Abstract
Functional lateralization can be an indicator of brain maturation. We have consistently shown that, in the adult brain, proprioceptive processing of muscle spindle afferents generating illusory movement of the right hand activates inferior frontoparietal cortical regions in a right-side dominant manner in addition to the cerebrocerebellar motor network. Here we provide novel evidence regarding the development of the right-dominant use of the inferior frontoparietal cortical regions in humans using this task. We studied brain activity using functional magnetic resonance imaging while 60 right-handed blindfolded healthy children (8–11 years), adolescents (12–15 years), and young adults (18–23 years) (20 per group) experienced the illusion. Adult-like right-dominant use of the inferior parietal lobule (IPL) was observed in adolescents, while children used the IPL bilaterally. In contrast, adult-like lateralized cerebrocerebellar motor activation patterns were already observable in children. The right-side dominance progresses during adolescence along with the suppression of the left-sided IPL activity that emerges during childhood. Therefore, the neuronal processing implemented in the adult's right IPL during the proprioceptive illusion task is likely mediated bilaterally during childhood, and then becomes right-lateralized during adolescence at a substantially later time than the lateralized use of the cerebrocerebellar motor system for kinesthetic processing.
Keywords: functional magnetic resonance imaging, human development, inferior frontoparietal cortices, proprioceptive awareness, right-hemispheric dominance
Introduction
The human cerebral cortex is composed of 2 distinct (left and right) hemispheres, each of which may exhibit bilateral asymmetry in structure and/or function. The typical example of this phenomenon is language lateralization to the left, or “dominant,” hemisphere (Springer et al. 1999; Catani et al. 2007; Hickok and Poeppel 2007; Willems et al. 2014). The right hemisphere is considered the “non-dominant” hemisphere, and its assigned functions are not yet fully understood.
In our series of studies (Naito et al. 2016a), we have consistently shown that, in the adult brain, proprioceptive (kinesthetic) processing of muscle spindle afferent inputs, which generate somatic sensation (kinesthetic illusion) of right-hand flexion, activates inferior frontoparietal cortical regions (especially cytoarchitectonic areas 44, PFt, PF, and PFm) in a right-side dominant manner, in addition to the cerebrocerebellar motor network. These frontoparietal regions are likely connected by the inferior branch of the superior longitudinal fasciculus tract (SLF III) (Amemiya and Naito 2016; Morita et al. 2017). Unlike the cerebrocerebellar motor network, the exact roles of the right inferior frontoparietal cortical regions during proprioceptive illusion are not well known, although neuroimaging findings show high consistency with motor and bodily awareness deficits following right-hemispheric damage (Berlucchi and Aglioti 1997; Berti et al. 2005; Moro et al. 2016). However, a growing body of evidence indicates that right inferior frontoparietal activity is somehow associated with proprioceptive/motor awareness of limb movement (Desmurget et al. 2009; Cignetti et al. 2014; Amemiya and Naito 2016). Specifically, the right inferior parietal lobule (IPL) is likely an important node capable of accessing the brain network associated with proprioceptive/motor awareness of limb movement (Desmurget et al. 2009). Despite evidence obtained from the adult brain, then manner in which the human brain develops the right-dominance of the inferior frontoparietal cortical regions during proprioceptive illusion accompanied by bodily awareness is unknown.
In general, the development of left-lateralized functions (mainly language) is relatively well-documented (Holland et al. 2001, 2007; Balsamo et al. 2002; Szaflarski et al. 2006a, 2006b, 2012). However, studies addressing the development of right-lateralized functions are as yet limited (Everts et al. 2009; Nagel et al. 2013). Everts et al. (2009) have shown that the right-hemispheric lateralization of visuospatial (visual search) functions likely progresses through childhood and adolescence (from 8 to 15 years). However, when we consider the data carefully, the majority (8 out of 9) of adolescents aged 12–15 years display right-lateralization, while more than half (5 out of 9) children aged 8–12 years have either left-lateralization or no lateralization (bilaterality). Thus, right-hemispheric lateralization appears to become robust at around 12–15 years of age. If the right-side dominance of the inferior frontoparietal cortices during proprioceptive illusion also develops over the same time-course, we may hypothesize that the right-hemispheric dominance observed during the illusion emerges and becomes robust during adolescence (12–15 years). Children (8–12 years) would thus have a bilateral pattern of brain activation.
If this is the case, it is important to understand how the dominance of the right-sided cortical regions emerges. It is generally conceivable that right-hemispheric lateralization is achieved by augmentation of right-side activity or suppression or the absence of substantial recruitment of its homotopic left-side activity. We further address this important issue by carefully investigating developmental changes in brain activity during illusion in adolescents and young adults when compared to children.
We studied brain activity (blood oxygenation level-dependent [BOLD] signal) using functional magnetic resonance imaging (fMRI) while 60 right-handed healthy children (aged 8–11 years), adolescents (aged 12–15 years), and young adults (aged 18–23 years) (20 participants per group) performed a proprioceptive illusion task. During the illusion task, we vibrated the tendons of the wrist extensor muscles of the relaxed right hand of blindfolded participants. The participants experienced the proprioceptive sensation of the flexion of the right wrist, which is elicited by the muscle spindle afferent inputs from the hand (Naito et al. 2016a). We then examined when and how the human brain begins to predominantly use the right inferior frontoparietal cortical regions during proprioceptive illusion. We used cytoarchitectonic probability maps and the tract probability map for the SLF III of the human brain for the anatomical identification of the activated brain regions, and for the anatomical definition of region of interest (ROI) for area 44 (see Materials and Methods).
Materials and Methods
Participants
Twenty healthy right-handed children (CH; mean age, 9.6 ± 0.9 years, ranging from 8 years and 7 months to 11 years and 3 months), 20 adolescents (ADO; mean age, 13.3 ± 0.7 years, ranging from 12 years and 8 months to 15 years), and 20 young adults (AD; mean age, 20.9 ± 1.4 years, ranging from 18 years and 10 months to 23 years and 7 months) participated in the study. Each group (CH, ADO, or AD) consisted of 10 male and 10 female participants. The participants’ right-handedness was confirmed using the Edinburgh Handedness Inventory (Oldfield 1971). No participant had a history of neurological or psychiatric disorder. The protocol used for this study was approved by the ethics committees of the University of Fukui and the National Institute of Information and Communications Technology. We explained the details of the study to the participants before the start of the experiment. All participants then provided written informed consent. We also obtained written informed consent from the legal guardians of the children and adolescents. The experiment was carried out following the principles and guidelines of the Declaration of Helsinki (1975).
Proprioceptive Illusion Task
Before we started the fMRI experiment, we provided the participants with instructions. The participants experienced the illusion task outside the scanner, so that they were familiar with the task before they entered the magnetic resonance imaging (MRI) room. The participants subsequently lay in the MRI scanner. At this time, their heads were immobilized using sponge cushions and their ears were plugged. We asked the participants to relax their entire body without producing unnecessary movements and to think only of things relevant to the tasks assigned.
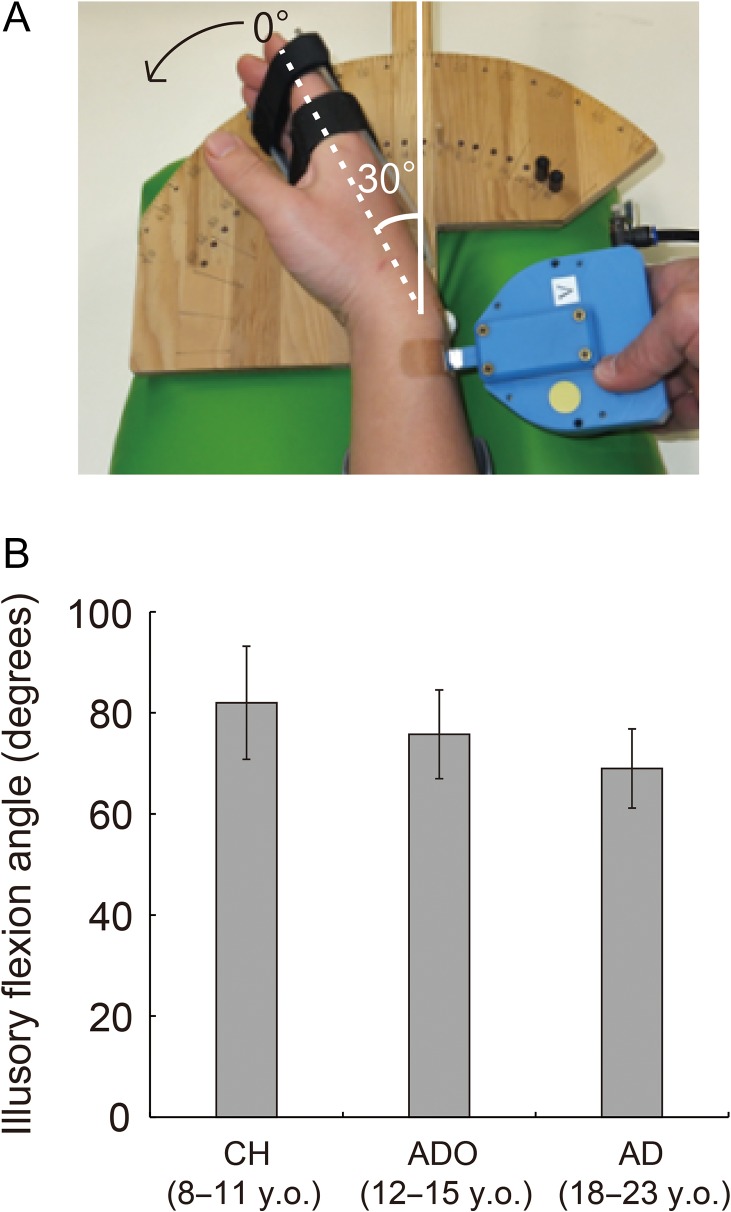
In the task, the participants closed their eyes and relaxed their limbs. Both the left and right arms of the participants were naturally semipronated and extended in front of them. The participants’ right hands were fixed onto a wooden apparatus using a hook-and-loop fastener (Fig. 1A), flexed at a 30° angle, and relaxed in this position (Fig. 1A).
Figure 1.
Experimental setup (A) and mean maximum illusory flexion angles reported by the participants in each group (B). (A) During the vibration, the participants flexed their right wrists at a 30° angle and relaxed in this position. When evaluating the maximum illusory angle after an experimental run, we measured the angle as a change from the original (30° flexion) position. (B) No significant group differences were observed. Bars indicate standard errors of means across participants. CH, child group; ADO, adolescent group; AD, adult group; y.o., years old.
Each participant completed 1 experimental run of this task. Of the case, 1 run was composed of 6 tendon-vibration epochs, each of which lasted for 15 s. During each epoch, we vibrated the tendon of the extensor carpi ulnaris muscle of the right wrist (Fig. 1A), which elicited an illusory flexion of the stationary right hand (Naito et al. 2002, 2005, 2007, 2011, 2016a). This illusion is elicited because the tendon-vibration excites the muscle spindle afferent fibers (Goodwin et al. 1972; Roll and Vedel 1982; Roll et al. 1989), and the brain receives and processes the proprioceptive (kinesthetic) inputs.
The tendon-vibration epochs were separated by 15-s baseline periods. During these periods, we vibrated the skin surface over a nearby bone (i.e., the processus styloideus ulnae of the hand just beside the tendon) using the same stimulus. Based on our series of studies, we know that this bone-vibration stimulus mainly elicits vibration sensations without generating any reliable (vivid and substantial) illusions. However, it is also true that the bone-vibration sometimes causes weak (unsubstantial) illusions probably owing to the spread of vibration from the bone to the surrounding wrist muscles (Naito et al. 2002). The participants usually have difficulty determining the directions of movement of these weak illusions. The bone-vibration stimuli are thus good controls for the tendon-vibration illusion, as they controls for attention to the vibrated hand, monitoring of possible illusory movements during the vibration, and the effects of the cutaneous afferent inputs from the vibrated skin around the wrist. Each run also included a 15-s period before the start of the first epoch and another 15-s period after the end of the last epoch. We performed the bone-vibration during these periods so that the stimulus was applied during all baseline periods. By examining the increase in brain activity during the tendon-vibration epochs comparing it to the baseline periods, we were able to evaluate the effect purely associated with the proprioceptive processing of muscle spindle afferent inputs, which generates the somatic sensation (kinesthetic illusion) of right wrist flexion by eliminating the effect of cutaneous vibro-tactile processing. Thus, we may consider brain activity identified in this manner as illusion-related activity not merely explained by attentional factors (see above).
We used a non-magnetic vibrator (110 Hz; Illusor; Umihira Ltd.; Kyoto, Japan; Fig. 1A) driven by constant air pressure provided by an air compressor (Amemiya and Naito 2016). We used vibration stimuli with amplitudes of approximately 3.5 mm. The contact surface on the skin was approximately 1 cm2. One experimenter (EN) operated the vibrator by manually applying it to the skin using light pressure. Computer-generated visual cues were displayed to the experimenter to instruct him regarding the timing of the tendon and bone vibrations. The blindfolded participants were unable to see these cues.
During the task, we asked the participants to be aware of movement sensations from the vibrated hand (during both tendon-vibration and bone-vibration). This task was thus a purely somatic perception (proprioceptive awareness) task wherein the blindfolded participants were aware of the change in hand posture. After the experimental run, we asked the participants whether they felt the illusion during the tendon-vibration and the bone-vibration. To verify that the participants in fact experienced the substantial proprioceptive illusion of right-hand flexion during the tendon-vibration task, we asked them to remember the maximum illusory flexion angle experienced during the run and to indicate the maximum angle after the run. In the scanner, we showed the participants a protractor on which a hand-shape indicator was mounted. This indicator was first set at the 30° flexion (original) position, which corresponded to the actual position of the participants’ relaxed hands (Fig. 1A). From this position, we began flexing the indicator. When the participants believed that the indicator had reached the maximum illusory angle that they experienced, they were asked to say “stop.” We measured this angle as a change from the original position. We calculated mean maximum illusory angle for all participants in each group. We performed a 1-way analysis of variance (ANOVA; age groups: CH, ADO, and AD [3]) for statistical analysis.
fMRI Data Acquisition
Functional images were acquired using T2*-weighted gradient-echo echo-planar imaging (EPI) sequences obtained using a 3-Tesla MRI machine (Discovery MR750; GE Healthcare; Milwaukee, WI) and a 32-channel array coil. We collected 82 volumes in total. Each volume consisted of 40 slices acquired in ascending order. The slices were 3.5 mm thick and had 0.5-mm gaps. We thus covered the entire brain. The time interval between 2 successive acquisitions from the same slice (TR) was 2500 ms. We used an echo time (TE) of 30 ms and a flip angle (FA) of 83°. The field of view (FOV) was 192 × 192 mm, and the matrix size was 64 × 64. Eventually, we had voxel dimensions of 3 × 3 × 3.5 mm in the x-, y-, and z-axes.
Imaging Data Analysis
Pre-processing
The first 4 volumes of each fMRI run were discarded because of unsteady magnetization. Imaging data were analyzed using Statistical Parametric Mapping (SPM8; The Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks, Sherborn, MA). Initially, EPI images were realigned to the first image and then to the mean image. All participants had less than 3 mm of maximum (cut-off) motion in every plane (x, y, z) during the fMRI run. In the CH group, average displacement within the run was 0.08 mm (ranging from 0.01 to 0.29 mm), 0.13 mm (0.02–0.94 mm), and 0.33 mm (0.03–1.52 mm) in x-, y-, and z-axis direction, respectively. These values were 0.05 mm (0.01–0.19 mm), 0.11 mm (0.02–0.86 mm), and 0.20 mm (0.03–1.82 mm) in the ADO group, and 0.04 mm (0.01–0.18 mm), 0.08 mm (0.02–0.33 mm), and 0.09 mm (0.02–1.25 mm) in the AD group. Thus, no data were excluded from the following analysis. The realigned images were normalized to the Montreal Neurological Institute (MNI) space (Evans et al. 1994). By comparing functional activation foci in children and adults within a common stereotaxic space, Kang et al. (2003) provided an empirical validation of normalization for analysis of fMRI data obtained from school-aged children. Finally, the spatially normalized functional images were filtered using a Gaussian kernel with a full-width-at-half-maximum (FWHM) of 4 mm in the x-, y-, and z-axes.
Analysis of Illusion-related Activity in Each Group
After pre-processing, we evaluated illusion-related activity using a general linear model (GLM; Friston et al. 1995; Worsley and Friston 1995) for each participant. The design matrix contained a boxcar function for the tendon-vibration (illusion) epoch, which was convolved with a canonical hemodynamic response function. To correct for residual motion-related variance after realignment, the 6 realignment parameters were also included in the design matrix as regressors of no interest. We first constructed an appropriate contrast image to examine brain regions showing illusion-related activity (tendon-vibration vs. bone-vibration [baseline]). We used this individual image in the following analyses in the present study. To accommodate inter-participant variability, the contrast images from all participants were entered into a second-level random effects group analysis (Holmes and Friston 1998). A one-sample t-test was then performed. This was done for each group separately. In the second-level analyses, we first generated a voxel-cluster image using an uncorrected voxel-wise threshold of P < 0.005 in each group. For statistical inference, we used a threshold of P < 0.05 at the cluster level after correction for multiple comparisons using the family-wise error rate (FWE) in the whole brain space. We adopted this threshold because it was used in a relevant previous study (Fontan et al. 2017) and we wanted to compare our results to those of previous studies. We consistently used this threshold in the subsequent analyses in the present study, except for the right-dominant area 44 activity (see below). We referred to the cytoarchitectonic probability maps in the MNI standard brain of the SPM anatomy toolbox v1.8 (Eickhoff et al. 2005) for anatomical identification of the activated brain regions.
Evaluation of Hemispheric Dominance in Each Group (Flip Analysis)
We examined hemispheric dominance in the illusion-related activity by adopting an approach employed by Shulman et al. (2010). In this analysis, during pre-processing, the original EPI images for each participant were first flipped across the midline to generate left-right reversed images (flipped EPI images). These flipped images were then realigned and normalized to the MNI space (Evans et al. 1994). Thus, the right (left) hemisphere was transformed in the best-fitting manner to the left (right) hemisphere. Finally, the normalized images were spatially smoothed using a 4-mm FWHM isotropic Gaussian kernel in the x-, y-, and z-axes.
For each participant, in addition to the original GLM built for the analysis of the original EPI images (see above), we also constructed a second GLM for the flipped EPI images. For images obtained during the illusion, we generated 2 contrast images showing illusion-related activity (tendon vs. bone) obtained from the original GLM and from the second (flipped) GLM, respectively. In the second-level group analysis, we performed a paired t-test using these images obtained from all participants. This was done for each (CH, ADO, AD) group. This analysis allowed us to perform voxel-wise comparisons between the original and flipped images in the MNI space. This in turn enabled us to perform a direct comparison between left and right hemisphere activation patterns in a more anatomically precise manner (Morita et al. 2017).
We also generated a voxel-cluster image using the uncorrected voxel-wise threshold of P < 0.005 in each group. For statistical inference, we used the FWE-corrected cluster-wise threshold of P < 0.05 for the entire brain space. We used the image of illusion-related activity obtained from the original image (uncorrected voxel-wise threshold of P < 0.05) as an inclusive mask. Using this mask image, we identified lateralized activation within brain regions in which activity increased during the tendon-vibration (illusion) by eliminating the possibility that the lateralized activation was merely caused by deactivation in the corresponding brain region in the opposite hemisphere.
We found right-side dominant activity in area 44 in adults and adolescents, although this activity did not reach the significance level in the entire brain space. However, in our series of proprioceptive illusion studies in adults, we have consistently reported right area 44 activity during illusion (Naito et al. 2005, 2007) and its right-side dominance (Amemiya and Naito 2016; Morita et al. 2017). Thus, right-side dominant area 44 activity during illusion was highly expected and biologically plausible in the present study. Then, we used small volume correction when we identified the significance of the spatial extent of the right-side dominant area 44 activity using a ROI. The ROI was defined by the cytoarchitectonic area 44 map of the right hemisphere, which is available in the SPM anatomy toolbox v1.8 (Eickhoff et al. 2005). We used the FWE-corrected cluster-wise threshold of P < 0.05 in the ROI (small volume correction).
Comparisons Between Groups
When evaluating hemispheric dominance, we found adult-like right-side dominant activity in the IPL in adolescents, but not in children (Fig. 2A). In order to further investigate whether the emergence of right-side dominant IPL activity in the adolescents was due to augmentation of right-side activity or suppression (or the absence of substantial recruitment) of left-side activity when compared to the children, we performed comparisons between the groups. We first compared illusion-related activity in children (CH) with that in adolescents (ADO) to determine whether there is greater left IPL activity in children (CH vs. ADO). Here, we used the image of illusion-related activity obtained from the children (uncorrected voxel-wise threshold of P < 0.05) as an inclusive mask. We also performed the opposite comparison (ADO vs. CH) to determine whether there was greater right IPL activity in adolescents using an inclusive mask image of the adolescents’ illusion-related activity (uncorrected voxel-wise threshold of P < 0.05). We also compared CH versus AD and AD versus CH. In these analyses, we first generated a voxel-cluster image using the uncorrected voxel-wise threshold of P < 0.005 for each contrast. Then, we used the FWE-corrected cluster-wise threshold of P < 0.05 in the entire brain space.
Figure 2.
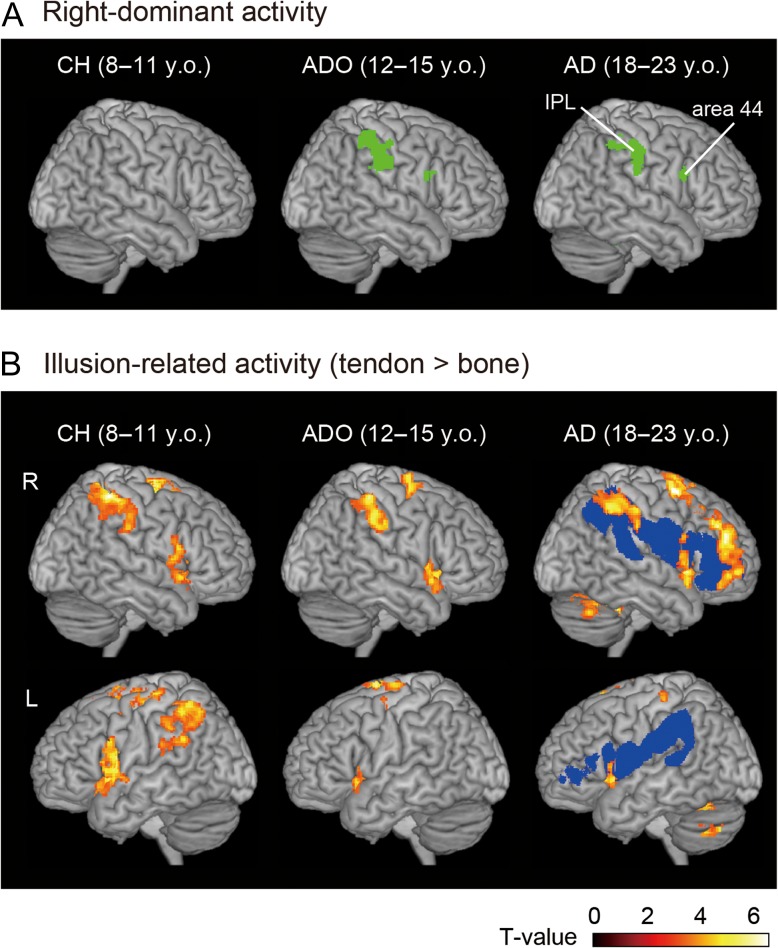
Brain regions with right-hemispheric-dominant activity during the proprioceptive illusion of right-hand flexion (results from flip analysis, A) and brain regions with illusion-related activity in each group (B). In each panel, brain activation patterns are rendered onto the MNI standard brain. Blue sections in Panel B indicate brain regions that are likely connected by the SLF III (50% probability map) in the adult brains (cf. Parlatini et al. 2017; Morita et al. 2017). The right inferior frontoparietal activations are located in these brain regions. CH, child group; ADO, adolescent group; AD, adult group; y.o., years old.
Correlation Analysis
We examined the manner in which adult-like right-dominant IPL activity emerges during development course from childhood to adolescence by performing a correlation analysis. Using age (months) as a covariate, we examined brain regions in which illusion-related activity was correlated with age in the 40 children and adolescents. We tested both negative and positive correlations. For each analysis, we generated a cluster image of voxels wherein activity was correlated with age using the uncorrected voxel-wise threshold of P < 0.005. For statistical inference, we used the FWE-corrected cluster-wise threshold of P < 0.05 in the entire brain space.
Results
Behavioral Results
After the experimental run wherein we asked the participants regarding their illusory experiences, all of the participants reported that they experienced vivid movement sensations of right-hand flexion when we vibrated the tendon of the wrist extensor muscle (during the tendon-vibration). Some participants in each group also reported that they felt weak movement sensations of the right hand toward a range of directions (supination, pronation, and their combinations) even during the bone-vibration (baseline) periods. However, when we asked them specifically about these sensations, they reported that the sensations were distinguishable from the movement sensations of right-hand flexion during the tendon-vibration. During the scan, we visually checked that the hand was not actually moving during the tendon-vibration. It is thus fair to say that the participants experienced substantial illusory flexion of the right stationary hand. Surprisingly, some of the participants reported that they experienced an illusory flexion angle beyond the natural endpoint of wrist flexion during the tendon-vibration. This finding indicates that our body is represented as flexible in our brain and that an illusory experience may sometimes evoke a physically impossible limb position.
The reported mean maximum illusory flexion angle was 82.0° in children (ranging from 20° to 200°), 75.8° in adolescents (ranging from 25° to 170°), and 69.0° in young adults (ranging from 25° to 165°) (Fig. 1B). The ANOVA revealed no differences between the age groups [F2,57 = 0.48]. This finding is in line with the report that adult-like vibration frequency-dependent illusory experience emerges between 7 and 9 years of age (Redon et al. 1994), although kinesthetic acuity continuously develops throughout adolescence (Visser and Geuze 2000; Goble et al. 2005; Holst-Wolf et al. 2016; Marini et al. 2017).
Lateralized Illusion-related Activity
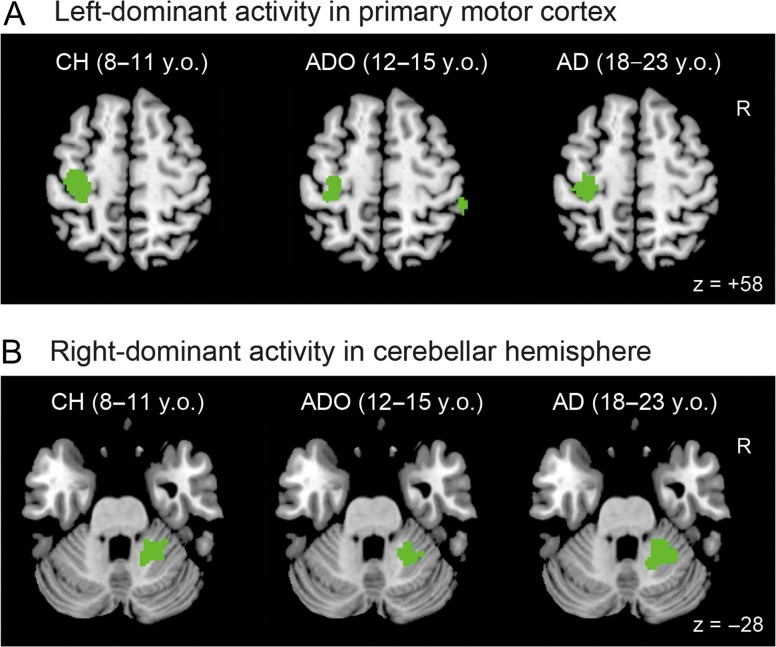
In the young adults, when we examined the lateralized illusion-related activity in the whole brain using voxel-wise comparisons between the 2 hemispheres, we found right-side dominant activity in the IPL (activation peaks in cytoarchitectonic areas PFt and hIP1, and the supramarginal gyrus; Fig. 2A and Table 1). This activity was present even when the participants experienced the illusion in the right hand. We also found right-side dominant activity (53 voxels) in the inferior frontal gyrus (peak in area 44), which became significant after the small volume correction (see Materials and Methods). In addition to the right-lateralized cerebral activity, we also found lateralized activities in the cerebrocerebellar motor network (Fig. 3 and Table 1). Specifically, we found left-dominant activity in the hand section of the primary sensorimotor cortices (SM1: peaks in areas 4a, 3a, 4p, and 3b; Fig. 3A) and right-dominant activity in the hand section of the cerebellar hemisphere (peaks in lobules VI and V, Fig. 3B, Grodd et al. 2001) and in the cerebellar vermis and paravermis (peaks in lobules VI and V, Table 1).
Table 1.
Lateralized activity during illusion in each group
| Children | Adolescences | Adults | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T | Size | Area | x | y | z | T | Size | Area | x | y | z | T | Size | Area | ||
| Right inferior frontoparietal network | |||||||||||||||||||
| Inferior frontal cluster | |||||||||||||||||||
| 50 | 8 | 22 | 3.69 | 59* | Area 44 | 54 | 12 | 24 | 3.96 | 53* | Area 44 | ||||||||
| Inferior parietal cluster | |||||||||||||||||||
| 58 | −26 | 32 | 7.51 | 725 | Area PF | 54 | −24 | 38 | 4.85 | 434 | Area PFt | ||||||||
| 58 | −28 | 44 | 5.89 | Area PFt | 62 | −24 | 46 | 4.61 | Supramarginal gyrus | ||||||||||
| 40 | −38 | 44 | 4.66 | Area hIP2 | 48 | −38 | 46 | 3.69 | IPL | ||||||||||
| 50 | −34 | 50 | 4.63 | Area PFt | 40 | −48 | 44 | 3.56 | Area hIP1 | ||||||||||
| 26 | −46 | 48 | 4.59 | Area 7PC | |||||||||||||||
| 54 | −42 | 56 | 3.46 | IPL | |||||||||||||||
| Motor network | |||||||||||||||||||
| Left primary motor cluster | |||||||||||||||||||
| −32 | −24 | 54 | 7.84 | 569 | Area 4p | −30 | −24 | 54 | 5.65 | 332 | Area 4p | −34 | −26 | 62 | 7.07 | 710 | Area 4a | ||
| −30 | −40 | 64 | 2.93 | Area 1 | −28 | −18 | 72 | 4.59 | Area 6 | −36 | −24 | 46 | 5.09 | Area 3a | |||||
| −30 | −30 | 68 | 3.31 | Area 4a | −24 | −26 | 54 | 4.15 | Area 4p | ||||||||||
| −30 | −36 | 64 | 3.87 | Area 3b | |||||||||||||||
| −24 | −26 | 74 | 3.84 | Postcentral gyrus | |||||||||||||||
| Right-cerebellar cluster | |||||||||||||||||||
| 30 | −44 | −32 | 4.32 | 341 | Lobule VI | 20 | −48 | −26 | 5.42 | 290 | Lobule VI | 22 | −48 | −26 | 6.47 | 708 | Lobule VI | ||
| 10 | −52 | −20 | 5.40 | Lobule V | 4 | −66 | −22 | 5.96 | Lobule VI (vermis) | ||||||||||
| 32 | −46 | −30 | 3.26 | Lobule VI | 12 | −48 | −18 | 5.77 | Lobule V | ||||||||||
| 28 | −42 | −32 | 5.02 | Lobule VI | |||||||||||||||
| 6 | −58 | −12 | 4.38 | Lobule V (paravermis) | |||||||||||||||
| 6 | −70 | −12 | 3.32 | Lobule VI (paravermis) | |||||||||||||||
| Other regions | |||||||||||||||||||
| −50 | −40 | 18 | 5.10 | 690 | STG | ||||||||||||||
| −28 | −6 | −2 | 5.01 | Putamen | |||||||||||||||
| −32 | −26 | 4 | 4.67 | Insula (lg1) | |||||||||||||||
| −40 | −14 | 12 | 4.67 | Area OP3 | |||||||||||||||
| −28 | −12 | 10 | 4.66 | Putamen | |||||||||||||||
Note: Uncorrected height threshold, P < 0.005; extent threshold, P < 0.05, FWE-corrected. Size = number of active voxels. For anatomical identification of peaks, we only considered cytoarchitectonic areas available in the anatomy toolbox that had probabilities greater than 30%. The cytoarchitectonic area with the highest probability was reported for each peak. When cytoarchitectonic areas with probabilities higher than 30% were not available to determine a peak, we simply provided the anatomical location of the peak. In each cluster, we reported peaks that were more than 10 mm apart from each other in order of increasing T-value. *Uncorrected height threshold, P < 0.005; extent threshold, P < 0.05, FWE-corrected in the ROI of area 44 in the right hemisphere (see text). STG, superior temporal gyrus.
Figure 3.
Lateralized activity in the left primary motor cortex (A) and in the right-cerebellar hemisphere (B) during illusion of the right hand. These activities were identified by flip analysis in each group. The activities are superimposed in horizontal sections of z = +58 (A) and z = −28 (B) in the MNI standard brain. CH, child group; ADO, adolescent group; AD, adult group; y.o., years old.
Highly similar patterns of lateralized activity were observed in adolescents (Fig. 2A and Table 1). We observed right-dominant activity in the IPL (activation peaks in cytoarchitectonic areas PF, PFt, hIP2, and 7PC; Table 1). We also found right-side dominant activity (59 voxels) in the inferior frontal gyrus (area 44), which became significant after the small volume correction. In the cerebrocerebellar motor network, we found left-dominant activity in the hand section of the primary motor cortex (M1; peaks in areas 4p, 4a, and 6; Fig. 3A) and right-dominant activity in the hand section of the cerebellar hemisphere (peaks in lobules VI and V, Fig. 3B).
In contrast, we found no right-dominant activity in the cerebral cortex in children (Fig. 2A and Table 1). However, we found adult-like lateralized activation in the cerebrocerebellar motor network. We found left-dominant activity in the hand section of SM1 (peaks in areas 4p and 1, Fig. 3A) and right-dominant activity in the hand section of the cerebellar hemisphere (1 peak in lobule VI, Fig. 3B).
We thus found conspicuous right-side dominant illusion-related activity in the IPL in the adult brain. The adult-like right-dominant recruitment of the IPL was present in adolescents, but was absent in the children, although the adult-like lateralized activation patterns in the left-M1-right-cerebellar motor network were already observable in the children.
Changes in right-lateralized IPL activity in the 3 groups were consistent with our observations when we assessed the illusion-related (tendon > bone) activity in each group (Fig. 2B). In all groups, the illusion-related activity was observed in the right inferior frontoparietal cortices, which are likely connected by the inferior branch of the superior longitudinal fasciculus (SLF III). However, in adults and adolescents, the inferior frontoparietal activity was dominant in the right hemisphere while the left cortical regions (except for the anterior insula) were silent. In contrast, illusion-related inferior frontoparietal activity was observed bilaterally in children (Fig. 2B).
When analyzing lateralized illusion-related activity in the children, we found a cluster of left-dominant activity (peaks in the superior temporal cortex, putamen, posterior insula, and area OP3; Table 1). However, since these cortical regions were not substantially active during the illusion in the children, the left-lateralized activity was likely due to the combinatory effects of subthreshold increases in brain activity in these areas and decreases in brain activity in the same regions on the right side.
Comparisons Among Groups and Correlations
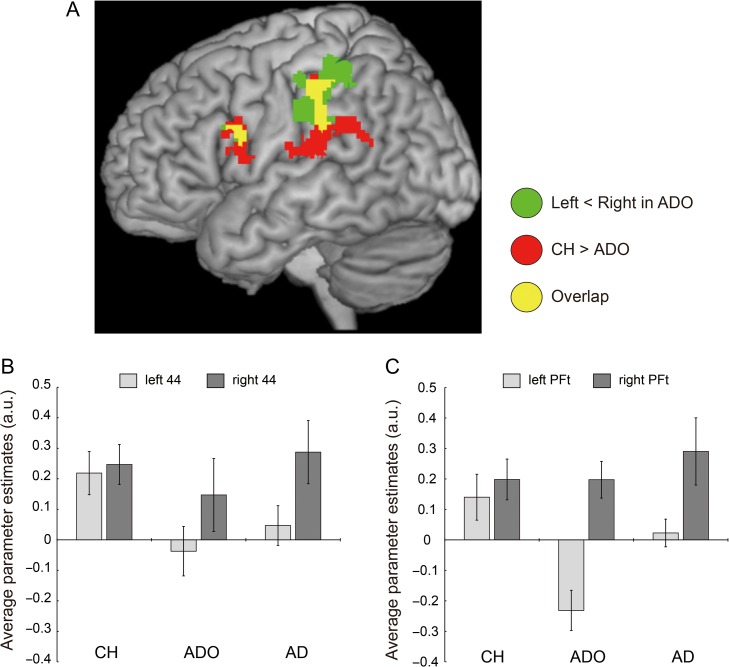
We examined whether the right-side dominant IPL activity observed in the adolescents and adults was due to augmentation of right-side activity or suppression (or the absence of substantial recruitment) of left-side activity when compared to children. When we directly compared the illusion-related activity observed in the children to that observed in the adolescents (CH vs. ADO), we found significantly greater activity in the inferior frontoparietal cortical regions of the left hemisphere in children (Fig. 4A and Table 2). These regions overlapped with the left-side regions whose corresponding regions on the right side had right-lateralized illusion-related activity in adolescents (Table 1 and Fig. 2A). When we identified activity peaks in such overlapping regions on the left, we found a peak voxel in area PFt (MNI coordinates [x, y, z] = [−54, −28, 34]) for the left IPL cluster (Table 2). A similar peak voxel for the left inferior frontal cluster was identified in area 44 (peak coordinates = [−48, 8, 16]). Importantly, these regions on the left side corresponded well to those with illusion-related activity in the children (Fig. 2B). In contrast, when we made the opposite comparison (ADO vs. CH), we did not find significantly greater activity in the adolescents anywhere in the brain. This indicates that the right-side IPL activity was not augmented in the adolescents when compared to the children.
Figure 4.
Comparisons among groups. (A) Greater left inferior frontoparietal activity in children (red and yellow sections) when comparing CH to ADO. Green and yellow sections indicate left-side regions whose corresponding right-side regions have right-lateralized illusion-related activity (Fig. 2A and Table 1). Yellow sections signify overlapped regions between the red and green regions. The activity peak in the left IPL cluster was located in area PFt (−54, −28, 34) and that in the left inferior frontal cluster was identified in area 44 (−48, 8, 16). (B) and (C): Illusion-related activity in bilateral areas 44 (B) and in bilateral areas PFt (C) in each group. In each panel, light gray bars indicate data obtained from the left-side cortex and dark gray bars indicate data obtained from the right-side cortex. In Panel B, we extracted parameter estimates from a sphere 4 mm in radius around the left area 44 peak (−48, 8, 16) and around the corresponding peak of the right hemisphere. In Panel C, the same was done for the left area PFt peak (−54, −28, 34) and for its corresponding right peak. In each case, the right peak was identified based on the flip analysis. This was performed for each participant, and the mean was calculated for the participants. Bars indicate the standard error of the mean. CH, child group; ADO, adolescent group; AD, adult group.
Table 2.
Greater illusion-related left inferior frontoparietal activity in children when compared to adolescents
| Cluster | Size | x | y | z | T | Anatomical identification |
|---|---|---|---|---|---|---|
| Left inferior parietal cluster | 953 | −54 | −28 | 34 | 5.00 | Area PFt |
| −62 | −24 | 42 | 4.13 | Area PFt | ||
| −52 | −26 | 44 | 4.06 | Area 2 | ||
| Left inferior frontal cluster | 303 | −48 | 8 | 16 | 3.52 | Area 44 |
| −56 | 10 | 26 | 3.28 | Area 44 |
When we compared the illusion-related activity observed in children to that observed in adults (CH vs. AD), we found no significantly greater activity in the children. This indicated that substantial (drastic) changes in illusion-related activity in the left inferior frontoparietal regions occur from childhood to adolescence. When we made the opposite comparison (AD vs. CH), no significantly greater activity was observed in the right IPL. This again indicated that there was no augmentation of right IPL activity in adults. We however found significantly greater activity in the right-cerebellar paravermis (249 voxels, peak coordinates = [10, −76, −14]) in adults (not shown in figure). This region overlapped with the region displaying illusion-related activity in adults (Table 1). There was no significant difference between the ADO and AD groups.
When we checked the activity in areas PFt and 44 to verify the above-described results, the activity in these areas increased bilaterally during the illusion in children (Fig. 4B, C). In contrast, in adolescents and adults, we observed no substantial increases (or observed decreases) in left-side activity, while the intensity of right-side activity was approximately the same as that observed in the children. Importantly, the decrease in illusion-related activity (bone > tendon) in adolescents was conspicuous in the left area PFt when compared to the left area 44 (Fig. 4B, C). One may assume that fMRI deactivation (negative BOLD) is associated with neuronal suppression (Smith et al. 2004; Shmuel et al. 2006). Our data thus suggest that the right-side dominant IPL activity observed in adolescents and adults during the illusion is likely not due to augmentation of right-side activity, but to the suppression or absence of substantial recruitment of left-side activity when compared to children.
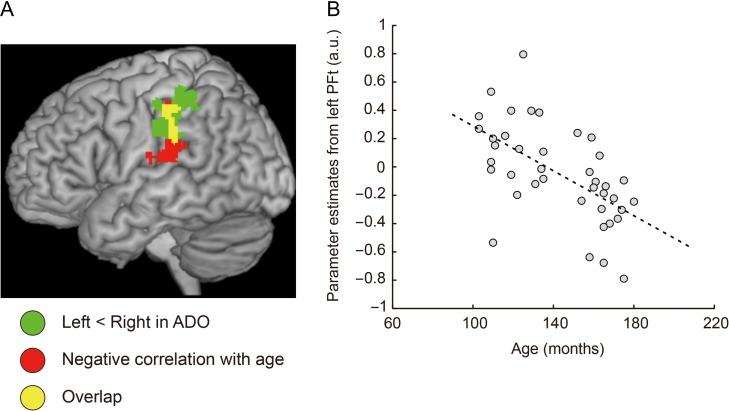
Finally, when we performed a correlation analysis using age (months) as a covariate in the population of 40 children and adolescents, we found that the activity of the left IPL cluster (759 voxels) decreased with age (Fig. 5). This was the only region in the entire brain where we made this observation. No such correlation was observed in area 44. This suggests that there were more notable developmental changes in the IPL. The left IPL corresponded well to the region whose counterpart on the right had right-lateralized illusion-related activity in adolescents (Fig. 2A). We found the voxel with the strongest negative correlation (T-value = 4.69) in the left area PFt (−54, −28, 32). This voxel was adjacent to the peak voxel identified in the above CH versus ADO comparison. Finally, we found no regions wherein activity was increased with age. The right-side dominant IPL (area PFt) activity during the illusion thus appeared to emerge in adolescents most likely due to the gradual age-dependent suppression of left IPL activity from childhood to adolescence.
Figure 5.
(A) Left IPL region (red and yellow sections), where activity was negatively correlated with age (months) in the 40 children and adolescents. Green and yellow sections indicate the left IPL regions whose corresponding right regions had right-lateralized illusion-related activity (Fig. 2A). Yellow sections indicate the overlapped regions between the red and the green regions. The voxel with the strongest negative correlation was located in left area PFt (−54, −28, 32). This voxel was adjacent to the peak voxel identified in the above CH vs. ADO comparison (Fig. 4). (B) Negative correlation (r = −0.59) between age (months, horizontal axis) and mean activity (parameter estimates) in a sphere 4 mm in radius around the voxel (−54, −28, 32). Each dot represents data obtained from each participant and the dashed line indicates a regression line. a.u., arbitrary units.
Discussion
We demonstrated that the adult-like right-side dominant use of the IPL during proprioceptive illusion of the right hand emerges in adolescents. The children recruited the IPL bilaterally, even though they experienced the same degree of illusion. This is in clear contrast with the finding that the adult-like lateralized activation patterns in the left-M1-right-cerebellar motor network during the illusion were already observable in the children. We further showed that the right-side dominant IPL activity in the adolescents and adults is most likely associated with the suppression of left-side activity or the absence of its recruitment when compared to the children. In adolescents, right-side dominant IPL (area PFt) activity during the illusion was most likely due to the gradual age-dependent suppression of left IPL activity from childhood to adolescence. Our results thus provide novel evidence that neuronal processing in the adult right IPL during proprioceptive illusion is first mediated by the bilateral IPL during childhood and then becomes right-lateralized in association with suppression of left-side activity during adolescence. This is the first study to reveal when and how the human brain develops the right-dominant use of the IPL during proprioceptive (kinesthetic) illusion.
General
The illusion-related activity (tendon > bone) in adults (including bilateral insular activity, Fig. 2B) had a highly similar pattern to those consistently shown in our series of studies (Naito et al. 2016a). The right-side dominant inferior frontoparietal activity in the adults (Fig. 2A) also replicated previous findings (Naito et al. 2005, 2007; Hagura et al. 2009; Amemiya and Naito 2016). These lines of evidences seem to indicate the reliability of the present results.
Interestingly, our results are not perfectly consistent with those reported in previous studies (bilateral IPL activation; Cignetti et al. 2017; Fontan et al. 2017), even though we used the same statistical threshold. One major reason for this discrepancy may be the use of different control conditions. We used vibration of the bone just beside the tendon at the same frequency as a control. We thus identified the illusion-related activity by eliminating the effects of cutaneous afferent inputs from the skin around the vibrated wrist by contrasting the tendon-vibration with the bone-vibration. On the other hand, in the previous studies, 30-Hz vibration was used as a control when identifying illusion-related activity using 100-Hz tendon-vibration. In this condition, one cannot eliminate the effects of cutaneous afferent inputs, as the most sensitive receptors for the 30-Hz vibration are the Meissner corpuscles, while those sensitive to the 100-Hz vibration are the Pacinian corpuscles (Mountcastle et al. 1972). Indeed, we have reported a bilateral pattern of IPL activity as the main effect of skin vibration (Naito et al. 1999; 2007). Therefore, the contrast image of 100-Hz tendon-vibration versus 30-Hz tendon-vibration still includes brain activities associated with cutaneous afferent processing derived from Pacinian receptors, as opposed to Meissner receptors.
In contrast to the fact that the adult-like right-hemispheric dominant use emerged in the adolescents (Fig. 2A), the adult-like lateralized activation patterns in the motor (left-M1-right-cerebellar) network were already observable in the children (Fig. 3). As we have carefully discussed in our series of studies, the cerebrocerebellar motor network most likely involves the kinesthetic processing of muscle spindle afferent inputs during the illusion of the right hand (Naito et al. 2016a). Thus, our findings indicate that the right-side dominant use of the inferior frontoparietal cortical regions during proprioceptive (kinesthetic) illusion develops later than the lateralized use of the motor system for kinesthetic processing. This is consistent with the general notion that cortical motor and sensory areas mature first and higher-order association areas mature later (Gogtay et al. 2004; Casey et al. 2005).
The greater illusion-related activity in the right-cerebellar vermal section in adults was a new finding. The children did not substantially use this section during the illusion (Table 1). This region is considered a part of the spinocerebellum, which receives somatosensory (proprioceptive and cutaneous) afferent inputs (Bosco and Poppele 2001). We have in fact reported illusion-related spinocerebellar activity in our series of adult studies (Naito et al. 2016a). The spinocerebellum is normally considered a phylogenetically (and ontogenetically) older region, even within the cerebellum (Tiemeier et al. 2010). However, our recent anatomical and functional studies have shown that the spinocerebellar afferent tract and the use of the vermis for motor function are not yet fully mature in 8–11-year-old children when compared to 18–23-year-old adults (Amemiya et al. 2016; Naito et al. 2016b). Thus, the present results seem consistent with the observation of immaturity in the use of the cerebellar vermis during childhood, which might be related to lower proprioceptive acuity in children (Marini et al. 2017).
Inferior Parietal Lobule
Among the inferior frontoparietal cortical regions, we observed the most notable developmental changes in the IPL (area PFt). It is unlikely that the observed right IPL activity is merely associated with attention, as this region is not a main locus of goal-directed and/or stimulus-driven attention (Corbetta and Shulman 2002; Corbetta et al. 2008). In addition, attentional factors implicated in the monitoring of movement sensations were equally required during both the tendon-vibration and bone-vibration in the present study (see Materials and Methods). We may assume that the present right IPL activity is associated with higher-order proprioceptive processing, which may generate the somatic sensation (kinesthetic illusion) of right-hand movement, as suggested in previous studies (Desmurget et al. 2009; Cignetti et al. 2014; Amemiya and Naito 2016), though further studies are still required to verify this claim. The present findings suggest that similar processing in the adult right IPL is likely mediated bilaterally during childhood and then becomes right-lateralized during adolescence (Fig. 2).
Another important finding of our study was that the emergence of right-dominant illusion-related IPL activity during adolescence (12–15 years) is likely not due to augmentation of right-side activity, but was in fact to age-dependent suppression of left-side activity from childhood to adolescence (Figs 4C and 5B). These findings were further corroborated by the observation that the left IPL was one of the brain regions that showed significant illusion-related deactivation (bone > tendon) in the adolescents (deactivation peak in area PFt: −60, −18, 28). No such deactivation was observed in the adults (see Supplementary Material). Our results thus seem to support the uniqueness of adolescence during the development of the human central nervous system (cf. Casey et al. 2005; Blakemore 2012; Morita et al. 2016a), and provide new evidence that right-hemispheric functional lateralization for higher-order proprioceptive processing conspicuously progresses during adolescence.
We speculate that the suppression of left-side IPL activity during adolescence (Figs 4C and 5B) reflects the brain's strategy to affirmatively promote a shift from bilateral use of the IPL during childhood to its right-dominant use during adulthood. By doing so, the brain can promote functional segregation between the right and left IPL.
It might be worth discussing some potential functions of the left IPL in humans. One of the well-known functions of the adult left IPL is the association between kinesthetic-motor internal representations and representations of external objects and tools. This enables us to pantomime and actually use objects and tools, as several human lesion (apraxia) and neuroimaging studies have suggested (Niessen et al. 2014). Consistent with these findings, we have shown that the left IPL is predominantly activated when adult blindfolded participants experience the kinesthetic illusion of a hand exclusively when the hand touches an external object, irrespective of the side of stimulation (Naito and Ehrsson 2006). Thus, the association between internal and external representations may be one function of the left IPL in the adult brain.
In the present study, among several cytoarchitectonic sub-areas in the human IPL (Caspers et al. 2006, 2013), right-hemispheric dominance during the illusion seems to be particularly promoted in area PFt (rostral area of IPL) during adolescence (Figs 4 and 5). An activation peak in area PFt is also reported in the illusion-related right IPL activity identified in our previous adult study (Amemiya and Naito 2016). In addition to putative higher-order proprioceptive functions, area PFt seems to be an important brain structure for action observation and imitation function, as it is shown to be the most consistently activated brain region in 139 neuroimaging studies of action observation and imitation (Caspers et al. 2010). Importantly, area PFt in the left (but not right) hemisphere of the adult brain is a human-specific brain region active during observation of actions with tools (Peeters et al. 2009). More importantly, the left PFt region (−60, −21, 31) was highly similar to the regions wherein we observed the suppression of illusion-related activity during adolescence (Figs 4 and 5). Since the action observation brain network also seems to change from a bilateral network during childhood to a left-lateralized network during adulthood (Biagi et al. 2016), it might be the case that the function of observation (and imitation) of actions with tools is predominantly implemented in the left PFt region during human development.
In the experiment wherein we scanned brain activity while the same participants performed continuous alternating flexion-extension movements of the right wrist at 1 Hz (see Supplementary Material), we found that the similar region in the left (contralateral to the hand) rostral IPL (areas 2 and PFt) had significant movement-related activity in adults and adolescents, but not in the children (Supplementary Fig. 1A). The movement-related activity gradually increased from childhood to adulthood, although this activity was not left-side dominant in any group (Supplementary Fig. 1B). These results at least provide evidence that the left rostral IPL (area PFt) gradually strengthens its involvement in sensory-motor control of right-hand movement from childhood to adulthood, which made a clear contrast to the finding that the left area PFt weakens its involvement in genuine proprioceptive processing during the illusion after adolescence (Fig. 4C).
As described in the introduction, the right IPL seems to be a particularly important node that has access to the brain network associated with proprioceptive/motor awareness of limb movement. In the adult brain, the right inferior frontoparietal cortical regions active during the illusion are likely connected by the SLF III (Fig. 2). The human right SLF III has greater volume than the left SLF III (Thiebaut de Schotten et al. 2011). This anatomical feature appears to be specific to humans (Hecht et al. 2015). The greater tract volume is suitable for the speedy processing of massive amounts of complex information derived from our body. Thus, we assume that the high-capacity information processing of the right SLF III may underlie right-hemispheric dominance during proprioceptive illusion. It might thus be possible that the shift from bilateral to right-lateralized use of the inferior frontoparietal cortical regions during proprioceptive illusion is associated with such a structural maturation of the SLF III (cf. Giorgio et al. 2008; Asato et al. 2010; Lebel et al. 2008, 2012; Lebel and Beaulieu 2011; Peters et al. 2012).
Development of Hemispheric Asymmetry
Left-hemispheric dominance is already observable at 5 years of age, particularly with respect to the language production function (verb generation task; Szaflarski et al. 2006b; Holland et al. 2007). However, in the present study, right-hemispheric dominance in the proprioceptive illusion task emerged during adolescence (12–15 years). The right-hemispheric dominance seems also to become apparent at similar ages for the visual search function (Everts et al. 2009) and for self-face recognition (Morita et al. 2016b). Thus, the right-hemispheric lateralization of certain functions appears to develop during adolescence. This is substantially later than the left-hemispheric lateralization of language (production) function, which is already acquired during early childhood.
This time lag in development may allow the human brain to preserve the right “non-dominant” hemisphere as a spare for the left “dominant” hemisphere to subserve the function of the left hemisphere in its malfunction. Indeed, studies in pediatric patients with epilepsy (8–18 years old) that left-dominant activation patterns normally observed when right-handed healthy controls perform a language production (verb generation) task are shifted towards bilateral or right-dominant patterns (Yuan et al. 2006). In addition, lateralization of language function can be shifted from the left hemisphere (at 6.8 years of age) to the right hemisphere (at 10.5 years of age) after left hemispherotomy at 9 years of age (Hertz-Pannier et al. 2002). These clinical findings suggest that the brain has an innate bilateral functional network, which allows it to reorganize a pre-existing bilateral network in cases of hemispheric malfunction.
A developmental shift from more bilateral use of the cerebral cortex during childhood to its lateralized use during adulthood has been reported in the intact brain (e.g., visuospatial function: Everts et al. 2009; and action observation: Biagi et al. 2016). The present study generally supported these findings by demonstrating a shift from more bilateral use of the IPL during childhood to right-lateralized use of this structure during adolescence and adulthood in response to proprioceptive illusion accompanied by bodily awareness.
Supplementary Material
Supplementary Material
Funding
This work was supported by a Grant-in-Aid for Specially Promoted Research (No. 24000012), by Scientific Research on Innovative Areas “Embodied-brain” (JSPS KAKENHI Grant Number JP26120003), by a Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant Number JP17H02143) to E.N., and by a Grant-in-Aid for Young Scientists (B) to T.M. (JSPS KAKENHI Grant Number 15K21138).
References
- Amemiya K, Morita T, Saito D, Ban M, Shimada K, Okamoto Y, Kosaka H, Okazawa H, Asada M, Naito E. 2016. Maturation of cerebellar afferent and efferent tracts in typically developed brains. 22nd Annual Meeting of the Organization for Huma Brain Mapping (Geneva).
- Amemiya K, Naito E. 2016. Importance of human right inferior frontoparietal network connected by inferior branch of superior longitudinal fasciculus tract in corporeal awareness of kinesthetic illusory movement. Cortex. 78:15–30. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. 2010. White matter development in adolescence: a DTI study. Cereb Cortex. 20:2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, Elliott TK, Gaillard WD. 2002. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol. 59:1168–1174. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S. 1997. The body in the brain: neural bases of corporeal awareness. Trends Neurosci. 20:560–564. [DOI] [PubMed] [Google Scholar]
- Berti A, Bottini G, Gandola M, Pia L, Smania N, Stracciari A, Castiglioni I, Vallar G, Paulesu E. 2005. Shared cortical anatomy for motor awareness and motor control. Science. 309:488–491. [DOI] [PubMed] [Google Scholar]
- Biagi L, Cioni G, Fogassi L, Guzzetta A, Sgandurra G, Tosetti M. 2016. Action observation network in childhood: a comparative fMRI study with adults. Dev Sci. 19:1075–1086. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. 2012. Imaging brain development: the adolescent brain. Neuroimage. 61:397–406. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. 2001. Proprioception from a spinocerebellar perspective. Physiol Rev. 81:539–568. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. 2005. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 9:104–110. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. 2006. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 33:430–448. [DOI] [PubMed] [Google Scholar]
- Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, Zilles K. 2013. Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex. 23:615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. 2010. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 50:1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Allin MPG, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. 2007. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 104:17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignetti F, Fontan A, Menant J, Nazarian B, Anton JL, Vaugoyeau M, Assaiante C. 2017. Protracted development of the proprioceptive brain network during and beyond adolescence. Cereb Cortex. 27:1285–1296. [DOI] [PubMed] [Google Scholar]
- Cignetti F, Vaugoyeau M, Nazarian B, Roth M, Anton JL, Assaiante C. 2014. Boosted activation of right inferior frontoparietal network: a basis for illusory movement awareness. Hum Brain Mapp. 35:5166–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron. 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3:201–215. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. 2009. Movement intention after parietal cortex stimulation in humans. Science. 324:811–813. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D. 1994. An MRI-based probabilistic atlas of neuroanatomy In: Shorvon SD, editor. Magnetic resonance scanning and epilepsy. New York: Plenum Press; p. 263–274. [Google Scholar]
- Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, Perrig W, Steinlin M. 2009. Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Hum Brain Mapp. 30:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan A, Cignetti F, Nazarian B, Anton JL, Vaugoyeau M, Assaiante C. 2017. How does the body representation system develop in the human brain? Dev Cogn Neurosci. 24:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. 1995. Analysis of fMRI time-series revisited. Neuroimage. 2:45–53. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. 2008. Changes in white matter microstructure during adolescence. Neuroimage. 39:52–61. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Hurvitz EA, Brown SH. 2005. Development of upper limb proprioceptive accuracy in children and adolescents. Hum Mov Sci. 24:155–170. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. 1972. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 175:1382–1384. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. 2001. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 13:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagura N, Oouchida Y, Aramaki Y, Okada T, Matsumura M, Sadato N, Naito E. 2009. Visuokinesthetic perception of hand movement is mediated by cerebro-cerebellar interaction between the left cerebellum and right parietal cortex. Cereb Cortex. 19:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht EE, Gutman DA, Bradley BA, Preuss TM, Stout D. 2015. Virtual dissection and comparative connectivity of the superior longitudinal fasciculus in chimpanzees and humans. Neuroimage. 108:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaque I, Renaux-Kieffer V, Van de Moortele PF, Delalande O, Fohlen M, Brunelle F, Le Bihan D. 2002. Late plasticity for language in a child's non-dominant hemisphere. A pre- and post-surgery fMRI study. Brain. 125:361–372. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. 2007. The cortical organization of speech processing. Nat Rev Neurosci. 8:393–402. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS. 2001. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 14:837–843. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, Schmithorst VJ, Yuan W, Plante E, Byars AW. 2007. Functional MRI of language lateralization during development in children. Int J Audiol. 46:533–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. 1998. Generalisability, random effects and population inference. Neuroimage. 7:S754. [Google Scholar]
- Holst-Wolf JM, Yeh IL, Konczak J. 2016. Development of proprioceptive acuity in typically developing children: normative data on forearm position sense. Front Hum Neurosci. 10:438 10.3389/fnhum.2016.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggara BL. 2003. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 19:16–28. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 60:340–352. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieua C. 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Marini F, Squeri V, Morasso P, Campus C, Konczak J, Masia L. 2017. Robot-aided developmental assessment of wrist proprioception in children. J Neuroeng Rehabil. 14:3 10.1186/s12984-016-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro V, Pernigo S, Tsakiris M, Avesani R, Edelstyn NM, Jenkinson PM, Fotopoulou A. 2016. Motor versus body awareness: voxel-based lesion analysis in anosognosia for hemiplegia and somatoparaphrenia following right hemisphere stroke. Cortex. 83:62–77. [DOI] [PubMed] [Google Scholar]
- Morita T, Asada M, Naito E. 2016. a. Contribution of neuroimaging studies to understanding development of human cognitive brain functions. Front Hum Neurosci. 10:464 10.3389/fnhum.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Saito DN, Ban M, Shimada K, Okamoto Y, Kosaka H, Okazawa H, Asada M, Naito E. 2016. b. Development of right inferior front-parietal cortices associated with self-face recognition. 22nd Annual Meeting of the Organization for Huma Brain Mapping (Geneva).
- Morita T, Saito DN, Ban M, Shimada K, Okamoto Y, Kosaka H, Okazawa H, Asada M, Naito E. 2017. Self-face recognition shares brain regions active during proprioceptive illusion in the right inferior frontoparietal superior longitudinal fasciculus III network. Neuroscience. 348:288–301. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, LaMotte RH, Carli G. 1972. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanorecetive afferent nerve fibers innervating monkey hand. J Neurophysiol. 35:122–136. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Herting MM, Maxwell EC, Bruno R, Fair D. 2013. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 82:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH. 2006. Somatic sensation of hand-object interactive movement is associated with activity in the left inferior parietal cortex. J Neurosci. 26:3783–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE. 1999. Illusory arm movements activate cortical motor areas: A positron emission tomography study. J Neurosci. 19:6134–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Matsumoto R, Hagura N, Oouchida Y, Tomimoto H, Hanakawa T. 2011. Importance of precentral motor regions in human kinesthesia: a single case study. Neurocase. 17:133–147. [DOI] [PubMed] [Google Scholar]
- Naito E, Morita T, Amemiya K. 2016. a. Body representations in the human brain revealed by kinesthetic illusions and their essential contributions to motor control and corporeal awareness. Neurosci Res. 104:16–30. [DOI] [PubMed] [Google Scholar]
- Naito E, Morita T, Asada M. 2016. b. Immature cerebro-cerebellar interaction for timing motor control in children. 22nd Annual Meeting of the Organization for Huma Brain Mapping (Geneva).
- Naito E, Nakashima T, Kito T, Aramaki Y, Okada T, Sadato N. 2007. Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur J Neurosci. 25:3476–3487. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Ehrsson HH. 2002. I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron. 36:979–988. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Grefkes C, Choi HJ, Eickhoff S, Geyer S, Zilles K, Ehrsson HH. 2005. Dominance of the right hemisphere and role of area 2 in human kinesthesia. J Neurophysiol. 93:1020–1034. [DOI] [PubMed] [Google Scholar]
- Niessen E, Fink GR, Weiss PH. 2014. Apraxia, pantomime and the parietal cortex. Neuroimage Clin. 5:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Parlatini V, Radua J, Dell'Acqua F, Leslie A, Simmons A, Murphy DG, Catania M, Thiebaut de Schotten M. 2017. Functional segregation and integration within frontoparietal networks. Neuroimage. 146:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. 2009. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 29:11523–11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Szeszko1 PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, et al. 2012. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 38:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redon C, Hay L, Rigal R, Roll JP. 1994. Contribution of the propriomuscular channel to movement coding in children: a study involving the use of vibration-induced kinaesthetic illusion. Hum Mov Sci. 13:95–108. [Google Scholar]
- Roll JP, Vedel JP. 1982. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 47:177–190. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. 1989. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res. 76:213–222. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. 2006. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 9:569–577. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. 2010. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 30:3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AT, Williams AL, Singh KD. 2004. Negative BOLD in the visual cortex: evidence against blood stealing. Hum Brain Mapp. 21:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM. 1999. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 122:2033–2045. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. 2006. a. An fMRI study of language lateralization in children and adults. Hum Brain Mapp. 27:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK. 2006. b. A longitudinal fMRI study of language development in children age 5–11. Ann Neurol. 59:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, Schapiro MB, Plante E, Holland SK. 2012. Left-handedness and language lateralization in children. Brain Res. 1433C:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M. 2011. A lateralized brain network for visuospatial attention. Nat Neurosci. 14:1245–1246. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. 2010. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 49:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J, Geuze RH. 2000. Kinaesthetic acuity in adolescent boys: a longitudinal study. Dev Med Child Neurol. 42:93–96. [DOI] [PubMed] [Google Scholar]
- Willems RM, Van der Haegen L, Fisher SE, Francks C. 2014. On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nat Rev Neurosci. 15:193–201. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. 1995. Analysis of fMRI time-series revisited-again. Neuroimage. 2:173–181. [DOI] [PubMed] [Google Scholar]
- Yuan WH, Szaflarski JP, Schmithorst VJ, Schapiro M, Byars AW, Strawsburg RH, Holland SK. 2006. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 47:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.