Abstract
The onset of motor deficits in parkinsonism is thought to result from dopamine (DA) loss-induced corticostriatal disruption and the development of excessive cortico-basal ganglia synchronization. To gain insights into the mechanisms underlying such corticostriatal dysfunction, we conducted local field potential (LFP) recordings in rats and measured how striatal manipulations of DA, cyclic guanosine monophosphate (cGMP), and gamma-aminobutyric acid- A receptor (GABA-AR) signaling impact corticostriatal transmission at specific oscillatory frequencies. Results indicate that the degree of 6-hydroxydopamine-induced DA lesion and subsequent changes in striatal DA, cGMP, and GABA-AR signaling contribute to impair LFP suppression such that the DA-depleted striatum becomes more permissive to cortically driven oscillations at 10–20 Hz, and to a lesser extent, at 40 Hz. Notably, the corticostriatal dysfunction at 40 Hz emerged only when the degree of chronic DA lesion surpassed 90%, which coincides with the appearance of severe forelimb stepping deficits. Collectively, these results indicate that several mechanisms contribute to suppress LFP within the 10–20 Hz range, yet a critical level of striatal GABAergic activity is required for sustaining corticostriatal inhibition at 40 Hz. Both the degree and chronicity of DA lesion are major contributing factors to the severity of motor and striatal GABAergic deficits that could only be reversed by strengthening local GABA-AR function.
Keywords: basal ganglia, electrophysiology, local field potentials, oscillations, parkinsonism, plasticity
Introduction
Dopamine (DA) loss in Parkinson's disease (PD) and the subsequent development of motor deficits has been associated with the emergence of excessive synchronization of neuronal activity within the cortico-basal ganglia loop (Murer et al. 2002; Brown 2003, 2007; Trottenberg et al. 2006; Hammond et al. 2007; Walters et al. 2007). Such facilitation of synchronized activity has been found to occur primarily within the frequency range of alpha (8–12 Hz) and beta (15–30 Hz) oscillations, typically in association with the onset of motor deficits (Brown 2003; Gatev et al. 2006; Hammond et al. 2007; McCarthy et al. 2011). It has been proposed that these changes in oscillatory activity contribute to disrupt motor function by locking the frequency-dependent modulation of interconnected functional domains in the basal ganglia (Murer et al. 2002; Walters et al. 2007). Yet, the underlying mechanisms that bias the cortico-basal ganglia dynamics into a state of increased synchronization after chronic DA loss remain unclear.
Of particular interest are the DA-dependent disruptions of striatal cyclic-GMP (cGMP) and GABAergic signaling (West and Tseng 2011; Gittis and Kreitzer 2012), two major nondopaminergic mechanisms known to fine-tune the gain of corticostriatal transmission for sustaining proper striatal integration of cortical activity (West and Grace 2004; Mallet et al. 2005; Padovan-Neto et al. 2015). In this regard, the characteristic hyperexcitable and permissive striatal output in response to cortical drive observed following chronic nigrostriatal DA lesion (Tseng et al. 2001) may emerge as a result of DA depletion-induced striatal elevation of cGMP signaling (Chalimoniuk et al. 2004; Tseng et al. 2011) and local impairment of GABAergic inhibition (Mallet et al. 2006; Gittis et al. 2011). It is therefore likely that, in addition to DA deficits, converging dysregulation of local striatal cGMP- and GABA-mediated control of cortical inputs is required to enable a state of frequency-dependent disinhibition of corticostriatal transmission that ultimately contributes to sustain aberrant synchronous activity in the parkinsonian basal ganglia.
Given the above, the goal of the present study is to determine whether local striatal DA, cGMP, and GABA-AR signaling exert distinct roles in the regulation of corticostriatal transmission in vivo. Here, we combined striatal local field potential (LFP) recordings with local infusions of agonists and antagonists to identify the mechanisms by which the healthy/DA-intact and DA-depleted striatum respond differently to cortical activity driven at specific oscillatory frequencies. We used the unilateral 6-hydroxydopamine (6-OHDA) model of experimental parkinsonism (Schwarting and Huston 1996) to assess how chronic DA lesion of the nigrostriatal pathway and subsequent changes in striatal DA, cGMP, and GABA-AR signaling contribute to disrupt the overall pattern of striatal inhibitory control of corticostriatal transmission at 10, 20, and 40 Hz. These frequencies of train stimulation were chosen to represent the different ongoing cortical states often seen in association with changes in motor functions (e.g., Brittain and Brown 2014).
Materials and Methods
All experimental procedures were carried out in adult male Sprague-Dawley rats (Harlan) according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee. In the present study, rats were group-housed (2 per cage) under conditions of constant temperature (21–23°C) and humidity in a 12:12-h light/dark cycle with food and water available ad libitum. All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) except for the selective soluble guanylyl cyclase (sGC) inhibitor 1H-[1,2,4] oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ), which was purchased from Tocris Bioscience (Ellisville, MO, USA).
Unilateral Dopamine Lesion by 6-OHDA, Forelimb Stepping Behavior, and Tyrosine Hydroxylase Immunohistochemistry
Male adult rats (250–280 g) were deeply anesthetized using isoflurane vapor (2–5%) and randomly assigned to receive a microinfusion of either vehicle (0.1% ascorbic acid; sham group) or 6-OHDA (8 μg/4 μL of vehicle) into the medial forebrain bundle as previously described (Tseng et al. 2011). In the present study, the forelimb stepping test was used to evaluate the degree of DA lesion at 6 weeks postsurgery (Olsson et al. 1995; Tseng et al. 2005). Typically, when a rat is moved with one paw touching the surface of the table (1 m in length), 10–12 adjustment steps are made. Following unilateral 6-OHDA lesion, the number of forelimb adjustments made by the contralateral paw decreases to <3 steps when the degree of DA lesion in the substantia nigra (SN) is >90% (Tseng et al. 2005, 2011). Here, the number of tyrosine hydroxylase (TH)-positive neurons estimated by means of unbiased stereology (Stereo Investigator, MBF Biosciences, VT, USA) was used to determine the level of DA lesion in the SN (6 rostrocaudal levels at 200-µm interval from bregma −5.0 to −6.2) as previously described (Zhang et al. 2013). Briefly, rats were transcardially perfused with ice-cold saline and 4% paraformaldehyde (PFA) at the end of the recording session, the brains removed and postfixed in 4% PFA for 24 h before transferring them into 30% sucrose solution for 72 h. Coronal sections (50 µm thick) containing the SN were obtained using a freezing microtome (SM-2000R, Leica Microsystems) and incubated in rabbit anti-TH (1:2000; Pel-Freez Biologicals, AR, USA) for 48 h followed by 2-h incubation in biotinylated goat anti-rabbit IgG (1:500; Vector Laboratories, CA, USA). The bound complexes were then visualized with 3,3′-diaminobenzidine and hydrogen peroxide tablets as previously described (Tseng et al. 2011).
In Vivo Recordings of Corticostriatal LFP Responses
All recordings of striatal LFP responses evoked by cortical train stimulation were conducted following the same experimental procedure as described previously (Thomases et al. 2013), typically within the 7th to 8th week post-6-OHDA or sham lesion. Briefly, rats were deeply anesthetized with 8% chloral hydrate (400 mg/kg i.p.), placed in a stereotaxic apparatus (ASI instruments, MI, USA), and maintained at 37–38°C (TCAT-2LV, Physitemp Instruments). Anesthesia supplements (8% chloral hydrate, 400 μL/h) were then delivered and maintained throughout the entire recording session using an i.p. cannula (26G) attached to a syringe minipump (BASi Baby Bee Syringe Drives). Burr holes were then drilled in the skull for electrode placement in the motor cortex (B: +3.5 mm; L: 2.0 mm; V: 2.0 mm from the cortical surface) and striatum (B: +0.7 mm; L: 3.4 mm; V: 4.5 mm from the cortical surface) as previously described (Tseng et al. 2011). All striatal LFP recordings were obtained using a concentric bipolar electrode (SNE-100X 50 mm; Rhodes Medical Instruments, Inc.), amplified (gain: 500; Cygnus Technology, Inc.), filtered (bandwidth: 1–100 Hz), and digitized (Digidata 1440A; Molecular Devices) at a sampling rate of 10 kHz. A second concentric bipolar electrode (NE-100X 50 mm; Rhodes Medical Instruments, Inc.) was placed in the motor cortex for stimulation. Both single and trains of electrical pulses (300-μs square pulses) were delivered every 15 s through a computer-controlled pulse generator (Master-8 Stimulator, AMPI). Given the ongoing slow-wave corticostriatal oscillations (often referred as up-down transitions), any sweeps of cortically evoked LFP were excluded for analyses if the onset of the first-evoked LFP response coincided precisely to a suspected up or down state as determined by the 150-ms trajectory of the slow-wave oscillation preceding the onset of the first pulse. Data from our current sampling indicate that the onset of the first LFP response for all sweeps of cortically evoked LFP included in the present study occurred within the −0.6 to +0.6 mV range surrounding the 0 mV value (i.e., transition zone). In each experiment, the intensity of stimulation was chosen from the minimal current within the 0.25–1.0 mA range needed to evoke a reliable striatal LFP with <15% variability in amplitude and slope, and an average onset latency within the 9–12 ms range. Typically, a current intensity within 0.6–0.75 mA is required to evoke such a corticostriatal LFP response. Each set of trains is comprised of 10 pulses delivered at 10, 20, and 40 Hz. The pattern of the evoked response at a given frequency was estimated by averaging the amplitude (from the onset to the peak response) of each of the evoked LFP obtained from 10 to 15 sweeps of train stimulation and normalized to the first-evoked response. Pilot studies conducted from both controls and DA-depleted rats revealed that this pattern of train stimulation (i.e., pulses/train, intertrain interval, number of repetitions) does not elicit enduring changes in cortical excitability as determined by LFP recordings. The exact locations of the electrode placements were determined at the end of the recording session by means of Nissl staining as previously described (Thomases et al. 2013).
Local Striatal Microinfusions of Agonists and Antagonists
All microinfusion procedures were performed following the same experimental design used in Thomases et al. (2013). Briefly, a 28G stainless steel cannula was attached to the recording electrode with an offset of 0.7 mm dorsally from the tip of the electrode. Before lowering the recording electrode, the cannula was filled with artificial cerebrospinal fluid (aCSF)-containing vehicle [0.1% dimethyl sulfoxide (DMSO)] SCH23390 (10 μM), eticlopride (20 μM), SKF38393 (5 μM), quinpirole (5 μM), picrotoxin (50–100 μM/0.1% DMSO), indiplon (5 μM), 8-bromoguanosine 3′5′-cyclic monophosphate sodium salt (8-Br-cGMP; 20 nM), or ODQ (50 μM/0.1% DMSO). The chemical composition of the aCSF solution is (in mM): 122.5 NaCl, 3.5 KCl, 25 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1 MgCl2, 20 glucose, 1 ascorbic acid (pH: 7.40, 295–305 mOsm). All microinfusions (1 μL) were performed at a rate of 0.1 μL/min and changes in cortically evoked striatal LFP were determined within the 15–40-min postinfusion period.
Statistical Analysis
Data were summarized as mean ± standard error of the mean and differences among experimental groups were considered statistically significant at P < 0.05. Student's t-test was used for two-group comparison involving a single continuous variable, whereas one-way and two-way ANOVA were applied for determining the significant effects along 3 or more variables (StatSoft, Tulsa, OK, USA). The Kruskal–Wallis ANOVA by ranks was preferred for datasets that were not normally distributed or had unequal variances. Normality and homogeneity of variances were determined by the Kolmogorov–Smirnov and Levene's tests, respectively.
Results
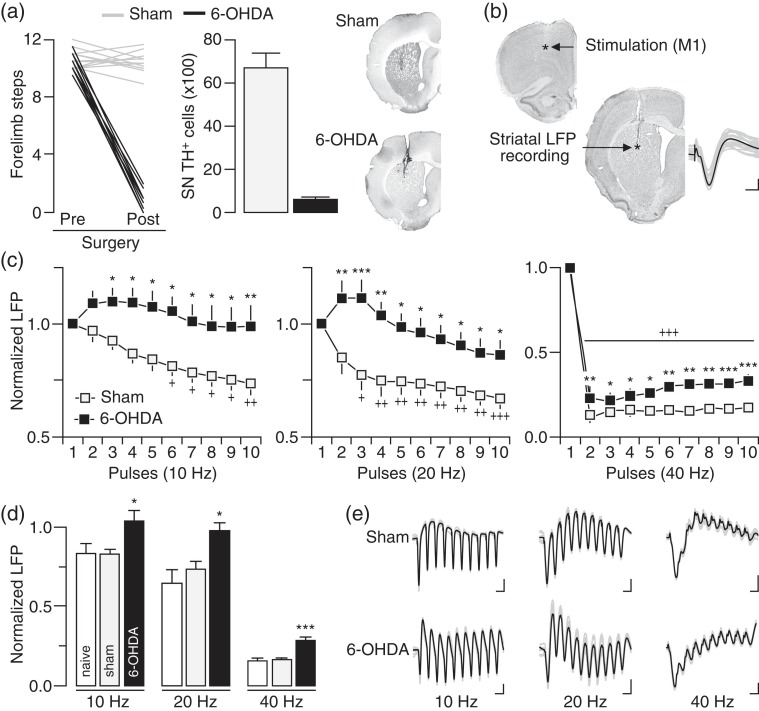
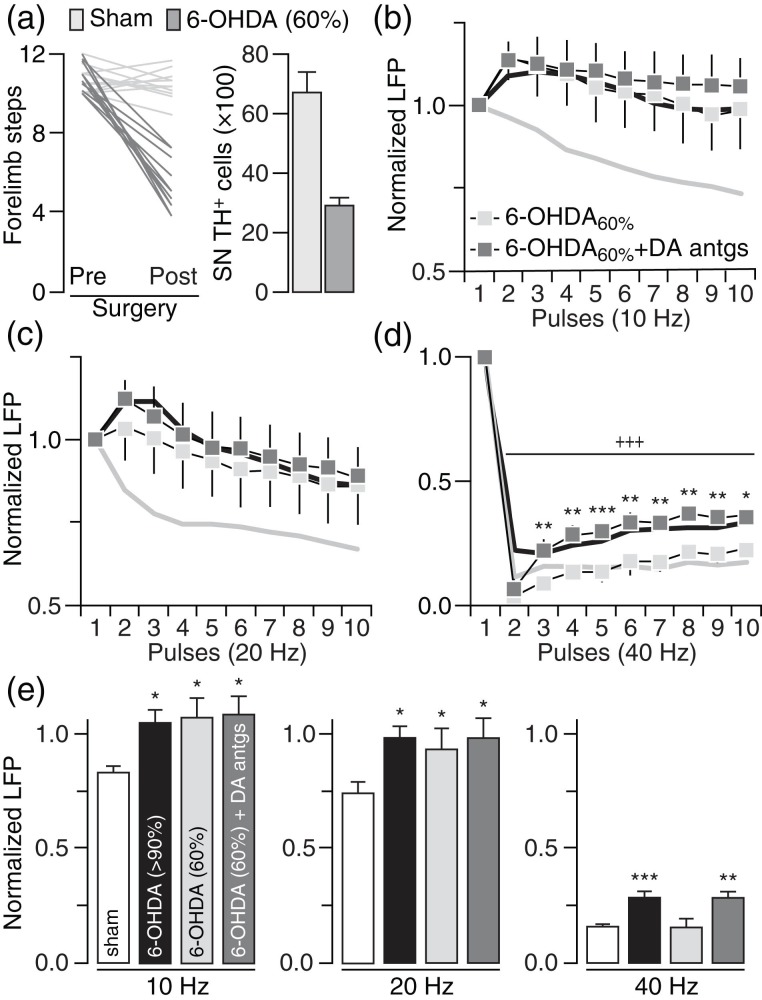
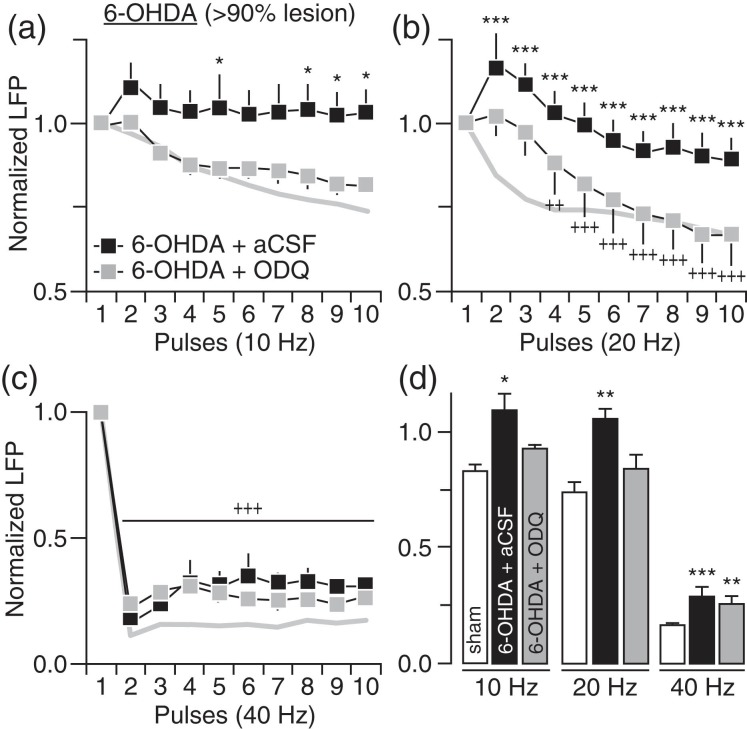
We first determined if chronic lesion of the nigrostriatal DA pathway disrupts corticostriatal transmission in a frequency-dependent manner in vivo (Fig. 1). Typically, a significant stepping deficit emerges in the contralateral paw (i.e., <3 steps) of the 6-OHDA infusion side when the degree of DA lesion in the SN is >90% (Fig. 1a). Using LFP recordings and cortical train stimulation (Fig. 1b), we found that the normal pattern of cortically evoked striatal LFP suppression observed in sham controls is lacking or attenuated in rats with a DA lesion (Fig. 1c) despite that the intensity of cortical stimulation (sham: 0.68 ± 0.03 mA; 6-OHDA: 0.67 ± 0.03 mV) and the amplitude of the first striatal response (sham: 5.35 ± 0.47 mV; 6-OHDA: 5.42 ± 0.31 mV) were similar among sham and 6-OHDA-treated rats. While cortical stimulation at 10 and 20 Hz elicited a pattern of incremental attenuation of LFP responses in the DA-intact striatum of sham animals, a lack of LFP suppression emerged in the DA-depleted striatum of 6-OHDA-treated rats (Fig. 1c–e). At 40 Hz, cortical train stimulation markedly suppressed the amplitude of striatal LFP in both sham and 6-OHDA rats (Fig. 1c). While the overall pattern of striatal response at 40 Hz remains largely unaffected by the DA lesion, a significant attenuation of the LFP inhibition emerged in the DA-depleted striatum of 6-OHDA-treated rats (Fig. 1c–e). We next asked whether the degree of nigrostriatal DA lesion (and striatal DA) contributes to the development of striatal LFP disinhibition. Toward this goal, we conducted recordings from rats that received 6-OHDA, but failed to show >90% DA cell loss in the SN (Fig. 2a). Results revealed a similar pattern of striatal LFP disinhibition following ∼60% DA cell loss that resembles that seen in the >90% DA lesion group, especially, in response to cortical drive at 10 and 20 Hz (Fig. 2b,c). However, the magnitude of striatal LFP suppression at 40 Hz in the ∼60% DA lesion group was indistinguishable from sham controls (Fig. 2d). It was only after the infusion of DA D1 and D2 receptor antagonists (10 µM SCH23390 + 20 µM eticlopride) into the partially DA-depleted striatum that the attenuated LFP inhibition at 40 Hz became apparent (Fig. 2d,e). Together, these results indicate that the degree of striatal LFP inhibition resulting from cortical stimulation is frequency-dependent and highly sensitive to the level of striatal DA depletion.
Figure 1.
Disinhibition of striatal local field potential (LFP) response to cortical train stimulation following chronic nigrostriatal DA lesion. (a) Forelimb stepping test assessed presurgery and 6 weeks postsurgery revealed that 6-OHDA injection into the medial forebrain bundle (n = 13) resulted in marked stepping deficits in the contralateral forelimb and >90% TH-positive (DA) cell loss in the ipsilateral substantia nigra (SN) relative to sham controls (n = 11). Insets show examples of TH staining illustrating the extent of DA lesion in the striatum. (b) Coronal sections of Nissl staining showing the location of the stimulating and recording electrodes. Inset shows traces of cortically evoked striatal LFP responses (calibration: 2 mv/25 ms). (c) Cortical train stimulation typically suppresses striatal LFP in sham controls. This inhibitory response was lacking or attenuated following DA lesion as revealed by two-way ANOVA and significant main effects of treatment at 10 Hz (F1,220 = 49.8, P < 0.0005), 20 Hz (F1,220 = 62.3, P < 0.0005), and 40 Hz (F1,220 = 71.4, P < 0.0005). Post hoc analyses showed a lack of LFP inhibition at 10 and 20 Hz, and an attenuated LFP inhibition at 40 Hz in the 6-OHDA group relative to sham controls (+P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, *P < 0.05/**P < 0.005/***P < 0.0005 vs. sham, LSD post hoc test). (d) Summary of the mean LFP response calculated from pulses 2 to 10 (*P < 0.05/***P < 0.0005 vs. sham or naïve, LSD post hoc test, one-way ANOVA; 10 Hz: F2,27 = 4.2, P < 0.05; 20 Hz: F2,27 = 5.4, P < 0.05; 40 Hz: F2,27 = 14.1, P < 0.0005). (e) Traces of cortically evoked striatal LFP illustrating the effects of 6-OHDA lesion (calibration: 2 mV/100 ms at 10 Hz, 2 mV/50 ms at 20 Hz, 2 mV/25 ms at 40 Hz).
Figure 2.
The degree of nigrostriatal DA lesion determines the level of striatal LFP disinhibition. (a) Cohort of 6-OHDA-treated rats (n = 12) with 60% TH-positive cell loss in the SN and moderate forelimb stepping deficits. The mean forelimb steps and TH-positive cell count from sham controls were included for comparison. (b) Relative to sham controls (gray line), a pattern of striatal LFP disinhibition was observed in the 60% DA lesion group at 10 Hz (6-OHDA60%; n = 7; main effect of treatment: F1,160 = 47.1, P < 0.0005, two-way ANOVA) similar to that seen in rats with >90% DA lesion (black line). Striatal infusion of D1 (10 µM SCH23390) + D2 (20 µM eticlopride) receptor antagonists (6-OHDA60%+ DA antgs; n = 5) failed to further change the LFP disinhibition. (c) Similarly, a pattern of LFP disinhibition was found in the striatum of 60% DA lesion rats at 20 Hz (main effect of treatment: F1,160 = 22.9, P < 0.0005, two-way ANOVA, sham vs. 6-OHDA60%), a disruption that remained unchanged following infusion of DA antagonists. (d) At 40 Hz, the magnitude of LFP inhibition recorded in the striatum of rats with 60% DA lesion was similar to that of sham controls. However, the inclusion of DA antagonists significantly attenuated the level of LFP inhibition (main effect of treatment: F1,100 = 71.7, P < 0.0005) in the 60% DA lesion group (*P < 0.05/**P < 0.005/***P < 0.0005 vs. 6-OHDA60%, +++P < 0.0005 vs. 1st pulse LSD post hoc test, two-way ANOVA) to the same degree seen in rats with >90% DA lesion. (e) Summary of the mean LFP response calculated from pulses 2 to 10 (*P < 0.05/**P < 0.005/***P < 0.0005 vs. sham, LSD post hoc test, one-way ANOVA; 10 Hz: F3,32 = 3.0, P < 0.05; 20 Hz: F3,32 = 2.9, P < 0.05; 40 Hz: F3,32 = 11.1, P < 0.0005).
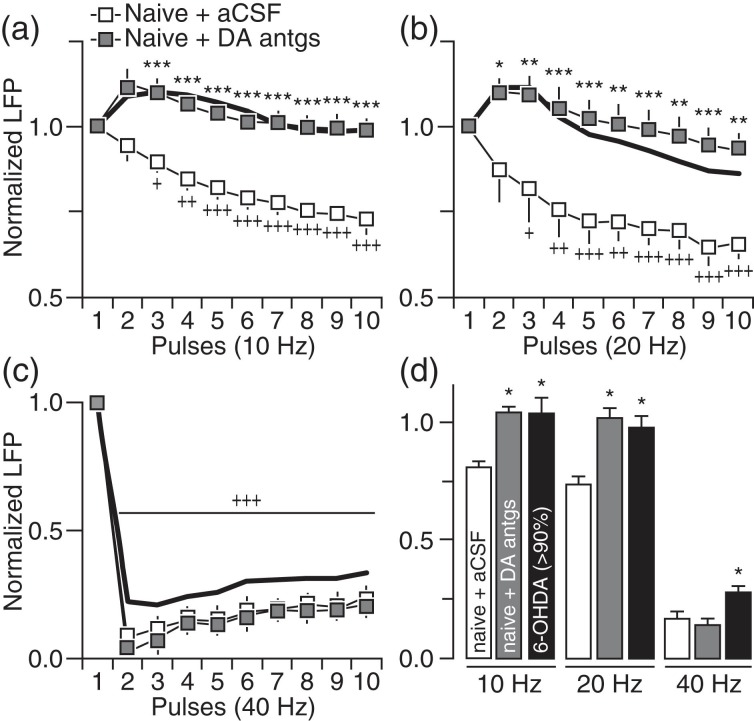
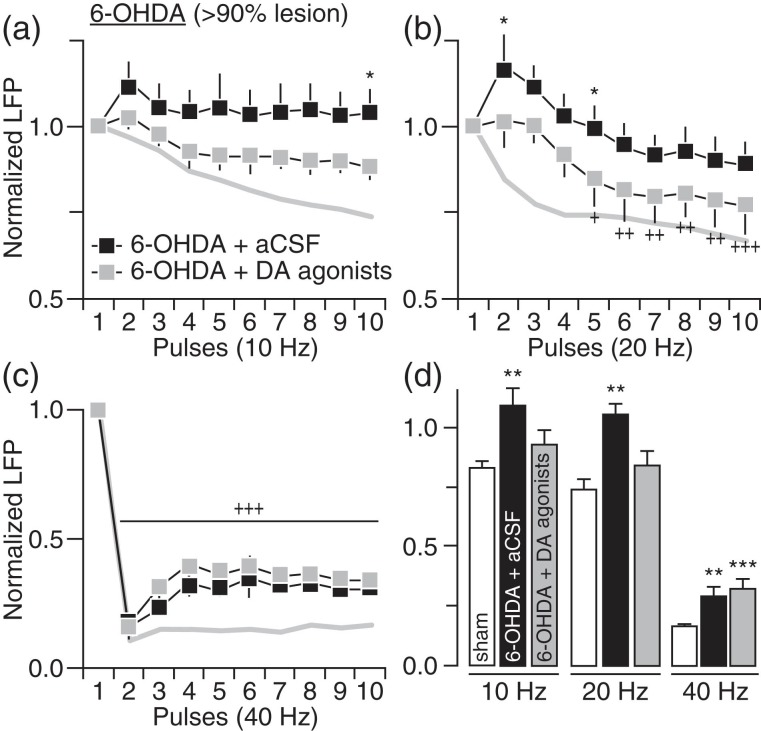
We next asked whether the pattern of striatal LFP suppression observed following chronic DA lesion can be reproduced acutely by direct blockade of striatal DA transmission in adult naïve rats. Using local infusion of DA antagonists, we found that acute striatal blockade of D1 (10 µM SCH23390) + D2 (20 µM eticlopride) receptors was sufficient to induce a pattern of attenuated LFP suppression at 10 and 20 Hz resembling that seen in the 6-OHDA group (Fig. 3a,b). However, DA receptor blockade failed to attenuate striatal LFP inhibition at 40 Hz (Fig. 3c,d). Similarly, acute infusion of D1 (5 µM SKF38393) + D2 (5 µM quinpirole) receptor agonists directly into the DA-depleted striatum of 6-OHDA-treated rats significantly increased the level of LFP suppression at 10 and 20 Hz (Fig. 4a,b) without normalizing the response at 40 Hz (Fig. 4c,d). These results in conjunction with those shown in Figure 3 emphasize a critical role of striatal DA in regulating corticostriatal transmission at 10 and 20 Hz, and highlight the possibility that a nondopaminergic mechanism underlies the LFP suppression at 40 Hz.
Figure 3.
Striatal blockade of DA receptors in naïve rats failed to fully induce the pattern of LFP disinhibition observed in 6-OHDA-treated rats. (a) Relative to aCSF (naïve + aCSF; n = 8), striatal infusion of D1 + D2 receptor antagonists (10 µM SCH23390 + 20 µM eticlopride) in naïve rats (naïve + DA antgs; n = 7) was sufficient to induce a pattern of LFP disinhibition at 10 Hz (main effect of treatment: F1,130 = 192.7, P < 0.0005; ***P < 0.0005 vs. naïve + aCSF, +P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, LSD post hoc test, two-way ANOVA) resembling that seen in 6-OHDA rats with >90% DA lesion (black line). (b) Similarly, a pattern of LFP disinhibition emerged at 20 Hz following striatal infusion of DA antagonists (main effect of treatment: F1,130 = 97.5 P < 0.0005; *P < 0.05/**P < 0.005/***P < 0.0005 vs. naïve + aCSF, +P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, LSD post hoc test, two-way ANOVA). (c) However, striatal infusion of DA antagonists failed to alter the pattern of LFP inhibition at 40 Hz in naïve rats (+++P < 0.0005 vs. 1st pulse, LSD post hoc test after significant main effect of pulse, F9,130 = 177.0, P < 0.0005, two-way ANOVA). (d) Summary of the mean LFP response calculated from pulses 2 to 10 (*P < 0.05 vs. naïve + aCSF, LSD post hoc test, one-way ANOVA; 10 Hz: F2,25 = 4.8, P < 0.05; 20 Hz: F2,25 = 5.4, P < 0.05; 40 Hz: F2,25 = 7.2, P < 0.005).
Figure 4.
Striatal infusion of D1 + D2 receptor agonists in 6-OHDA-treated rats partially rescued the pattern of striatal LFP inhibition. (a) Relative to aCSF (6-OHDA + aCSF; n = 6), striatal infusion of D1 + D2 receptor agonists (5 µM SKF38393 + 5 µM quinpirole) in 6-OHDA rats with >90% DA lesion (6-OHDA + DA agonists; n = 6) significantly increased the level of LFP inhibition at 10 Hz (main effect of treatment: F1,100 = 20.3, P < 0.0005; *P < 0.05 vs. 6-OHDA + aCSF, LSD post hoc test). The pattern of LFP inhibition from sham controls (gray line) was included for comparison. (b) Two-way ANOVA also revealed a significant increase in LFP inhibition at 20 Hz following striatal infusion of DA agonists (main effect of treatment: F1,100 = 25.1, P < 0.0005; +P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, *P < 0.05 vs. 6-OHDA + aCSF, LSD post hoc test). (c) At 40 Hz however, striatal infusion of DA agonists failed to normalize the level of LFP suppression in 6-OHDA-treated rats (+++P < 0.0005 vs. 1st pulse, LSD post hoc test after significant main effect of pulse, F9,100 = 49.1, P < 0.0005, two-way ANOVA). (d) Summary of the mean LFP response calculated from pulses 2 to 10 (**P < 0.005/***P < 0.0005 vs. sham, LSD post hoc test, one-way ANOVA; 10 Hz: F2,20 = 5.2, P < 0.05, 20 Hz: F2,20 = 5.2, P < 0.05, 40 Hz: F2,20 = 16.4, P < 0.0005).
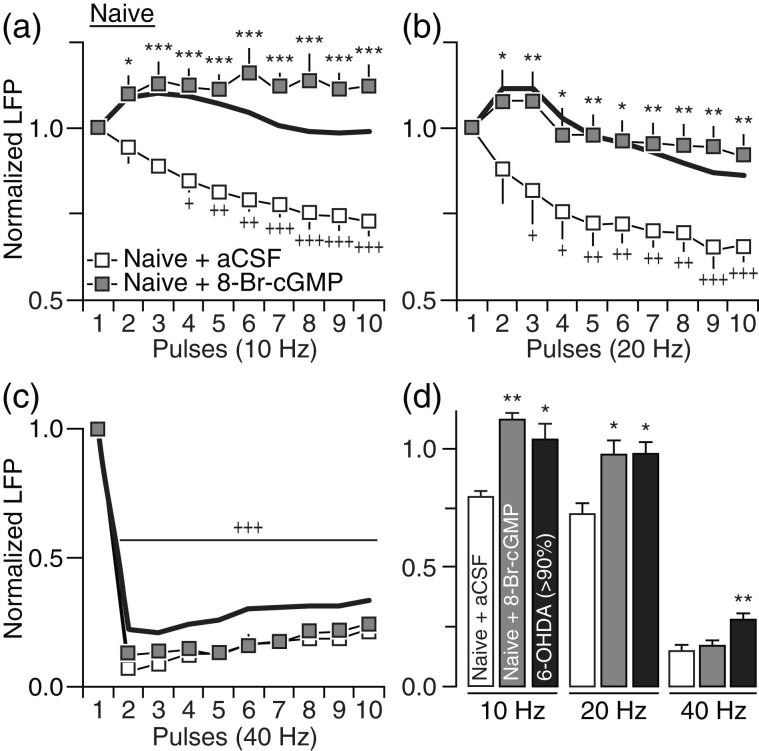
The above findings prompted us to examine nondopaminergic mechanisms underlying the altered striatal LFP response triggered by chronic DA lesion. Of particular interest is the sGC-cGMP signaling pathway, which has been repeatedly shown to be abnormally upregulated in the DA-depleted striatum and is thought to contribute to the enhanced corticostriatal transmission observed following chronic DA lesion (Chalimoniuk et al. 2004; Tseng et al. 2011). We first tested the impact of elevating striatal cGMP levels in adult naïve rats by locally infusing the cGMP analog 8-Br-cGMP (20 nM) and found that only the response pattern at 10 and 20 Hz became disinhibited (Fig. 5a,b). However, the normal suppression of the striatal LFP response at 40 Hz remained unaltered following administration of 8-Br-cGMP (Fig. 5c,d). Accordingly, infusion of the sGC inhibitor ODQ directly into the DA-depleted striatum of 6-OHDA-treated rats failed to normalize the LFP response at 40 HZ despite that it effectively restored that pattern of LFP inhibition at 10 and 20 Hz (Fig. 6). Collectively, these results indicate that the disruption of LFP suppression observed in 6-OHDA-treated rats at 10–20 Hz is causally linked to DA lesion-induced striatal elevations in cGMP levels. However, a contribution of striatal cGMP signaling in modulating corticostriatal transmission at 40 Hz is not apparent.
Figure 5.
Striatal infusion of 8-Br-cGMP in naïve rats failed to fully induce the pattern of LFP disinhibition observed in 6-OHDA-treated rats. (a) Relative to aCSF infusion (naïve + aCSF; n = 8), striatal elevation of cGMP via infusion of 20 nM 8-Br-cGMP in naïve rats (naïve + 8-Br-cGMP; n = 8) resulted in marked disinhibition of LFP at 10 Hz (main effect of treatment: F1,140 = 223.4, P < 0.0005; *P < 0.05/***P < 0.0005 vs. naïve + aCSF, +P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, LSD post hoc test, two-way ANOVA) resembling the pattern seen in rats with >90% DA lesion (black line). (b) A similar LFP disinhibition was observed at 20 Hz following striatal infusion of 8-Br-cGMP in naïve rats (F1,140 = 64.4, P < 0.0005; +P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, *P < 0.05/**P < 0.005 vs. naïve + aCSF, LSD post hoc, two-way ANOVA). (c) However, striatal infusion of 8-Br-cGMP in naïve rats failed to alter the pattern LFP inhibition at 40 Hz (+++P < 0.0005 vs. 1st pulse, LSD post hoc test after significant main effect of pulse: F9,140 = 195.6, P < 0.0005, two-way ANOVA). (d) Summary of the mean LFP responses calculated from pulses 2 to 10 (*P < 0.05/**P < 0.005 vs. naïve + aCSF, LSD post hoc test, one-way ANOVA; 10 Hz: F2,26 = 6.6, P < 0.005, 20 Hz: F2,26 = 4.6, P < 0.05, 40 Hz: F2,26 = 9.7, P < 0.005).
Figure 6.
Blockade of striatal sGC-cGMP signaling in 6-OHDA-treated rats partially rescued the pattern of striatal LFP inhibition. (a) Relative to aCSF (6-OHDA + aCSF; n = 6), infusion of the sGC inhibitor ODQ (50 μM) into the DA-depleted striatum of 6-OHDA-treated rats (6-OHDA + ODQ; n = 7) significantly increased the level of LFP inhibition at 10 Hz (main effect of treatment: F1,100 = 32.5, P < 0.0005; *P < 0.05 vs. 6-OHDA + aCSF, LSD post hoc test, two-way ANOVA). The pattern of LFP inhibition from sham controls (gray line) was included for comparison. (b) Two-way ANOVA also revealed a marked enhancement of LFP inhibition at 20 Hz following striatal infusion of ODQ in 6-OHDA-treated rats (main effect of treatment: F1,100 = 164.8, P < 0.0005; ***P < 0.0005 vs. aCSF, ++P < 0.005/+++P < 0.0005 vs. 1st pulse, LSD post hoc test) resembling the pattern of inhibition seen in sham controls (gray line). (c) However, striatal infusion of ODQ failed to normalize the level of LFP suppression observed in 6-OHDA-treated rats at 40 Hz (+++P < 0.0005 vs. 1 pulse, LSD post hoc test after significant main effect of pulse: F9,100 = 35.3, P < 0.0005, two-way ANOVA). (d) Summary of the mean LFP response calculated from pulses 2 to 10 (*P < 0.05/**P < 0.005/***P < 0.0005 vs. sham, LSD post hoc test, one-way ANOVA; 10 Hz: F2,22 = 4.5, P < 0.05, 20 Hz: (2,22) = 6.2, P < 0.005, 40 Hz: F2,22 = 14.5, P < 0.0005).
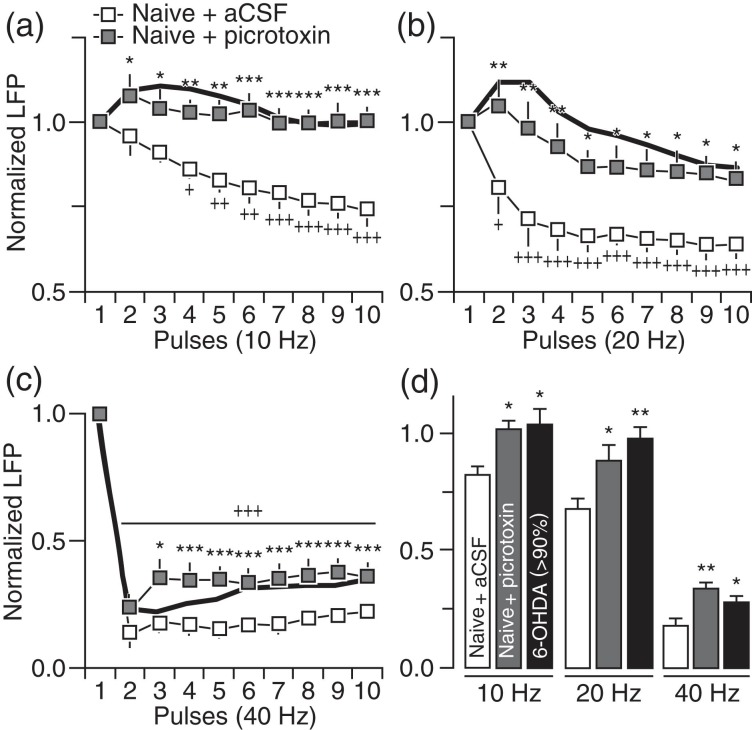
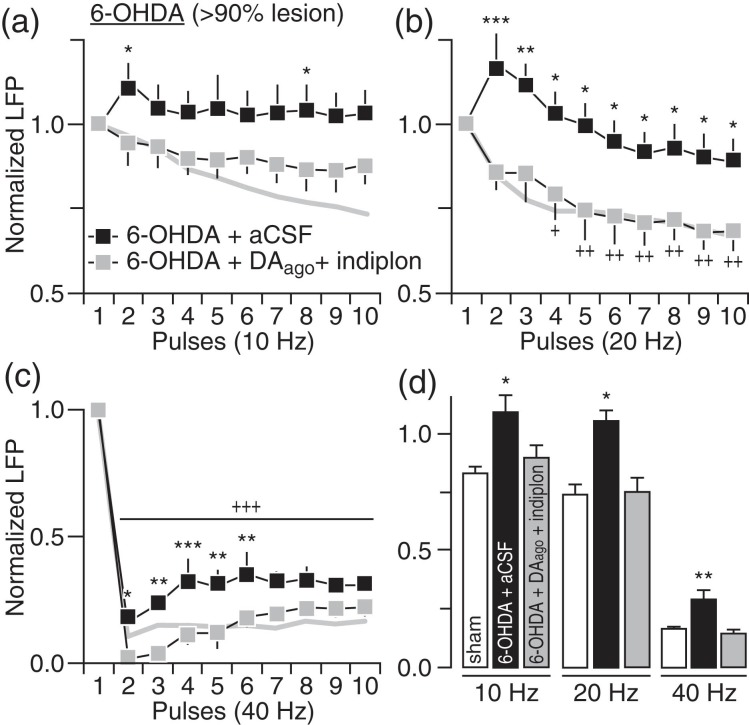
In addition to striatal DA and cGMP signaling, local GABAergic transmission and its regulation of feedforward inhibition of corticostriatal responses is also impaired in the DA-depleted striatum (Mallet et al. 2006). Thus, changes in striatal LFP suppression induced following local infusion of the GABA-AR antagonist picrotoxin were determined in naïve rats to assess the contribution of striatal GABAergic deficits in mediating the pattern of LFP disruption observed in 6-OHDA-treated rats. Striatal infusion of picrotoxin (50–100 μM) effectively disrupted corticostriatal transmission such that the patterns of LFP response at 10, 20, and 40 Hz resemble those seen in the DA-depleted striatum (Fig. 7). We next tested whether the inclusion of the GABA-AR-positive allosteric modulator indiplon into the DA-depleted striatum could supplement the effects of DA agonists in restoring the pattern of LFP inhibition in 6-OHDA-treated rats. Results show that co-infusions of indiplon (5 μM) with the DA agonists fully normalized the pattern of striatal LFP response including that observed at 40 Hz (Fig. 8). Notably, we also observed that indiplon alone was sufficient to increase the level of LFP inhibition in the DA-depleted striatum of 6-OHDA-treated rats to such extent that the resulting patterns of corticostriatal response at 10, 20, and 40 Hz fully resemble those observed in sham controls (Fig. 9). Together, the above findings converge to indicate that, while several striatal mechanisms contribute to enable LFP suppression at 10 and 20 Hz, an exclusive role for local GABAergic transmission is required for sustaining corticostriatal inhibition at 40 Hz.
Figure 7.
Blockade of striatal GABA-A receptors in naïve rats fully reproduced the pattern of LFP disinhibition induced by chronic DA lesion. (a) Relative to aCSF (naïve + aCSF; n = 8), striatal infusion of the GABA-A receptor antagonist picrotoxin (100 μM) in naïve rats (naïve + picrotoxin; n = 8) resulted in LFP disinhibition at 10 Hz (main effect of treatment: F1,140 = 104.2, P < 0.0005; *P < 0.05/**P < 0.005/***P < 0.0005 vs. naïve + aCSF, +P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, LSD post hoc test, two-way ANOVA) that resembles the response pattern seen in rats with >90 DA lesion (black line). (b) A similar LFP disinhibition was observed at 20 Hz following striatal infusion of picrotoxin in naïve rats (main effect of treatment: F1,140 = 67.4, P < 0.0005; *P < 0.05/**P < 0.005 vs. naïve + aCSF, +P < 0.05/++P < 0.005/+++P < 0.0005 vs. 1st pulse, LSD post hoc test, two-way ANOVA). (c) Striatal infusion of picrotoxin in naïve rats also resulted in marked attenuation of the evoked LFP inhibition at 40 Hz (main effect of treatment: F1,140 = 109.1, P < 0.0005; *P < 0.05/***P < 0.0005 vs. naïve + aCSF, +++P < 0.0005 vs. 1st pulse, LSD post hoc test, two-way ANOVA). (d) Summary of the mean LFP response calculated from pulses 2 to 10 (*P < 0.05/**P < 0.005 vs. naïve + aCSF, LSD post hoc test, one-way ANOVA; 10 Hz: F2,26 = 4.0, P < 0.05, 20 Hz: F2,26 = 6.5, P < 0.005, 40 Hz: F2,26 = 9.3, P < 0.005).
Figure 8.
Striatal infusion of DA agonists + indiplon fully normalized the pattern of LFP response in the DA-depleted striatum of 6-OHDA-treated rats. (a) Relative to aCSF (6-OHDA + aCSF; n = 6), co-infusion of DA agonists (same as in Fig. 4) + indiplon (5 μM) into the DA-depleted striatum (6-OHDA + DAago+ indiplon; n = 7) significantly increased the level of LFP inhibition at 10 Hz (main effect of treatment: F1,110 = 25.5, P < 0.0005; *P < 0.05 vs. 6-OHDA + aCSF, LSD post hoc test, two-way ANOVA). The pattern of LFP inhibition from sham controls (gray line) was included for comparison. (b) The magnitude of LFP inhibition at 20 Hz was also increased following DA agonists + indiplon co-infusion into the DA-depleted striatum (main effect of treatment: F1,110 = 55.7, P < 0.0005; +P < 0.05/++P < 0.005 vs. 1st pulse, *P < 0.05/**P < 0.005/***P < 0.0005 vs. 6-OHDA + aCSF, LSD post hoc test, two-way ANOVA) such that it closely resembled the pattern of inhibition observed in sham controls (gray line). (c) Notably, co-infusion of DA agonists + indiplon into the DA-depleted striatum fully normalized the level of LFP suppression at 40 Hz (main effect of treatment: F1,110 = 50.0, P < 0.0005; +++P < 0.005 vs. 1st pulse, *P < 0.05/**P < 0.005/***P < 0.0005 vs. 6-OHDA + aCSF, LSD post hoc test, two-way ANOVA). (d) Summary of the mean LFP response calculated from pulses 2 to 10 (*P < 0.05/**P < 0.005 vs. sham, LSD post hoc test, one-way ANOVA; 10 Hz: F2,21 = 4.4, P < 0.05, 20 Hz: F2,21 = 5.1, P < 0.05, 40 Hz: F2,21 = 11.7, P < 0.005).
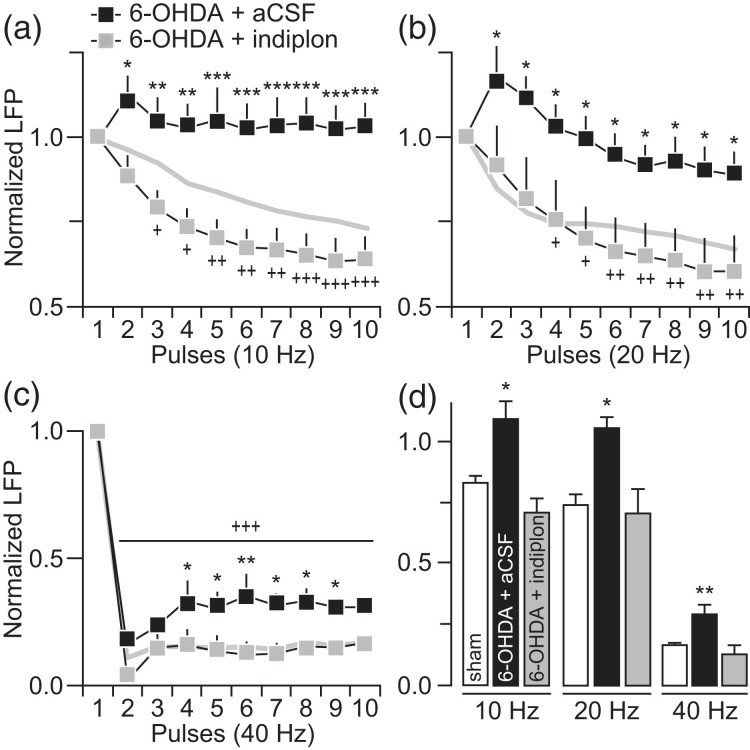
Figure 9.
Striatal infusion of indiplon alone effectively increased the magnitude of striatal LFP inhibition in the DA-depleted striatum to sham control levels. (a) Relative to aCSF (6-OHDA + aCSF; n = 6), infusion of indiplon alone (5 μM) into the DA-depleted striatum of 6-OHDA-treated rats (6-OHDA + indiplon; n = 5) was sufficient to restore the normal pattern of LFP inhibition at 10 Hz (main effect of treatment: F1,90 = 112.8, P < 0.0005; +P < 0.05/++P < 0.005/+++P < 0.005 vs. 1st pulse, *P < 0.05/**P < 0.005/ ***P < 0.0005 vs. aCSF, LSD post hoc test, two-way ANOVA). The pattern of LFP inhibition from sham controls (gray line) was included for comparison. (b) A similar restoration of the LFP response to sham control levels (gray line) was observed at 20 Hz following striatal infusion of indiplon alone (main effect of treatment: F1,90 = 50.8, P < 0.0005; +P < 0.05/++P < 0.005 vs. 1st pulse, *P < 0.05 vs. 6-OHDA + aCSF, LSD post hoc test after significant two-way ANOVA). (c) At 40 Hz, the infusion of indiplon sufficiently increased the magnitude of striatal LFP inhibition (main effect of treatment: F1,90 = 37.6, P < 0.0005, +++P < 0.005 vs. 1st pulse, *P < 0.05/**P < 0.005 vs. 6-OHDA + aCSF, LSD post hoc test after significant two-way ANOVA) resembling that seen in sham controls (gray line). (d) Summary of the mean LFP response calculated from pulses 2 to 10 (*P < 0.05/**P < 0.005 vs. sham, LSD post hoc test, one-way ANOVA; 10 Hz: F2,19 = 8.1, P < 0.005, 20 Hz: F2,19 = 4.7, P < 0.05, 40 Hz: F2,19 = 8.5, P < 0.005).
Discussion
In the present study, we found that both dopaminergic and nondopaminergic mechanisms are required to sustain the frequency-dependent incremental suppression of cortically evoked striatal LFP in vivo. This inhibitory regulation of striatal LFP is highly sensitive to disruptions resulting from chronic lesion of the nigrostriatal DA pathway such that the magnitude of LFP suppression is markedly diminished in the DA-depleted striatum of 6-OHDA-treated rats. Results indicate that the degree of DA lesion and subsequent changes in striatal DA, cGMP, and GABA-AR signaling contribute to impair the normal pattern of LFP suppression at 10 and 20 Hz. However, an attenuation of LFP inhibition at 40 Hz emerged only when the degree of chronic DA lesion surpassed 90%, and is solely manifested upon a downregulation of striatal GABAergic function. Hence, several mechanisms contribute to the normal pattern of LFP suppression within the 10–20 Hz range, yet a critical level of striatal GABAergic activity has to be recruited for sustaining proper regulation of corticostriatal inhibition at 40 Hz.
It is generally accepted that the onset and degree of motor deficits in PD are positively correlated with the degree of nigrostriatal DA lesion and striatal DA depletion (Ma et al. 1997; Lindner et al. 1999). To what extent the magnitude of such dysregulation results from a disruption of corticostriatal processing remains unclear. Data from the present study indicate that a critical level of striatal DA is needed to sustain proper inhibitory control of corticostriatal transmission at 10 and 20 Hz. However, the degree of motor deficits and impaired striatal suppression of cortical drive within this frequency range appears to be unrelated. While rats with ∼60% and >90% DA cell loss exhibited similar patterns of striatal LFP disruption at 10 and 20 Hz, forelimb stepping deficits become more pronounced as the extent of DA lesion increases. These results point to an interesting possibility that the lack of inhibitory control observed in the DA-depleted striatum at 10 and 20 Hz could be mechanistically linked to the onset, but not to the degree of motor deficits in PD. It is therefore conceivable that a striatal network with insufficient DA levels will become increasingly permissive to cortical drive (Bamford et al. 2004), in particular to synchronous activity occurring within the frequency range of alpha (8–12 Hz) and beta (15–30 Hz) oscillations as seen in association with the emergence of motor deficits in both PD (Brown 2003; Hammond et al. 2007; McCarthy et al. 2011) and animal models of experimental parkinsonism (Hutchison et al. 2004; Avila et al. 2010; McCarthy et al. 2011). Future studies are warranted to identify the precise underlying mechanism that enables such a state of increased synchronization within the cortico-basal ganglia loop.
In addition to the diminished inhibitory regulation of cortically evoked striatal LFP at 10 and 20 Hz, an attenuation of corticostriatal suppression at 40 Hz becomes apparent when the degree of nigral DA lesion is >90%. At first glance, these results suggest that a higher level of striatal DA depletion is required to disrupt the inhibitory control of striatal response to cortical drive at higher frequencies. However, this was not the case since acute blockade of striatal DA receptors in naïve control rats failed to reproduce the corticostriatal dysfunction at 40 Hz found in the DA-depleted striatum of 6-OHDA-treated rats. Contrary to the disruption observed at 10 and 20 Hz following acute blockade of striatal DA receptors, the attenuated LFP inhibition at 40 Hz becomes apparent only after a chronic loss of DA has occurred. Similarly, infusion of DA receptor agonists into the DA-depleted striatum of 6-OHDA-treated rats selectively restored the pattern of LFP suppression at 10 and 20 Hz without normalizing the level of corticostriatal inhibition observed at 40 Hz. These results converge to indicate that, in addition to the extent of striatal DA depletion, the chronicity of DA lesion is one key contributing factor for sustaining a nondopaminergic mechanism of local dysfunction capable of progressively shifting the striatal network into a more permissive state dominated by cortical synchronous drive, most notably at higher frequency ranges including that at 40 Hz.
Chronic DA lesion has been shown to trigger nondopaminergic dysregulation within the striatum including an upregulation of the sGC-cGMP signaling pathway (Chalimoniuk et al. 2004; Tseng et al. 2011), which is predominantly localized in striatal output neurons (Ariano and Matus 1981). As stimulation of the sGC-cGMP pathway facilitates the cortical excitatory drive onto striatal output neurons (Threlfell and West 2013), the increased cGMP levels observed in the DA-depleted striatum is expected to enhance the transmission of cortical activity to downstream nuclei of the basal ganglia (West and Tseng 2011). Results from the present study expand this view by showing that acute pharmacological reversal of elevated cGMP levels in the DA-depleted striatum (with intrastriatal ODQ infusion; Tseng et al. 2011) effectively restored the normal inhibitory control of corticostriatal transmission at 10 and 20 Hz without altering the disruption at 40 Hz. A similar frequency-dependent normalization of striatal LFP inhibition was observed following striatal infusions of DA agonists. This could be explained by the fact that striatal cGMP levels are intrinsically coupled to local changes in DA and nitric oxide signaling (Altar et al. 1990; Garthwaite and Boulton 1995; Di Stefano et al. 2005; Padovan-Neto et al. 2015). Accordingly, acute striatal elevation of cGMP using the analog 8-Br-cGMP was sufficient to elicit a pattern of reduced LFP suppression at 10 and 20 Hz that resembles the diminished inhibitory response observed following acute striatal blockade of DA receptors in naïve controls. Thus, a proper balance of striatal DA transmission and cGMP signaling is required for mediating normal inhibitory control of corticostriatal drive within the 10–20 Hz range. The fact that acute striatal manipulations of neither DA nor cGMP signaling can alter the pattern of striatal LFP response to cortical drive at 40 Hz indicates that distinct striatal mechanisms contribute to the regulation of corticostriatal transmission in a frequency-dependent manner.
Data obtained from adult naïve rats revealed that a critical level of striatal GABAergic activity has to be recruited to sustain the characteristic suppression of corticostriatal transmission at 40 Hz. Such 40 Hz dependency on striatal GABAergic function was not observed at 10 and 20 Hz since local elevation of cGMP or blockade of DA and GABA-A receptors alone elicited similar patterns of striatal LFP disinhibition at these lower frequencies that were indistinguishable from each other. At the cellular level, over 95% of striatal neurons are GABAergic output cells (Oorschot 1996), yet it is the local GABAergic interneuronal population that provides the primary feedforward inhibitory regulation of striatal responses to cortical excitation (Koos and Tepper 1999; Koos et al. 2004; Mallet et al. 2005). Among the different populations of striatal GABAergic interneurons, a remodeling of fast-spiking neuronal connectivity has been observed in the DA-depleted striatum in a manner that amplifies synchronous striatal output through a disinhibitory mechanism (Mallet et al. 2006; Gittis et al. 2011). In this regard, cortical stimulation at 40 Hz may preferentially engage striatal fast-spiking interneurons (Beatty et al. 2014) and empower this interneuronal population for regulating striatal suppression of cortical synchronous drive including that at gamma frequencies. Thus, a functional downregulation of striatal fast-spiking interneurons may underlie the onset of attenuated inhibitory control of corticostriatal transmission observed at 40 Hz when the degree of chronic DA lesion surpasses 90%. Notably, the level of forelimb stepping impairments also become more pronounced as the extent of DA lesion increases. It is therefore possible that the severity of motor deficits typically associated with the chronicity of DA lesion is mechanistically linked to an impaired striatal GABAergic inhibition from fast-spiking interneurons and the development of corticostriatal dysfunction at 40 Hz. In addition, a disruption of nonfast-spiking interneurons in the DA-depleted striatum may also contribute to impair the inhibitory control of corticostriatal transmission at 10 and 20 Hz as a subset of these interneurons can be easily recruited by cortical inputs at such frequency ranges (Beatty et al. 2014).
In summary, results from the present study reveal that proper balance of DA, cGMP signaling, and GABAergic transmission converge at the striatal level to sustain the distinct pattern of frequency-dependent incremental suppression of corticostriatal LFP. A global state of striatal disinhibition emerges following chronic nigrostriatal lesion such that the DA-depleted striatum becomes particularly susceptible to following synchronized cortical activity within the alpha–beta frequency range, and to a lesser extent, at 40 Hz. Both the level and chronicity of DA lesion are major contributing factors associated with the severity of motor deficits and the onset of a striatal GABAergic disruption that, interestingly, could only be reversed by strengthening local GABA-AR activation. Although the use of chloral hydrate may confound the interpretation of this finding, its known facilitatory action on brain's GABA-AR transmission (Lovinger et al. 1993) points to a GABAergic impairment underlying the frequency-dependent corticostriatal deficits observed following chronic DA lesion. The fact that strengthening striatal GABA-Aα1 function can normalize the pattern of corticostriatal LFP response further indicates that striatal GABAergic inhibition is compromised by chronic DA depletion. Future studies are warranted to test whether a full behavioral recovery can be achieved by restoring the balance of striatal DA-cGMP-GABA-AR signaling in the parkinsonian brain.
Funding
Supported by the Parkinson's Disease Foundation, National Institutes of Health NS088502 and NS088554, Ply Gift Award, and the Parkinson's Research Institute Innovation Grant.
Notes
Conflict of Interest: None declared.
References
- Altar CA, Boyar WC, Kim HS. 1990. Discriminatory roles for D1 and D2 dopamine receptor subtypes in the in vivo control of neostriatal cyclic GMP. Eur J Pharmacol. 181:17–21. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Matus AI. 1981. Ultrastructural localization of cyclic GMP and cyclic AMP in rat striatum. J Cell Biol. 91:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila I, Parr-Brownlie LC, Brazhnik E, Castaneda E, Bergstrom DA, Walters JR. 2010. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp Neurol. 221:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. 2004. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 42:653–663. [DOI] [PubMed] [Google Scholar]
- Beatty JA, Song SC, Wilson CJ. 2014. Cell-type-specific resonances shape the responses of striatal neurons to synaptic input. J Neurophysiol. 113:688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Brown P. 2014. Oscillations and the basal ganglia: motor control and beyond. Neuroimage. 85(Pt 2):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. 2007. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 17:656–664. [DOI] [PubMed] [Google Scholar]
- Brown P. 2003. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 18:357–363. [DOI] [PubMed] [Google Scholar]
- Chalimoniuk M, Langfort J, Lukacova N, Marsala J. 2004. Upregulation of guanylyl cyclase expression and activity in striatum of MPTP-induced parkinsonism in mice. Biochem Biophys Res Commun. 324:118–126. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Sozio P, Cacciatore I, Cocco A, Giorgioni G, Costa B, Montali M, Lucacchini A, Martini C, Spoto G et al. 2005. Preparation and pharmacological characterization of trans-2-amino-5(6)-fluoro-6(5)-hydroxy-1-phenyl-2,3-dihydro-1H-indenes as D2-like dopamine receptor agonists. J Med Chem. 48:2646–2654. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. 1995. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 57:683–706. [DOI] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. 2006. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 21:1566–1577. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Hang GB, LaDow ES, Shoenfeld LR, Atallah BV, Finkbeiner S, Kreitzer AC. 2011. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 71:858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Kreitzer AC. 2012. Striatal microcircuitry and movement disorders. Trends Neurosci. 35:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. 2007. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 30:357–364. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. 2004. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. J Neurosci. 24:9240–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. 1999. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 2:467–472. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. 2004. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 24:7916–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD, Cain CK, Plone MA, Frydel BR, Blaney TJ, Emerich DF, Hoane MR. 1999. Incomplete nigrostriatal dopaminergic cell loss and partial reductions in striatal dopamine produce akinesia, rigidity, tremor and cognitive deficits in middle-aged rats. Behav Brain Res. 102:1–16. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Zimmerman SA, Levitin M, Jones MV, Harrison NL. 1993. Trichloroethanol potentiates synaptic transmission mediated by gamma-aminobutyric acid-A receptors in hippocampal neurons. J Pharmacol Exp Ther. 264:1097–1103. [PubMed] [Google Scholar]
- Ma SY, Roytta M, Rinne JO, Collan Y, Rinne UK. 1997. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson's disease using disector counts. J Neurol Sci. 151:83–87. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. 2006. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 26:3875–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. 2005. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 25:3857–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Moore-Kochlacs C, Gu X, Boyden ES, Han X, Kopell N. 2011. Striatal origin of the pathologic beta oscillations in Parkinson's disease. Proc Natl Acad Sci USA. 108:11620–11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer MG, Tseng KY, Kasanetz F, Belluscio M, Riquelme LA. 2002. Brain oscillations, medium spiny neurons, and dopamine. Cell Mol Neurobiol. 22:611–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Bjorklund A. 1995. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 15:3863–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot DE. 1996. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol. 366:580–599. [DOI] [PubMed] [Google Scholar]
- Padovan-Neto FE, Sammut S, Chakroborty S, Dec AM, Threlfell S, Campbell PW, Mudrakola V, Harms JF, Schmidt CJ, West AR. 2015. Facilitation of corticostriatal transmission following pharmacological inhibition of striatal phosphodiesterase 10A: role of nitric oxide-soluble guanylyl cyclase-cGMP signaling pathways. J Neurosci. 35:5781–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. 1996. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 50:275–331. [DOI] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Tseng KY. 2013. Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. J Neurosci. 33:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, West AR. 2013. Review: modulation of striatal neuron activity by cyclic nucleotide signaling and phosphodiesterase inhibition. Basal Ganglia. 3:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottenberg T, Fogelson N, Kuhn AA, Kivi A, Kupsch A, Schneider GH, Brown P. 2006. Subthalamic gamma activity in patients with Parkinson's disease. Exp Neurol. 200:56–65. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Caballero A, Dec A, Cass DK, Simak N, Sunu E, Park MJ, Blume SR, Sammut S, Park DJ et al. 2011. Inhibition of striatal soluble guanylyl cyclase-cGMP signaling reverses basal ganglia dysfunction and akinesia in experimental parkinsonism. PLoS One. 6:e27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Kargieman L, Gacio S, Riquelme LA, Murer MG. 2005. Consequences of partial and severe dopaminergic lesion on basal ganglia oscillatory activity and akinesia. Eur J Neurosci. 22:2579–2586. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. 2001. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 21:6430–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA. 2007. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 144:762–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA. 2004. The nitric oxide-guanylyl cyclase signaling pathway modulates membrane activity states and electrophysiological properties of striatal medium spiny neurons recorded in vivo. J Neurosci. 24:1924–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Tseng KY. 2011. Nitric oxide-soluble guanylyl cyclase-cyclic GMP signaling in the striatum: new targets for the treatment of Parkinson's disease? Front Syst Neurosci. 5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K. 2013. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J Neurosci. 33:11655–11667. [DOI] [PMC free article] [PubMed] [Google Scholar]