Abstract
Deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VC/VS) is an investigational therapy for treatment-resistant obsessive-compulsive disorder. The ability of VC/VS DBS to evoke spontaneous mirth in patients, often accompanied by smiling and laughter, is clinically well documented. However, the neural correlates of DBS-evoked mirth remain poorly characterized. Patients undergoing VC/VS DBS surgery underwent intraoperative evaluation in which mirth-inducing and non-mirth-inducing stimulation localizations were identified. Using dynamic causal modeling (DCM) for fMRI, the effect of mirth-inducing DBS on functional and effective connectivity among established nodes in limbic cortico-striato-thalamo-cortical (CSTC) circuitry was investigated. Both mirth-inducing and non-mirth-inducing VC/VS DBS consistently resulted (conjunction, global null, family-wise error-corrected P < 0.05) in activation of amygdala, ventral striatum, and mediodorsal thalamus. However, only mirth-inducing DBS resulted in functional inhibition of anterior cingulate cortex. Dynamic causal modeling revealed that mirth-inducing DBS enhanced effective connectivity from anterior cingulate to ventral striatum, while attenuating connectivity from thalamus to ventral striatum relative to non-mirth-inducing stimulation. These results suggest that DBS-evoked mood elevation is accompanied by distinct patterns of limbic thalamocortical connectivity. Using the novel combination of DBS-evoked mood alteration and functional MRI in human subjects, we provide new insights into the network-level mechanisms that influence affect.
Keywords: DBS, fMRI, mood, nucleus accumbens, obsessive compulsive disorder
Introduction
In 2009, deep brain stimulation (DBS) of the ventral capsule and ventral striatum (VC/VS) became an FDA-approved therapeutic option for patients with treatment-resistant obsessive-compulsive disorder (OCD) under a Humanitarian Device Exemption (Greenberg et al. 2006, 2010). Originally based on the surgical technique of lesioning the anterior limb of the internal capsule, the DBS target has recently been optimized to reside at the caudal border of the nucleus accumbens (Greenberg et al. 2010). Beyond the effects on OCD symptoms, VC/VS DBS is known for its ability to exert reversible effects on mood including the induction of mirthful smiling and laughter, as well as despair and tearfulness (Okun et al. 2007; Greenberg et al. 2010; Kohl et al. 2014). These effects cease immediately when the stimulation parameters are adjusted or the device is turned off (Okun et al. 2007). Chronic VC/VS stimulation can also exert positive effects on mood: DBS at this target has been successful in treating comorbid depression in patients with OCD and can reduce symptom severity in patients with treatment-refractory major depressive disorder (Abelson et al. 2005; Bewernick et al. 2010, 2012; Schlaepfer et al. 2013). Although controversial, preliminary studies have indicated that acute DBS-evoked mood elevation may represent a useful clinical sign that, while not necessarily predicting optimal stimulation parameters, could potentially indicate the proximity of the DBS electrode to the chosen target (Haq et al. 2011).
The neural basis of mood elevation induced by electrical stimulation has yet to be fully characterized. However, important clues have recently emerged from studies in nonhuman primates and in epilepsy patients undergoing intracranial monitoring. Electrical stimulation of anterior cingulate (ACC) (Sperli et al. 2006; Caruana et al. 2015) and ventral insula (Caruana et al. 2011) can induce social behaviors of positive valence such as mirthful laughter (humans) and lip-smacking (nonhuman primates). In addition, several cases have been reported in which laughter was induced by stimulation of temporal regions (Arroyo et al. 1993; Satow et al. 2003; Yamao et al. 2015), supporting a potential role for the amygdala (Yamao et al. 2015). Combined with evidence accumulated in human functional neuroimaging studies, these results strongly implicate ACC, amygdala, and insula in the experience of pleasure and elevated mood (Kringelbach and Berridge 2009; Berridge and Kringelbach 2015; Lindquist et al. 2015).
Due to its established role as a relay station within the limbic cortico-striato-thalamo-cortical (CSTC) loop (Alexander et al. 1986), the VC/VS occupies a privileged position from which to modulate prefrontal and mesial temporal cortex. The ACC, amygdala, and insula all project to VS, which in turn sends direct and indirect projections to the mediodorsal (MD) thalamus by way of the ventral pallidum and substantia nigra pars reticulata (Alexander et al. 1986). In addition, the VC contains an array of white matter tracts connecting midline thalamus and brainstem with prefrontal cortex via direct reciprocal glutamatergic projections (Lehman et al. 2011).
Studying the effect of mood-altering DBS on functional connectivity within these circuits has thus far proven challenging. However, combining functional magnetic resonance imaging (fMRI) with DBS gives rise to a powerful method for studying brain connectivity and testing the modulatory effects of electrical stimulation on a neuronal network (Kahan et al. 2012, 2014; Min et al. 2012, 2014; Knight et al. 2015). The method allows one to compare widespread patterns of brain activity resulting from differing DBS contacts and stimulation parameters in a single, relatively short session (Kim et al. 2013). In addition, recent advancements in fMRI analysis allow one not only to identify brain areas affected by DBS (functional connectivity), but also to infer causality (effective connectivity) among those areas.
Within each of the subjects in this study, we identified a DBS setting that caused patients to report beneficial effects on mood, anxiety, and energy level, accompanied by spontaneous smiling and laughter. This same high-frequency DBS setting was then applied using a different active contact configuration that did not cause any significant observed or reported beneficial effects on mood or anxiety. Using both univariate fMRI analysis and dynamic causal modeling (DCM) (Friston et al. 2003), we tested the hypothesis that these 2 stimulation localizations would differentially affect functional and effective connectivity within established limbic CSTC circuits. While common networks appear to be affected by both mirth-inducing and non-mirth-inducing DBS, we observed a functional inhibition of anterior cingulate cortex and found that effective connectivity among the ACC, ventral striatum, and mediodorsal thalamus was modulated during mirth-inducing DBS relative to non-mirth-inducing stimulation. These observations may represent novel neural correlates of the human experience of mirth and may provide important clues regarding the mechanism of action by which VC/VS DBS may achieve its therapeutic effects in patients with psychiatric disorders.
Methods and Materials
Patients
After obtaining approval from the Mayo Clinic Institutional Review Board in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki), a series of 4 patients (3 male, 1 female) with medically refractory OCD were enrolled (see Supplementary Table 1). Three patients were part of a multicenter NIMH trial (NCT00640133) and 1 patient was enrolled into a clinical protocol under the Humanitarian Device Exemption indication. Inclusion and exclusion criteria were the same for both protocols (see Supplementary Information). The clinical summary of each subject was discussed and approved by the Mayo Clinic DBS Committee, which is composed of neurologists, neurosurgeons, psychiatrists, neuropsychologists, speech pathologists, and a biomedical ethicist.
Operative Approach
Each patient was secured within the LeksellTM stereotactic headframe (Elekta, Stockholm, Sweden) and a 1.5T structural MRI (General Electric. Milwaukee, WI, Signa HDx, 16× software) scan was performed prior to implantation using magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (Knight et al. 2015). Quadripolar DBS electrodes (3387 Medtronic, Inc., Minneapolis, MN, USA) were implanted bilaterally in the VC/VS (2.5 mm anterior to the anterior border of the anterior commissure, 2–4 mm inferior to the intercommisural line, 7.5 mm lateral to midline) under local anesthesia. The surgical trajectory followed the course of the anterior limb of the internal capsule as visualized in the coronal plane. Intraoperative microelectrode recordings were used to identify the nucleus accumbens. Lead placement was confirmed by computed tomography (Sensation 64, Siemens, Munich, Germany): image resolution: 0.59 × 0.59 × 1.00 mm.
Intraoperative Testing of DBS Electrodes
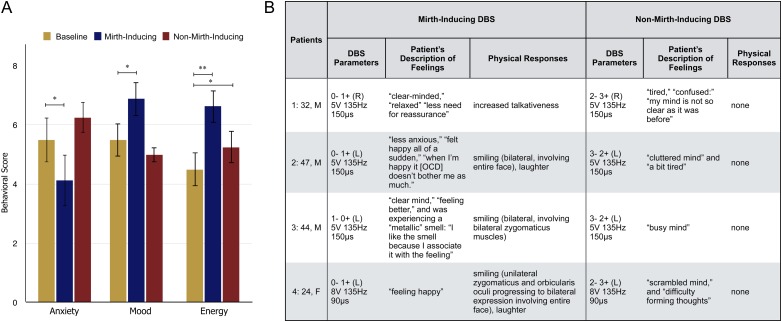
Unilateral monopolar stimulation at 135 Hz was performed to determine the thresholds of beneficial and adverse effects. At each of the 8 contacts, voltage was increased in 2 V increments from 0 to 8 V, with pulse width held at 90 µs, and then increased in 1 V increments from 0 to 5 V at 150 µs for each contact. By convention, the 4 DBS contacts are labeled Contact 0 through 3, with “−” denoting the cathode and “+” denoting the anode. Each setting was maintained for 30–60 s, during which the patient was asked to rate his/her mood, anxiety, and energy in a scale from 0 to 10 where 0 is lowest and 10 is highest mood, anxiety, or energy level, in accordance with the intraoperative clinical evaluation protocol used during the NIMH-sponsored clinical trial. The patient was also asked to describe whether he/she experienced any changes in OCD symptoms. Stimulation settings that elicited obvious mood elevation were repeated, confirmed with sham stimulation, and verified using bipolar stimulation (e.g., 2 to 3+). This was done because our experimental paradigm only allows for the application of bipolar stimulation during fMRI. Patients’ responses to mood, anxiety, energy, and OCD questions as well as any spontaneous utterances or behaviors were documented by an independent recorder. Each testing session lasted approximately 90 min and was video recorded. In each subject, the setting and contact configuration that resulted in the most robust patient-reported improvements in anxiety, mood, and energy levels was identified (“mirth-inducing DBS”). “Nonmirth-inducing DBS” is defined as DBS at the same voltage, pulse-width, and frequency as mirth-inducing DBS, but at different active contacts on the quadripolar DBS lead (e.g., from 0–1+ to 2–3+), during which no positive effects on mood or anxiety relative to baseline were observed (Fig. 1A).
Figure 1.
Intraoperative behavioral responses to mirth-inducing and non-mirth-inducing DBS. (A) Average changes in patient-reported anxiety, mood, and energy levels during DBS *P < 0.05, **P < 0.01 (paired t-test). (B) Stimulation parameters and observed behavioral responses for each patient.
fMRI Data Acquisition
Immediately following intraoperative testing, each patient underwent 4 runs of DBS during fMRI: 2 runs during DBS at the selected mirth-inducing stimulation localization and 2 runs at the nonmirth-inducing stimulation localization. Intraoperative 1.5T fMRI was performed under general anesthesia, using methods described in detail elsewhere (Knight et al. 2015). For all sequences, a manufacturer's standard transmit/receive RF head coil was used (1.5-T quad head coil, model 46-28211862; GE Healthcare). The fMRI was acquired using 2-dimensional echo-echo planar imaging (GE-EPI): repetition time/echo time, 3000/50; flip angle, 90°; field of view, 22 × 22 cm; matrix, 64 × 64; slice thickness, 3.5 mm with a 0-mm gap thickness. For each acquisition, 135 volumes (the first 5 volumes were discarded for scanner equilibration) were acquired using a block paradigm, with five 6 s stimulation periods (2 volumes) alternated with six 60 s rest periods (20 volumes), for a total time of 6 min 45 s per run. The DBS stimulation parameters applied for each patient are listed in Figure 1B. Average head-specific absorption rate (SAR) values of <0.1 W/kg were recorded during the fMRI study in all the patients, and a board-certified MRI physicist with expertise in MRI for patients with implanted electronic devices was present during all the sessions (Gorny et al. 2013). Following fMRI, patients were returned to the operating room for pulse generator implantation surgery.
fMRI Data Analysis
The fMRI data were subjected to standard preprocessing steps implemented using BrainVoyager QX software (version 2.8; Brain Innovation), including slice scan time correction, 3-dimensional motion correction, temporal filtering (high-pass filter: Fourier basis set with 5 cycles; and low-pass filter: Gaussian filter with full width at half maximum, 3.1 s), and spatial smoothing (Gaussian filter with full width at half maximum: 2.0 pixels). The data were then co-registered to the anatomical MP-RAGE images using corresponding points-based alignment and normalized to the Talairach brain coordinate system. Data from Subject 1 (right-sided stimulation) were mirrored to enable grouping with left-sided stimulation data. Double-gamma hemodynamic response function (onset = −1 s, time to response peak = 7 s, time to undershoot peak = 20 s) correlated voxel-wise BOLD signal changes with the given stimulus protocol were calculated using the general linear model. Fixed-effects group analysis was used to generate statistical parametric maps representing the effect of mirth-inducing DBS versus baseline, and nonmirth-inducing DBS versus baseline (false discovery rate corrected P < 0.001), and global masking was applied to exclude voxels outside of the brain. A fixed-effects analysis was used, because data from 4 subjects are not sufficient to conduct a traditional random-effects analysis. A fixed-effects analysis assumes equal variances among subjects, and therefore only allows for inference regarding the subjects studied, and does not allow for population-level inference. To identify nodes within limbic CSTC circuitry that were affected by DBS with a higher level of between-subject consistency, and therefore appropriate to include in our DCM analysis, we performed multisubject conjunction analyses (Friston et al. 1999) for mirth-inducing and non-mirth-inducing DBS using SPM12 [global null, family-wise error-corrected P < 0.05 (Wellcome Trust Centre for Neuroimaging, London, UK]. In brief, a multisubject conjunction analysis is a form of fixed-effects analysis that is particularly suited for studies with small sample sizes. This analysis tests for effects that are consistent over all subjects studied while addressing the multiple comparison problem implicit in imaging analyses. The DBS electrode resulted in an artifact surrounding the electrode tip, and the subgaleal electrode connector caused signal loss over unilateral parietal cortex (see Supplementary Fig. 1). Despite this artifact, BOLD signal was detected in ventral striatum (see Supplementary Figs. 1 and 3), in line with previous findings (Figee et al. 2013).
Dynamic Causal Modeling
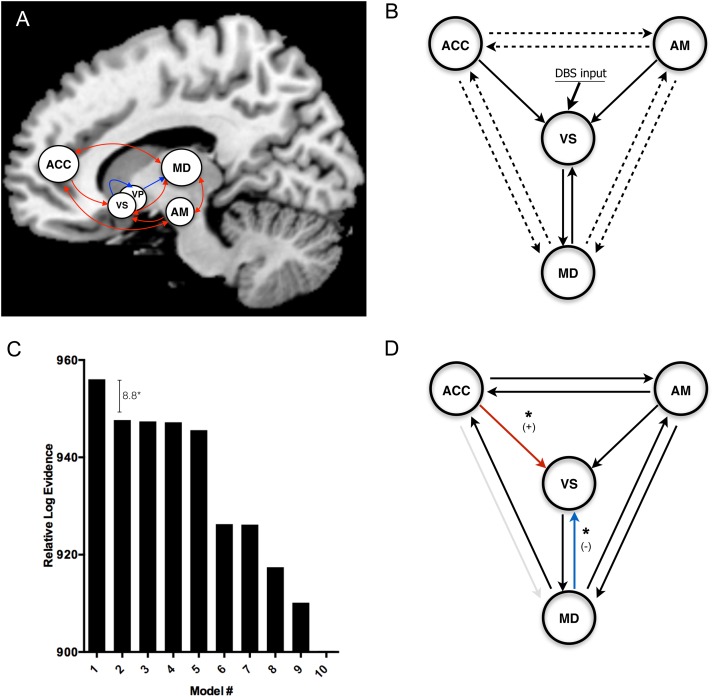
Deterministic, 1-state DCM for fMRI is used for investigating causal relationships among detected regions of neural activation (see Supplementary Information for a description of the method). To implement DCM, data were reanalyzed in SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk) and subjected to multisubject conjunction analysis (see Supplementary Information). Regions of interest (ROIs) corresponding to ACC, amygdala, ventral striatum, and MD thalamus were selected for each subject. Each ROI included voxels within a 10 mm range of the subject-specific peak voxel, which was constrained to a 12 mm radius of the group maximum voxel. For ACC, the ROI was centered upon the average location of active contacts reported to cause stimulation-evoked mirth in a series of 5 cases reported by Caruana and colleagues (TAL -6 28 25) (Caruana et al. 2015). Locations of the centers of selected regions of interest for each subject are included in Supplementary Table 2, and the average location of the peak voxel for each ROI with respect to the group activation map is shown in Supplementary Figure 4. Justification for choosing this network is provided in Supplementary Information. Briefly, these regions were chosen because they are 1) established nodes within limbic CSTC circuitry with known roles in the generation of positive mood states and 2) consistently affected by DBS among our subjects, as determined by conjunction analysis. For each subject, the BOLD time-course data from these ROIs were concatenated across all 4 runs (2 non-mirth-inducing DBS and 2 mirth-inducing DBS). For each subject, 128 competing models were constructed in which all models contained the same intrinsic connections (DCM A Matrix), reflective of the known connectivity of the limbic CSTC loop (Fig. 4A). The effect of electrical stimulation on the system that was common to both mirth-inducing and non-mirth-inducing DBS was modeled as a single driving input on VC/VS (DCM C Matrix). Effects that were unique to each of the 2 stimulation localizations were modeled as modulatory effects on between-region connections, with mirth-inducing and non-mirth-inducing DBS represented by separate DCM B Matrices (B1 and B2, respectively). Since the source of stimulation is known a priori (VS), and robust activation was observed in MD, amygdala, and ACC, we made the assumption that direct connections between these 3 structures and VS were affected in all models. To assess the likelihood of DBS modulating other between-region connections (thalamo-striatal, cortico-cortical, and thalamo-cortical), models in which DBS affected all possible combinations of these connections constituted our model space (128 models × 4 subjects = 512 models total) (Fig. 4B). Variational free energy values were estimated for each model for each subject using generalized filtering (Friston et al. 2003; Li et al. 2011). The posterior probabilities of competing models were computed using Bayesian model selection (fixed-effects assumptions) (Penny et al. 2004; Stephan et al. 2009). Interregional connectivity parameters from the winning model were then subjected to pairwise comparison using bootstrap random sampling with replacement displaying confidence intervals of 95% (Efron and Tibshirani 1994). Although this post hoc analysis is rather inefficient to implement across only 4 subjects, it enables the quantification of effect sizes across the small sample and the assessment of these effects in relation to intersubject variability.
Figure 4.
Dynamic causal modeling. (A) Established connectivity of the limbic cortico-striato-thalamo-cortical (CSTC) loop, with ACC and amygdala projecting to MD thalamus by way of ventral striatum (VS) and ventral pallidum (VP) (excitatory projection = red; inhibitory projection = blue). (B) Simplified limbic CSTC loop that forms the basis of our model space, in which the inhibitory projections from VS to VP to MD are modeled as a single excitatory projection from VS to MD. The arrows represent the anatomical priors that are held constant across model space. For solid arrows, the modulatory effect of DBS is present throughout model space. For dotted arrows, the presence of a modulatory effect due to DBS is varied across model space (128 possible permutations = 128 models). (C) The top 10 models (fixed-effects Bayesian model selection) and their respective log evidences relative to the least likely model. The winning model displayed log evidence of 8.8 greater than the closest competitor, corresponding to a posterior probability of >99.9%. (D) The winning model determined by Bayesian model selection (solid arrows indicate the presence of a modulatory effect due to DBS; red and blue arrows represent connections in which connectivity mirth-inducing DBS exerted a significant enhancement (+) or attenuation (−) of interregional connectivity relative to non-mirth-inducing DBS, respectively (see Supplementary Fig. 5). (*P < 0.05 indicates significant difference between mean interregional connectivity parameters [DCM B Matrix values]: bootstrap random sampling with replacement).
Results
Intraoperative Trial Stimulation
In each of the 4 subjects, stimulation parameters were identified that resulted in consistent improvements in patient-reported ratings of anxiety, mood, and energy level relative to baseline (paired t-test) (Fig. 1A). This response occurred during high amplitude (5–8 V), high frequency (135 Hz; 90–150 μs) unilateral DBS (right-sided in Patient 1, left-sided in Patients 2–4). Spontaneous bilateral smiling was observed in 3 of 4 patients (Fig. 1B). In Patient 2, this smile was bilateral, involving the entire face. In Patient 3, the smile involved bilateral zygomaticus muscles, but it was unclear whether the orbicularis oculi muscles were involved. In Patient 4, the smile began as a unilateral “hemi-smile” involving contralateral zygomaticus and orbicularis oculi muscles, and progressed to involve the entire face. In Patients 2 and 4, smiling was accompanied by spontaneous laughter. Upon review of videotape, only Patient 1 was found to not exhibit obvious signs of smiling or laughter. However, at the chosen setting, he reported decreased anxiety (−1) and increased mood (+1) and energy levels (+1.5), and he reported feeling “a lot better,” “relaxed,” “clear-headed,” and “at ease” with “less need for reassurance” and he began to spontaneously engage in conversation with the surgical team. Other patients used words to describe their state of mind at the mirth-inducing setting as “clear-minded” (Patients 1 and 3); “less anxious” (Patient 2); “happy” (Patients 2 and 4). While the effects on OCD symptoms were not explicitly evaluated, Patient 2 stated during mirth-inducing DBS that “when I'm happy, it [OCD] doesn't bother me as much” (see Supplementary Video) and Patient 1 reported experiencing feeling “less need for reassurance.” Notably, Patient 3 experienced an olfactory side effect during mirth-inducing DBS. He reported experiencing a “metallic” smell that accompanied the feeling of well-being. Upon cessation of stimulation, he asked whether the experimenters could “bring that smell back,” since he associated it with pleasant feelings.
In each patient, response to stimulation at the same amplitude, frequency, and pulse-width as the mirth-inducing setting was evaluated using a more dorsally located contact configuration (i.e., 3–2+ as opposed to 0–1+). This stimulation localization did not result in significant effects on anxiety or mood relative to baseline, though a positive effect on reported energy levels was observed (Fig. 1A). No mirthful responses were observed or reported at this stimulation localization, which we therefore refer to as “non-mirth-inducing.” However, patients did report vague adverse sensations that were difficult to describe. Descriptions included feeling “confused,” “cluttered,” “busy-minded,” and “scrambled” (Fig. 1B).
Anatomical Modeling of the Volume of Tissue Activated by DBS
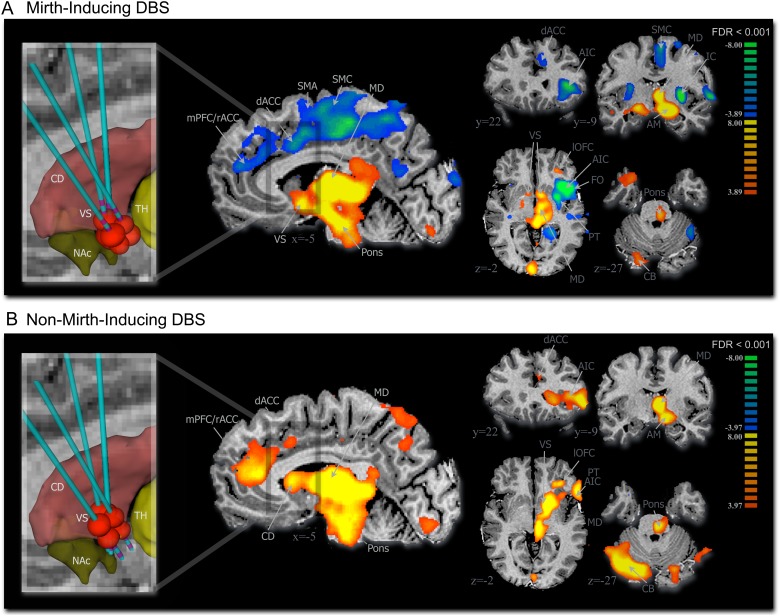
The anatomical location of current spread due to DBS or “volume of tissue activated” (VTA) by mirth-inducing and non-mirth-inducing DBS was modeled using methods described by McIntyre and colleagues (see Supplementary Information) (Lujan et al. 2011). Localizations of VTAs for mirth-inducing and non-mirth-inducing DBS with respect to subcortical structures (nucleus accumbens, ventral striatum, and caudate) are shown in the inset of Figure 2, and localizations of VTAs with respect to the subcortical anatomy of each subject are shown in Supplementary Figure 3. This analysis revealed that the VTA for mirth-inducing DBS consistently bordered on the posterolateral portion of the nucleus accumbens. By virtue of the more dorsally located active contacts used in the non-mirth-inducing DBS configuration, the VTA in all 4 subjects was predicted to reside dorsolateral to NAc, largely occupying the ventral portion of the anterior limb of the internal capsule.
Figure 2.
VC/VS DBS-evoked BOLD signal. (A) Mirth-inducing DBS and (B) non-mirth-inducing DBS (n = 4, 2 runs per subject for each DBS contact configuration) (multisubject analysis, false discovery rate corrected, P < 0.001). Inset shows approximate location of the volume of tissue activated (red ellipsoids) by the active DBS electrode relative to subcortical structures including striatum (red), nucleus accumbens (green), and thalamus (yellow). AIC, anterior insular cortex; AM, amygdala; CB, cerebellum (contralateral); CD, caudate; dACC, dorsal anterior cingulate cortex; IC, insular cortex; MD, mediodorsal thalamus; mPFC/rACC, medial prefrontal cortex/rostral anterior cingulate cortex; NAc, nucleus accumbens; PT, putamen; SMA, supplementary motor area; SMC, sensorimotor cortex; TH, thalamus; VS, ventral striatum.
fMRI Results
Group fMRI analysis of mirth-inducing and non-mirth-inducing VC/VS DBS showed that both contact configurations evoked positive BOLD signal in MD thalamus, pons, lateral orbitofrontal cortex, amygdala, and contralateral cerebellum (pFDR <0.001; Fig. 2). For both stimulation localizations, the DBS-evoked global peak t-value was located within MD thalamus (Table 1). Only mirth-inducing DBS elicited a negative BOLD signal within supplementary motor, cingulate, medial prefrontal cortex, anterior insula, frontal operculum, and putamen (Fig. 2A), while non-mirth-inducing DBS elicited positive BOLD within medial and ventrolateral prefrontal cortex (Fig. 2B). Multisubject conjunction analysis (global null, pFWE <0.05) confirmed that in all 4 subjects, both mirth-inducing and non-mirth-inducing DBS resulted in consistent BOLD responses in regions that included anterior cingulate cortex, amygdala, ventral striatum, and MD thalamus, while insular activation did not survive conjunction analysis (see Supplementary Fig. 2 and Table 1). Other regions of interest to survive conjunction analysis included sensorimotor and supplementary motor cortex (mirth-inducing DBS); ventrolateral and medial prefrontal cortex, posterior cingulate cortex, and pons (non-mirth-inducing DBS); lateral orbitofrontal cortex, putamen, and contralateral cerebellum (both contact configurations) (see Supplementary Fig. 2 and Table 2).
Table 1.
Areas of significant BOLD signal—region of interest statistics
| Location | Brodmann areas | Coordinates (x y z) | Max t-score | Cluster size (mm3) | |
|---|---|---|---|---|---|
| Mirth-inducing | Thalamus (I)a | −3 −16 8 | 13.48 | 4147 | |
| Amygdala (I)a | −9 −10 −10 | 13.89 | 4837 | ||
| Pons (I)a | −4 −21 −18 | 11.18 | 2524 | ||
| Ventral striatum (I) | −8 3 −7 | 9.65 | 2324 | ||
| Amygdala (C)a | 18 −9 −9 | 8.11 | 2902 | ||
| Fusiform gyrus (C) | 37 | 61 −58 9 | 7.58 | 3118 | |
| Cerebellum (C) | 20 −80 −27 | 6.40 | 2747 | ||
| Fusiform gyrus (I) | 37 | −45 −49 −17 | 6.05 | 717 | |
| Ventral striatum (C) | 9 4 −5 | 5.92 | 1240 | ||
| Temporal pole (C) | 38 | 28 9 −27 | 5.81 | 1062 | |
| Lateral orbitofrontal (I)a | 11 | −27 34 −3 | 5.73 | 300 | |
| Insula/claustrum (I) | 13, 14, 15, 16 | −36 14 1 | −12.00 | 10 104 | |
| Frontoparietal operculum (I) | 40, 41, 42, 43, 44, 45 | −45 11 0 | −10.75 | 8197 | |
| Putamen (I)a | −28 1 1 | −9.54 | 5184 | ||
| Middle cingulate (I) | 24, 31 | −8 −21 42 | −7.95 | 3694 | |
| Sensorimotor (I)a | 3, 4 | −4 −27 49− | −7.80 | 5911 | |
| Lingual (I)a | 18,19 | −21 −46 −14 | −7.73 | 7933 | |
| Precuneus (I) | 7 | −18 −52 56 | −7.41 | 11 274 | |
| Supplementary motor (I)a | 6 | −16 5 64 | −7.22 | 4168 | |
| Superior frontal (I) | 10 | −20 44 23 | −6.72 | 3483 | |
| Dorsal anterior cingulate (I)a | 24, 32 | −3 2 42 | −6.84 | 3794 | |
| Rostral anterior cingulate (I)a | 24, 32 | −7 44 20 | −5.74 | 2035 | |
| Medial prefrontal (I) | 9, 10 | −15 43 25 | −5.67 | 1593 | |
| Non-mirth-inducing | Thalamus (I)a | −3 −17 7 | 16.87 | 7621 | |
| Cerebellum (C)a | 29 −69 −33 | 13.74 | 27 021 | ||
| Caudate (I) | −19 14 6 | 12.07 | 4260 | ||
| Putamen (I)a | −21 8 4 | 12.05 | 3992 | ||
| Ventral striatum (I)a | 15 0 −3 | 11.62 | 1477 | ||
| Amygdala (I)a | −14 −5 −5 | 10.38 | 1803 | ||
| Frontoparietal operculum (I) | 40, 41, 42, 43, 44, 45 | −51 24 2 | 10.28 | 6868 | |
| Ventrolateral prefrontal (I)a | 46,47 | 50 26 4 | 9.97 | 9056 | |
| Posterior cingulate (I)a | 24,31 | −4 −39 6 | 9.95 | 637 | |
| Pons (I)a | −3 −24 −24 | 9.88 | 4850 | ||
| Cerebellar tonsil (C)a | 8 −53 −43 | 9.23 | 3336 | ||
| Insula/claustrum (I) | 13, 14, 15, 16 | −25 12 5 | 8.87 | 5191 | |
| Fusiform gyrus (C) | 37 | 34 −74 −21 | 8.81 | 4543 | |
| Rostral anterior cingulate (I)a | 24,32 | −5 33 15 | 8.35 | 3043 | |
| Lateral orbitofrontal (I)a | 11,47 | −31 30 −2 | 7.82 | 2002 | |
| Medial prefrontal (I)a | 9, 10 | −6 41 15 | 7.45 | 2129 | |
| Cerebellum (I) | −22 −73 −29 | 6.79 | 581 | ||
| Fusiform gyrus (I)a | 37 | −51 −57 −21 | 6.74 | 3737 | |
| Dorsal anterior cingulate (I) | 24, 32 | −7 13 36 | 5.09 | 323 |
Note: Coordinates in Talariach space (mm): x = mediolateral, y = rostrocaudal, and z = dorsoventral.
C, Contralateral; I, Ipsilateral.
aSurvived conjunction analysis (global null, family-wise error corrected P < 0.05).
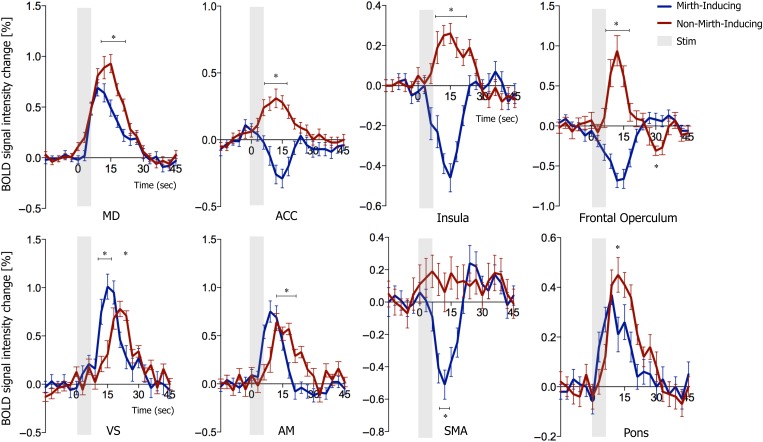
Time-course BOLD data revealed that the 2 contact configurations yielded opposite BOLD responses in the rostral, pregenual ACC (Fig. 3). Both stimulation localizations resulted in positive BOLD responses in MD, VS, and amygdala. The BOLD % change due to mirth-inducing DBS was greater in amygdala and ventral striatum, while the change due to non-mirth-inducing DBS was greater in MD thalamus and pons (Fig. 3, P < 0.001, t-test). Time-course BOLD data from insula and frontal operculum reflected the differential effect described above (Fig. 3) and confirmed that the negative signal in supplementary motor area was unique to mirth-inducing DBS (Fig. 3).
Figure 3.
BOLD signal change resulting from mirth-inducing and non-mirth-inducing DBS at peak regions of DBS-evoked activation in mediodorsal thalamus, ventral striatum, anterior cingulate cortex, amygdala, insula, frontal operculum, supplementary motor area, and pons (2-sample t-test, n = 40 stimulations per group, 2-sample t-test with Holm–Sidak correction for multiple comparisons, α = 5.0%).
DCM: Bayesian Model Selection
The winning model by BMS (fixed-effects assumptions) displayed the greatest log-evidence over the nearest competitor by 8.8, corresponding to a >99% posterior probability (Fig. 4C). In this model, DBS modulated all interregional connections in the limbic CSTC system, except for the connection from MD to ACC (Fig. 4D). In the subjects studied, winning model parameters revealed that mirth-inducing DBS resulted in a significant enhancement of interregional connectivity from ACC to VS, and an attenuation of connectivity from MD thalamus to VS (P < 0.05) (Fig. 4D). The computed values for interregional connectivity parameters are reported as 95% confidence intervals in Supplementary Fig. 5.
Discussion
Functional MRI analysis showed a pattern of modulation resulting only from mirth-inducing DBS that was common to all 4 subjects (conjunction analysis with family-wise error-corrected P < 0.05). Only during mirth-inducing DBS did we observe a negative BOLD signal that spanned supplementary motor, sensorimotor, and anterior cingulate regions. This result supports the theory that anterior cingulate and motor regions play a central role in the emergence of mirth. Recently, Caruana et al. reported that direct electrical stimulation of the rostral, pregenual anterior cingulate cortex resulted in mirthful laughter in 5 epilepsy patients undergoing intracranial monitoring (Caruana et al. 2015). In addition to its projections to mediodorsal thalamic nucleus and amygdala, this region contributes to corticobulbar projections that innervate bilateral frontalis and orbicularis oculi muscles, as well as the contralateral lower facial muscles (Morecraft et al. 2001). The ACC also projects to the reticular formation of the brainstem, a key regulator of the autonomic component of emotions (Porrino and Goldman-Rakic 1982). It is therefore highly likely that the observed BOLD effect within ACC played a role in mediating both the emotional and motor components of the mirthful response to DBS observed in our subjects.
The nature of the differential BOLD effect that was observed merits discussion, particularly with reference to the anterior cingulate cortex. While controversy surrounds the neurophysiological basis of this signal, several studies have correlated the negative BOLD signal with decreases in neuronal activity or neuronal inhibition (Shmuel et al. 2002, 2006; Devor et al. 2007; Pasley et al. 2007), as well as increased local concentration of the inhibitory neurotransmitter GABA (Northoff et al. 2007). The pregenual anterior cingulate is also known for its role in fear extinction (Büchel et al. 1998; Phelps et al. 2004) and error detection and monitoring (Gehring and Knight 2000; van Veen and Carter 2002), functions that are thought to be dysregulated in OCD (Maltby et al. 2005; Schlösser et al. 2010). The differential modulation of ACC that we observed might indicate that hyperactive error monitoring and/or fear conditioning mechanisms are selectively normalized by mirth-inducing VC/VS DBS. Indeed, stereotactic lesioning of the dorsal anterior cingulate has been found to ameliorate OCD symptoms (Dougherty et al. 2002), and decreased anterior cingulate metabolism is associated with successful response to OCD treatment by serotonin reuptake inhibitors (Perani et al. 1995).
DBS is known to result in simultaneous excitation and inhibition of neural circuits (McIntyre et al. 2004). In addition, it has been suggested that nucleus accumbens DBS may drive recurrent inhibition in prefrontal cortex through antidromic activation of corticostriatal axon collaterals (McCracken and Grace 2007). Therefore, we must also consider the possibility that the negative signal represents compensatory local inhibition in response to DBS-driven neural excitation. Notably, activation of the pregenual ACC occurs in response to a variety of positively valenced stimuli, including visual and language-based humor (Watson et al. 2007), pleasant touch (Lindgren et al. 2012), placebo and opioid analgesia (Petrovic et al. 2002). Therefore, while compensatory inhibition in response to local activation may explain the phenomenon of negative BOLD observed here, further study will be needed to elucidate its neurophysiological basis.
In this study, the active contacts during non-mirth-inducing DBS were located within the dorsolateral portion of the ventral striatum, while the active contacts during the mirth-inducing stimulation resided within the medial ventral striatum and nucleus accumbens (Fig. 2, inset; see Supplementary Fig. 3). A tracing study in nonhuman primates (Kunishio and Haber 1994) revealed the topography of corticostriatal afferents from ACC. The medial aspect of the ventral striatum and nucleus accumbens was shown to receive input from rostral ACC, while the dorsal sensorimotor striatum is innervated by the dorsal ACC. Interestingly, our results do not appear to reflect this topography, since the more ventral contact configuration (mirth-inducing) resulted in peak BOLD within the pregenual rostral ACC and dorsal ACC (Fig. 2A and see Supplementary Fig. 2A), while the more dorsal configuration (non-mirth-inducing) resulted in peak BOLD within the rostral, perigenual ACC (Fig. 2B and Supplementary Fig. 2B). Of note, the BOLD effect due to the 2 contact configurations overlapped at the pregenual ACC, and this was the region from which the differential BOLD signal was extracted for the DCM analysis. This is also the region in which electrical stimulation was reported to induce mirth in 5 epilepsy patients (Caruana et al. 2011). The observed pattern suggests that DBS-evoked activation in ACC may not have occurred simply as a result of antidromic activation of corticostriatal afferents and may rather have resulted from a polysynaptic mechanism involving cortico-striato-thalamo-cortical circuitry. We note that while our DCM analysis revealed that mirth-inducing DBS modulated effective connectivity among limbic CTSC nodes including ACC, the analysis cannot address the directionality (antidromic vs. orthodromic) by which these changes in connectivity are mediated. Therefore, while our results support the notion that DBS modulates effective connectivity throughout the limbic CTSC loop involving ACC, these effects may be mediated by orthodromic and/or antidromic activation.
During both mirth-inducing and non-mirth-inducing DBS, the global peak activation was observed within the mediodorsal thalamus. This region is well established as the primary output nucleus of the limbic corticostriatal loop, which travels from limbic cortical structures including ACC and amygdala to the MD thalamus by way of the VS and ventral pallidum (Alexander et al. 1986). It was recently shown that mediodorsal thalamus can drive feed-forward inhibition in ACC (Delevich et al. 2015). Importantly, our DCM results show a relative attenuation of connectivity from MD to VS, suggesting that this mechanism may be at play in DBS-evoked mood regulation. Due to the established connectivity of the MD thalamus with the ventral striatum, the anatomical location of the observed midline thalamic activation, and the known ability of VS DBS to modulate neuronal activity in MD thalamus (McCracken and Grace 2009), we concluded that this region of interest is highly likely to represent activation in MD thalamus. However, we note that our 3.5 mm isovoxel resolution is not sufficient to differentiate between individual midline and intralaminar thalamic nuclei, and it is quite possible that VC/VS DBS also resulted in activation of other nuclei including paraventricular and centromedian-parafascicular nuclei. Notably, both of these nuclei are extensively connected with the amygdala (Sadikot et al. 1992; Benarroch 2008), and we therefore note that these structures may also play a role in the observed modulation of thalamocortical connectivity between thalamus and amygdala.
While our effective connectivity analysis did not detect consistent changes in the CTSC circuit involving amygdala during mirth-inducing DBS, this structure was nevertheless robustly activated (conjunction, pFWE <0.05) during this stimulation condition. The amygdala is a critical structure in the regulation of affect (Höistad and Barbas 2008). It is activated in conjunction with the mesolimbic dopaminergic reward system during humor appreciation (Mobbs et al. 2003; Watson et al. 2007), and electrical stimulation of basal temporal structures can also elicit mirth, suggestive of an amygdala-dependent mechanism (Arroyo et al. 1993; Satow et al. 2003; Yamao et al. 2015). The structural and functional connectivity of the amygdala with the medial thalamus is well established (Robinson et al. 2010; Child and Benarroch 2013; Mitchell and Chakraborty 2013). The MD thalamus sends extensive projections to Layer I cortical neurons, which are known to drive synchrony across cortical regions (Haber and McFarland 2001). It is therefore highly possible that amygdala activation by this route could drive downstream activation in affective processing nodes, such as anterior cingulate, prefrontal cortex, and insula. Beyond its role in mood elevation, it is possible that the amygdala plays a role in the therapeutic effects of the stimulation. A wealth of evidence has implicated the amygdala in OCD pathology. In patients with OCD, symptom provocation results in heightened amygdala activity (Breiter et al. 1996; Mataix-Cols et al. 2004), and patients exhibit abnormal amygdala responses to emotional stimuli unrelated to symptom provocation (Cannistraro et al. 2004; Britton et al. 2010; Cardoner et al. 2011). Further investigation into the effects of VC/VS DBS on connectivity of the amygdala is warranted.
Though our analysis of effective connectivity was limited to the established limbic CSTC circuitry, our data also suggest that motor circuits also may play a role in the emergence of mirth. Conjunction analysis revealed that mirth-inducing DBS resulted in negative BOLD within sensorimotor and supplementary motor areas in all 4 patients. Electrical stimulation of supplementary motor area has been shown to elicit mirthful laughter (Fried et al. 1998) as well as laughter without emotional content (Schmitt et al. 2006). Notably, our fMRI experiment was performed with the subjects under general anesthesia. Therefore, we show that mirth-inducing DBS modulates motor circuitry in the absence of sensorimotor feedback. This result highlights the possibility that during mirth, activation of motor regions may occur in parallel with limbic and cognitive activation. Motor priming experiments have demonstrated the ability of induced facial expressions to modulate emotional experiences (Strack et al. 1988; Hennenlotter et al. 2009), and our result provides further evidence for the central role of motor circuitry in mirth. The established role of the VS, in particular the nucleus accumbens, as the limbic-motor interface (Groenewegen et al. 1996; Goto and O'Donnell 2002) is supported by anatomical data from nonhuman primates, which suggests that there are multi-synaptic projections from the ventral putamen to motor cortical areas (Kelly and Strick 2004). Our VTA analysis suggests that mirth-inducing DBS affected the posterolateral portion of nucleus accumbens. The observed BOLD data in motor-related cortical regions may therefore be explained by activation of these fibers by the more ventral stimulation localization. Since MD thalamus does not project to motor cortex, it is likely that the observed motor responses occurred by a distinct circuit mechanism than that which we explored in our DCM analysis. More comprehensive analysis that takes into account the interplay of motor and limbic circuits is therefore warranted in future work.
While it did not survive conjunction analysis, a negative BOLD effect was also observed within the anterior insula and adjoining frontal operculum during mirth-inducing DBS (Fig. 2). This region has been proposed as the neural center of “interoception,” or awareness of physical and emotional sensations, and direct electrical stimulation of this structure induces behavior of positive valence in nonhuman primates (Caruana et al. 2011), indicating that modulation of this structure may play a role in DBS-evoked mirth. Insula is activated in the context of a variety of sensory stimuli (Craig 2009), and OCD patients display hyperactive insula responses when presented with disgust-inducing cues (Stein et al. 2006). Again, while a negative BOLD signal in this region during mirth-inducing DBS may suggest that the stimulation results in insular inhibition, we cannot rule out the possibility that there is an excitatory component to this effect. In support of the latter hypothesis, the frontal operculum is activated during the observation and the subsequent voluntary imitation of smiles (Leslie et al. 2004; Hennenlotter et al. 2005; van der Gaag et al. 2007), and during the production of voluntary, involuntary, and inhibited laughter (Wattendorf et al. 2013).
Our observations above support the notion that DBS can exert robust effects on pathological coupling within emotion systems. While the study was carried out in patients with treatment refractory psychiatric illness, it remains possible that our results could have implications for the functional anatomy of emotion in normal subjects. According to the theory of interoceptive inference (Seth 2013; Barret and Satpute 2013; Gu and FitzGerald 2014), emotional states such as mirth may result from a predictive coding mechanism, in which higher order cortical areas generate predictive models that interpret emotionally salient sensory input. The anterior cingulate is known to play a key role in error prediction (Carter et al. 1998) and is also crucial for integrating prediction errors from exteroceptive (e.g., visual, auditory stimuli via the thalamus) and interoceptive (e.g., anterior insula) inputs in order update representations of the state of the self and the external world. Under a predictive coding paradigm, a crucial component for the appropriate generation of emotional states is the precision or gain assigned to ascending prediction errors. Our results suggest that mirth-inducing DBS alters connectivity between ACC and thalamus, and therefore could induce mirth by altering the sensitivity of ACC to ascending prediction errors.
Several limitations must be acknowledged. Notably, subjects were under general anesthesia during the fMRI experiments and therefore did not experience mirth during the scan itself. The effects of general anesthesia therefore represent a potential confounder. However, we note that while the effects of general anesthesia on the DBS-evoked BOLD signal have yet to be completely characterized, our preliminary results comparing MRI during DBS in awake versus anesthetized patients (Knight et al. 2015) suggest that anesthesia does not have a major effect on the pattern of DBS-evoked activation, but may affect the signal strength. In addition, this experiment involved the short-term manipulation of DBS parameters in an intraoperative setting. Therefore, the neural responses that we observed may be different from those induced by chronic therapeutic stimulation. Finally, while our VTA analysis suggests that activated neural tissue was located primarily within VS, NAc, and the anterior limb of the internal capsule, it remains likely that collateral activation of fibers of passage may have partially contributed to the observed patterns of activation. The internal capsule in particular carries a variety fiber tracts that connect prefrontal structures with several thalamic thalamic nuclei and brainstem structures (Lehman et al. 2011). While our conjunction analysis identified specific subcortical regions (mediodorsal thalamus, pons, and amygdala) that were activated with a high level of between-subject consistency, we cannot discount the potential contribution of “off target” activation due to current spread.
Until now, studies that have employed either direct electrical stimulation or functional imaging of changes in affect have provided the most compelling insights regarding the circuitry of human mood. We show that intraoperative imaging of patients undergoing psychiatric DBS provides an unprecedented opportunity to combine these 2 techniques, enabling us to dissect the network-level mechanisms that mediate the emergence of human emotion. However, due to the stringent inclusion and exclusion criteria for psychiatric DBS, it is extremely difficult to recruit large sample sizes for these studies. Nevertheless, we show that the effects of DBS that we observe with fMRI are highly robust. These effects can be detected using effective connectivity analyses, providing yet another avenue by which to investigate the mechanisms of emotion. While the present study focuses on the neural correlates of an acute DBS-evoked behavioral effect, it remains possible that some of the DBS-evoked alterations in effective and functional connectivity observed here may be predictive of therapeutic responses to psychiatric DBS. Future studies will be necessary to investigate the relationship between the neural correlates of these acute effects and those of therapeutic efficacy.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the National Institutes of Health (R01 NS 70872 awarded to K.H.L.) and by The Grainger Foundation.
Supplementary Material
Notes
We thank the patients for their willingness to participate in this study. We thank Cynthia (Cindy) J. Stoppel for her efforts in clinical coordination, Penelope Duffy, PhD, for her editorial contributions, J. Luis Lujan, PhD, for his assistance with modeling the volume of tissue activated by DBS, and Dong-Pyo Jang, PhD, for his helpful comments. Conflict of Interest: None declared.
References
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, Martis B, Giordani B. 2005. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 57:510–516. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 9:357–381. [DOI] [PubMed] [Google Scholar]
- Arroyo S, Lesser RP, Gordon B, Uematsu S, Hart J, Schwerdt P, Andreasson K, Fisher RS. 1993. Mirth, laughter and gelastic seizures. Brain. 116:757–780. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. 2013. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr Opin Neurobiol. 23:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. 2008. The midline and intralaminar thalamic nuclei: anatomic and functional specificity and implications in neurologic disease. Neurology. 71:944–949. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. 2015. Pleasure systems in the brain. Neuron. 86:646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX et al. 2010. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 67:110–116. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. 2012. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 37:1975–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS et al. 1996. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 53:595–606. [DOI] [PubMed] [Google Scholar]
- Britton JC, Stewart SE, Killgore WDS, Rosso IM, Price LM, Gold AL, Pine DS, Wilhelm S, Jenike MA, Rauch SL. 2010. Amygdala activation in response to facial expressions in pediatric obsessive-compulsive disorder. Depress Anxiety. 27:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. 1998. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 20:947–957. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Wright CI, Wedig MM, Martis B, Shin LM, Wilhelm S, Rauch SL. 2004. Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry. 56:916–920. [DOI] [PubMed] [Google Scholar]
- Cardoner N, Harrison BJ, Pujol J, Soriano-Mas C, Hernández-Ribas R, López-Solá M, Real E, Deus J, Ortiz H, Alonso P et al. 2011. Enhanced brain responsiveness during active emotional face processing in obsessive compulsive disorder. World J Biol Psychiatry. 12:349–363. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 280:747–749. [DOI] [PubMed] [Google Scholar]
- Caruana F, Avanzini P, Gozzo F, Francione S, Cardinale F, Rizzolatti G. 2015. Mirth and laughter elicited by electrical stimulation of the human anterior cingulate cortex. Cortex. 71:323–331. [DOI] [PubMed] [Google Scholar]
- Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. 2011. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol. 21:195–199. [DOI] [PubMed] [Google Scholar]
- Child ND, Benarroch EE. 2013. Anterior nucleus of the thalamus: functional organization and clinical implications. Neurology. 81:1869–1876. [DOI] [PubMed] [Google Scholar]
- Craig ADB. 2009. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 10:59–70. [DOI] [PubMed] [Google Scholar]
- Delevich K, Tucciarone J, Huang ZJ, Li B. 2015. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci. 35:5743–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EMC, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. 2007. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 27:4452–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Baer L, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, Jenike MA, Rauch SL. 2002. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 159:269–275. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. 1994. An introduction to the bootstrap, monograph in applied statistics and probability, No. 57. New York: (NY: ): Chapman and Hall. [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, de Koning P, Vulink N et al. 2013. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 16:386–387. [DOI] [PubMed] [Google Scholar]
- Fried I, Wilson CL, MacDonald KA, Behnke EJ. 1998. Electric current stimulates laughter. Nature. 391:650. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. 2003. Dynamic causal modelling. NeuroImage. 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Büchel C, Worsley KJ. 1999. Multisubject fMRI studies and conjunction analyses. NeuroImage. 10:385–396. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. 2000. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 3:516–520. [DOI] [PubMed] [Google Scholar]
- Gorny KR, Presti MF, Goerss SJ, Hwang SC, Jang DP, Kim I, Min H-K, Shu Y, Favazza CP, Lee KH et al. 2013. Measurements of RF heating during 3.0-T MRI of a pig implanted with deep brain stimulator. Magnetic Reson Imaging. 31:783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O'Donnell P. 2002. Timing-dependent limbic-motor synaptic integration in the nucleus accumbens. PNAS. 99:13189–13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Gabriëls LA, Malone DA, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS et al. 2010. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 15:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA. 2006. Three-year outcomes in deep brain stimulation for highly resistant obsessive–compulsive disorder. Neuropsychopharmacology. 31:2384–2393. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer A. 1996. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 107:485–511. [DOI] [PubMed] [Google Scholar]
- Gu X, FitzGerald THB. 2014. Interoceptive inference: homeostasis and decision-making. Trends Cogn Sci. 18:269–270. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR. 2001. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 7:315–324. [DOI] [PubMed] [Google Scholar]
- Haq IU, Foote KD, Goodman WG, Wu SS, Sudhyadhom A, Ricciuti N, Siddiqui MS, Bowers D, Jacobson CE, Ward H et al. 2011. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. NeuroImage. 54:S247–S255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenlotter A, Dresel C, Castrop F, Ceballos-Baumann AO, Wohlschlager AM, Haslinger B. 2009. The link between facial feedback and neural activity within central circuitries of emotion - new insights from botulinum toxin-induced denervation of frown muscles. Cerebral Cortex. 19:537–542. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, Lange KW, Ceballos-Baumann AO. 2005. A common neural basis for receptive and expressive communication of pleasant facial affect. NeuroImage. 26:581–591. [DOI] [PubMed] [Google Scholar]
- Höistad M, Barbas H. 2008. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. NeuroImage. 40:1016–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J, Mancini L, Urner M, Friston K, Hariz M, Holl E, White M, Ruge D, Jahanshahi M, Boertien T et al. 2012. Therapeutic subthalamic nucleus deep brain stimulation reverses cortico-thalamic coupling during voluntary movements in Parkinson's disease. PLoS ONE. 7:e50270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J, Urner M, Moran R, Flandin G, Marreiros A, Mancini L, White M, Thornton J, Yousry T, Zrinzo L et al. 2014. Resting state functional MRI in Parkinson's disease: the impact of deep brain stimulation on ‘effective’ connectivity. Brain. 137:1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. 2004. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 143:449–459. [DOI] [PubMed] [Google Scholar]
- Kim JP, Min H-K, Knight EJ, Duffy PS, Abulseoud OA, Marsh MP, Kelsey K, Blaha CD, Bennet KE, Frye MA et al. 2013. Centromedian-parafascicular deep brain stimulation induces differential functional inhibition of the motor, associative, and limbic circuits in large animals. Biol Psychiatry. 74:917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EJ, Testini P, Min H-K, Gibson WS, Gorny KR, Favazza CP, Felmlee JP, Kim I, Welker KM, Clayton DA et al. 2015. Motor and nonmotor circuitry activation induced by subthalamic nucleus deep brain stimulation in patients with Parkinson disease: intraoperative functional magnetic resonance imaging for deep brain stimulation. Mayo Clinic Proc. 90:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Schönherr DM, Luigjes J, Denys D, Mueller UJ, Lenartz D, Visser-Vandewalle V, Kuhn J. 2014. Deep brain stimulation for treatment-refractory obsessive compulsive disorder: a systematic review. BMC Psychiatry. 14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC. 2009. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 13:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishio K, Haber SN. 1994. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 350:337–356. [DOI] [PubMed] [Google Scholar]
- Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. 2011. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 31:10392–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie KR, Johnson-Frey SH, Grafton ST. 2004. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 21:601–607. [DOI] [PubMed] [Google Scholar]
- Li B, Daunizeau J, Stephan KE, Penny W, Hu D, Friston K. 2011. Generalised filtering and stochastic DCM for fMRI. NeuroImage. 58:442–457. [DOI] [PubMed] [Google Scholar]
- Lindgren L, Westling G, Brulin C, Lehtipalo S, Andersson M, Nyberg L. 2012. Pleasant human touch is represented in pregenual anterior cingulate cortex. NeuroImage. 59:3427–3432. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. 2015. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb Cortex. doi:10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed]
- Lujan JL, Chaturvedi A, Malone DA, Rezai AR, Machado AG, McIntyre CC. 2011. Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Hum Brain Mapp. 33:958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA. 2005. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. NeuroImage. 24:495–503. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. 2004. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 61:564–576. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. 2007. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci.. 27:12601–12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. 2009. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 29:5354–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. 2004. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 115:1239–1248. [DOI] [PubMed] [Google Scholar]
- Min H-K, Hwang S-C, Marsh MP, Kim I, Knight E, Striemer B, Felmlee JP, Welker KM, Blaha CD, Chang S-Y et al. 2012. Deep brain stimulation induces BOLD activation in motor and non-motor networks: an fMRI comparison study of STN and EN/GPi DBS in large animals. NeuroImage. 63:1408–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H-K, Ross EK, Lee KH, Dennis K, Han SR, Jeong JH, Marsh MP, Striemer B, Felmlee JP, Lujan JL et al. 2014. Subthalamic nucleus deep brain stimulation induces motor network BOLD activation: use of a high precision MRI guided stereotactic system for nonhuman primates. Brain Stimul. 7:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Chakraborty S. 2013. What does the mediodorsal thalamus do? Front Syst Neurosci. 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Greicius MD, Abdel-Azim E, Menon V, Reiss AL. 2003. Humor modulates the mesolimbic reward centers. Neuron. 40:1041–1048. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. 2001. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 124:176–208. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Beoeker H, Grimm S, Boesiger P. 2007. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 10:1515–1517. [DOI] [PubMed] [Google Scholar]
- Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, Knight W, Martin P, Goodman WK. 2007. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. J Neurol Neurosurg Psychiatr. 78:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. 2007. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. NeuroImage. 36:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. 2004. Comparing dynamic causal models. NeuroImage. 22:1157–1172. [DOI] [PubMed] [Google Scholar]
- Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, Bellodi L, Smeraldi E, Fazio F. 1995. [18F]FDG PET study in obsessive-compulsive disorder. A clinical/metabolic correlation study after treatment. Br J Psychiatry. 166:244–250. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. 2002. Placebo and opioid analgesia - imaging a shared neuronal network. Science. 295:1737–1740. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 43:897–905. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Goldman-Rakic PS. 1982. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Compar Neurol. 205:63–76. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. 2010. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 31:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Francois C. 1992. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Compar Neurol. 315:137–159. [DOI] [PubMed] [Google Scholar]
- Satow T, Usui K, Matsuhashi M, Yamamoto J, Begum T, Shibasaki H, Ikeda A, Mikuni N, Miyamoto S, Hashimoto N. 2003. Mirth and laughter arising from human temporal cortex. J Neurol Neurosurg Psychiatr. 74:1004–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA. 2013. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 73:1204–1212. [DOI] [PubMed] [Google Scholar]
- Schlösser RGM, Wagner G, Schachtzabel C, Peikert G, Koch K, Reichenbach JR, Sauer H. 2010. Fronto-cingulate effective connectivity in obsessive compulsive disorder: a study with fMRI and dynamic causal modeling. Hum Brain Mapp. 31:1834–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JJ, Janszky J, Woermann F, Tuxhorn I, Ebner A. 2006. Laughter and the mesial and lateral premotor cortex. Epilepsy Behavior. 8:773–775. [DOI] [PubMed] [Google Scholar]
- Seth AK. 2013. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci. 17: 565–573. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. 2006. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 9:569–577. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. 2002. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 36:1195–1210. [DOI] [PubMed] [Google Scholar]
- Sperli F, Spinelli L, Pollo C, Seeck M. 2006. Contralateral smile and laughter, but no mirth, induced by electrical stimulation of the cingulate cortex. Epilepsia. 47:440–443. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Arya M, Pietrini P, Rapoport JL, Swedo SE. 2006. Neurocircuitry of disgust and anxiety in obsessive-compulsive disorder: a positron emission tomography study. Metab Brain Dis. 21:267–277. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. 2009. Bayesian model selection for group studies. NeuroImage. 46:1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack F, Martin L, Stepper S. 1988. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Personality Social Psychol. 54:768–777. [DOI] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. 2007. Facial expressions: what the mirror neuron system can and cannot tell us. Soc Neurosci. 2:179–222. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. 2002. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 77:477–482. [DOI] [PubMed] [Google Scholar]
- Watson KK, Matthews BJ, Allman JM. 2007. Brain activation during sight gags and language-dependent humor. Cereb Cortex. 17:314–324. [DOI] [PubMed] [Google Scholar]
- Wattendorf E, Westermann B, Fiedler K, Kasa E, Lotze M, Celio MR. 2013. Exploration of the neural correlates of ticklish laughter by functional magnetic resonance imaging. Cereb Cortex. 23:1280–1289. [DOI] [PubMed] [Google Scholar]
- Yamao Y, Matsumoto R, Kunieda T, Shibata S, Shimotake A, Kikuchi T, Satow T, Mikuni N, Fukuyama H, Ikeda A et al. 2015. Neural correlates of mirth and laughter: a direct electrical cortical stimulation study. Cortex. 66:134–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.