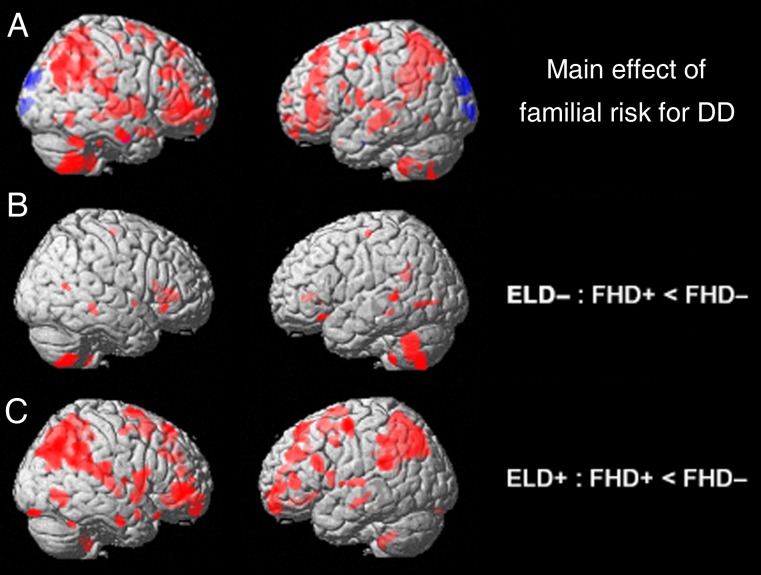
A main effect of familial risk for dyslexia was observed in superior/inferior/middle frontal gyri, including the anterior cingulate; in occipitotemporal brain regions, including the fusiform gyrus/cuneus; in parietal brain regions, including the precuneus and supramarginal and angular gyri; and in temporal brain regions, including the superior/middle temporal gyrus, in correspondence with previous publications (Fig. 3A; Raschle et al. 2011). Post hoc investigations of the effects of familial risk of dyslexia in children with a retrospective report of early language delay compared with those without indicated reduced gray matter volume in both overlapping and distinct regions of the brain. In particular, children without an early language delay but with a familial risk for dyslexia displayed gray matter volume reductions in temporoparietal and occipitotemporal regions when compared with children with neither a retrospective report of early language delay nor a familial risk for dyslexia (Fig. 3B). In children with a retrospective report of early language delay, these alterations (gray matter volume reductions) in children with a familial risk for dyslexia compared with those without a familial risk for dyslexia were much more prominent and widespread but similarly include posterior dorsal and ventral components of the reading network (Fig. 3C).
Figure 3.
(A) Gray matter volume alterations for the main effect of familial risk for dyslexia (P = 0.005; red: gray matter volume decrease, blue: gray matter volume increase); gray matter volume reductions in children with a familial risk for dyslexia compared with without, divided by retrospective report of early language delay (with (B) showing those with early language delay and (C) showing those without). ELD, early language delay; FHD, familial risk of dyslexia; + = with; − = without.
Interaction of Early Language Delay and Familial Risk for Dyslexia
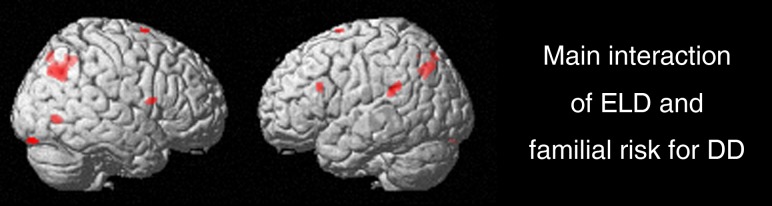
While main effects of familial risk for dyslexia and main effect of early language delay are able to indicate brain regions independently affected by each factor, a significant interaction effect highlights those areas that are affected through both familial risk and language factors combined. Here we observed a significant interaction effect for familial risk for dyslexia and a retrospective report of early language delay in brain areas including bilateral temporoparietal (inferior/middle occipital gyrus), occipital (inferior occipital gyrus), and frontal (inferior/middle frontal gyrus) regions (Fig. 4 and Table 5).
Figure 4.
Statistical parametric maps showing the main interaction of a retrospective report of early language delay and familial risk for dyslexia in areas including temporoparietal, occipital, and frontal brain regions (P = 0.005). ELD, early language delay; DD, developmental dyslexia.
Table 5.
Peak coordinates representing cortical areas with a significant interaction effect of language delay and familial risk for dyslexia
| Region | k | (Zo) | x | y | z |
|---|---|---|---|---|---|
| Interaction: language delay × familial risk | |||||
| Frontal lobe | |||||
| Inferior/middle frontal gyrus [L] | 39 | 2.84 | −46 | 21 | 25 |
| Precentral/inferior frontal gyrus [R] | 62 | 2.84 | 46 | 12 | 13 |
| Superior frontal gyrus [R] | 56 | 3.12 | 9 | 5 | 72 |
| Temporal lobe | |||||
| Inferior temporal/middle occipital gyrus [R] | 62 | 2.95 | 40 | −69 | −3 |
| Occipital lobe | |||||
| Inferior occipital gyrus [R] | 86 | 3.64 | 34 | −90 | −21 |
| Parietal lobe | |||||
| Inferior parietal lobule/superior temporal gyrus [L] | 104 | 2.87 | −50 | −45 | 25 |
| Precuneus [R] | 112 | 3.42 | 15 | −57 | 45 |
| Precuneus/angular gyrus [R] | 502 | 3.36 | 28 | −61 | 37 |
| Precuneus [L] | 164 | 2.95 | −27 | −69 | 40 |
| Precuneus [R] | 60 | 3.56 | 2 | −75 | 51 |
Note: L, left; R, right.
Discussion
The present study demonstrates that children with a retrospective report of early language delay in infancy behaviorally display reduced performance on language and reading-related measures (e.g., expressive and receptive language). In terms of brain structure, children with a retrospective report of early language delay exhibit alterations in gray matter volume in the left middle temporal, occipital, and frontal regions when compared with typically developing control children. When considering familial risk for dyslexia within the same analysis, our results indicate that a retrospective report of early language delay similarly affects gray matter volume indices in middle temporal brain regions in both children with a familial risk for dyslexia and those without. However, children with both a familial risk for dyslexia and a retrospective report of early language delay additionally display gray matter volume reductions in language-related brain areas, including temporoparietal and occipitotemporal brain regions. Furthermore, we replicate findings of structural brain alterations in left-hemispheric language- and reading-related brain regions in children at familial risk for dyslexia (Gabrieli 2009; Raschle et al. 2011). However, effects of familial risk for dyslexia are more prominent in children with a retrospective report of language delay, as opposed to the effect of familial risk for dyslexia in children without an early language delay. Finally, an interaction effect of a retrospective report of early language delay and familial risk for dyslexia was observed in temporoparietal and inferior occipital brain regions. Our findings are in line with and support studies suggesting cumulative effects of early behavioral and genetic risk factors on brain structure (Henrichs et al. 2011).
Effects of a Retrospective Report of Early Language Delay and Dyslexia Risk on Preschool Abilities
Our whole-group behavioral subtype analysis in 114 children revealed that children with a retrospective report of early language delay performed significantly lower than control children on several language-related measures, including expressive and receptive language skills, core language, and language structure. Our results align with various longitudinal behavioral research studies that indicate deficits in expressive language skills in children with late language onset (Scarborough 1990; Rescorla 2000; Rescorla et al. 2000a; Rescorla 2002; Lyytinen et al. 2005; Puolakanaho et al. 2008; Rescorla 2009; Torppa et al. 2010; McBride-Chang et al. 2011). Within the group of children with a familial risk for dyslexia tested here, we found significantly lower scores on nonverbal IQ and letter knowledge/identification as opposed to their typically developing peers. This is in line with a range of previous publications on children with dyslexia compared with those without or children with a familial risk for dyslexia compared with those without a risk (Raschle et al. 2011; Norton and Wolf 2012; O'Brien et al. 2012; Raschle, Zuk, Gaab 2012; Raschle et al. 2014; Thompson et al. 2015). Finally, previous findings demonstrate that a combination of familial risk variables combined with early language delay most strongly impacts reading-related skills (Lyytinen et al. 2005; Duff et al. 2015). However, the multivariate analysis for covariance model (2 × 2 MANCOVA) failed to detect an interaction effect. Interestingly, the effect sizes for the behavioral language assessments are lower in the VBM subgroups than those in the overall behavioral group (see Fig. 1), which may be due to overall better scores in children with higher language scores or a self-selection bias for the imaging part of the study. While the effects are substantial, the VBM results should nevertheless be interpreted with some caution.
Research has previously indicated that maternal and family variables, such as socioeconomic status or home literacy environment, may play a role in the onset of language in young children (Zubrick et al. 2007), though results are controversial and no consensus exists (Rescorla and Alley 2001; Zubrick et al. 2007). Since we did not observe differences in socioeconomic status or home language environment across the groups of children with a retrospective report of early language delay compared with those without, we assume that the observed differences are not due to familial background variables. However, a potential bias of the current group is the overall high socioeconomic status of all children participating. Moreover, it is notable that early language delay and early motoric delays often co-exist during infancy (Trauner et al. 2000). Our results corroborate this finding and support a link between early language and early motor development (Hill 2001; Webster et al. 2005; Viholainen et al. 2006).
Main Effects of a Retrospective Report of Early Language Delay on Brain Structure
Brain areas that show a main effect of retrospective report of early language delay include left-hemispheric middle temporal/limbic (fusiform/parahippocampal gyrus and caudate), occipital (middle occipital gyrus/cuneus), and frontal (middle frontal/precentral gyrus) brain regions, as well as the left putamen. Research has demonstrated that structural and functional alterations in regions including inferior frontal and temporal areas or nucleus caudatus can differentiate adults with speech and language disorders from typical controls (Watkins et al. 2002; Badcock et al. 2012). For example, SLI in adulthood has been linked to gray matter volume reductions in the head of the caudate bilaterally (Watkins et al. 2002). In line with our findings, studies using VBM or diffusion tensor imaging in individuals with SLI have demonstrated both gray matter volume decreases and increases related to language deficits (Jancke et al. 2007; Soriano-Mas et al. 2009; Badcock et al. 2012), in the putamen (Badcock et al. 2012) and bilateral superior temporal cortex (Soriano-Mas et al. 2009; Badcock et al. 2012). During typical development, brain maturation is reflected by gray matter volume increases in frontal brain regions caused by an initial overproliferation due to axonal pruning (Huttenlocher and Dabholkar 1997). We thus hypothesize that comparable mechanisms, in particular a disturbance in early development, may hence be responsible for the alterations within frontal brain regions, as suggested by others before (Badcock et al. 2012).
Functional MRI evidence demonstrates that the majority of brain regions impacted here play a significant role in skilled reading (for reviews, see Schlaggar and McCandliss 2007; Peterson and Pennington 2012; Price 2012) and language (e.g., De Guibert et al. 2010; Turken and Dronkers 2011). For example, middle temporal brain areas of the left hemisphere are similarly recruited when accessing semantics, building semantic associations (Meyer et al. 2005), listening passively (Binder et al. 1996), or comprehending sentences (Bottini et al. 1994; Jobard et al. 2007). The parahippocampal gyrus, involved in social causality/context (Ethofer et al. 2011; Baetens et al. 2013) and recognition of scenes (Aguirre et al. 1996), has also been linked to semantic production and recognition (Binder et al. 2009; Karunanayaka et al. 2011). Additionally, occipitotemporal brain areas have been found to be crucial for visual (Cohen et al. 2002) or auditory word processing (Cohen et al. 2004) and object naming (Price et al. 2006) and are suggested loci for the so-called visual word form area, activated during skilled reading (Cohen et al. 2002; Jobard et al. 2003).
Our findings of a main effect of a retrospective report of early language delay on brain structure in left-hemispheric middle temporal, occipital, and frontal brain regions are also in line with functional neuroimaging evidence during speech- and language-related tasks in typically and atypically developing children and adolescents. For example, Preston et al. (2010) demonstrated that neuronal activation patterns during speech comprehension and reading were reduced in bilateral thalamus and putamen, left insula, and superior temporal gyrus in elementary school children with a report of early language delay. In line with this finding, Pecini et al. (2011) revealed that individuals with dyslexia and a history of early language delay presented reduced neuronal activation patterns in left inferior and medial frontal gyrus during rhyme generation (Pecini et al. 2011) compared with those with dyslexia without early language delay. Combining previous functional neuroimaging findings with the present structural results, we conclude that the functional characteristics reported previously (Preston et al. 2010, 2012) may originate from the here identified structural deficits in middle temporal brain regions.
Main Effects of Familial Risk for Dyslexia on Brain Structure
In the current study, we identified main effects of familial risk for dyslexia on gray matter volume in widespread bilateral cortex areas, with the strongest effects throughout language and reading-related brain regions (McCandliss and Noble 2003), in alignment with previous work (Specht et al. 2009; Blau et al. 2010; Brem et al. 2010; Raschle et al. 2011; Yamada et al. 2011; Raschle, Zuk, Gaab 2012; Raschle et al. 2014). Interestingly, when dividing the groups with and without a familial risk for dyslexia further into those with a retrospective report of early language delay and those without, the most prominent deficits due to familial risk for dyslexia seem to exist in children who also have a report of early language delay. This finding is in line with studies in Italian-speaking children with a reported early language delay, where the research team observed more profound deficits in the neuronal representation of phonological processing in children with a diagnosis of dyslexia and language delay (Pecini et al. 2011).
Interaction Effects of Language Delay and Dyslexia Risk on Brain Structure
It has been demonstrated that a combination of reported early language delay and familial risk for dyslexia is strongly associated with a subsequent dyslexia diagnosis, beyond the 2 risk factors individually (McBride-Chang et al. 2011). In particular, the risk of developing a dyslexia diagnosis for children with a familial risk for dyslexia increases from 50% up to 62% if language delay is diagnosed as well (McBride-Chang et al. 2011). Here, we show an interaction effect of early language delay and familial risk for dyslexia in temporoparietal and inferior occipital brain regions. Both brain regions have repeatedly been shown to be impacted in children at familial risk for dyslexia (gray matter volume reductions or functional hypoactivations) and are similarly affected in individuals with language deficits (Specht et al. 2009; Blau et al. 2010; Brem et al. 2010; Pecini et al. 2011; Raschle et al. 2011; Yamada et al. 2011; Raschle, Zuk, Gaab 2012; Raschle et al. 2014). Here, we replicate reports that temporoparietal and inferior occipital brain regions are affected as a consequence of genetic vulnerability to dyslexia. Furthermore, these effects are boosted if early language delay is added as a risk variable.
A Cumulative Risk Model of Early Language Delay and Familial Risk for Dyslexia
Speech and language disabilities are known to occur in a variety of childhood disorders and exhibit very complex, multifaceted etiologies (Bishop 2009). In the current study, we have demonstrated that familial risk for dyslexia and a retrospective report of early language delay are both risk variables associated with later structural brain alterations. Both behavioral and neuroimaging work have led to the conclusion that individual differences in language skills arise from various causal factors (e.g., genetic or environmental causes [Rescorla and Achenbach 2002; Oliver et al. 2004]). However, population-based models (e.g., Rescorla 2002; Bishop et al. 2003; Reilly et al. 2007; Henrichs et al. 2011) testing predictors of early language delay have led to varying findings and much variance remains unexplained. For that reason, Henrichs et al. (2011) suggest a cumulative risk model for language disabilities in which single factors may have a small impact on the persistence of expressive language difficulties, but progressive accumulation of genetic, behavioral, and environmental risk factors may increase the risk for language difficulties exponentially (Henrichs et al. 2011). Our results are in favor of such a cumulative model, demonstrating the strongest impact on brain structure through an accumulation of early language delay and familial risk factors. While genetic impacts with different trajectories of brain development can be speculated (Galaburda et al. 2006; Giedd and Rapoport 2010; Raschle et al. 2011), only longitudinal study designs starting in early infancy can test when exactly these alterations manifest, how they develop and whether biological variables, such as early brain structure, will add to the predictive value of current models as indicated by previous studies (Hoeft et al. 2011).
Limitations
There are a number of limitations to this research study. First, language delay and familial risk studies have previously either used self-report or objective measures in order to determine risk status (Nash et al. 2013). Similar to previous reports, the current paper describes children with a retrospective report of early language delay and children without (as employed in Rescorla and Alley 2001; Zubrick et al. 2007; Preston et al. 2010; Preston et al. 2012) and self-reported familial risk of dyslexia (as employed in Brem et al. 2010; Richardson 2009; Raschle, Zuk, Ortiz-Mantilla et al. 2012; Im et al. 2015). It must be noted that the children have no current diagnosis of either language delay or developmental dyslexia. An infant's language delay, as a study construct, is commonly quantified by expressive variables (e.g., first words/first sentences spoken, vocabulary size, or mean length of utterance), or receptive language measures (e.g., following simple commands or phonological processing skills as assessed via head-turn or sucking rates; Zubrick et al. 2007; Rice et al. 2008; Preston et al. 2010; Beckage et al. 2011), which are known to predate later language and literacy skills (Scarborough 1990; Lyytinen et al. 2001; Lyytinen et al. 2005; Torppa et al. 2010; McBride-Chang et al. 2011). However, no linear developmental trajectory of early behavioral characteristics and later language abilities exists (Scarborough 1990; Storch and Whitehurst 2002; McGuiness 2005; Flax et al. 2009; Torppa et al. 2010; Rescorla 2011). Specific long-term impacts of early language delay are subtle and at times difficult to isolate (Paul 1996, 2000; Rescorla 2000, 2009, 2011; Thal et al. 2005; Ellis Weismer 2007, 1994; Moyle et al. 2007; Rice et al. 2008; Preston et al. 2010). Only longitudinal designs will allow characterization of the precise developmental trajectories and thus any of the current findings and interpretations is limited to the identified groups.
Second, we report data on 2 groups of children. A behavioral group (N = 114) and a subgroup of children with neuroimaging assessments, additionally matched for familial risk for dyslexia and retrospective report of early language delay (N = 46). This investigation leads to relatively small sample sizes in each subgroup; therefore, the representative extent of the smaller sample (“VBM”) relative to the larger group (“BEH”) may be questioned. Furthermore, even though a retrospective report of early language delay results in significantly lower scores on behavioral measures, the scores of those children with a retrospective report of early language delay are not below average performance and thus not clinically significant (below 1 standard deviation from the mean). It also needs to be noted that we do not yet know how many of the children at risk will develop a clinical diagnosis of dyslexia. However, we see here an effect in a group of children at risk for dyslexia, of which only ∼50% will receive a clinical diagnosis (Pennington and Gilger 1996). This highlights the possibility that the observed differences might be even stronger when excluding those children who do not develop a diagnosis later on.
With consideration of the given limitations, an increased understanding of the neuronal characteristics of children with a retrospective report of early language delay compared with those without can nevertheless inform us about the observed variances in the behavioral phenotype across development and may further complement our understanding of language and literacy development in children at risk for developmental disabilities. We aim to facilitate the understanding of the neuronal basis of typical and atypical language development and improve understanding of the etiology and complex relationship of different language disorders, ultimately leading to improvements in diagnosis and treatment for individuals with a clinical diagnosis of such.
Supplementary Material
Supplementary material can be found here.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (1R01HD065762 to N.G.); Charles H. Hood Foundation (to N.G.); Boston Children's Hospital Pilot Grant (to N.G.); the Swiss National Foundation (to N.M.R), and the Janggen-Pöhn Stiftung (to N.M.R.).
Notes
Conflict of Interest: None declared.
Supplementary Material
References
- Aguirre GK, Detre JA, Alsop DC, D'Esposito M. 1996. The parahippocampus subserves topographical learning in man. Cereb Cortex. 6(6):823–829. [DOI] [PubMed] [Google Scholar]
- Badcock NA, Bishop DV, Hardiman MJ, Barry JG, Watkins KE. 2012. Co-localisation of abnormal brain structure and function in specific language impairment. Brain Lang. 120(3):310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetens K, Ma N, Steen J, Van Overwalle F. 2013. Involvement of the mentalizing network in social and non-social high construal. Soc Cogn Affect Neurosci. 96:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckage N, Smith L, Hills T. 2011. Small worlds and semantic network growth in typical and late talkers. PLoS One. 6(5):e19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG. 2003. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp. 18(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 19(12):2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. 1996. Function of the left planum temporale in auditory and linguistic processing. Brain. 119(Pt 4):1239–1247. [DOI] [PubMed] [Google Scholar]
- Bishop DV. 2009. Genes, cognition, and communication: insights from neurodevelopmental disorders. Ann N Y Acad Sci. 1156:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV, Price TS, Dale PS, Plomin R. 2003. Outcomes of early language delay: II. Etiology of transient and persistent language difficulties. J Speech Lang Hear Res. 46(3):561–575. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Snowling MJ. 2004. Developmental dyslexia and specific language impairment: same or different? Psychol Bull. 130(6):858–886. [DOI] [PubMed] [Google Scholar]
- Blau V, Reithler J, van Atteveldt N, Seitz J, Gerretsen P, Goebel R, Blomert L. 2010. Deviant processing of letters and speech sounds as proximate cause of reading failure: a functional magnetic resonance imaging study of dyslexic children. Brain. 133(Pt 3):868–879. [DOI] [PubMed] [Google Scholar]
- Blau V, van Atteveldt N, Ekkebus M, Goebel R, Blomert L. 2009. Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Curr Biol. 19(6):503–508. [DOI] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, Frith CD. 1994. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain. 117(Pt 6):1241–1253. [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. 2010. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc Natl Acad Sci USA. 107(17):7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MM, Plante E. 1998. Morphology of the inferior frontal gyrus in developmentally language-disordered adults. Brain Lang. 61(2):288–303. [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. 2004. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage. 23(4):1256–1270. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. 2002. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 125(Pt 5):1054–1069. [DOI] [PubMed] [Google Scholar]
- Costa A, Sebastian-Galles N. 2014. How does the bilingual experience shape the brain? Nat Rev Neurosci. 15:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guibert C, Maumet C, Ferre JC, Jannin P, Biraben A, Allaire C, Barillot C, Le Rumeur E. 2010. FMRI language mapping in children: a panel of language tasks using visual and auditory stimulation without reading or metalinguistic requirements. Neuroimage. 51(2):897–909. [DOI] [PubMed] [Google Scholar]
- Duff FJ, Reen G, Plunkett K, Nation K. 2015. Do infant vocabulary skills predict school-age language and literacy outcomes?. J Child Psychol Psychiatry. 56(8):848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Weismer S. 2007. Typical talkers, late talkers, and children with specific language impairment: a language endowment spectrum? In: Paul R, editor. The influence of developmental perspectives on research and practice in communication disorders: a festschrift for Robin S. Chapman. Mahwah, NJ: Lawrence Erlbaum Associates; p. 83–101. [Google Scholar]
- Ellis Weismer S, Murray-Brainch J, Miller J. 1994. A prospective longitudinal study of language development in late talkers. J Speech Hearing Res. 37:852–867. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Gschwind M, Vuilleumier P. 2011. Processing social aspects of human gaze: a combined fMRI-DTI study. Neuroimage. 55(1):411–419. [DOI] [PubMed] [Google Scholar]
- Fernald A, Marchman VA. 2012. Individual differences in lexical processing at 18 months predict vocabulary growth in typically developing and late-talking toddlers. Child Dev. 83(1):203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flax JF, Realpe-Bonilla T, Roesler C, Choudhury N, Benasich A. 2009. Using early standardized language measures to predict later language and early reading outcomes in children at high risk for language-learning impairments. J Learn Disabil. 42(1):61–75. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE. 2009. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 325(5938):280–283. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. 2006. From genes to behavior in developmental dyslexia. Nat Neurosci. 9(10):1213–1217. [DOI] [PubMed] [Google Scholar]
- Gallagher A, Frith U, Snowling MJ. 2000. Precursors of literacy delay among children at genetic risk of dyslexia. J Child Psychol Psychiatry. 41(2):203–213. [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. 2010. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 67(5):728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppanen PH, Hamalainen JA, Eklund KM, Lyytinen HJ. 2010. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. J Learn Disabil. 43(5):391–401. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppanen PH, Poikkeus AM, Eklund KM, Lyytinen P, Lyytinen H. 2005. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 41(3):291–303. [DOI] [PubMed] [Google Scholar]
- Hayiou-Thomas ME, Harlaar N, Dale PS, Plomin R. 2010. Preschool speech, language skills, and reading at 7, 9, and 10 years: etiology of the relationship. J Speech Lang Hear Res. 53(2):311–332. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Rescorla L, Schenk JJ, Schmidt HG, Jaddoe VW, Hofman A, Raat H, Verhulst FC, Tiemeier H. 2011. Examining continuity of early expressive vocabulary development: the generation R study. J Speech Lang Hear Res. 54(3):854–869. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, Bakardjiev AI, Hodgson J, Takeoka M, Makris N et al. . 2005. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 128(Pt 1):213–226. [DOI] [PubMed] [Google Scholar]
- Hill EL. 2001. Non-specific nature of specific language impairment: a review of the literature with regard to concomitant motor impairments. Int J Lang Commun Disord. 36(2):149–171. [DOI] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Lyytinen H, Whitfield-Gabrieli S, Glover GH, Reiss A et al. . 2011. Neural systems predicting long-term outcome in dyslexia. PNAS. 108(1):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz SM, Irwin JR, Briggs-Gowan MJ, Bosson Heenan JM, Mendoza J, Carter AS. 2003. Language delay in a community cohort of young children. J Am Acad Child Adolesc Psychiatry. 42(8):932–940. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Compar Neurol. 387(2):167–178. [DOI] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Grant PE, Gaab N. 2015. Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cereb Cortex. 10.1093/cercor/bhu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Siegenthaler T, Preis S, Steinmetz H. 2007. Decreased white-matter density in a left-sided fronto-temporal network in children with developmental language disorder: evidence for anatomical anomalies in a motor-language network. Brain Lang. 102(1):91–98. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. 2003. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 20(2):693–712. [DOI] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Mazoyer B, Tzourio-Mazoyer N. 2007. Impact of modality and linguistic complexity during reading and listening tasks. Neuroimage. 34(2):784–800. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. KBIT-2: Kaufman brief intelligence test, 2nd ed Minneapolis, MNP: NCS Pearson, Inc., 1997. [Google Scholar]
- Karunanayaka P, Kim KK, Holland SK, Szaflarski JP. 2011. The effects of left or right hemispheric epilepsy on language networks investigated with semantic decision fMRI task and independent component analysis. Epilepsy Behav. 20(4):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir T. 2009. How research in the cognitive neuroscience sheds lights on subtypes of children with dyslexia: implications for teachers. Cortex. 45(4):558–559. [DOI] [PubMed] [Google Scholar]
- Koster C, Been PH, Krikhaar EM, Zwarts F, Diepstra HD, Van Leeuwen TH. 2005. Differences at 17 months: productive language patterns in infants at familial risk for dyslexia and typically developing infants. J Speech Lang Hear Res. 48(2):426–438. [DOI] [PubMed] [Google Scholar]
- Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. 2003. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 126(Pt 11):2455–2462. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Given B, Virginia B, Eden G. 2006. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 129(Pt 12):3329–3342. [DOI] [PubMed] [Google Scholar]
- Lyytinen H, Ahonen T, Eklund K, Guttorm TK, Laakso ML, Leinonen S, Leppanen PH, Lyytinen P, Poikkeus AM, Puolakanaho A et al. . 2001. Developmental pathways of children with and without familial risk for dyslexia during the first years of life. Dev Neuropsychol. 20(2):535–554. [DOI] [PubMed] [Google Scholar]
- Lyytinen P, Eklund K, Lyytinen H. 2005. Language development and literacy skills in late-talking toddlers with and without familial risk for dyslexia. Ann Dyslexia. 55(2):166–192. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Brandeis D. 2003. Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. Neuroreport. 14(17):2245–2250. [DOI] [PubMed] [Google Scholar]
- Maurer U, Schulz E, Brem S, der Mark S, Bucher K, Martin E, Brandeis D. 2011. The development of print tuning in children with dyslexia: evidence from longitudinal ERP data supported by fMRI. Neuroimage. 57(3):714–722. [DOI] [PubMed] [Google Scholar]
- McBride-Chang C, Lam F, Lam C, Chan B, Fong CY, Wong TT, Wong SW. 2011. Early predictors of dyslexia in Chinese children: familial history of dyslexia, language delay, and cognitive profiles. J Child Psychol Psychiatry. 52(2):204–211. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Noble KG. 2003. The development of reading impairment: a cognitive neuroscience model. Ment Retard Dev Disabil Res Rev. 9(3):196–204. [DOI] [PubMed] [Google Scholar]
- McGuiness D. 2005. Language development and learning to read: the scientific study of how language development affects reading skill. Cambridge, MA: MIT Press. [Google Scholar]
- Meyer M, Zysset S, von Cramon DY, Alter K. 2005. Distinct fMRI responses to laughter, speech, and sounds along the human peri-sylvian cortex. Brain Res Cogn Brain Res. 24(2):291–306. [DOI] [PubMed] [Google Scholar]
- Moyle MJ, Ellis Weismer S, Evans JL, Lindstrom MJ. 2007. Longitudinal relationships between lexical and grammatical development in typical and late-talking children. J Speech Lang Hear Res. 50(2):508–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash HM, Hulme C, Gooch D, Snowling MJ. 2013. Preschool language profiles of children at family risk of dyslexia: continuities with specific language impairment. J Child Psychol Psychiatry. 54(9):958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Monaco AP. 2010. Genetic advances in the study of speech and language disorders. Neuron. 68(2):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton ES, Wolf M. 2012. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annu Rev Psychol. 63:427–452. [DOI] [PubMed] [Google Scholar]
- O'Brien BA, Wolf M, Lovett MW. 2012. A taxometric investigation of developmental dyslexia subtypes. Dyslexia. 18(1):16–39. [DOI] [PubMed] [Google Scholar]
- Oliver B, Dale PS, Plomin R. 2004. Verbal and nonverbal predictors of early language problems: an analysis of twins in early childhood back to infancy. J Child Lang. 31(3):609–631. [DOI] [PubMed] [Google Scholar]
- Paul R. 1996. Clinical implications of the natural history of slow expressive language development. Am J Speech-Lang Pathol. 5:5–30. [Google Scholar]
- Paul R. 2000. Predicting outcomes of early expressive delay: Ethical implications. In: Leonard L, Bishop DVM, editors. Speech and language impairments in children: causes, characteristics, intervention and outcome. Philadelphia: Psychology Press. [Google Scholar]
- Pecini C, Biagi L, Brizzolara D, Cipriani P, Di Lieto MC, Guzzetta A, Tosetti M, Chilosi AM. 2011. How many functional brains in developmental dyslexia? When the history of language delay makes the difference. Cogn Behav Neurol. 24(2):85–92. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Bishop DV. 2009. Relations among speech, language, and reading disorders. Annu Rev Psychol. 60:283–306. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Gilger J. 1996. In: Chase CH, Rosen GD, Sherman GF, editors. Developmental dyslexia: neural, cognitive, and genetic mechanisms. York: Baltimore; p. 41–61. [Google Scholar]
- Pennington BF, Lefly DL. 2001. Early reading development in children at family risk for dyslexia. Child Dev. 72(3):816–833. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. 2012. Developmental dyslexia. Lancet. 379(9830):1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante E, Swisher L, Vance R, Rapcsak S. 1991. MRI findings in boys with specific language impairment. Brain Lang. 41(1):52–66. [DOI] [PubMed] [Google Scholar]
- Preis S, Jancke L, Schittler P, Huang Y, Steinmetz H. 1998. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia. 36(9):849–855. [DOI] [PubMed] [Google Scholar]
- Preston JL, Felsenfeld S, Frost SJ, Mencl WE, Fulbright RK, Grigorenko EL, Landi N, Seki A, Pugh KR. 2012. Functional brain activation differences in school-age children with speech sound errors: speech and print processing. J Speech Lang Hear Res. 55(4):1068–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JL, Frost SJ, Mencl WE, Fulbright RK, Landi N, Grigorenko E, Jacobsen L, Pugh KR. 2010. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain. 133(Pt 8):2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JL, Molfese PJ, Mencl WE, Frost SJ, Hoeft F, Fulbright RK, Landi N, Grigorenko EL, Seki A, Felsenfeld S et al. . 2014. Structural brain differences in school-age children with residual speech sound errors. Brain Lang. 128(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. 2012. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 62(2):816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, McCrory E, Noppeney U, Mechelli A, Moore CJ, Biggio N, Devlin JT. 2006. How reading differs from object naming at the neuronal level. Neuroimage. 29(2):643–648. [DOI] [PubMed] [Google Scholar]
- Puolakanaho A, Ahonen T, Aro M, Eklund K, Leppanen PH, Poikkeus AM, Tolvanen A, Torppa M, Lyytinen H. 2008. Developmental links of very early phonological and language skills to second grade reading outcomes: strong to accuracy but only minor to fluency. J Learn Disabil. 41(4):353–370. [DOI] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, Gaab N. 2012. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann N Y Acad Sci. 1252:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. 2011. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 57(3):742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Lee M, Buechler R, Christodoulou JA, Chang M, Vakil M, Stering PL, Gaab N. 2009. Making MR imaging child's play—pediatric neuroimaging protocol, guidelines and procedure. J Vis Exp. 29:e1309. 10.3791/1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Stering PL, Meissner SN, Gaab N. 2014. Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cerebral Cortex. 24(9):2489–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. 2012. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci USA. 109(6):2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S, Wake M, Bavin EL, Prior M, Williams J, Bretherton L, Eadie P, Barrett Y, Ukoumunne OC. 2007. Predicting language at 2 years of age: a prospective community study. Pediatrics. 120(6):e1441–9. [DOI] [PubMed] [Google Scholar]
- Reilly S, Wake M, Ukoumunne OC, Bavin E, Prior M, Cini E, Conway L, Eadie P, Bretherton L. 2010. Predicting language outcomes at 4 years of age: findings from early language in victoria study. Pediatrics. 126(6):e1530–e1537. [DOI] [PubMed] [Google Scholar]
- Rescorla L. 2000. Do late-talking toddlers turn out to have reading difficulties a decade later? Ann Dyslexia. 50:87–102. [DOI] [PubMed] [Google Scholar]
- Rescorla L. 2002. Language and reading outcomes to age 9 in late-talking toddlers. J Speech Lang Hear Res. 45(2):360–371. [DOI] [PubMed] [Google Scholar]
- Rescorla L. 2005. Age 13 language and reading outcomes in late-talking toddlers. J Speech Lang Hear Res. 48(2):459–472. [DOI] [PubMed] [Google Scholar]
- Rescorla L. 2009. Age 17 language and reading outcomes in late-talking toddlers: support for a dimensional perspective on language delay. J Speech Lang Hear Res. 52(1):16–30. [DOI] [PubMed] [Google Scholar]
- Rescorla L. 2011. Late talkers: do good predictors of outcome exist? Dev Disabil Res Rev. 17(2):141–150. [DOI] [PubMed] [Google Scholar]
- Rescorla L, Achenbach TM. 2002. Use of the language development survey (LDS) in a national probability sample of children 18 to 35 months old. J Speech Lang Hear Res. 45(4):733–743. [DOI] [PubMed] [Google Scholar]
- Rescorla L, Alley A. 2001. Validation of the language development survey (LDS): a parent report tool for identifying language delay in toddlers. J Speech Lang Hear Res. 44(2):434–445. [DOI] [PubMed] [Google Scholar]
- Rescorla L, Dahlsgaard K, Roberts J. 2000. a. Late-talking toddlers: MLU and IPSyn outcomes at 3;0 and 4;0. J Child Lang. 27(3):643–664. [DOI] [PubMed] [Google Scholar]
- Rescorla L, Mirak J, Singh L. 2000. b. Vocabulary growth in late talkers: lexical development from 2;0 to 3;0. J Child Lang. 27(2):293–311. [DOI] [PubMed] [Google Scholar]
- Rice ML, Redmond SM, Hoffman L. 2006. Mean length of utterance in children with specific language impairment and in younger control children shows concurrent validity and stable and parallel growth trajectories. J Speech Lang Hear Res. 49(4):793–808. [DOI] [PubMed] [Google Scholar]
- Rice ML, Taylor CL, Zubrick SR. 2008. Language outcomes of 7-year-old children with or without a history of late language emergence at 24 months. J Speech Lang Hear Res. 51(2):394–407. [DOI] [PubMed] [Google Scholar]
- Richardson U, Kulju P, Nieminen L, Torvelainen P. 2009. Early signs of dyslexia from the speech and language processing of children. Int J Speech Lang Pathol. 115:366–380. [Google Scholar]
- Scarborough HS. 1990. Very early language deficits in dyslexic children. Child Dev. 61(6):1728–1743. [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. 2007. Development of neural systems for reading. Annu Rev Neurosci. 30:475–503. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. 2004. Clinical evaluation of language fundamentals, preschool: second edition (CELF-Preschool-2). San Antonio, TX: The Psychological Corporation, Inc. [Google Scholar]
- Sheng L, McGregor KK. 2010. Lexical-semantic organization in children with specific language impairment. J Speech Lang Hear Res. 53(1):146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbe LE, Grimm KJ, Stanton-Chapman TL, Justice LM, Pence KL, Bowles RP. 2008. Reading trajectories of children with language difficulties from preschool through fifth grade. Lang Speech Hear Serv Sch. 39(4):475–486. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, Gallagher A, Frith U. 2003. Family risk of dyslexia is continuous: individual differences in the precursors of reading skill. Child Development. 74:358–373. [DOI] [PubMed] [Google Scholar]
- Soriano-Mas C, Pujol J, Ortiz H, Deus J, Lopez-Sala A, Sans A. 2009. Age-related brain structural alterations in children with specific language impairment. Hum Brain Mapp. 30(5):1626–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht K, Hugdahl K, Ofte S, Nygard M, Bjornerud A, Plante E, Helland T. 2009. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children. Scand J Psychol. 50(1):79–91. [DOI] [PubMed] [Google Scholar]
- Storch SA, Whitehurst GJ. 2002. Oral language and code-related precursors to reading: evidence from a longitudinal structural model. Dev Psychol. 38(6):934–947. [PubMed] [Google Scholar]
- Stothard SE, Snowling MJ, Bishop DVM, Chipchase BB, Kaplan CA. 1998. Language-impaired preschoolers: a follow-up into adolescence. J Speech Lang Hear Res. 41:407–418. [DOI] [PubMed] [Google Scholar]
- Thal DJ, Miller S, Carlson J, Vega MM. 2005. Nonword repetition and language development in 4-year-old children with and without a history of early language delay. J Speech Lang Hear Res. 48(6):1481–1495. [DOI] [PubMed] [Google Scholar]
- Thompson PA, Hulme C, Nash HM, Gooch D, Hayiou-Thomas E, Snowling MJ. 2015. Developmental dyslexia: predicting individual risk. J Child Psychol Psychiatry. 569:976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torppa M, Lyytinen P, Erskine J, Eklund K, Lyytinen H. 2010. Language development, literacy skills, and predictive connections to reading in Finnish children with and without familial risk for dyslexia. J Learn Disabil. 43(4):308–321. [DOI] [PubMed] [Google Scholar]
- Trauner D, Wulfeck B, Tallal P, Hesselink J. 2000. Neurological and MRI profiles of children with developmental language impairment. Dev Med Child Neurol. 42(7):470–475. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. 2011. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M et al. . 1998. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci USA. 95(21):12695–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viholainen H, Ahonen T, Lyytinen P, Cantell M, Tolvanen A, Lyytinen H. 2006. Early motor development and later language and reading skills in children at risk of familial dyslexia. Dev Med Child Neurol. 48(5):367–373. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. 1999. The comprehensive test of phonological processing. Austin: PRO-ED, Inc. [Google Scholar]
- Watkins KE, Gadian DG, Vargha-Khadem F. 1999. Functional and structural brain abnormalities associated with a genetic disorder of speech and language. Am J Hum Genet. 65(5):1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RS, Mishkin M, Gadian DG. 2002. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 125(Pt 3):465–478. [DOI] [PubMed] [Google Scholar]
- Webster RI, Majnemer A, Platt RW, Shevell MI. 2005. Motor function at school age in children with a preschool diagnosis of developmental language impairment. J Pediatr. 146(1):80–85. [DOI] [PubMed] [Google Scholar]
- Wolf M, Denckla MB. 2005. RAN/RAS: rapid automatized naming and rapid alternating. Austin, TX: PRO-ED, Inc. [Google Scholar]
- Woodcock RW. 1998. Woodcock reading mastery tests—revised. Minneapolis, MN: NCS Pearson, Inc. [Google Scholar]
- Yamada Y, Stevens C, Dow M, Harn BA, Chard DJ, Neville HJ. 2011. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study. Neuroimage. 57(3):704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AR, Beitchman JH, Johnson C, Douglas L, Atkinson L, Escobar M, Wilson B. 2002. Young adult academic outcomes in a longitudinal sample of early identified language impaired and control children. J Child Psychol Psychiat. 43(5):635–645. [DOI] [PubMed] [Google Scholar]
- Zubrick SR. 2007. Commentary: area social cohesion, deprivation and mental health--does misery love company? Int J Epidemiol. 36(2):345–347. [DOI] [PubMed] [Google Scholar]
- Zubrick SR, Taylor CL, Rice ML, Slegers DW. 2007. Late language emergence at 24 months: an epidemiological study of prevalence, predictors, and covariates. J Speech Lang Hear Res. 50(6):1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.