Abstract
Adolescents born preterm (PT) with no evidence of neonatal brain injury are at risk of deficits in visual memory and fine motor skills that diminish academic performance. The association between these deficits and white matter microstructure is relatively unexplored. We studied 190 PTs with no brain injury and 92 term controls at age 16 years. The Rey–Osterrieth Complex Figure Test (ROCF), the Beery visual-motor integration (VMI), and the Grooved Pegboard Test (GPT) were collected for all participants, while a subset (40 PTs and 40 terms) underwent diffusion-weighted magnetic resonance imaging. PTs performed more poorly than terms on ROCF, VMI, and GPT (all P < 0.01). Mediation analysis showed fine motor skill (GPT score) significantly mediates group difference in ROCF and VMI (all P < 0.001). PTs showed a negative correlation (P < 0.05, corrected) between fractional anisotropy (FA) in the bilateral middle cerebellar peduncles and GPT score, with higher FA correlating to lower (faster task completion) GPT scores, and between FA in the right superior cerebellar peduncle and ROCF scores. PTs also had a positive correlation (P < 0.05, corrected) between VMI and left middle cerebellar peduncle FA. Novel strategies to target fine motor skills and the cerebellum may help PTs reach their full academic potential.
Keywords: cerebellum white matter, diffusion tensor imaging, mediator model, preterm, visual-motor integration
Introduction
Preterm (PT) birth is a major public health concern, whose scope has only increased as improvements in medical care have yielded better survival rates of infants born very premature. Worldwide, in 2010 alone there were an estimated 15 million preterm births, with 10.4% of these born at 28–31 weeks gestational age (Blencowe et al. 2013). The consequences of surviving very preterm birth are extensive, with nearly 30% of survivors experiencing long-term neurodevelopmental disability or delay (Blencowe et al. 2013). As they grow, PT children can experience persistent cognitive and executive function deficits as well as behavioral problems that can lead to difficulties in school-age (Allin et al. 2008; Boyle et al. 2011; Wong et al. 2014; Joseph et al. 2016), adolescence, and young adulthood (Lefebvre et al. 2005; Hille et al. 2007; Luu et al. 2011; Taylor et al. 2015).
Perinatal brain injury, including intraventricular hemorrhage, white matter injury, and low-pressure ventriculomegaly, likely causes a significant degree of neurocognitive deficits in the PT population at large (Volpe 1998; O'Shea et al. 2012). However, even in those individuals who survive premature birth with no clinically measurable neurological damage, neurodevelopmental consequences of prematurity can persist, and these may represent the more prevalent mild sequelae of premature birth. In particular, deficits in visual memory and visual-spatial skills are common in the PT population (Taylor et al. 2004; Van Braeckel et al. 2008; Butcher et al. 2012; Molloy et al. 2014). Likewise, motor impairment is common in PTs, and even without severe impairments such as cerebral palsy, PTs are at higher risk of motor deficit, with mild-moderate impairment occurring with a pooled estimated prevalence of 40.5/100 (95% confidence interval 32.1–48.9/100) (Williams et al. 2010). The cause of the increased prevalence of fine motor, visual-spatial, and visual memory impairment in PT populations remains elusive, although several etiologies have been implicated, such as persistent white matter derangement or reductions in cerebellar volume (Peterson et al. 2000; Allin et al. 2001; Tam et al. 2009; Van Braeckel and Taylor 2013; Sripada et al. 2015; Zwicker et al. 2016).
Diffusion tensor imaging (DTI) is a common Magnetic Resonance Imaging (MRI) technique used to assess the microstructure of white matter tracts. Microstructure investigations of white matter tracts have demonstrated white matter difference or derangement of white matter development in premature infants as compared with age-equivalent term infants (Anjari et al. 2007), and these changes persist into childhood and even adolescence (Constable et al. 2008; Mullen et al. 2011; Li et al. 2015). However, few groups have explored the microstructural changes that underlie deficits in visual-spatial skills and visual memory in the PT population (Sripada et al. 2015).
The relationship between fine motor performance, visual-spatial skills, visual memory, and white matter microstructure has been underexplored in PT birth. Thus, the current study seeks to evaluate these functions in PT adolescents by examining the relationships between a widely used test of visual-spatial constructional ability and visual memory, the Rey–Osterrieth Complex Figure Test (ROCF), and measures of both motor function and visual-motor integration (VMI and GPT). Given the observed significant correlations between ROCF scores, motor function, and VMI score, we hypothesize that deficits in fine motor ability would mediate the group differences seen in more complex tests of visual-motor and visual memory abilities. Mapping the influence that motor impairment has on other realms increases the field's knowledge regarding the functional impact of poor motor skills on cognitive outcomes in PT-born adolescents. We further hypothesize that PT-born 16-year olds with no evidence of neonatal brain injury will have deficits in the microstructure of white matter tracts that correlate with deficits in visual memory.
Methods
This study was performed at the Yale University School of Medicine in New Haven, CT, the Warren Alpert Medical School of Brown University in Providence, RI, and Maine Medical Center, Portland, ME, and the protocols were approved by the institutional review boards at each institution. Parent (s) provided written consent for the study while children provided written assent. Scans were obtained and analyzed at Yale University.
Participants
The PT cohort consisted of the 437 surviving PT patients enrolled in the follow-up component of the “Multicenter Randomized Indomethacin IVH Prevention Trial” (Ment et al. 1994a, 1994b). By the age of 16, 223 PT participants either were lost to follow-up (N = 100), were excluded from analysis if they had either perinatal evidence of intraventricular hemorrhage, low-pressure ventriculomegaly or periventricular leukomalacia (N = 92), or were excluded if they had incomplete testing on either the ROCF, the Beery Developmental Test of Visual-Motor Integration (VMI), or the Grooved Pegboard Test (GPT) (N = 31). From these participants, an additional 24 PTs who scored at least 1.5 interquartile range below the first quartile or above the third quartile on ROCF, VMI, or GPT were labeled as outliers and excluded from analysis. In total, 190 PT participants were included in this study.
Term controls were enrolled at age 8 years; these participants were zip code matched to PT participants, and every attempt was made to match participants on sex, race, and ethnicity. Of 102 term controls, 92 were analyzed after excluding 4 for missing data and 6 for outlier scores.
Finally, PT and term participants from the neurocognitive cohort were sequentially recruited for the MRI study when they reached 16 years of age. In total, 40 PT and 40 term participants were included in the MRI study.
Neurocognitive Testing
The PT and term control participants underwent neurocognitive testing with the ROCF (Rey 1941; Osterrieth 1944; Shin et al. 2006), a measure of visual-spatial skills and visual memory, the VMI (Beery 1989), which assesses visual-motor integration, and the GPT (Matthews and Klove 1964), which is used to evaluate fine motor dexterity of fingers and wrists and allows for assessment of fine motor function. For ROCF and VMI, higher scores indicate better performance, while, for GPT, higher scores indicate poorer performance (i.e., longer time to complete peg placement task). Participants underwent evaluation with the Wechsler Intelligence Scale for Children third edition (Wechsler 1991) (WISC-III), and the measures of Full Scale IQ, Freedom from Distractibility IQ, Verbal Scale IQ, and Performance IQ are reported here.
Diffusion Tensor Imaging
Imaging was performed on a Siemens Sonata 1.5 T scanner. DTI data were measured using a double spin echo Echo Planar Imaging sequence with 32 directions, one b-value (1000 s/mm2) in addition to b = 0, and one average with time echo = 87, time repetition = 6200, 128 Å~128 acquisition matrix, Bandwidth1630, Flip Angle 90°, Field of View = 20 Å~20 cm, with 40 slices, 3 mm thick, skip 0 mm. BioImage Suite (Joshi et al. 2011) was used to calculate fractional anisotropy (FA) values from the tensor data, and the FA data were nonlinearly registered to the Johns Hopkins University (JHU) white matter atlas (Mori et al. 2009) for both groups of participants. Participant scans were masked based on white matter tracts from the JHU atlas.
Statistical Methods
Of this, 2 × 2 contingency tables were used to assess significance in categorical measures. Standard chi-squared statistics were used to analyze categorical data for sample size greater than 5; otherwise, P values were calculated using the Fisher Exact Test. Group differences in continuous-valued data such as neurocognitive testing scores or FA values were assessed using t-tests. Pearson correlations were employed to examine the relationships between the ROCF, GPT, and VMI scores within the PT and term groups. P values of < 0.05 were considered significant. Monte Carlo simulations as implemented in AFNI (Cox 1996) were used to correct for multiple comparisons for all imaging data. False discovery rate (FDR) was used to correct for multiple comparisons for non-imaging data. All analyses were performed using MatLab (MATLAB and Statistics Toolbox Release 2015a, The MathWorks, Inc.) for neurocognitive data, and BioImage Suite for imaging data.
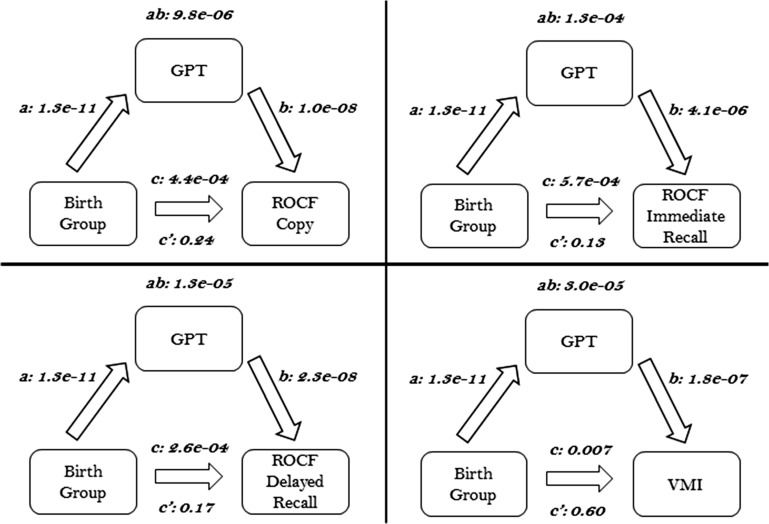
Mediator Model
We chose GPT scores as the mediating variable as the ROCF and VMI examine more complex neuropsychological functions that build on the simpler motor skills tested in the GPT. In the mediation model established by Baron and Kenny (1986), Judd and Kenny (1981) (see Fig. 1), the following criteria must be met: 1) There must be a significant P value (P < 0.05) describing the relationship between the independent variable (i.e., birth group) and the outcome variable (i.e., ROCF score), represented by path c; 2) There must be a significant P value (P < 0.05) describing the relationship between the independent variable and the potential mediating variable (i.e., GPT), represented by path a; 3) The mediating variable must have a significant relationship (P < 0.05) to the outcome variable when the independent variable is controlled for, as represented by path b; and 4) The P value associated with path c must be more significant (i.e., lower in absolute value) than the P value associated with path c’. Path c’ is the relationship of the independent variable (birth group) to the outcome variable (ROCF score) while controlling for the mediator. Moreover, if the P value associated with the multiplied coefficient a × b (ab) is significant (P < 0.05) then the mediation effect is significant. If there is a significant mediation effect and the c’ coefficient P value is statistically significant, then there is partial mediation; if c’ P > 0.05 then there is complete mediation.
Figure 1.
Performance on the GPT mediates group difference in visual-motor integration and visual memory. Significant pair wise associations were observed for birth group, GPT, VMI, and ROCF scores (see paths a, b, and c, P < 0.01, for all). GPT was a significant mediator of group differences in VMI and ROCF scores (see path ab, P < 0.001 for all). Additionally, the direct effect of group differences in VMI and ROCF was reduced for all (path c’ < path c) when the indirection mediation path (path ab) was included in the model.
Meditation analyses were performed using the Multilevel Mediation and Moderation (M3) Toolbox (Atlas et al. 2010) to investigate the observed relations between birth group, GPT, and ROCF scores. P values for the mediating effects were determined through nonparametric bootstrapping. In separate analyses, participant sex or WISC-III Freedom from Distractibility IQ score were used as covariates in the mediator model to control for these parameters.
Results
Study Population
Table 1 shows the neonatal findings of the PT neurocognitive testing and imaging cohorts. The PT neurocognitive testing cohort was larger and included the imaging participants. These participants had an average gestational age of 28.1 ± 2.0 weeks and an average birthweight of 976.8 ± 172 g. Similar to the neurocognitive testing cohort, PT imaging participants had an average gestational age of 28.2 ± 2.0 weeks, with a mean birthweight of 990.7 ± 191 g. All participants with neonatal findings of brain injury in addition to those with outlier scores on neurocognitive testing were excluded from analysis and are not shown in the table.
Table 1.
Preterm neonatal data
| PT neurocognitive | PT imaging | P value | |
|---|---|---|---|
| N | 190 | 40 | — |
| Birthweight, g, mean ± SD | 976.8 ± 172 | 990.7 ± 191 | 0.67 |
| Gestational age, weeks, mean ± SD | 28.1 ± 2.0 | 28.2 ± 2.0 | 0.77 |
| Bronchopulmonary dysplasia, N (%) | 76 (40)a | 10 (26)b | 0.10 |
| Necrotizing enterocolitis, N (%) | 14 (7.4) | 4 (10) | 0.74 |
| Any confirmed episode sepsis, N (%) | 37 (20)c | 10 (26)d | 0.45 |
| Retinopathy of prematurity (ROP) any grade, N (%) | 70 (43)e | 14 (50)f | 0.47 |
| ROP either eye, grade 2 or 3, N (%) | 19 (12)e | 2 (7.1)f | 0.55 |
| Received any morphine, N (%) | 9 (4.7) | 1 (2.5) | 0.7 |
| Received any postnatal steroids, N (%) | 77 (41) | 18 (45) | 0.63 |
| If received postnatal steroids, average number of days, mean ± SD | 2.6g ± 2.9 | 2.05 ± 1.8 | 0.45 |
aData available for 189/190 subjects.
bData available for 39/40 subjects.
cData available for 183/190 subjects.
dData available for 39/40 subjects.
eData available for 164/190 subjects.
fData available for 28/40 subjects.
gData available for 76/77 subjects.
Demographic data for the PT and term cohorts are presented in Table 2. Among the neurocognitive cohort, there was no significant difference between the PT and term groups in the number of males, non-white status, or mother's education less than high school. PT participants exhibited significant lower full scale, verbal, and performance IQ (P < 0.001) compared with term participants. The PT and term imaging cohorts are representative of their respective encompassing neurocognitive cohort (see Table 2). Similar to the participants in the neurocognitive cohort, PT imaging participants exhibited significant lower full scale, verbal, and performance IQ (P = 0.03) compared with term imaging participants.
Table 2.
Demographic data for study participants
| Neurocognitive testing cohort | Imaging cohort | |||||
|---|---|---|---|---|---|---|
| Preterm (N = 190) |
Term (N = 92) |
P value | Preterm (N = 40) |
Term (N = 40) |
P value | |
| Male, N (%) | 99 (52) | 44 (48) | 0.50 | 24 (60) | 19 (48) | 0.26 |
| Age at scan, years, mean, ± SD | — | — | — | 16.3 ± 0.3 | 16.3 ± 0.3 | 0.98 |
| Right-handed, N (%) | 152 (80) | 83 (90) | 0.03 | 35 (87) | 36 (90) | 0.75 |
| Non-white, N (%) | 39 (21) | 25 (27) | 0.21 | 13 (33) | 13 (33) | 1.0 |
| Maternal education less than high school, N (%) | 17 (9)a | 4 (4) | 0.15 | 2 (5)b | 2 (5) | 1.0 |
| Full scale IQ, mean ± SD | 94.0 ± 14c | 104 ± 15d | <0.001 | 97.0 ± 12 | 104.0 ± 16b | 0.03 |
| Freedom from distractibility IQ, mean ± SD | 94.0 ± 16c | 101 ± 15d | <0.001 | 95.2 ± 15 | 101.7 ± 14b | 0.05 |
| Verbal scale IQ, mean ± SD | 94.8 ± 14c | 103.6 ± 14d | <0.001 | 98.4 ± 14 | 102.5 ± 15b | 0.21 |
| Performance scale IQ, mean ± SD | 93.9 ± 14c | 104.2 ± 16d | <0.001 | 96.2 ± 13 | 105.1 ± 17b | 0.01 |
aData available for 184/190 subjects.
bData available for 38/40 subjects.
cData available for 188/190 subjects.
dData available for 90/92 subjects.
Group Differences in Neurocognitive Performance (Total Sample)
Group means and standard deviations for selected neurocognitive testing performed on the total PT and term neurocognitive testing cohorts are shown in Table 3. PT participants significantly underperformed term controls on all ROCF measures, including the Copy score (P < 0.001), Immediate Recall score (P < 0.001), and Delayed Recall score (P < 0.001). PT participants also scored significantly poorer on the VMI (P < 0.01) and the GPT (P < 0.001). GPT score was negatively correlated with all measures of ROCF and the VMI (P < 0.001). Therefore, improved fine motor dexterity on the GPT (lower score, meaning shorter time to task completion) correlated with improved (higher) ROCF scores. Among term participants, GPT was not significantly correlated with ROCF scores but did have a negative correlation with VMI score (P < 0.01).
Table 3.
Neurocognitive testing scores
| Neurocognitive testing cohort | Imaging cohort | |||||
|---|---|---|---|---|---|---|
| Preterm (N = 190) |
Term (N = 92) |
P value correcteda | Preterm (N = 40) |
Term (N = 40) |
P value correcteda |
|
| ROCF copy, mean ± SD | 31.7 ± 3.7 | 33.3 ± 2.9 | <0.001 | 30.6 ± 4.2 | 33.5 ± 2.6 | <0.001 |
| ROCF immediate recall, mean ± SD | 19.0 ± 7.7 | 22.4 ± 8.2 | <0.001 | 18.8 ± 6.9 | 20.7 ± 7.9 | 0.26 |
| ROCF delayed recall, mean ± SD | 18.6 ± 7.7 | 22.3 ± 8.2 | <0.001 | 17.2 ± 7.1 | 20.7 ± 8.3 | 0.06 |
| GPT, mean ± SD | 76.0 ± 14 | 64.7 ± 9.0 | <0.001 | 78.1 ± 13 | 63.5 ± 7.8 | <0.001 |
| VMI, mean ± SD | 78.3 ± 12 | 82.5 ± 12 | <0.01 | 77.4 ± 13 | 84.7 ± 11 | 0.02 |
aCorrected for multiple comparisons using FDR.
GPT as Mediator of ROCF: GPT Mediates Group Difference in ROCF Visual Memory Scores
Additionally, GPT scores significantly mediated the differences between PT and term neurocognitive testing cohorts in ROCF Copy, ROCF Immediate Recall, ROCF Delayed Recall and VMI (P < 0.01 for all, see path ab in Fig. 1). GPT score was labeled a complete mediator for ROCF Copy, ROCF Immediate Recall, ROCF Delayed Recall and VMI as the bivariate associations were no longer significant in the mediator model. No significant effects of sex or WISC-III Freedom from Distractibility IQ score on mediation analyses were observed (see Supplementary Figs S2 and S3). When controlling for verbal IQ, only group differences in ROCF Copy were still significantly mediated by GPT scores and group differences in ROCF Delayed Recall showed trend level significance (Supplementary Fig. S4).
Microstructural Differences Between Groups: Whole-Brain Subtraction Analysis
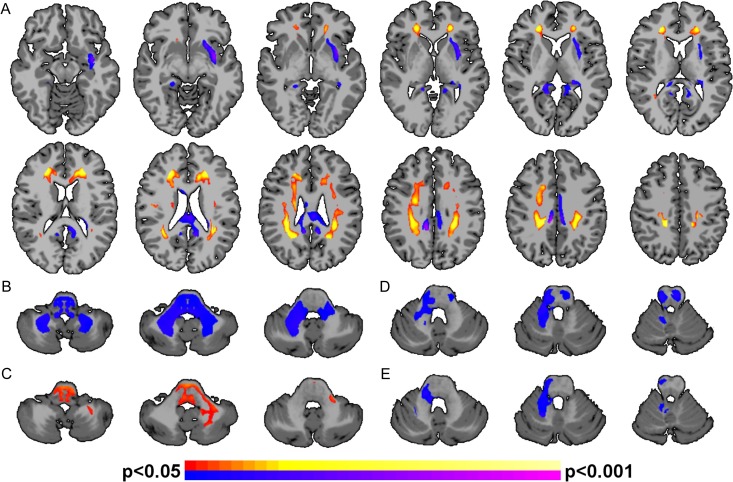
Voxel-by-voxel whole-brain subtraction comparing FA values between the PT and term participants in the imaging cohort is shown in Fig. 2A. PT participants exhibited significantly (P < 0.05, corrected) higher FA when compared with term controls in 8 white matter tracts: bilateral posterior, mid-, and anterior corona radiata and forceps minor. PT participants had (P < 0.05, corrected) lower FA when compared with term controls in 3 tracts: left external capsule, corpus body, and corpus splenium.
Figure 2.
Alterations in white matter for PT participants. (A) PT participants exhibited significantly (P < 0.05, corrected) altered FA in several white matter tracts. Regions of significantly greater FA for the PT participants compared with terms include bilateral posterior, mid-, and anterior corona radiata and forceps minor (shown in hot colors); regions of significantly reduced FA for the PT participants compared with terms include the left external capsule, corpus body, and corpus splenium (shown in cool colors). (B) GPT scores, (C) VMI, (D) ROCF Copy, and (E) ROCF Immediate scores were significantly correlated (P < 0.05, corrected) with FA in the cerebellum white matter for PT participants. Higher FA were associated with lower (improved) GPT scores, higher (improved) VMI scores, lower (worse) ROCF Copy, and lower (worse) ROCF Immediate scores. Positive correlations between neurocognitive testing and FA are shown in hot colors; negative correlations between neurocognitive testing and FA are shown in cool colors. No correlations in the cerebellum were observed for the term participants.
Voxel-Wise Correlational Analysis
PT participants showed a negative correlation (P < 0.05, corrected) between FA of cerebellar white matter including the bilateral middle cerebellar peduncles and GPT score (Fig. 2B), with higher FA correlating to lower (improved) GPT score. PT participants demonstrated a positive correlation (P < 0.05, corrected) between VMI and left middle cerebellar peduncle FA (Fig. 2C) with higher FA correlating with higher (improved) VMI score. PT participants demonstrated a negative correlation (P < 0.05, corrected) between FA of the right superior cerebellar peduncle and ROCF Copy score (Fig. 2D), and ROCF Immediate Recall (Fig. 2E), with higher FA correlating to lower (worse) ROCF score. To test the hypothesis that there were hemispheric asymmetries, correlations between cognitive scores and cerebellar FA in the right and left hemispheres were performed. (Table S1). These analyses show no significant differences between the hemispheres (P > 0.69, for all). Finally, voxel-wise analysis of term participants showed no significant relationships between FA and GPT, ROCF, or VMI score.
Discussion
Using neuropsychological and diffusion-weighted imaging data in PT adolescents with no evidence of neonatal brain injury, we highlight the importance of fine motor dexterity in more complex neuropsychological constructs and provide preliminary evidence suggesting white matter in the cerebellum as a novel correlate of these measures. Deficits in fine motor dexterity mediate the significant disparities in visual memory scores between PT and term participants at adolescence. Thus, deficits in simple fine motor ability underlie the deficits seen in much more complex visual memory tasks. Moreover, using whole-brain voxel-wise analysis, we demonstrate significant relationships between visual motor, visual memory, and fine motor skill and white matter coherence of the cerebellar peduncles. Together, these results implicate fine motor ability and cerebellar white matter contributions to visual memory deficits in PT adolescents.
These neuropsychological data provide novel insights into a previously observed deficit in PT performance on measures of fine motor ability and visual-spatial integration (Taylor et al. 2004; Van Braeckel et al. 2008; Williams et al. 2010; Butcher et al. 2012; Husby et al. 2013; Molloy et al. 2014). We show that deficits in fine motor ability mediate deficits in visual-motor integration or visual memory for PT participants, suggesting that the indirect path (PT birth results in deficits in fine motor which, in turn, results in deficits in visual-motor integration or visual memory) is a better explanation than the direct path (PT birth results in deficits in visual-motor integration or visual memory). As such, fine motor skills may represent a target for intervention that could lead to improved performance in other, more complex domains. Further, given the reported cerebellar correlates and rapid development of the cerebellum in infants born prematurely (Volpe 2009; Limperopoulos 2016; Stoodley and Limperopoulos 2016), intervention during infancy when these circuits develop may be the most impactful. However, we cannot make strong claims of causality (i.e., fine motor deficits cause visual memory deficits) as mediation does not imply causality unless both the independent variable and the mediator are experimentally manipulated (Sobel 2008). Finally, the deficits we describe may represent the more prevalent, mild sequelae of preterm birth that have significant implications for the school performance of PT participants in academic disciplines requiring the use of visual memory and visual-motor integration.
Despite no difference in FA values, our findings show an association between middle and superior cerebellar peduncle FA, visual memory, visual motor, and fine motor ability, providing preliminary evidence that cerebellar white matter may underlie visual memory in PT individuals. The cerebellar peduncles are part of a complex motor network (Schmahmann and Pandya 1997) and, given that we observe motor abilities mediating higher order cognitive measures in PT individuals, it is not surprising to observe neuro-correlates of these measures in traditional motor networks. As only PT participants showed these associations, these cerebellar tracts may subserve alternate or “reserve” pathways that the PT participants employ. Likewise, our previous functional connectivity results in PT participants at young adulthood also implicate the cerebellum as a potential mediator of reserve pathways (Constable et al. 2013). Alternative explanations include decreased power for between-group comparisons and delays in maturation for PT participants. As these are hypothesis-generating results, further replication study is needed.
Our data come in the midst of a shift in understanding of the cerebellum's role in brain function and behavior (Buckner Randy 2013). The cerebellum has conventionally been associated with coordination of motor function, but more recently has been implicated in language pathways (Constable et al. 2013; Shany et al. 2016), cognition (Allin et al. 2001; Ravizza et al. 2006; Rosenberg et al. 2016; Shany et al. 2016), and spatial processing (Stoodley 2012). For the first time, our results show that the cerebellum may also play a role in visual memory, if only in a specific clinical population. However, the fact that our PT participants showed no evidence of neonatal brain injury may increase the generalizability of our findings to other populations or neurodevelopmental measures.
The combination of neuropsychological and imaging data provides a unique framework with which to explore deficits in the so-called “healthy” PT participants. While several studies show deficits in PT or very low birthweight adolescents and young adults compared with terms in gross and fine motor dexterity (Husby et al. 2013), cognition (Allin et al. 2008; Breeman et al. 2015), and visual memory, with delayed visual memory being especially problematic (Molloy et al. 2014), others demonstrate that cerebellar injury adversely affects neurodevelopmental outcomes in this population (Limperopoulos et al. 2014; Brossard-Racine et al. 2015). A single study addresses white matter microstructure of the cerebellar peduncles and outcome in the prematurely born (Shany et al. 2016). While other studies establish the presence of impairment in PT adolescents, this study is able to parse the contribution of possible neonatal brain injury to these deficits by including only PT with no evidence of brain injury. Finally, emerging studies suggest that other neonatal health and treatment factors in addition to brain injury are also associated with alterations in cerebellum volume and visual-motor outcomes (Ranger et al. 2015; Zwicker et al. 2016).
The strengths of this study include a PT cohort with extensive neonatal radiographic testing showing no evidence of neonatal brain injuries commonly found in PT populations. However, this population represents only a subset of the PT cohort, and thus findings may not be representative of PT outcomes as a more general demographic category.
Nevertheless, this study has several limitations. As we did not collect neuropsychological measures of visual processing or perception, we could not model VMI and ROCF as a function of visual skill as we did with fine motor skills. However, there were no differences between study groups in visual acuity or percent wearing corrective lens, suggesting that basic vision problems did not contribute to our findings. Moreover, emerging evidence suggest that neonatal factors are associated with reduced cerebellar volume at age 7 years (Ranger et al. 2015). Comparable in-depth data from the neonatal period are not available for this cohort. Therefore, it is important to note that what this study describes as the broad category of “preterm birth” is in fact a constellation of many specific perinatal factors that may individually or cumulatively work to alter cerebellar microstructure. Finally, although we did not find a single unifying model including group status, FA, and neurocognitive scores, these data demonstrate several important associations between fine motor ability, visual memory, and cerebellar FA in PT participants.
The increasing survival of prematurely born neonates demands improved neurodevelopmental interventions for this vulnerable population, and it is critical to have assessment tools that provide meaningful insights on subtle deficits. In this study, the differential scores seen in ROCF and VMI between PT and term adolescents are mediated by group differences in fine motor ability, and preliminary data suggest brain correlates in the cerebellar white matter. As diminished academic performance may be linked to poor visual-spatial awareness and visual-spatial integration (Carlson et al. 2013), these findings pave the way for intervention studies. Novel strategies to improve fine motor skills in the prematurely born may help PT adolescents to reach their full academic potential.
Supplementary Material
Supplementary material are available at Cerebral Cortex online.
Supplementary Material
Notes
We thank Dr R. Todd Constable PhD, Department of Radiology and Biomedical Imaging, and Neurosurgery, for his considerable comments on the results and manuscript. Conflict of Interest: None declared.
Funding
This work was supported by NS27116 and the Vernon W. Lippard, M.D., Student Summer Research Fellowship.
References
- Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MH, Stewart AL, Rifkin L, Murray RM. 2001. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 124:60–66. [DOI] [PubMed] [Google Scholar]
- Allin M, Walshe M, Fern A, Nosarti C, Cuddy M, Rifkin L, Murray R, Rushe T, Wyatt J. 2008. Cognitive maturation in preterm and term born adolescents. J Neurol Neurosurg Psychiatry. 79:381–386. [DOI] [PubMed] [Google Scholar]
- Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. 2007. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 35:1021–1027. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. 2010. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 30:12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. 1986. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- Beery KE. 1989. Developmental test of visual-motor integration. 3rd rev Cleveland, Ohio: Modern Curriculum Press. [Google Scholar]
- Blencowe H, Lee ACC, Cousens S, Bahalim A, Narwal R, Zhong N, Chou D, Say L, Modi N, Katz J, et al. . 2013. Preterm birth–associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 74:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MH, Miskovic V, Van Lieshout R, Duncan L, Schmidt LA, Hoult L, Paneth N, Saigal S. 2011. Psychopathology in young adults born at extremely low birth weight. Psychol Med. 41:1763–1774. [DOI] [PubMed] [Google Scholar]
- Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D. 2015. Preterm cognitive function into adulthood. Pediatrics. 136:415–423. [DOI] [PubMed] [Google Scholar]
- Brossard-Racine M, du Plessis AJ, Limperopoulos C. 2015. Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum. 14:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner Randy L. 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 80:807–815. [DOI] [PubMed] [Google Scholar]
- Butcher PR, Bouma A, Stremmelaar EF, Bos AF, Smithson M, Van Braeckel KN. 2012. Visuospatial perception in children born preterm with no major neurological disorders. Neuropsychology. 26:723–734. [DOI] [PubMed] [Google Scholar]
- Carlson AG, Rowe E, Curby TW. 2013. Disentangling fine motor skills’ relations to academic achievement: the relative contributions of visual-spatial integration and visual-motor coordination. J Genet Psychol. 174:514–533. [DOI] [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer RJ, et al. . 2008. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 121:306–316. [DOI] [PubMed] [Google Scholar]
- Constable RT, Vohr BR, Scheinost D, Benjamin JR, Fulbright RK, Lacadie C, Schneider KC, Katz KH, Zhang H, Papademetris X, et al. . 2013. A left cerebellar pathway mediates language in prematurely-born young adults. Neuroimage. 64:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Hille ET, Weisglas-Kuperus N, van Goudoever JB, Jacobusse GW, Ens-Dokkum MH, de Groot L, Wit JM, Geven WB, Kok JH, de Kleine MJ, et al. . 2007. Functional outcomes and participation in young adulthood for very preterm and very low birth weight infants: the Dutch Project on Preterm and Small for Gestational Age Infants at 19 years of age. Pediatrics. 120:e587–e595. [DOI] [PubMed] [Google Scholar]
- Husby IM, Skranes J, Olsen A, Brubakk AM, Evensen KA. 2013. Motor skills at 23 years of age in young adults born preterm with very low birth weight. Early Hum Dev. 89:747–754. [DOI] [PubMed] [Google Scholar]
- Joseph RM, O'Shea TM, Allred EN, Heeren T, Hirtz D, Jara H, Leviton A, Kuban KC. 2016. Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics. 137:e20154343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, Papademetris X. 2011. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 9:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. 1981. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 5:602–619. [Google Scholar]
- Lefebvre F, Mazurier E, Tessier R. 2005. Cognitive and educational outcomes in early adulthood for infants weighing 1000 grams or less at birth. Acta Paediatr. 94:733–740. [DOI] [PubMed] [Google Scholar]
- Li K, Sun Z, Han Y, Gao L, Yuan L, Zeng D. 2015. Fractional anisotropy alterations in individuals born preterm: a diffusion tensor imaging meta-analysis. Dev Med Child Neurol. 57:328–338. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C. 2016. The vulnerable immature cerebellum. Semin Fetal Neonatal Med. 21:293–294. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. 2014. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex. 24:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, Ment L, Allan W, Schneider K, Vohr BR. 2011. Executive and memory function in adolescents born very preterm. Pediatrics. 127:e639–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Klove H. 1964. Instruction manual for the adult neuropsychology test battery. Madison, WI: University of Wisconsin Medical School; p. 36. [Google Scholar]
- Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, Duncan CC, Scott DT, Taylor KJ, Katz KH, et al. . 1994. a. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 93:543–550. [PubMed] [Google Scholar]
- Ment LR, Oh W, Ehrenkranz RA, Phillip AGS, Vohr B, Allan W, Makuch RW, Taylor KJW, Schneider KC, Katz KH, et al. . 1994. b. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 124:951–955. [DOI] [PubMed] [Google Scholar]
- Molloy CS, Wilson-Ching M, Doyle LW, Anderson VA, Anderson PJ. 2014. Visual memory and learning in extremely low-birth-weight/extremely preterm adolescents compared with controls: a geographic study. J Pediatr Psychol. 39:316–331. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Faria AV. 2009. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol. 22:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR. 2011. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 54:2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea TM, Allred EN, Kuban KC, Hirtz D, Specter B, Durfee S, Paneth N, Leviton A. 2012. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. J Child Neurol. 27:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth PA. 1944. Le test de copie d'une figure complexe; contribution à l’étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory.]. Archiv Psychol. 30:206–356. [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, et al. . 2000. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 284:1939–1947. [DOI] [PubMed] [Google Scholar]
- Ranger M, Zwicker JG, Chau CM, Park MT, Chakravarthy MM, Poskitt K, Miller SP, Bjornson BH, Tam EW, Chau V, et al. . 2015. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J Pediatr. 167:292–298.e291. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. 2006. Cerebellar damage produces selective deficits in verbal working memory. Brain. 129:306–320. [DOI] [PubMed] [Google Scholar]
- Rey A. 1941. L'examen psychologique dans les cas d'encéphalopathie traumatique. (Les problems.). [The psychological examination in cases of traumatic encepholopathy. Problems.]. Archiv Psychol. 28:215–285. [Google Scholar]
- Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM. 2016. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 19:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. 1997. The cerebrocerebellar system. Int Rev Neurobiol. 41:31–60. [DOI] [PubMed] [Google Scholar]
- Shany E, Inder TE, Goshen S, Lee I, Neil JJ, Smyser CD, Doyle LW, Anderson PJ, Shimony JS. 2016. Diffusion tensor tractography of the cerebellar peduncles in prematurely born 7-year-old children. Cerebellum. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Park SY, Park SR, Seol SH, Kwon JS. 2006. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc. 1:892–899. [DOI] [PubMed] [Google Scholar]
- Sobel ME. 2008. Identification of causal parameters in randomized studies with mediating variables. J Educ Behav Stat. 33:230–251. [Google Scholar]
- Sripada K, Lohaugen GC, Eikenes L, Bjorlykke KM, Haberg AK, Skranes J, Rimol LM. 2015. Visual-motor deficits relate to altered gray and white matter in young adults born preterm with very low birth weight. Neuroimage. 109:493–504. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. 2012. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 11:352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Limperopoulos C. 2016. Structure-function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med. 21:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EW, Ferriero DM, Xu D, Berman JI, Vigneron DB, Barkovich AJ, Miller SP. 2009. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 66:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Margevicius S, Schluchter M, Andreias L, Hack M. 2015. Persisting behavior problems in extremely low birth weight adolescents. J Dev Behav Pediatr. 36:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. 2004. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 10:149–163. [DOI] [PubMed] [Google Scholar]
- Van Braeckel K, Butcher PR, Geuze RH, van Duijn MA, Bos AF, Bouma A. 2008. Less efficient elementary visuomotor processes in 7- to 10-year-old preterm-born children without cerebral palsy: an indication of impaired dorsal stream processes. Neuropsychology. 22:755–764. [DOI] [PubMed] [Google Scholar]
- Van Braeckel KN, Taylor HG. 2013. Visuospatial and visuomotor deficits in preterm children: the involvement of cerebellar dysfunctioning. Dev Med Child Neurol. 55(Suppl 4):19–22. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. 1998. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 5:135–151. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. 2009. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 24:1085–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1991. Wechsler scale of intelligence for children. 3rd ed New York, NY: Psychological Corporation Harcourt Brace Co. [Google Scholar]
- Williams J, Lee KJ, Anderson PJ. 2010. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. 52:232–237. [DOI] [PubMed] [Google Scholar]
- Wong T, Taylor HG, Klein N, Espy KA, Anselmo MG, Minich N, Hack M. 2014. Kindergarten classroom functioning of extremely preterm/extremely low birth weight children. Early Hum Dev. 90:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, Liu M, Synnes A, Poskitt KJ, Stiver ML, et al. . 2016. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. 172:81–87.e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.