Abstract
The dopamine D3 receptor (D3R) belongs to the dopamine D2-like receptor family and is principally located in the ventral striatum. However, previous studies reported D3R overexpression in the dorsal striatum following l-DOPA treatment in parkinsonian animals. This fact has drawn attention in the importance of D3R in l-DOPA-induced dyskinesia (LID). Here, we used D3R knockout mice to assess the role of D3R in LID and rotational sensitization in the hemiparkinsonian model. Mice lacking D3R presented a reduction in dyskinesia without interfering with the antiparkinsonian l-DOPA effect and were accompanied by a reduction in the l-DOPA-induced rotations. Interestingly, deleting D3R attenuated important molecular markers in the D1R-neurons such as FosB, extracellular signal-regulated kinase, and histone-3 (H3)-activation. Colocalization studies in D1R-tomato and D2R-green fluorescent protein BAC-transgenic mice indicated that l-DOPA-induced D3R overexpression principally occurs in D1R-containing neurons although it is also present in the D2R-neurons. Moreover, D3R pharmacological blockade with PG01037 reduced dyskinesia and the molecular markers expressed in D1R-neurons. In addition, this antagonist further reduced dyskinetic symptoms in D1R heterozygous mice, indicating a direct interaction between D1R and D3R. Together, our results demonstrate that D3R modulates the development of dyskinesia by targeting D1R-mediated intracellular signaling and suggest that decreasing D3R activity may help to ameliorate LID.
Keywords: abnormal involuntary movements, basal ganglia, behavioral sensitization, Parkinson disease, striatonigral
Introduction
Dopamine (DA) depletion is the main neuropathological feature of Parkinson disease (PD), and the current standard treatment for PD focuses on restoring dopaminergic neurotransmission by l-3,4-dihydroxyphenylalanine (l-DOPA). However, long-term exposure to this drug causes a decrease in therapeutic efficacy and the appearance of l-DOPA-induced dyskinesia (LID) (Obeso et al. 2000). Although the pathophysiology of this side effect remains unclear, it has been shown that prolonged stimulation of D1 dopamine receptor (D1R) results in the development of LID (Darmopil et al. 2009). The resulting increase in the D1R signaling pathway leads to increased activation of the extracellular signal-regulated kinase (ERK) pathway (Pavón et al. 2006; Santini et al. 2007; Westin et al. 2007; Fasano et al. 2010) and the subsequent activation of molecular markers of dyskinesia such as FosB and histone3 (H3) (Andersson et al. 1999; Santini et al. 2009).
The dopamine receptors are divided into 2 major groups, the dopamine D1-like (D1 and D5) receptors and the dopamine D2-like (D2, D3 and D4) receptors (Beaulieu and Gainetdinov 2011). We demonstrated that D1R but not D2R is critical for the development of LID (Darmopil et al. 2009; Murer and Moratalla 2011). However, previous research has suggested that the dopamine D3 receptor (D3R) may also play a role in LID, since D3R activation can potentiate D1R-mediated signaling in the striatum (Fiorentini et al. 2008; Marcellino et al. 2008) and regulates D1R internalization (Berthet et al. 2009).
The D3R is mainly expressed in the nucleus accumbens, olfactory tubercle, islands of Calleja, and at low levels in the dorsal striatum (Sokoloff et al. 1990; Flores et al. 1996; Xu et al. 1997). l-DOPA treatment induces an increase in D3R expression in the dorsal striatum in rats with 6-hydroxydopamine (6-OHDA) lesion (Bordet et al. 1997; Guillin et al. 2001), and this increase correlates with LID (Guigoni et al. 2005). Other previous studies have also provided evidence supporting an important role for D3R in LID (Bézard et al. 2003). Recent experimental findings drew attention to the potential use of D3R-preferring antagonists in the treatment of LID since these antagonists potentiate the effect of l-DOPA as well as decrease dyskinesia (Kumar et al. 2009; Visanji et al. 2009). However, some studies found no reduction in LID after D3R blockade (Silverdale et al. 2004; Mela et al. 2010). This variability may be due to the lack of specificity of pharmacological approaches, which do not irrefutably distinguish between the D2 and D3 receptor. To address this discrepancy and conclusively stabilize the role of D3R, we determined how eliminating D3R expression impacts the development of LID.
We used D3 knockout (D3−/−) mice to study the development of LID as well as rotational responses to repeated l-DOPA in hemiparkinsonian mice. In addition, we investigated FosB, H3, and ERK activation in the striatum. To complete our understanding, we evaluated the effect of pharmacological blockade of D3R in the development and expression of LID in wild-type (WT) and in dopamine D1 heterozygous (D1+/−) mice. We also examined the expression of D3R in the dorsolateral striatum in dyskinetic mice using bacterial artificial chromosome (BAC)-transgenic-D1R-tomato (D1R-tomato) and BAC-transgenic D2R-enhanced green fluorescent protein (D2R-GFP) mice.
Materials and Methods
Animals
This study was carried out in female and male mice lacking D3R generated by homologous recombination as described previously (Xu et al. 1997). Wild-type and homozygous D3−/− mutant mice were obtained from mating heterozygous mice. The genotype of D3−/− and WT mice was determined by the Southern blotting method (Xu et al. 1997). D1R-tomato and D2R-GFP mice (Suárez et al. 2014) were used to study D3R localization in the dorsal striatum. WT mice were also used to study the pharmacological blockade of D3R. D1+/− mice were used to further investigate the interaction between D3R and D1R in the dyskinetic response (Table 1). The maintenance of animals followed guidelines from European Union Council Directive (86/609/European Economic Community), and experimental protocols were approved by the CSIC Ethics Committee.
Table 1.
Experimental groups
| Group | Genotype | n/group | IHC | THa |
|---|---|---|---|---|
| Effect of D3R deletion on LID | ||||
| Saline | WT | 10 | TH, FosB, pAcH3, pERK | 43.3 ± 2.9 |
| D3−/− | 3 | 48.0 ± 2.3 | ||
| l-DOPA | WT | 10 | 46.0 ± 1.9 | |
| D3−/− | 10 | 45.2 ± 2.5 | ||
| Expression of D3R in the striatum | ||||
| Intact | D1R-tomato | 3 | TH, D3R, FosB | NA |
| Parkinsonian | 3 | 49.8 ± 6.2 | ||
| Dyskinetic | 4 | 50.4 ± 1.9 | ||
| Dyskinetic | D2R-GFP | 4 | TH, D3R | 44.9 ± 2.4 |
| Effect of PG01037 on the development of LID | ||||
| l-DOPA+ saline | WT | 9 | TH, FosB, pAcH3 | 45.0 ± 3.0 |
| l-DOPA+PG01037 | 9 | 47.8 ± 1.9 | ||
| Effect of PG01037 on the expression of LID | ||||
| l-DOPA+saline | WT | 9 | TH, FosB, pAcH3 | 47.5 ± 2.3 |
| l-DOPA+PG01037 | 8 | 45.7 ± 2.7 | ||
| Interaction between the D1R and D3R on LID | ||||
| l-DOPA | WT | 10 | TH, FosB, pAcH3 | 47.4 ± 2.2 |
| D1+/− | 7 | 49.3 ± 3.1 | ||
| l-DOPA+PG01037 | WT | 6 | 46.1 ± 2.6 | |
| D1+/− | 7 | 48.8 ± 3.1 | ||
Note: IHC: immunohistochemistry; NA, not applicable.
aExtent of striatal lesions assessed by percentage of striatal volume completely denervated.
Drugs
l-DOPA, benserazide, and desipramine were purchased from Sigma–Aldrich (Spain), and PG01037 was purchased from Tocris-Bioscience (UK). All drugs were freshly prepared in 0.9% NaCl before use and injected i.p. in a volume of 10 mL/kg.
6-OHDA Lesion and Treatment
For the stereotaxic procedure, animals (weighing 25–30 g) were deeply anesthetized with isofluorane anesthesia. As previously described (Ruiz-DeDiego et al. 2015), striatal lesions were performed by unilateral injection of 6-OHDA hydrobromide (20 mmol/L, containing 0.02% ascorbic acid; Sigma–Aldrich, Spain) into striatum at the following coordinates (millimeter) from bregma and dura: anteroposterior (+0.65), lateral (−2.0), and dorsoventral (−4.0 and −3.5). Before neurotoxin injection, all mice received desipramine (20 mg/kg) to protect norepinephrine neurons. Three weeks after lesion, all mice started a 2-week course of daily i.p. injections of benserazide (10 mg/kg) followed by l-DOPA (10 mg/kg) 20 min later.
To assess the role of D3R blockade in LID, we injected the selective D3R antagonist PG01037 (10 mg/kg) or saline 15 min after l-DOPA in WT mice and in D1+/− mice. PG01037 is a highly selective D3R antagonist (Ki = 0.7 ± 0.1 nm) with 133-fold selectivity for D3R over D2R (Grundt et al. 2005). Pharmacological magnetic resonance imaging studies showed that PG01037 crosses the blood–brain barrier and localizes in D3R-rich regions such as the islands of Calleja and nucleus accumbens (Grundt et al. 2007). This compound displays plasma half-life t(1/2) of 1.83 h and has higher brain concentrations than plasma concentrations (Mason et al. 2010). The PG01037 dose and procedure were chosen according to previous studies (Kumar et al. 2009). To study the effect of pharmacological blockade of D3R on the development of LID, we daily co-administered l-DOPA plus PG01037 for 2 weeks. To assess the effect of D3R blockade on expression of LID, mice were rendered dyskinetic by 2 weeks of daily l-DOPA administration as described earlier and on Days 15–18 received l-DOPA plus PG01037.
Behavioral Assessment
To evaluate LID, mice were observed in clear-glass cylinders and were rated by a trained observer. During each rating period, individual dyskinesia severity scores ranging from 0 (not present) to 4 (severe) were given for axial, limb and orolingual dyskinesia. The 3 dyskinetic movement subtypes were summed to create a single score for data analysis. On Day 13 of treatment, we evaluated the LID every 20 for 160 min after l-DOPA injection. Circling behavior was measured as a measure of behavioral sensitization. All animals were video-recorded by a vertically mounted video camera for 15, 5 min after l-DOPA injection. Rotations were analyzed in Viewer2, Biobserve (GmbH). Only completed contralateral turns (360°) were counted.
Motor coordination was evaluated using the rotarod test following an accelerating protocol with increasing speed from 4 to 40 rpm over a 5-min period as described elsewhere (Ruiz-DeDiego et al. 2015). Animals were trained and evaluated before 6-OHDA lesion (prelesion), before the treatment of l-DOPA (pre-l-DOPA; Day 0), and on Day 14 or 16, 90 min after the l-DOPA injection (post-l-DOPA) to avoid the peak of dyskinetic symptoms. The variable analyzed was the latency to fall from the rotarod. For locomotor response experiments, animals were placed in Activity Cages (AccuScan Instruments, Inc.). The locomotor activity was measured and displayed as total distance (cm) over a 30-min period beginning 60 min after saline (pre-l-DOPA) or l-DOPA (post-l-DOPA) injection as described previously (Solís et al. 2015). All behavioral experiments were carried out with the experimenter blind to genotype and treatment.
Immunohystochemistry
The mice were deeply anesthetized with pentobarbital and perfused transcardially with cold saline, followed by a solution of 4% paraformaldehyde in phosphate-buffered saline 1 h after the last l-DOPA injection. Immunostaining was performed on free-floating coronal brain sections as described previously (Granado et al. 2011; Ares-Santos et al. 2014), with the following rabbit antisera: tyrosine hydroxylase (TH, 1:1000; Chemicon), FosB (1:7500; Santa Cruz Biotechnology), phospho-(Ser10)-acetyl (Lys14)-histone 3 (pAcH3; 1:1500; Upstate—Cell Signaling Solutions), pERK (1:250; Cell Signaling Technology), D3R (1:100; Alomone Labs), and Hoechst (1 mg/mL; Sigma–Aldrich). For immunofluorescence experiments, we used Alexa fluor 488- and 594-conjugated secondary antibodies (1:500; Invitrogen).
The extent of dopaminergic lesions was quantified using Stereoinvestigator (MBF Bioscience), depicting the border of striatal areas with complete loss of TH-immunoreactive fibers with a 4× lens using 7 serial rostrocaudal sections per animal. Quantification of FosB, pAcH3, and pERK immunoreactivity was carried out using Image J (Schneider et al. 2012). Immunostaining intensity and the number of immunolabeled nuclei/cells were determined for all animals in each group using 2 serial rostrocaudal sections per animal from both sides of the lesioned dorsolateral striatum for a total of 6 images per animal. Digital images were obtained under a Leica microscope using a 40× objective. The data are presented as the number of stained nuclei per square millimeter in the lesioned striatum.
Immunofluorescence images from dorsolateral part of the striatum were obtained using sequential laser scanning confocal microscopy (Leica) at 40× and 63× from 3 sections per animal. Neuronal quantification was performed on 2 images for each section. D3R colocalization data are expressed as % of D3R-positive cells in D1R-positive or -negative cells or as % of D3R-positive cells in D2R-positive or -negative cells. Expression of FosB in D1R-positive and D1R-negative striatal neurons was also studied. Images were quantified with the “cell counter” plug-in in ImageJ (NIH). The investigator performing immunohistochemistry quantification was blinded to sample identities.
Statistical Analysis
Behavioral data were analyzed by repeated-measures two-way analyses of variance (ANOVA) followed by Bonferroni post hoc test. Immunohistochemistry (FosB, pAcH3, and pERK) data were evaluated by unpaired t-test. Statistical comparison of D3R expression in the dorsal striatum was analyzed using two-way ANOVA followed by Bonferroni post hoc test. Analysis was performed with GraphPad Prism 5 (La Jolla). Data are expressed as the mean ± standard error of the mean (SEM) unless stated otherwise. The significance level was P < 0.05.
Results
Effect of D3R Deletion on Dyskinesia and Behavioral Sensitization Induced by l-DOPA
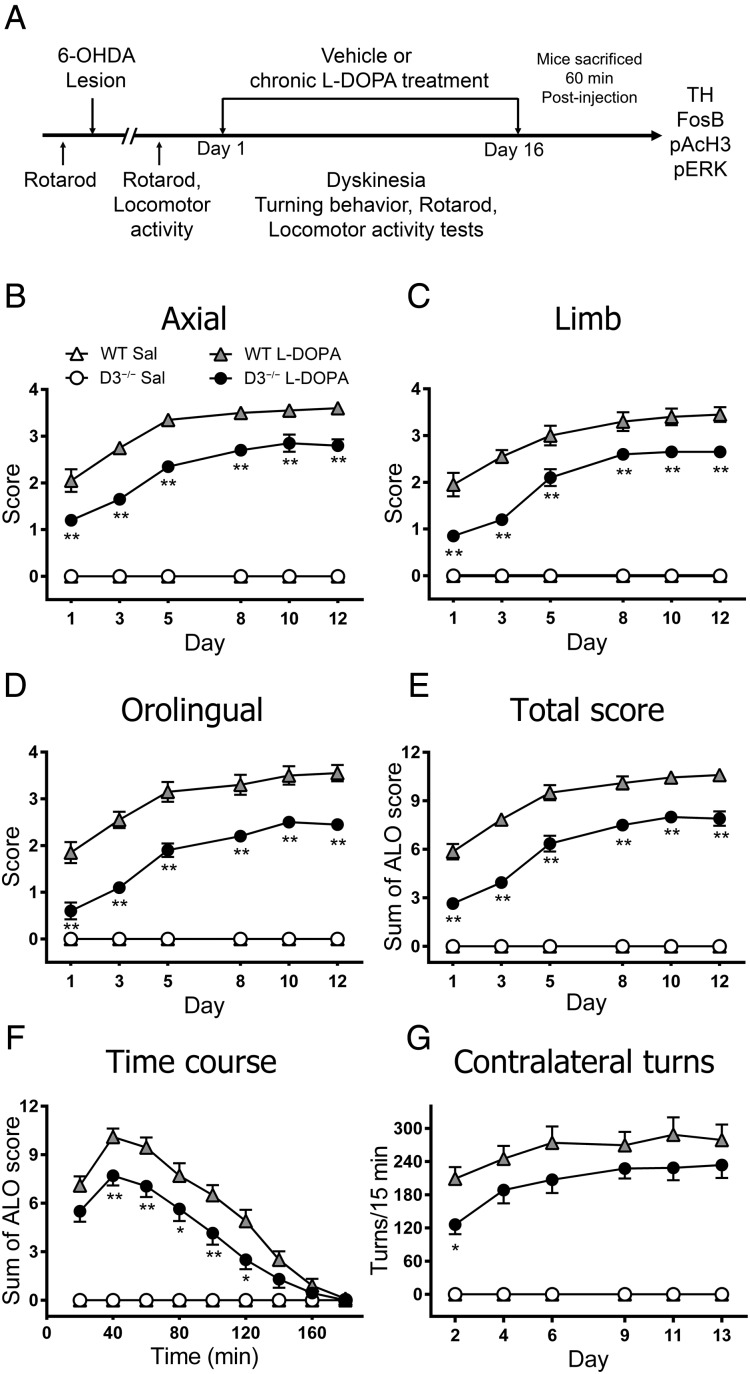
To explore the effect of D3R genetic inactivation on LID, 6-OHDA-lesioned WT and D3−/− mice were chronically treated with l-DOPA (Fig. 1A). We assessed 3 subtypes of dyskinetic movements (axial, limb, and orolingual) 40 min after l-DOPA injection as had been described in former work (Pavón et al. 2006). Dyskinetic movements appeared in hemiparkinsonian WT mice on the first day of l-DOPA treatment and increased progressively, reaching a plateau at Day 5 that was maintained until the end of treatment, consistent with previous results (Darmopil et al. 2009). Deletion of the D3R resulted in significant lower axial (P < 0.05), limb (P < 0.05), and orolingual (P < 0.01) (Fig. 1B–D) dyskinetic subtypes, as well as total dyskinesia compared with their WT counterparts (P < 0.01) (Fig. 1E). In addition, time course analysis carried out on Day 13 revealed that total dyskinesia was significantly attenuated in D3−/− mice assessed during the peak of LID (Fig. 1F). The hemiparkinsonian mice of both genotypes that received saline did not show any dyskinetic symptom. Together these data demonstrate that D3R deletion significantly attenuates LID.
Figure 1.
Effect of genetic deletion of D3R on LID and behavioral sensitization. (A) Schematic view of the experimental design. The D3−/− mice exhibited a reduction in axial (B), limb (C), and orolingual (D), as well as the cumulated total score (E) compared with littermate controls. Dyskinetic movements were evaluated 40 min after l-DOPA. Two-way ANOVA with repeated measures followed by a Bonferroni post hoc test showed significant differences for genotype (F3,150 = 414.3, P < 0.001 for axial; F3,150 = 212.6, P < 0.001 for limb; F3,150 = 187.9, P < 0.001 for orolingual; F3,150 = 375.5, P < 0.001 for total score) and day (F5,150 = 39.5, P < 0.001 for axial; F5,150 = 44.1, P < 0.001 for limb; F5,150 = 40.4, P < 0.001 for orolingual; F5,150 = 75.1, P < 0.001 for total score). The kinetic profile of dyskinetic symptoms was evaluated once every 20 min over 180 min on Day 13 of the l-DOPA treatment (F). Two-way ANOVA followed by a Bonferroni test showed significant differences for genotype (F3,270 = 294.4, P < 0.001) and time (F5,270 = 39.07, P < 0.001). Contralateral turns in hemiparkinsonian mice evaluated for 15 min on the indicated day (F). Two-way ANOVA with repeated measures followed by a Bonferroni test showed significant differences for genotype (F3,150 = 68.6, P < 0.001) and day (F5,150 = 5.6, P < 0.001). Data are expressed as the mean ± S.E.M. *P < 0.05 and **P < 0.01 versus WT + l-DOPA. n = 10 for each group, except in the D3−/− saline group, n = 3.
We evaluated circling behavior as a measure of sensitization (González-Aparicio and Moratalla 2014). Administration of chronic l-DOPA induced contralateral rotations in hemiparkinsonian mice, with D3−/− mice showing significant lower contralateral rotations on the first evaluation day compared with WT littermates (P < 0.05). This decrease continued during the next evaluation days, but it did not reach significance (P > 0.05; Fig. 1F). Saline-treated animals did not develop contralateral rotations.
D3R Deletion Does not Affect the Antiparkinsonian Efficacy of l-DOPA
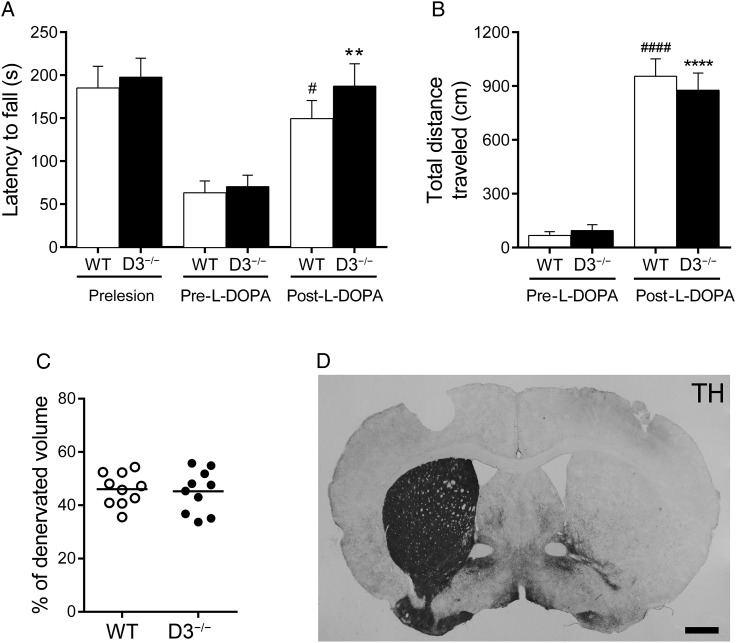
To rule out the possibility that the antidyskinetic effect in D3−/− mice was due to locomotor impairment, we measured motor coordination and locomotor activity. The D3−/− group animals did not show any significant difference to WT animals in the motor coordination (tested by rotarod) before (prelesion) and after the 6-OHDA lesion (pre-l-DOPA). The lesion affected motor coordination similarly in both genotypes. However, chronic l-DOPA treatment, tested on Day 14 (post-l-DOPA), significantly improved the time on the rotarod (P < 0.01) (Fig. 2A). Although latency to fall from the rotarod was longer in D3−/− mice, this increase was not statistically significant compared with WT littermates (P > 0.05). Locomotor activity in hemiparkinsonian WT and D3−/− mice significantly improves after chronic l-DOPA, tested on Day 15 (P < 0.0001). We did not find any difference in the locomotor response between WT and D3−/− mice (Fig. 2B). The results suggest that D3R genetic inactivation does not impair l-DOPA's antiparkinsonian effect.
Figure 2.
Genetic deletion of D3R does not affect the therapeutic effect of l-DOPA. (A) Motor coordination on the rotarod was evaluated in D3−/− and WT mice before 6-OHDA lesion (prelesion), 3 weeks after lesion (pre-l-DOPA), and on Day 14 of the chronic treatment, 90 min after the l-DOPA injection (post-l-DOPA). Two-way ANOVA, followed by Bonferroni's test. **P < 0.01 versus D3−/− (pre-l-DOPA) mice; #P < 0.05 versus WT (pre-l-DOPA) mice. (B) Total distance traveled (cm) during 30 min was measured in a multicage activity meter system. Two-way ANOVA, followed by Bonferroni's test. ****P < 0.0001 versus D3−/− (pre-l-DOPA) mice; ####P < 0.0001 versus WT (pre-l-DOPA) mice. (C) Scatter dot plot of the extent of striatal lesions assessed by percentage of striatal volume completely denervated. (D) Coronal section from a dyskinetic D3−/− mouse stained for TH. n = 10 for each group. Scale bar = 500 µm.
Previous evidence indicated that the extent of the dopaminergic lesion correlates with the severity of dyskinesia (Darmopil et al. 2009). To exclude the possibility of differences in lesion size between WT and D3−/− mice, we measured the volume in the ipsilateral striatum devoid of TH fibers and found no difference in the extent of the lesion between groups (P > 0.05; Fig. 2C,D)
Deleting D3R Regulates Molecular Markers of LID
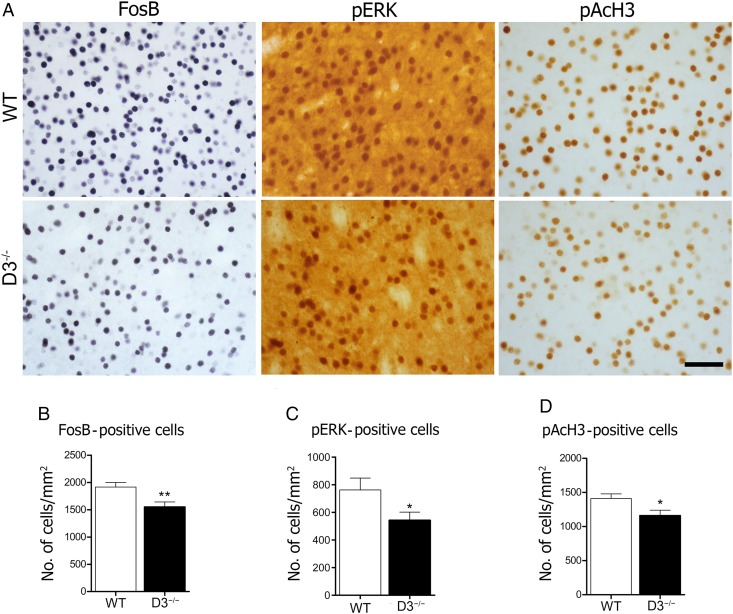
To explore potential molecular mechanisms underlying the effects of D3R deletion on LID, we measured the expression of FosB, pERK, and pAcH3 in the DA-denervated striatum (Fig. 3A). These have been implicated as key determinants in appearance of D1 dopamine receptor supersensitivity in LID (Pavón et al. 2006; Santini et al. 2007; Westin et al. 2007; Darmopil et al. 2009). Immunostaining revealed that repeated treatment with l-DOPA resulted in significant FosB expression in the lesioned striatum in WT mice, as expected. D3R deletion significantly decreased the number of FosB-positive cells (P < 0.01; Fig. 3B). It has been demonstrated that l-DOPA activates the ERK pathway (Pavón et al. 2006). Thus, we assessed pERK activation after l-DOPA treatment and found that pERK activation in the lesioned striatum was significantly diminished in the D3−/− mice compared with WT (P < 0.05; Fig. 3C). Next, we examined the effect of chronic l-DOPA treatment on the phosphoacetylation of striatal H3, a downstream target of ERK (Santini et al. 2007, 2009). As shown in Figure 3D, there was a significant increase in pAcH3 in the denervated striatum of WT mice. D3−/− mice also showed attenuated pAcH3 expression (P < 0.05). Our data indicate that D3R regulates FosB, pERK, and pAcH3 in the denervated striatum of dyskinetic mice.
Figure 3.
Genetic inactivation of D3R attenuates molecular markers of LID. Photomicrographs of l-DOPA-induced FosB, pERK, and pAcH3 expression in the dopamine-denervated dorsal striatum (A). Immunohistochemical analysis shows that the D3R deletion decreases FosB- (B), pERK- (C), and pAcH3- (D) positive cells induced by l-DOPA. *P < 0.05 and **P < 0.01 versus WT (unpaired t-test). n = 10 for each group. Scale bar = 50 µm.
l-DOPA-Induced D3R-Increased Expression Occurs in the Direct and Indirect Striatal Neurons in the Dorsal Striatum
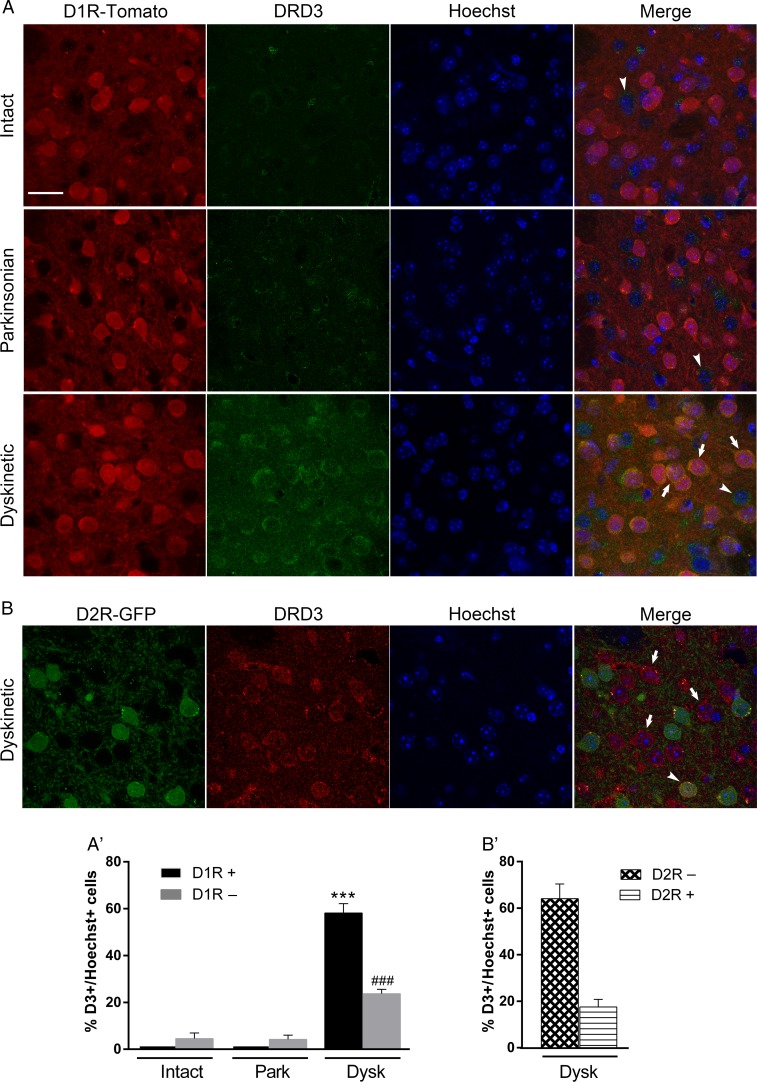
l-DOPA treatment causes an increase in D3R expression in the DA-denervated dorsal striatum (Bordet et al. 1997; Guigoni et al. 2005). However, to date there is no direct evidence that determines whether this expression occurs in striatonigral D1R or in striatopallidal D2R projection neurons. To address this issue, we used intact (unlesioned), hemiparkinsonian, and dyskinetic D1R-tomato mice to identify direct from indirect pathway neurons. In intact animals, there were some D1R-negative neurons that expressed the D3R but not in the D1R-positive neurons of the dorsal striatum (Fig. 4A). This pattern of expression was similar in the parkinsonian denervated dorsal striatum. Interestingly, we found that l-DOPA increased D3R expression in the hemiparkinsonian mice; this increase occurred in 58.1% of the D1R-positive cells and in 23.6% of the D1R-negative cells of the dorsal striatum (Fig. 4A′). This pattern of D3R-increased distribution in dyskinetic animals was further confirmed using (BAC)-transgenic D2R-GFP mice (Fig. 4B). As expected, D3R expression increased in D2R-negative cells (64%) and in D2R-positive cells (18%) (Fig 4B′). As additional controls, we used l-DOPA-treated D3−/− mice, and no D3R expression was observed (data not shown). Altogether, these results indicate that L-DOPA increases D3R expression in both striatonigral and striatopallidal neurons, although this increase is 2.5- to 3-fold larger in D1R than that in D2R-neurons in dyskinetic animals.
Figure 4.
Chronic l-DOPA increases D3R expression in the DA-depleted dorsal striatum. (A) Confocal images of the dorsal striatum of intact, parkinsonian, and dyskinetic D1R-BAC-transgenic mice illustrating D3R expression (green) in D1R-positive neurons (red-tomato) and in D1R-negative neurons. Nuclei are visualized via Hoechst staining. Arrows indicate examples of D3R-positive and D1R-positive neurons; arrow-heads point to D3R-positive and D1R-negative neurons. (A′) Graph showing the percentage of D3R-positive cells in D1R-positive or -negative cells. Data are expressed as the mean ± SEM. Two-way ANOVA, followed by Bonferroni's test. ***P < 0.001 versus intact D1R-positive neurons; ###P < 0.001 versus intact D1R-positive neurons. (B) Confocal images of the dorsal striatum of dyskinetic D2R-BAC-transgenic mice illustrating D3R expression (red) in D2R-positive neurons (green-GFP) and in D2R-negative neurons. Nuclei are visualized via Hoechst staining. Arrows indicate examples of D3R-positive and D2R-negative neurons; arrow-heads point to D3R-positive and D2R-positive neurons. (B′) Graph showing the percentage of D3R-positive cells in D2R-positive or negative cells. n = 3–4 for each group. Scale bar = 25 µm.
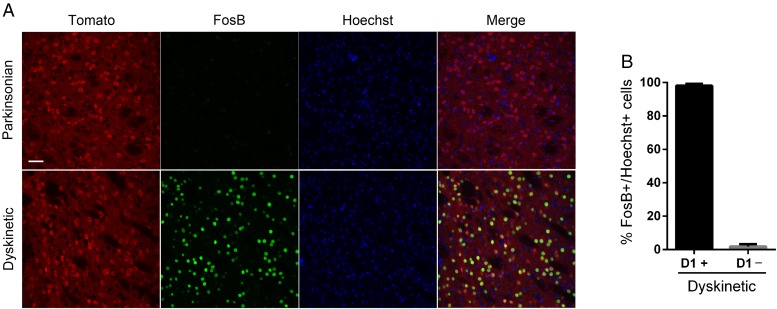
Despite the fact that D3R increases in both types of striatal neurons after l-DOPA, we found that FosB expression was induced in the D1R-positive neurons and was hardly detected in the D1R-negative neurons (Fig. 5), suggesting that FosB activation occurs in the striatonigral neurons, consistent with previous reports (Pavón et al. 2006; Darmopil et al. 2009). In parkinsonian mice, FosB expression was barely detected in the striatum.
Figure 5.
l-DOPA-induced FosB expression occurs in striatonigral projection neurons. (A) Confocal images of the dorsal striatum of parkinsonian and dyskinetic D1R-BAC-transgenic mice illustrating FosB expression (green) in D1R-positive neurons (red-tomato). Nuclei are visualized via Hoechst staining. (B) Graph showing the percentage of D3R-positive cells in D1R-positive or -negative cells. Data are expressed as the mean ± SEM. n = 3–4 for each group. Scale bar = 40 µm.
Effect of D3R Pharmacological Blockade on LID in WT Mice
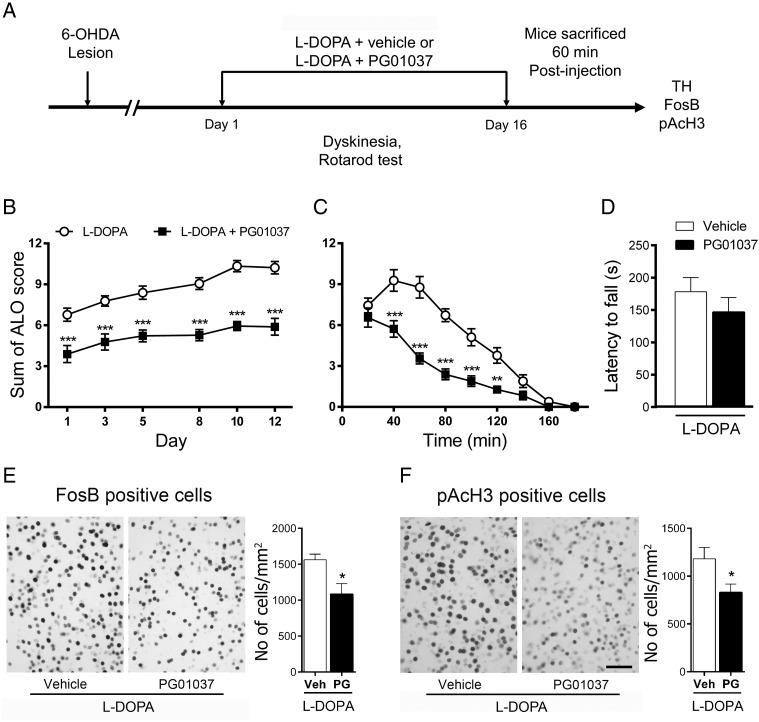
To rule out the possibility of compensatory mechanism in D3−/− during development that could be acting on LID, we used the selective D3R-preferring antagonist PG01037 to further investigate the role of D3R on the development and expression of LID. To assess the role of D3R in the development of LID, we administered PG01037 or saline to hemiparkinsonian mice daily for 2 weeks, 15 min after l-DOPA treatment (Fig 6A). Notably, the dyskinetic response of PG01037-treated mice upon initial exposure to l-DOPA was both smaller (P < 0.01; Fig. 6B) and of shorter duration (P < 0.01; Fig. 6C) than that of mice that received only l-DOPA plus saline over the 2 weeks of treatment. This treatment did not significantly reduce the therapeutic effect of l-DOPA as measured in the rotarod test (Day 14) (P > 0.05; Fig 6D). In agreement with the dyskinetic response, PG01037 significantly decreased FosB (P < 0.05) and pAcH3 (P < 0.05) expression induced by l-DOPA (Fig 6E,F).
Figure 6.
Effect of D3R-preferring antagonist PG01037 on the development of LID. (A) Schematic view of the experimental design. (B and C) The chronic coadministration of l-DOPA with PG01037 decreased the development of LID. **P < 0.01 and ***P < 0.001 versus l-DOPA plus saline, two-way repeated-measures ANOVA followed by Bonferroni post hoc test. (D) PG01037 cotreatment did not affect the latency to fall from the rotarod. P = 0.17 versus l-DOPA plus saline, unpaired t-test. Immunohistochemical analysis shows that PG01037 decreases FosB- (E) and pAcH3- (F) positive cells induced by l-DOPA. *P < 0.05 versus WT, unpaired t-test. All data are expressed as mean ± S.E.M. n = 9 for each group. Scale bar = 50 µm.
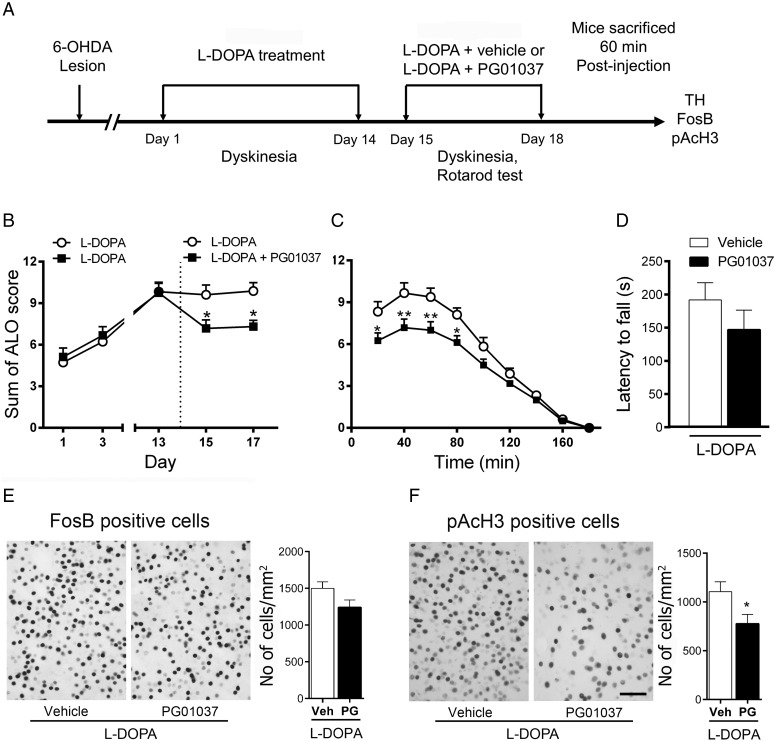
We next asked whether D3R pharmacological blockade had a role in the expression of LID. This was measured in mice with established dyskinesia (Fig 7A). We found that co-administration of l-DOPA plus PG01037 on Day 15–17 decreased LID compared with l-DOPA plus saline (P < 0.05) (Fig 7B,C). In addition, PG01037 did not significantly affect the antiparkinsonian efficacy of l-DOPA, indicated by the latency to fall in the rotarod test (Day 16) (P > 0.05; Fig. 6D). We then investigated FosB and pAcH3 activation and found that PG01037 diminish FosB and pAcH3 (P = 0.05) expression induced by l-DOPA, although this decrease was not significant for FosB (P = 0.08) (Fig 7D,E). This can be due to the longer half-life of FosB that gradually accumulates following chronic treatment (Hope et al. 1994; Moratalla et al. 1996a, 1996b). D3R antagonist can block new FosB expression but is unable to eliminate the accumulated FosB induced by the previous treatment. By contrast, H3 activation is transient, with maximal expression 60 min after injection and returns to baseline after 6 h (data not shown).
Figure 7.
Effect of D3R-preferring antagonist PG01037 on the expression of established LID. (A) The time line shows experimental design. (B and C) The administration of PG01037 diminished the expression of established LID. *P < 0.05 and **P < 0.01 versus l-DOPA plus saline, two-way repeated-measures ANOVA followed by Bonferroni post hoc test. (D) PG01037 cotreatment did not affect the latency to fall from the rotarod on established LID. P = 0.08 versus l-DOPA plus saline, unpaired t-test. Immunohistochemical analysis showed that coadministration of PG01037 does not significantly decrease FosB- (E) and pAcH3- (F) positive cells in mice with established LID (P = 0.08 and P = 0.22, respectively), unpaired t-test. All data are expressed as mean ± S.E.M. n = 8–9 for each group. Scale bar = 50 µm.
Interaction between D1R and D3R in Dyskinetic Mice
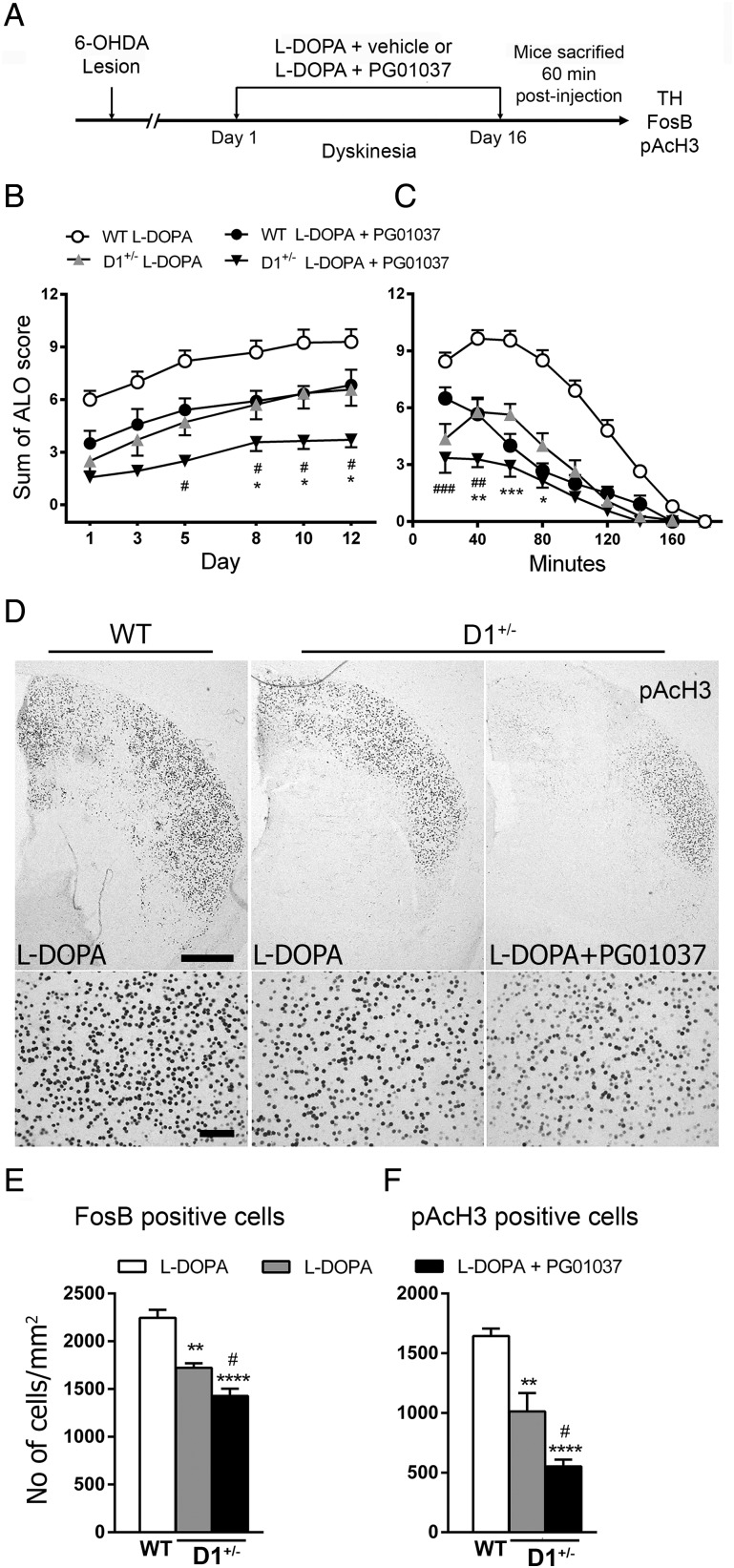
In this study, we show that genetic deletion of D3R decreases LID through attenuation of D1R signaling. Previous results of our laboratory demonstrated that the D1R is critical for the development of LID (Darmopil et al. 2009). To see whether D3R interacts with D1R to produce dyskinesias, we test whether D3R antagonist was able to further reduce D1R-mediated LID in D1+/− mice (Fig. 8A). As expected, D1+/− animals and PG01037 treatment in WT mice significantly reduced LID compared with l-DOPA-treated WT group (P < 0.01). Interestingly, the pharmacological blockade of D3R synergically reduced LID in D1+/− mice (P < 0.05) compared with l-DOPA-treated D1+/− and PG01037 plus l-DOPA WT animals (Fig. 8B,C). In agreement with the behavioral results, we found that D1+/− display lower FosB and pAcH3 expression after l-DOPA compared with WT mice, and this expression was further reduced by PG cotreatment with l-DOPA (Fig. 8D–F). Taken together, our results suggest a direct interaction between the D1R and the D3R in dyskinesia.
Figure 8.
Effect of coadministration of PG01037 plus l-DOPA in D1+/− mice. (A) The time line shows experimental design. (B) Total dyskinetic score was evaluated 40 min after l-DOPA administration at the indicated days. Two-way ANOVA followed by a Bonferroni test showed significant differences for genotype (F3,234 = 165.5, P < 0.001) and day (F8,234 = 108.6, P < 0.001). (C) Time course of dyskinetic symptoms evaluated once every 20 min during 180 min on Day 13 of l-DOPA treatment. Two-way ANOVA followed by a Bonferroni test showed significant differences for genotype (F3,156 = 71.04, P < 0.001) and time (F5,156 = 13.86, P < 0.001). (D) Immunostaining for pAcH3. Photomicrographs at high and low magnifications of coronal sections from the dopamine-denervated striatum of L-DOPA-treated WT, D1+/− mice, and L-DOPA plus PG01037-treated mice. Scale bar = 500 µm for low-magnification and 100 µm for high-magnification images. (E,F) Immunohistochemical analysis shows that the D3R blockade decreases FosB- and pAcH3-positive cells induced by l-DOPA in D1+/− mice. *P < 0.05, **P < 0.01, and ***P < 0.001 versus D1+/− + l-DOPA; #P < 0.05, ##P < 0.01, and ###P < 0.001. All data are expressed as mean ± S.E.M. n = 6–10 for each group.
Discussion
The aim of this study was to evaluate the role of the specific D3R on the development of LID and the expression of associated molecular markers in the denervated striatum of hemiparkinsonian mice. We demonstrated that LID was decreased in D3R−/− mice compared with their WT counterparts. These behavioral responses were accompanied by a reduction in the expression of l-DOPA-induced molecular markers. Furthermore, we demonstrated that l-DOPA treatment increased D3R expression in both D1R direct and D2R indirect pathway neurons, although the presence of D1R-neuron is 2.5- to 3-fold larger. Moreover, pharmacological blockade of D3R diminished LID, without affecting the antiparkinsonian efficacy of l-DOPA. Finally, our behavioral results using D1+/− hemiparkinsonian mice support the notion that the D3R directly interacts with the D1R in LID.
D3R is highly expressed in the limbic areas of the striatum and barely expressed in the dorsal striatum (Sokoloff et al. 1990; Xu et al. 1997). However, this pattern of expression decreases following DA depletion and increases in the denervated dorsal striatum after l-DOPA treatment (Bordet et al. 1997). Previous research has suggested that D3R is associated with behavioral sensitization and dyskinesia in PD (Bordet et al. 1997; Bézard et al. 2003). In support of this assertion, lentiviral-induced D3R overexpression in the dorsal striatum leads to the appearance of dyskinetic behaviors (Cote et al. 2014). Thus, it has been hypothesized that deleting D3R might ameliorate the motor side effects induced by l-DOPA.
In this study, D3−/− hemiparkinsonian mice exhibited a reduction in l-DOPA-induced behavioral sensitization compared with their WT counterparts. This finding is supported by previous studies that revealed that D3R plays an important role in behavioral sensitization after l-DOPA treatment (Bordet et al. 1997). Also, D3R antisense oligonucleotides blocked l-DOPA-induced behavioral sensitization (van Kampen and Stoessl 2003). In the current study, we showed a reduction in LID in the D3−/− mice without interfering with the antiparkinsonian effect of l-DOPA. This result is in agreement with previous work that showed that D3R-preferring antagonists are effective at decreasing LID in different animal models (Visanji et al. 2009). As a result, we looked at the effect of D3R blockade and observed a reduction in LID without affecting the benefits of l-DOPA, in accordance with previous studies in the dyskinetic rat model (Kumar et al. 2009). However, there has been some controversy regarding pharmacological blockade of D3R and LID with some groups showing no effect (Mela et al. 2010). These conflicting results may be due to different specificities of the D3R antagonists, none of which completely distinguish between the different D2-like receptors. Indeed, recent evidence has also shown an antidyskinetic role of blocking D4R (D2-like) with a D4R-preferring antagonist (Huot et al. 2012).
Previous evidence has shown that D3R stimulation potentiates D1R-mediated behavioral responses and adenyl cyclase-mediated intracellular responses (Fiorentini et al. 2008; Marcellino et al. 2008). Indeed, it was described that D3R contributes to D1R-agonist-induced c-Fos expression in the striatum (Jung and Schmauss 1999). In addition, D3R stimulation enhances the transmitter release induced by D1R activation in the substantia nigra reticulata (Cruz-Trujillo et al. 2013). Taken together, these data suggest a synergic effect of D1R and D3R. The precise mechanisms underlying LID reduction in D3−/− mice in our study remain unclear. Possible mechanisms include loss of synergy between D1R and D3R that may act on direct pathway neurons to promote the activation of immediate early gene expression. Nevertheless, it is noteworthy that intracellular signaling in the striatum is oppositely regulated by D1R and D3R following cocaine challenge (Zhang et al. 2004). The paradoxical difference of the antagonistic effect of D3R in the normosensitive striatum versus the synergic role of D3R in the lesioned striatum is currently uncertain. A likely explanation is suggested by work showing that the D3R responsiveness is increased in the medium spiny neurons in the DA-depleted striatum (Prieto et al. 2011), and this enhanced activity of D3R could induce synergic cross talk with D1R following chronic l-DOPA treatment (Farré et al. 2014). In line with this, we found that D3R blockade in D1+/− mice, the dyskinetic response was further reduced compared with dyskinetic animals that received PG01037 and those observed in D1+/− mice, suggesting a direct interaction between the D1R and the D3R in LID.
Previous studies have shown that l-DOPA-induced D3R mRNA was preferentially expressed in dynorphin mRNA-containing neurons in the denervated dorsal striatum (Bordet et al. 2000). In the present work, using BAC-transgenic -D1R-tomato and -D2R-GFP mice, we directly demonstrated that l-DOPA-induced D3R expression occurs in both types of neurons, although in D1R-neurons, D3R expression is 2.5- to 3-fold larger than that in D2R-neurons. The colocalization of D1R and D3R may allow interaction through heterodimerization and/or overactivation of adenyl cyclase (Fiorentini et al. 2008; Marcellino et al. 2008; Farré et al. 2014) and ERK signaling pathways (Guitart et al. 2014). It is widely accepted that l-DOPA treatment increases D1R signaling, leading to increased pERK levels (Pavón et al. 2006; Westin et al. 2007), which promote phosphoacetylation of H3 (Darmopil et al. 2009; Santini et al. 2009) at the FosB promoter (Feyder et al. 2014). Our data suggest that the capacity of l-DOPA to activate this transduction pathway is partially dependent on activation of dopamine D3 receptor, since we found a decrease in FosB, pERK, and pAcH3 expressed in D1R (Darmopil et al. 2009).
An important question that persists concerns the role of the D2R striatopallidal pathway in dyskinesia. Recent evidence has shown modest l-DOPA-induced increases in gene expression in D2R indirect pathway neurons (Heiman et al. 2014). Moreover, we demonstrated that l-DOPA selectively restores the number of the dendritic spines in the D2R medium spiny neurons, suggesting that these neurons play an important role in LID (Suárez et al. 2014). Interestingly, in the present study, we observed that l-DOPA increases D3R expression in the striatopallidal neurons in hemiparkinsonian mice. Although the role of D3R in these neurons in LID is currently unknown, previous studies showed an upregulation of D2R signaling in the striatopallidal neurons that is partially mediated by the D3R in parkinsonian mice, indicating that D3R contributes to D2R-supersensitive signaling (Prieto et al. 2011). Since D2R can be responsible for the abnormal expression of long-term depression in dyskinesia (Thiele et al. 2014), it is possible that D3R could participate in this abnormal plasticity in D2R-containing neurons. However, future experiments are needed to investigate these possibilities.
To the best of our knowledge, the present study is the first to investigate the effect of genetic inactivation of D3R in parkinsonian animal models of LID. We found that D3R deletion reduces dyskinesia and expression of associated molecular markers, supporting the therapeutic relevance of D3R as a potential target for treating or preventing LID. In addition, we provide direct evidence for the first time of the expression of the D3R in the striatonigral and striatopallidal projection neurons in LID.
Funding
This work was supported by grants from the Spanish Ministerios de Economía y Competitividad (SAF2013-48532-R) and of Sanidad Política Social e Igualdad (PNSD #2012/071), and Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CB06/05/0055) and Comunidad de Madrid ref. S2011/BMD-2336 to R.M. This work was supported by the National Institutes of Health (R21 DA036921A1 to M.X.). O.S. and J.R.G.M. acknowledge Consejo Nacional de Ciencia y Tecnología-Mexico and Secretaría de Ciencia, Tecnología e Innovación-Mexico, Distrito Federal for scholarship.
Notes
We thank Beatriz Pro and Emilia Rubio for excellent technical assistance. Conflict of Interest: None declared.
References
- Andersson M, Hilbertson A, Cenci MA. 1999. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 6:461–474. [DOI] [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Espadas I, Martinez-Murillo R, Moratalla R. 2014. Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining. Neuropsychopharmacology. 39:1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. 2011. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 63:182–217. [DOI] [PubMed] [Google Scholar]
- Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B. 2009. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci. 29:4829–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bézard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P. 2003. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med. 9:762–767. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. 1997. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci. 94:3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. 2000. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 12:2117–2123. [DOI] [PubMed] [Google Scholar]
- Cote SR, Chitravanshi VC, Bleickardt C, Sapru HN, Kuzhikandathil EV. 2014. Overexpression of the dopamine D3 receptor in the rat dorsal striatum induces dyskinetic behaviors. Behav Brain Res. 263:46–50. [DOI] [PubMed] [Google Scholar]
- Cruz-Trujillo R, Avalos-Fuentes A, Rangel-Barajas C, Paz-Bermúdez F, Sierra A, Escartín-Perez E, Aceves J, Erlij D, Florán B. 2013. D3 dopamine receptors interact with dopamine D1 but not D4 receptors in the GABAergic terminals of the SNr of the rat. Neuropharmacology. 67:370–378. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Martín AB, De Diego IR, Ares S, Moratalla R. 2009. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA–induced dyskinesia and histone activation. Biol Psychiat. 66:603–613. [DOI] [PubMed] [Google Scholar]
- Farré D, Muñoz A, Moreno E, Reyes-Resina I, Canet-Pons J, Dopeso-Reyes IG, Rico AJ, Lluís C, Mallol J, Navarro G et al. 2014. Stronger dopamine D1 receptor-mediated neurotransmission in dyskinesia. Mol Neurobiol. 10.1007/s12035-014-8936-x. [DOI] [PubMed] [Google Scholar]
- Fasano S, Bezard E, D'Antoni A, Francardo V, Indrigo M, Qin L, Doveró S, Cerovic M, Cenci MA, Brambilla R. 2010. Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with l-dopa–induced dyskinesia. Proc Natl Acad Sci. 107:21824–21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyder M, Södersten E, Santini E, Vialou V, LaPlant Q, Watts EL, Spigolon G, Hansen K, Caboche J, Nestler EJ et al. 2014. A role for mitogen- and stress-activated kinase 1 in L-DOPA-induced dyskinesia and ΔFosB expression. Biol Psychiatry. 10.1016/j.biopsych.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. 2008. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 74:59–69. [DOI] [PubMed] [Google Scholar]
- Flores G, Barbeau D, Quirion R, Srivastava LK. 1996. Decreased binding of dopamine D3 receptors in limbic subregions after neonatal bilateral lesion of rat hippocampus. J Neurosci. 16:2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Aparicio R, Moratalla R. 2014. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinsońs disease. Neurobiol Dis. 62:416–425. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, O'Shea E, Martin ED, Colado MI, Moratalla R. 2011. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis. 42:391–403. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. 2005. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 48:839–848. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. 2007. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 50:4135–4146. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Håkansson K et al. 2005. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat Dis. 11(Suppl 1):S25–S29. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. 2001. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 411:86–89. [DOI] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sanchez-Soto M, Kumar-Barodia S, Naidu YT, Mallol J, Cortes A et al. 2014. Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol Pharmacol. 86:417429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Heilbut A, Francardo V, Kulicke R, Fenster RJ, Kolaczyk ED, Mesirov JP, Surmeier DJ, Cenci MA, Greengard P. 2014. Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc Natl Acad Sci USA. 111:4578–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. 1994. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 13:1235–1244. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Koprich JB, Aman A, Fox SH, Brotchie JM. 2012. L-745,870 reduces L-DOPA-induced dyskinesia in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Pharmacol Exp Ther. 342:576–585. [DOI] [PubMed] [Google Scholar]
- Jung MY, Schmauss C. 1999. Decreased c-fos responses to dopamine D(1) receptor agonist stimulation in mice deficient for D(3) receptors. J Biol Chem. 274:29406–29412. [DOI] [PubMed] [Google Scholar]
- Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR. 2009. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 56:944–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A et al. 2008. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem. 283:26016–26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CW, Hassan HE, Kim KP, Cao J, Eddington ND, Newman AH, Voulalas PJ. 2010. Characterization of the transport, metabolism, and pharmacokinetics of the dopamine D3 receptor-selective fluorenyl- and 2-pyridylphenyl amides developed for treatment of psychostimulant abuse. J Pharmacol Experim Ther. 333:854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela F, Millan MJ, Brocco M, Morari M. 2010. The selective D(3) receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect L-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats. Neuropharmacology. 58:528–536. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel AM. 1996. a. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 17:147–156. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Vallejo M, Elibol B, Graybiel AM. 1996. b. D1-class dopamine receptors influence cocaine-induced persistent expression of Fos-related proteins in striatum. Neuroreport. 8:1–5. [DOI] [PubMed] [Google Scholar]
- Murer MG, Moratalla R. 2011. Striatal signaling in L-DOPA-induced dyskinesia: common mechanisms with drug abuse and long term memory involving D1 dopamine receptor stimulation. Front Neuroanat. 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Olanow CW, Nutt JG. 2000. Levodopa motor complications in Parkinson's disease. Trends Neurosci. 23:S2–S7. [DOI] [PubMed] [Google Scholar]
- Pavón N, Martín AB, Mendialdua A, Moratalla R. 2006. ERK Phosphorylation and FosB Expression Are Associated with L-DOPA-Induced Dyskinesia in Hemiparkinsonian Mice. Biol Psychiatry. 59:64–74. [DOI] [PubMed] [Google Scholar]
- Prieto GA, Perez-Burgos A, Palomero-Rivero M, Galarraga E, Drucker-Colin R, Bargas J. 2011. Upregulation of D2-class signaling in dopamine-denervated striatum is in part mediated by D3 receptors acting on Ca V 2.1 channels via PIP2 depletion. J Neurophysiol. 105:2260–2274. [DOI] [PubMed] [Google Scholar]
- Ruiz-DeDiego I, Mellstrom B, Vallejo M, Naranjo JR, Moratalla R. 2015. Activation of DREAM (downstream regulatory element antagonistic modulator), a calcium-binding protein, reduces L-DOPA-induced dyskinesias in mice. Biol Psychiatry. 77:95–105. [DOI] [PubMed] [Google Scholar]
- Santini E, Alcacer C, Cacciatore S, Heiman M, Hervé D, Greengard P, Girault JA, Valjent E, Fisone G. 2009. l DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 108:621–633. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Hervé D, Greengard P, Fisone G. 2007. Critical Involvement of cAMP/DARPP-32 and Extracellular Signal-Regulated Protein Kinase Signaling in l-DOPA-Induced Dyskinesia. J Neurosci. 27:6995–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverdale MA, Nicholson SL, Ravenscroft P, Crossman AR, Millan MJ, Brotchie JM. 2004. Selective blockade of D(3) dopamine receptors enhances the anti-parkinsonian properties of ropinirole and levodopa in the MPTP-lesioned primate. Exp Neurol. 188:128–138. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. 1990. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 347:146–151. [DOI] [PubMed] [Google Scholar]
- Solís O, Espadas I, Del-Bel EA, Moratalla R. 2015. Nitric oxide synthase inhibition decreases l-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3(-/-) aphakia mice. Neurobiol Dis. 73:49–59. [DOI] [PubMed] [Google Scholar]
- Suárez LM, Solís O, Caramés JM, Taravini IR, Solís JM, Murer MG, Moratalla R. 2014. L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry. 75:711–722. [DOI] [PubMed] [Google Scholar]
- Thiele SL, Chen B, Lo C, Gertler TS, Warre R, Surmeier JD, Brotchie JM, Nash JE. 2014. Selective loss of bi-directional synaptic plasticity in the direct and indirect striatal output pathways accompanies generation of parkinsonism and l-DOPA induced dyskinesia in mouse models. Neurobiol Dis. 71:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kampen JM, Stoessl AJ. 2003. Effects of oligonucleotide antisense to dopamine D3 receptor mRNA in a rodent model of behavioural sensitization to levodopa. Neuroscience. 116:307–314. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Fox SH, Johnston T, Reyes G, Millan MJ, Brotchie JM. 2009. Dopamine D3 receptor stimulation underlies the development of L-DOPA-induced dyskinesia in animal models of Parkinson's disease. Neurobiol Dis. 35:184–192. [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. 2007. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 62:800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. 1997. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 19:837–848. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. 2004. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 24:3344–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]