Abstract
Acclimation of leaves to high light (HL; 650 μmol m−2 s−1) was investigated in the long-lived epiphytic bromeliad Guzmania monostachia and compared with plants maintained under low light (LL; 50 μmol m−2 s−1). Despite a 60% decrease in total chlorophyll in HL-grown plants, the chlorophyll a/b ratio remained stable. Additionally, chloroplasts from HL-grown plants had a much lower thylakoid content and reduced granal stacking. Immunofluorescent labeling techniques were used to quantify the level of photosynthetic polypeptides. HL-grown plants had 30% to 40% of the content observed in LL-grown plants for the light-harvesting complex associated with photosystems I and II, the 33-kD photosystem II polypeptide, and Rubisco. These results were verified using conventional biochemical techniques, which revealed a comparable 60% decrease in Rubisco and total soluble protein. When expressed on a chlorophyll basis, the amount of protein and Rubisco was constant for HL- and LL-grown plants. Acclimation to HL involves a tightly coordinated adjustment of photosynthesis, indicating a highly regulated decrease in the number of photosynthetic units manifested at the level of the content of light-harvesting and electron transport components, the amount of Rubisco, and the induction of Crassulacean acid metabolism. This response occurs in mature leaves and may represent a strategy that is optimal for the resource-limited epiphytic niche.
The acclimation of higher plants to contrasting light regimes involves specific features of leaf structure and chloroplast composition (for reviews, see Boardman, 1977; Björkman, 1981; Anderson, 1986; Anderson et al., 1988, 1996). Leaves of shade plants are usually thinner than comparable sun leaves of the same species and have large and numerous chloroplasts, arranged in parallel to the leaf surface in order to maximize light absorption. In contrast, sun plants may have smaller and fewer chloroplasts arranged perpendicular to the surface. Shade chloroplasts have a high thylakoid membrane volume, and a large number of stacks per granum, whereas sun chloroplasts have a reduced thylakoid volume and granal stacking. In most cases, the chlorophyll a/b ratio is reduced and the PSII antenna size is increased in shade plants. This is coupled to a higher ratio of PSII/PSI and decreased levels of Rubisco. Acclimation to particular light environments within a species also involves comparable functional and compositional changes to the leaf morphology, thylakoid membrane composition, and enzyme complement. However, the same responses to photon flux density (PFD) are not universally observed in all species (Chow et al., 1991; McKiernan and Baker, 1991; Walters and Horton, 1995; Murchie and Horton, 1997).
Acclimation of photosynthesis has tended to be rationalized in terms of optimizing photosynthetic efficiency in sun and shade conditions: maximizing light capture in low light (LL) and photosynthetic capacity in high light (HL). However, when plants are exposed to light levels in excess of those that can be used in photosynthesis, there is a potential for photodamage to the proteins and pigments of the thylakoid membrane (Osmond, 1981; Powles, 1984). Therefore, it has been argued that some aspects of the long-term HL response are related to photoprotection (Anderson and Osmond, 1987; Horton, 1987; Anderson et al., 1996; Murchie and Horton, 1998), and acclimation is concerned with balancing efficient light utilization while protecting against photodamage. Many previous studies have been performed using crop plants under conditions that permit leaf expansion and development under the particular experimental regime, complicating interpretation. In this paper, data are presented regarding mature leaves in a plant species that is both slow growing and subject to large fluctuations in irradiance under natural conditions. Therefore, an extreme photoprotective strategy would be predicted.
Guzmania monostachia (L.) Rusby ex Mez var monostachia is an epiphytic bromeliad common throughout the middle to upper canopy in tropical forests in Trinidad (Pittendrigh, 1948; Griffiths and Smith, 1983). It has previously been demonstrated that acclimation to both HL and LL is rapid and reversible in this species (Maxwell et al., 1994, 1995). G. monostachia has a large potential for nonphotochemical dissipation of excess absorbed light energy (Ruban et al., 1993; Maxwell et al., 1994, 1995), a process induced when plants are exposed to prolonged light stress conditions. HL acclimation in G. monostachia is associated with a decrease in chlorophyll content and an increase in the xanthophyll cycle carotenoids coupled to metabolic changes, as shown by the induction of CAM (Maxwell et al., 1994).
In this paper we describe the changes in chloroplast structure and composition that accompany these functional changes. Immunolabeling of thin leaf sections has enabled localization of specific enzymes in different parts of the leaf and has even shown the subcellular distribution (Marrison and Leech, 1992; Marrison et al., 1993; Williams et al., 1998). In an extension of this technique and to develop the use of immunolabeling in the study of light acclimation, the amounts of individual proteins have been quantified in this study by determining the intensity of immunofluorescence (Leech and Marrison, 1996). Using improved image analysis, we describe the use of immunolabeling to quantify changes in the levels of photosynthetic enzymes and thylakoid membrane constituents in G. monostachia. This has allowed a complete analysis of the acclimation of mature leaves.
MATERIALS AND METHODS
Plant Material
Guzmania monostachia (L.) Rusby ex Mez var monostachia plants were collected from epiphytic sites in March, 1995. Plants were removed from deciduous hosts within Verdant Vale and the Simla Research Station, Trinidad, West Indies (grid reference: PS869823, location 10°41′N, 61°17′W). On return to the UK, the plants were maintained in a controlled-environment cabinet (Sanyo Gallenkamp, Loughborough, UK) over a 10-h photoperiod (8 am–6 pm). Day-night values for temperature and RH were 25°C/23°C and 65%/80%, respectively. Plants were subjected to a HL regime (650 μmol m−2 s−1) or a LL regime (50 μmol m−2 s−1) for at least 3 months prior to experimentation. The plants were watered and provided with a complete nutrient solution (BabyBio, Pan Britannica Industries, Hertfordshire, UK) every 2 d. The leaf material described was sampled from the center of the blade for leaves of the third innermost rosette. All tissues were sampled during the first 4 h of the photoperiod.
Chlorophyll Content
Five replicate leaf disc samples were taken and the extraction procedure was as described by Maxwell et al. (1994).
Transmission Electron Microscopy
Fresh leaf sections were cut by hand from the central portion of the leaf and immediately fixed in 3% (w/v) glutaraldehyde in 0.1 m phosphate buffer overnight. The samples were then washed in buffer, fixed in 2% (w/v) osmium tetroxide (aqueous) for 2 h, dehydrated with 75% (v/v) ethanol, then 95% (v/v) with three changes over 15 min, followed by absolute ethanol and then propylene oxide for two 10-min periods. The tissue was kept in Spurr's resin overnight and then for 3 d, with fresh resin applied on a daily basis, before fixing in fresh Spurr's resin. The sample was polymerized at 60°C overnight and 80-nm sections were cut on a ultramicrotome. The sections were mounted on copper grids and examined using a transmission electron microscope (model CM10, Philips, Eindhoven, The Netherlands).
Light Microscopy, Immunolabeling, and Quantification of Photosynthetic Proteins
Preparation of Leaf Tissue
Slices of leaf tissue (10 mm thick) were cut transversely from the mid portion of the leaf, and each slice was further dissected into five 2-mm, consecutive, transverse slices. Three leaves from a single plant at each light intensity were sampled. Slices of leaf tissue were fixed overnight in 3% (w/v) paraformaldehyde, 50% (v/v) ethanol, and 5% (v/v) acetic acid at room temperature and embedded in PEG 1500 (Fisons, Bellevue, WA) as previously described (Marrison and Leech, 1992). Transverse sections (10 μm) were cut on a microtome using a disposable steel blade, placed onto dampened polysine slides (BDH, Poole, UK), and left to dry on a hot plate overnight at 40°C. Ribbons of five tissue sections from leaves grown under each light intensity were analyzed together on a single microscope slide (total of 15 sections) to ensure uniformity of processing.
Immunolocalization
Immunolocalization was carried out as described by Marrison and Leech (1992) for the following antibodies: the major light-harvesting complex of PSII (LHCII), the light-harvesting complex of PSI (LHCI), the 33-kD component of the oxygen-evolving complex of PSII (OEC33), Rubisco, and PEP carboxylase (PEPc). Sections were incubated overnight at 4°C with 100 μL of primary antibody diluted in 0.5% (w/v) BSA/PBS. The antibodies used were gifts from Professor N.R. Baker (University of Essex, UK; LHCI), Dr. R. Nechushtai (The Hebrew University, Jerusalem; LHCII), Dr. A.J. Keys (Institute of Arable Crops Research, Harpenden, UK; Rubisco), Professor J. Barber (Imperial College, London; OEC33), and Professor H. Bohnert (University of Arizona, Tucson; PEPc). The sections were washed for 15 min in 0.5% (w/v) BSA/PBS, 0.01% (v/v) Tween 20/PBS, and PBS, and were then incubated for 1 h at room temperature with 100 μL of fluorescein isothiocyanate-conjugated goat-anti-rabbit antiserum (Sigma) diluted as recommended by the supplier. The sections were washed as above and mounted in Vectashield (Vectalabs, Burlingame, CA). Sections were viewed using a Nikon FXA microscope with an epifluorescence attachment, a high-pressure mercury lamp, and a filter combination of dichroic mirror 510, excitation filter 450 to 490 nm, and barrier filter 515IF. Photomicrographs were taken using Kodak Ektachrome 400 color slide film with automatic exposure setting.
Measurement of Chloroplast Size
The chloroplast cross-sectional area was measured after immunolocalization and photography (312× total magnification). Chloroplasts that appeared to have the largest cross-sectional area were measured from the 35-mm color slide film using a CCD TV camera (TM-560, PULNiX America, Sunnyvale, CA) with a zoom lens (model 18-108/2.5, PULNiX) and image analysis software (Seescan, Cambridge, UK). Mean chloroplast volume was calculated assuming the chloroplast cross-sectional plan area to be that of an oblate spheroid. Chloroplast volume was then calculated using the formula 4/3πr2(r/2), where r is the radius of the circular plan area.
Quantification of Protein Levels
To quantify LHCI, LHCII, Rubisco, and OEC33 protein levels in chloroplasts from plants grown at different light intensities, the level of the chloroplast immunofluorescence obtained after immunolocalization was measured using a microphotometry system attached to a fluorescence microscope and two-dimensional imaging software. Five tissue sections from leaves grown under each of the light intensities were analyzed together on a single microscope slide to ensure uniformity of processing. The sensitivity of the microphotometry system was set using tissue grown at a light intensity of 50 μmol photon m−2 s−1 and immunolocalization with Rubisco antisera and fluorescein isothiocyanate (i.e. from the most abundant protein at maximal expression). The maximum fluorescence value within 25 chloroplasts was recorded for each light intensity (five chloroplasts from five different sections). The product of the mean fluorescence value and the mean plastid volume was used to calculate the total fluorescence value per plastid for each PFD. This technique has recently been confirmed as an accurate procedure for the quantification of chloroplast proteins (Leech and Marrison, 1996).
Total Soluble Protein
Total soluble protein was calculated from five replicates taken from individual plants. Approximately 100 mg of tissue was ground in 0.5 mL of protein extraction buffer (450 mm Bicine, 50 mm 3-(cyclohexylamino)propanesulfonic acid [CAPS], pH 10.3, 1% [w/v] PEG 600, 1% [w/v] SDS, and 50 mm DTT). The samples were centrifuged at 13,000g for 5 min at 4°C. The supernatant (100 μL) was removed and added to 4 mL of Bradford's reagent. The mixture was incubated for 15 min at room temperature and the A595 was read. Protein content was calculated from a calibration curve (0−100 μg of BSA).
Western Blots
Leaf discs (2 cm2) were excised and homogenized in 500 μL of protein extraction buffer. The samples were centrifuged at 13,000g at 4°C for 5 min, and then the proteins were precipitated with 4 volumes of 80% (v/v) acetone. The samples were centrifuged at 4°C for 10 min, the supernatant was discarded, and the pellet was resuspended in Hammelis buffer and boiled for 3 min. The extracts were run on 10% (v/v) polyacrylamide gels and probed with Rubisco antisera using goat-anti-rabbit secondary antisera, following the procedure of Walker and Leegood (1996).
Carboxyarbanitol-1-Bisphosphate (14CABP) Binding
Leaf tissue (300 mg) was excised and protein extracted at 4°C in 2 mL of extraction buffer (350 mm HEPES-KOH, pH 8.0, 10 mm MgCl2, 5 mm EDTA, 14 mm β-mercaptoethanol, 3% [w/v] PVP 25, 15% [w/v] PEG 20,000, and 2.5% [v/v] Tween 20), 20 μL of 100 mm PMSF, and 200 mg of PVPP. Plant extract (200 mL) was mixed with 200 mL of 14CABP-binding solution (200 mm Na2SO4, 200 mm Bicine-NaOH, pH 8.0, 80 mm MgCl2, 20 mm NaHCO3, 100 mm β-mercaptoethanol, 0.3 mm 14CABP, and 1 pCi pmol−1). The protein was precipitated with 288 μL of 60% (w/v) PEG 3400 at 4°C for 30 min and resedimented by centrifugation at 10,000g for 10 min at 4°C. The pellet was resuspended in 400 μL of PEG on ice for 15 min and resedimented as above. Following a final PEG precipitation step, the pellet was resuspended in 1 mL of 1% (v/v) Triton X-100 and radioactivity was determined using a liquid scintillation counter. Calculations of Rubisco content were made assuming that the molecular mass of pure Rubisco is 550 kD with eight active sites per enzyme. The 14CABP was kindly provided by Dr. Martin Parry (IACR-Rothamstead, UK).
Photosynthetic Capacity
Photosynthetic capacity was assessed from the light- and CO2-saturated rate of O2 evolution measured using a leaf disc electrode system (LD2/2, Hansatech UK, King's Lynn, UK) as previously described (Maxwell et al., 1994). Measurements were made using five replicates at an actinic PFD of 400 μmol photon m−2 s−1, which was saturating for both HL- and LL-grown plants.
Titratable Acidity
The magnitude of CAM activity was assessed from the dawn-to-dusk level of titratable acidity (ΔH+). Leaf disc samples were frozen prior to extraction in 4 mL of boiling water. A 1-mL aliquot was titrated against 10 mm NaOH using phenolphthalein as an indicator.
RESULTS
Leaf Morphology
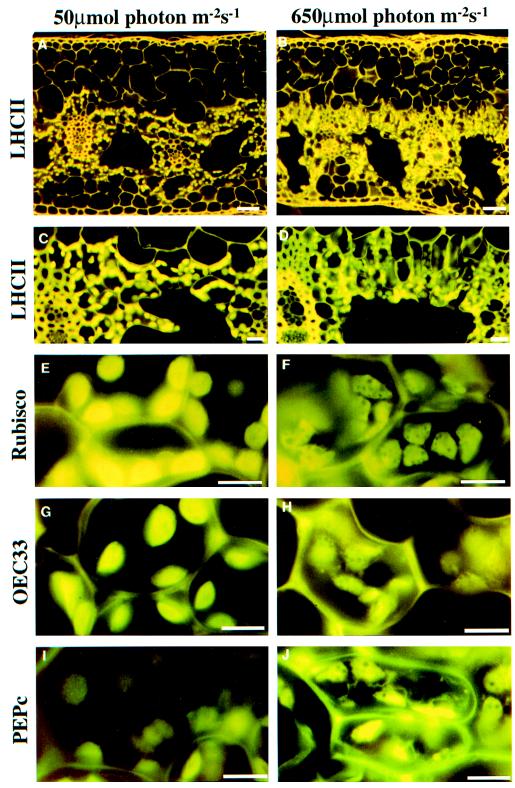
Light micrographs of LL and HL leaf sections of G. monostachia are shown in Figure 1. In these examples, the tissue shows immunofluorescent labeling for the LHCII, which was also used to derive quantitative data for this protein (see below). Strong labeling was observed despite the use of a heterologous antibody, and autofluorescence from the cell wall was also apparent. Irrespective of the growth regime, the chlorenchyma and chloroplasts were concentrated around and facing the air space, with the chloroplasts facing the air space. A stalk cell was observed on a number of chlorenchyma cells that extended into the air spaces (Fig. 1C). The number of chloroplasts per cell, the chloroplast cross-sectional area, and the chloroplast volume were all reduced under HL conditions (Table I).
Figure 1.
Immunolocalization of PEG-embedded G. monostachia leaf sections (10 μm thick). A, LL at low magnification; B, HL at low magnification; C, LL at high magnification; and D, HL at high magnification. Leaf sections were incubated with primary antisera to LHCII (A–D), Rubisco (E and F), OEC33 (G and H), and PEPc (I and J) followed by secondary goat anti-rabbit antisera conjugated to fluorescein isothiocyanate for LL plants (A, C, E, G, and I) and HL plants (B, D, F, H, and J). The scale bars represent 50 μm (A and B), 20 μm (C and D), and 10 μm (E–J).
Table I.
Chloroplast characteristics of HL- and LL-acclimated G. monostachia
| Treatment | Chloroplasts per Cell | Chloroplast Volume | Chloroplast Cross-Sectional Area | Thylakoid Volume | Stacks per Granum |
|---|---|---|---|---|---|
| no. | μm3 | μm2 | % | no. | |
| HL | 5 | 29 | 18 | 15 | 5 |
| LL | 12 | 54 | 27 | 38 | 14 |
The chloroplast data were calculated from 120 replicate sections taken from five plants. The sd was <5% of the mean in all cases.
Chloroplast Ultrastructure
All chloroplasts described were from spongy mesophyll cells located around the air spaces. The HL chloroplasts were longer and thinner, but smaller than the LL chloroplasts. The amount of thylakoid per chloroplast and the amount of membrane stacking was considerably reduced in HL plants. These differences were quantified and the results are shown in Table I. The volume of thylakoid was approximately 38% of the chloroplast in LL plants, while the thylakoid membrane accounted for only 15% in HL plants. The LL plants had an average of 14 stacks per granum but, in marked contrast, on average the number of stacks per granum was five in the HL plants (Table I).
Immunoquantitation of Chloroplast Components
At high magnification in immunolabeled fluorescent images, the individual chloroplasts are clearly seen and the chloroplasts of HL and LL plants are shown after labeling with antibodies to LHCII (Fig. 1, A–D), Rubisco (Fig. 1, E and F), and OEC33 (Fig. 1, G and H). Additional images were obtained for LHCI (data not shown). In each case there was a decrease in fluorescence intensity from LL to HL.
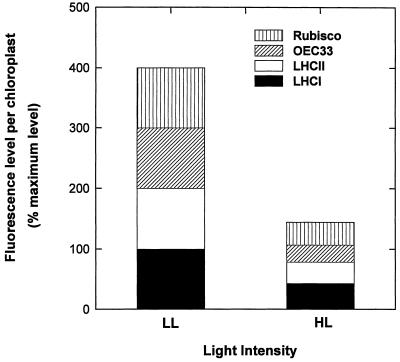
Measurement of the mean chloroplast cross-sectional area (Table I) allowed estimation of the chloroplast volume, which, together with the fluorescence intensity of the image, was used to calculate the relative contents of each antigen per chloroplast (Fig. 2). For the thylakoid components (LHCII, LHCI, and OEC33) and Rubisco, there was a parallel decrease of an approximately similar magnitude in all plants, with the content in HL plants being approximately 30% to 40% of that in LL plants (Fig. 2).
Figure 2.
Percentage maximum level of fluorescence per chloroplast for HL and LL plants normalized to 100% for each component under LL following immunolocalization using primary antisera to Rubisco, OEC33, LHCII, and LHCI.
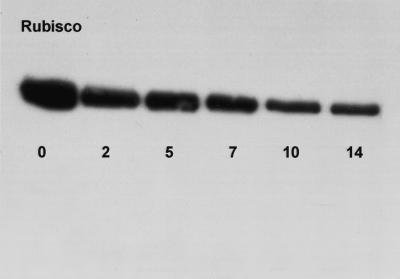
Total soluble protein was 3 and 9 mg g−1 fresh weight for HL and LL plants, respectively (Table II). Supporting data illustrating the loss of Rubisco under HL is provided in Figure 3 using western blotting. In this example, plants were transferred from LL to HL conditions over a 14-d period. When proteins were extracted and probed with an antibody to the Rubisco large subunit, it was apparent that over the experimental period, considerable degradation of Rubisco had occurred (Fig. 3). The amount of Rubisco was 1 mg g−1 fresh weight in HL and 2.7 mg g−1 fresh weight in LL plants, as quantified using 14CABP binding. Total chlorophyll was 233 and 639 μg g−1 fresh weight for HL and LL plants, respectively, while the chlorophyll a/b ratio remained constant (Table II). Similar results for pigment and protein data were obtained when determined on an area basis (data not shown). When expressed per unit chlorophyll, the amount of protein and Rubisco was comparable for HL and LL plants, while the ratio of Rubisco per unit protein was slightly higher under LL (Table II). The light- and CO2-saturated photosynthetic capacity (Pmax) was 6.4 and 3.2 μmol O2 m−2 s−1 for HL and LL plants, respectively. This is equivalent to the maximum photosynthetic rates of 84 nmol O2 mg−1 Chl for HL plants and 15.3 nmol O2 mg−1 Chl for LL plants.
Table II.
Chlorophyll, protein, and Rubisco content of HL- and LL-acclimated G. monostachia
| Treatment | Total Soluble Protein | Chlorophyll | Chlorophyll a/b | Rubisco | Protein per Unit Chlorophyll | Rubisco per Unit Chlorophyll | Rubisco per Unit Protein |
|---|---|---|---|---|---|---|---|
| mg g−1 fresh wt | μg g−1 fresh wt | mg g−1 fresh wt | |||||
| HL | 3.05 | 232.8 | 2.43 | 1.04 | 13.40 | 4.60 | 0.35 |
| (±0.19) | (±24.03) | (±0.08) | (±0.68) | (±1.40) | (±0.50) | (±0.04) | |
| LL | 9.25 | 639.05 | 2.68 | 2.73 | 14.50 | 4.81 | 0.47 |
| (±0.34) | (±0.02) | (±0.11) | (±0.11) | (±0.33) | (±0.06) | (±0.04) |
The pigment and protein data were calculated from five replicates sampled from individual plants. The data are provided as the means ± se.
Figure 3.
Western blots showing the abundance of Rubisco in leaves of G. monostachia over a 14-d transfer from LL to HL conditions.
Immunodetection of PEPc
The level of immunofluorescence obtained for PEPc was below the detection limit in the LL plants (Fig. 1I) but was clearly visible in the image of the HL leaf (Fig. 1J), although quantification of this diffuse image proved to be beyond the image analysis procedures. The magnitude of CAM activity assessed as the dawn-to-dusk difference in titratable acidity was 23 ± 1.8 and 115 ± 5.9 μg g−1 fresh weight for LL and HL plants, respectively.
DISCUSSION
Previously, the physiological basis of HL acclimation in leaves of G. monostachia has been described under field (Maxwell et al., 1992, 1995) and laboratory conditions (Maxwell et al., 1994). We are now able to rationalize these observations in the context of acclimation of the chloroplast in response to the light environment. We have demonstrated that HL acclimation involves a highly regulated adjustment in mature leaves at the level of chloroplast volume, thylakoid membrane composition, and complement of photosynthetic protein (specifically, the number of functional photosynthetic units).
Although alternative strategies are observed, a number of generalizations may be made regarding the acclimation of the photosynthetic apparatus to HL (Anderson et al., 1996). The underlying mechanisms result from the contrasting environmental pressures experienced in LL as opposed to HL. While success under shade requires maximal absorption and efficient transduction of light energy, plants in HL are optimized for maximizing photosynthetic light use, which may be accompanied by a parallel reduction in excitation energy capture and mechanisms that prevent long-term damage to the photosynthetic apparatus when energy capture exceeds the photosynthetic requirement. Increases in photosynthetic capacity are generally supported by adjustment of the composition of the thylakoid membrane proteins and chloroplast ultrastructure. HL plants tend toward a smaller light-harvesting complex relative to the PSII reaction center, which is manifested as a reduced chlorophyll content and an increased chlorophyll a/b ratio (Boardman, 1977; Anderson et al., 1988). However, it has been demonstrated that the chlorophyll content may be stable under contrasting light, while there is an increase in whole-chain electron transport rate and Rubisco activity in a strategy whereby light harvesting is constant but adjustments in the photosynthetic capacity alone permit successful HL acclimation (Chow and Anderson, 1987; McKiernan and Baker, 1991). Chow et al. (1991) demonstrated that the chlorophyll a/b ratio is stable under both HL and LL in Tradescantia albiflora despite a very significant increase in Rubisco activity, an example of a HL response dominated by acclimation within the stromal compartment of the chloroplast.
We have investigated chloroplastic acclimation to HL in leaves of G. monostachia. Acclimation to HL resulted in a significant reduction in chloroplast size, thylakoid volume, and the extent of granal stacking. This response was associated with a nearly parallel reduction in amounts of LHCI, LHCII, OEC33, and Rubisco. While the reduction of LHCII is a well-documented response to HL, the loss of OEC33 (indicating a reduction in PSII core subunits) and Rubisco were not predicted, since photosynthetic capacity was significantly higher in HL plants. It is likely that the induction of CAM functions to maintain or increase photosynthetic light use, and it is possible that the higher levels of Rubisco in LL plants may reflect a storage role for this protein in light-limited conditions.
It is therefore evident that the nature of acclimation to HL in G. monostachia is unlike many of the conventional processes that arise in response to HL as outlined above. In this species, a more extreme strategy is employed. When expressed per unit of chlorophyll, it is evident that the amounts of protein and Rubisco are remarkably constant in both HL and LL leaves, despite very significant absolute reductions in chlorophyll and protein per unit area or fresh weight. This observation, coupled to the stability of the chlorophyll a/b ratio, suggests that acclimation to HL involves the formation of fewer, but photosynthetically competent, photosynthetic units.
As a consequence of HL acclimation, approximately 60% total soluble protein and chlorophyll were degraded, indicating plastid protease activity. Regulatory proteolysis is crucial for correct chloroplast functioning in terms of chloroplast development, during stress, and for the removal of ill-conformed, damaged, or malfunctioning proteins (Schmidt and Mishkind, 1983; Desimone et al., 1998).
When evaluating the extreme features of the acclimation of G. monostachia to HL, several factors relating to the physiology and ecology of this species may be taken into consideration. At an ecological level, epiphytes are subject to a highly dynamic light environment with a prolonged period of exposure to HL (Maxwell et al., 1992, 1995). Successful acclimation to this light environment therefore requires both short-term strategies to dissipate excess excitation energy and long-term acclimation to HL. In addition, the epiphytic habitat is characterized by extreme resource limitation and relies on animal/plant detritus and leachate for nutrition. Therefore, epiphytes in general are slow-growing and have long-lived leaves (4–5 years).
Unlike many crop or ruderal plants, leaves of G. monostachia exhibit a massive loss of photosynthetically competent units rather than investing in photosynthetic proteins. It is possible that breakdown and subsequent re-allocation of photosynthetic protein is a critical component of HL acclimation in these and possibly other species native to nutrient-limited habitats. For example, we have demonstrated that the induction of CAM during HL acclimation in G. monostachia involves de novo synthesis of PEPc (Fig. 1j). It is entirely feasible that breakdown products could be re-utilized in the formation of this protein (Winter et al., 1982). Additionally, construction, maintenance, and repair costs are elevated under HL, and therefore a reduction in the number of functional units may reflect the limited carbon and nitrogen budgets available to epiphytic bromeliads. In this respect, it is noteworthy that constant values for chlorophyll a/b have been observed for a number of epiphytic bromeliads under contrasting light environments in the field (Griffiths and Maxwell, 1999), indicating that this acclimative strategy may be genetically conserved within the Bromeliaceae. Despite the apparent constraints imposed on epiphytes by resource-limited habitats, G. monostachia exhibits highly effective short- and long-term strategies in response to HL that have permitted exploitation of the epiphytic niche.
ACKNOWLEDGMENTS
We are to grateful Anne Borland (The University of Newcastle) and to Sasha Ruban and Robin Walters (University of Sheffield) for helpful comments and encouragement. Pauline Gaitens and John Proctor provided technical assistance with the transmission and scanning electron microscopy (University of Sheffield).
Footnotes
This work was funded by the Natural Environment Research Council, UK (grant no. GR3/8763).
LITERATURE CITED
- Anderson JM. Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136. [Google Scholar]
- Anderson JM, Chow WS, Goodchild DJ. Thylakoid membrane organization in sun/shade acclimation. Aust J Plant Physiol. 1988;15:11–26. [Google Scholar]
- Anderson JM, Chow WS, Park YI. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res. 1996;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Osmond CB. Shade-sun responses: compromises between acclimation and photoinhibition. In: Kyle DJ, Osmond CB, Arntzen DJ, editors. Photoinhibition, Topics in Photosynthesis, Vol 9. Amsterdam: Elsevier; 1987. pp. 1–38. [Google Scholar]
- Björkman O. Responses to different quantum flux intensities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of Plant Physiology, Vol 12A. Berlin: Springer-Verlag; 1981. pp. 57–107. [Google Scholar]
- Boardman NK. Comparative photosynthesis of sun and shade plants. Annu Rev Plant Physiol. 1977;28:355–377. [Google Scholar]
- Chow WS, Adamson HY, Anderson JM. Photosynthetic acclimation of Tradescantia albiflora to growth irradiance: lack of adjustment of light-harvesting components and its consequences. Plant Physiol. 1991;81:175–182. [Google Scholar]
- Chow WS, Anderson JM. Photosynthetic responses of Pisum sativum to an increase in irradiance during growth. I. Photosynthetic activities. Aust J Plant Physiol. 1987;14:1–8. [Google Scholar]
- Desimone M, Wagner E, Johanningmeier U. Degradation of active-oxygen modified ribulose-1,5-bisphosphate carboxylase/oxygenase by chloroplastic proteases requires ATP-hydrolysis. Planta. 1998;205:459–466. [Google Scholar]
- Griffiths H, Maxwell K. In memory of CS Pittendrigh: photoprotective strategies in epiphytic bromeliads in relation to exposure within the forest canopy. Funct Ecol. 1999;13:15–23. [Google Scholar]
- Griffiths H, Smith JAC. Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-form, habitat preference and the occurrence of CAM. Oecolgia. 1983;60:176–184. doi: 10.1007/BF00379519. [DOI] [PubMed] [Google Scholar]
- Horton P. Interplay between environmental and metabolic factors in the regulation of electron transport in higher plants. In: Biggins J, editor. Progress in Photosynthesis Research, Vol II. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 681–688. [Google Scholar]
- Leech RM, Marrison JL. Immunofluorescent quantitation of chloroplast proteins. Plant J. 1996;10:1169–1175. doi: 10.1046/j.1365-313x.1996.10061169.x. [DOI] [PubMed] [Google Scholar]
- Marrison JL, Leech RM. Co-immunolocalization of topoisomerase II and chloroplast DNA in developing, dividing and mature wheat chloroplasts. Plant J. 1992;2:783–790. [Google Scholar]
- Marrison JL, Onyeocha L, Baker A, Leech RM. Recognition of peroxisomes by immunofluorescence in transformed and untransformed tobacco cells. Plant Physiol. 1993;103:1055–1059. doi: 10.1104/pp.103.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C, Griffiths H, Borland AM, Broadmeadow MSJ, Fordham MC. Short term photosynthetic responses of the C3-CAM epiphyte Guzmania monostachia to simulated tropical seasonal transitions under field conditions. Aust J Plant Physiol. 1995;22:771–781. [Google Scholar]
- Maxwell C, Griffiths H, Borland AM, Broadmeadow MSJ, McDavid C. Photoinhibitory responses of Guzmania monostachia during the dry season in Trinidad maintain photochemical integrity under adverse conditions. Plant Cell Environ. 1992;15:37–47. [Google Scholar]
- Maxwell C, Griffiths H, Young AJ. Photosynthetic acclimation to light regime and water stress by the C3-CAM epiphyte Guzmania monostachia: gas exchange characteristics, photochemical efficiency and the xanthophyll cycle. Funct Ecol. 1994;8:746–754. [Google Scholar]
- McKiernan M, Baker NR. Adaptation to shade of the light-harvesting apparatus in Silene dioica. Plant Cell Environ. 1991;14:205–212. [Google Scholar]
- Murchie EH, Horton P. Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and growth preference. Plant Cell Environ. 1997;20:438–448. [Google Scholar]
- Murchie EH, Horton P. Contrasting patterns of photosynthetic acclimation to the light environment are dependent on the differential expression of the responses to altered irradiance and spectral quality. Plant Cell Environ. 1998;21:139–148. [Google Scholar]
- Osmond CB. Photorespiration and photoinhibition: some implications for the energetics of photosynthesis. Biochim Biophys Acta. 1981;639:77–98. [Google Scholar]
- Pittendrigh CS. The bromeliad-Anopheles-malaria complex in Trinidad. I. The bromeliad flora. Evolution. 1948;2:58–89. doi: 10.1111/j.1558-5646.1948.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- Ruban AV, Young AJ, Horton P. Induction of nonphotochemical energy dissipation and absorbance changes in leaves: evidence for changes in the state of the light harvesting system of photosystem II in vivo. Plant Physiol. 1993;102:741–750. doi: 10.1104/pp.102.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt GW, Mishkind ML. Rapid degradation of unassembled ribulose 1,5-bisphospahte carboxylase small subunits in chloroplasts. Proc Natl Acad Sci USA. 1983;80:2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RP, Leegood RC. Phosphorylation of phosphoenolpyruvate carboxykinase in plants. Biochem J. 1996;317:653–657. doi: 10.1042/bj3170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RG, Horton P. Acclimation of Arabidopsis thaliana to the light environment: regulation of chloroplast composition. Planta. 1995;197:475–481. doi: 10.1007/BF00196669. [DOI] [PubMed] [Google Scholar]
- Williams M, Robertson EJ, Leech RM, Harwood JL. The effects of elevated atmospheric CO2 on lipid metabolism in leaves from mature wheat (Triticum aestivum cv. Hereward) plants. Plant Cell Environ. 1998;21:927–936. [Google Scholar]
- Winter K, Foster JG, Schmitt MR, Edwards GE. Activity and quantity of ribulosebisphosphate carboxylase- and phosphoenolpyruvate carboxylase-protein in two Crassulacean acid metabolism plants in relation to leaf age, nitrogen nutrition and point in time during a day/night cycle. Planta. 1982;154:309–317. doi: 10.1007/BF00393908. [DOI] [PubMed] [Google Scholar]