Summary
A clinical case series is presented to characterize the interaction between carbapenem antibiotics and sodium valproate. Six illustrative cases are presented in which carbapenem therapy led to the rapid depletion of serum valproate levels, and one case is presented to demonstrate the difficulty of initiating valproate therapy in patients already on meropenem. The speed of valproate depletion after the initiation of carbapenem therapy, the effect of treatment duration, clinical manifestations, delay in valproate level normalization after carbapenem therapy, the efficacy of supplemental valproate doses, and the usefulness of valproate dose escalation are evaluated. Five out of the 7 patients became acutely symptomatic owing to their subtherapeutic valproate levels. The presented cases also highlight the relatively slow normalization of valproate levels after discontinuation of the antibiotic therapy. Our cases suggest that the interaction is not absorption‐mediated because all of our patients received intravenous valproate. We observed that the introduction of alternative antiepileptic drugs (AEDs) may be preferable to valproate dose escalation, which is ineffective in the presence of concomitant meropenem therapy. The characterization and recognition of this interaction have implications for the management of a particularly vulnerable patient cohort.
Keywords: Carbapenem, Meropenem, Valproate, Drug interaction, Pharmacokinetics
Carbapenem antibiotics, such as meropenem, are increasingly prescribed for the treatment of severe multidrug‐resistant bacterial infections. Despite its side effect profile, sodium valproate (VPA) remains widely used as an effective broad‐spectrum antiepileptic drug (AED) and is also prescribed for a range of psychiatric conditions. Although the interaction between VPA and carbapenems is relatively well described, the exact mechanism remains poorly understood.
Our objective is to present seven illustrative clinical cases of carbapenem‐VPA interaction, evaluate the speed of depletion and normalization of VPA levels, examine the effect of treatment duration, assess management options, and reflect on possible biochemical mechanisms underpinning this interaction.
Methods
Clinical cases were prospectively captured in a tertiary referral center during an 18‐month period between January 2015 and June 2016. The selection criteria were defined as coadministration of meropenem and VPA. A total of seven cases were ascertained with the help of the department of pharmacy in our center. Medical notes and electronic records were systematically reviewed with a focus on clinical background, epilepsy diagnosis, AEDs prior to admission, indication and duration of carbapenem therapy, VPA levels prior to and post‐meropenem therapy, VPA dose adjustments, and supplementation or introduction of an alternative AED (Table 1).
Table 1.
Summary of the demographic and clinical profile of the cases
| Case | Age | Sex | Pre‐meropenem VPA dose | Last pre‐meropenem VPA level | Duration of meropenem therapy | VPA measured after initiation of meropenem | VPA level during meropenem therapy | Patient symptomatic of low VPA | Intervention | Normalization of VPA levels post‐meropenem therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | Female | 800 mg BD | 19 | +14 days | 24 h | 8 | Yes; seizures | Increased dose + bolus + alternative AED | RIP |
| 2 | 42 | Male | 600 mg BD | 41 | 10 days | 24 h | <3 | No | No | 4 weeks |
| 3 | 24 | Female | 600 mg TDS | 45 | 3 days | 72 h | 9 | Yes; seizures | Increased dose + bolus + alternative AED | RIP |
| 4 | 42 | Male | 625 mg BD | N/A | 24 + 7 days | Meropenem introduced first | 6 | Yes; seizures | Increased dose + bolus | 4 weeks |
| 5 | 78 | Male | 600 mg BD | 27 | 3 days | 72 h | 9 | No, but intubated | Meropenem discontinued | RIP |
| 6 | 25 | Male | 1,300/1,200 mg | 106 | 7 days | 7 days | 11 | Yes; seizures | No | Checked 2 months later |
| 7 | 69 | Female | 300 mg BD | 40 | 10 days | 72 h | <3 | Yes; hypomania | Increased dose | 8 days |
AED, antiepileptic drug; BD, twice a day; RIP, patient deceased; TDS, three times a day.
Results
Case 1
A 55‐year‐old female patient with a background of localization‐related epilepsy (LRE), intellectual disability, schizophrenia, bronchiectasis, and chronic obstructive pulmonary disease was admitted to the hospital with aspiration pneumonia. She has been seizure free for years on VPA 800 mg twice a day (BD), and her serum VPA levels at admission were subtherapeutic: 19 mg/L. Her respiratory sepsis was initially treated with piperacillin/tazobactam. She subsequently developed type II respiratory failure and bilateral pulmonary embolism (PE); she was started on bilevel positive airway pressure (BIPAP) ventilation, and her antimicrobial therapy was changed to meropenem and vancomycin. Twenty‐four hours later, she had a cluster of seizures, and her serum VPA level was measured at 8 mg/L, which dropped further to 4 mg/L 2 days later despite supplemental intravenous (i.v.) VPA loading and dose escalation. Intravenous lacosamide was introduced, which has successfully controlled the seizures. The patient was treated with meropenem for a total of 14 days. VPA levels reached 19 mg/L only 3 days after the discontinuation of meropenem. The patient developed further medical complications in addition to her sepsis, bilateral PEs, and renal failure; she had an episode of hematemesis, and an underlying metastatic esophageal malignancy was identified. Palliative measures were subsequently introduced, and the patient died (Fig. 1).
Figure 1.
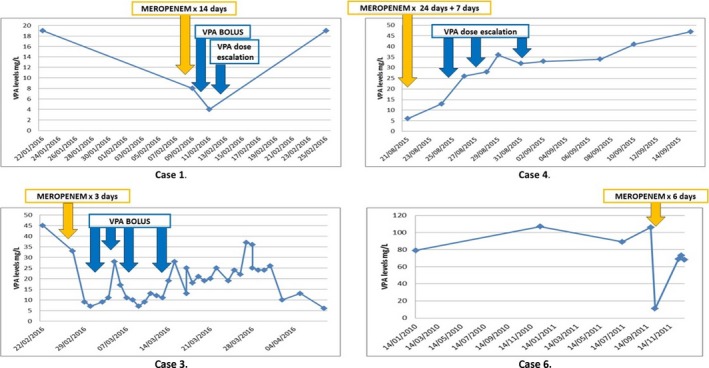
Graphical representation of serum sodium valproate (VPA) levels before and after meropenem therapy. Duration of meropenem therapy and VPA dose adjustments are indicated at the relevant time points. Yellow arrows indicate the starting date of meropenem therapy; a dose of 1 g eight hourly was given in each case.
Case 2
A 42‐year‐old male patient with a background of primary progressive multiple sclerosis, LRE, long‐term suprapubic catheter, intrathecal baclofen therapy, and recurrent lower respiratory tract infections was admitted to the ICU for the management of respiratory failure secondary to pneumonia. His LRE has been controlled with VPA 600 mg BD and phenytoin 300 mg once a day (OD). He received a 10‐day course of i.v. meropenem for the treatment of his recurrent respiratory sepsis, and his VPA levels became undetectable. It took more than 4 weeks for the serum VPA levels to normalize following meropenem therapy. He did not experience any seizures during this time.
Case 3
A 24‐year‐old female patient was admitted to the ICU with subarachnoid hemorrhage secondary to an arteriovenous malformation. She developed refractory status epilepticus despite multiple AEDs; lacosamide 100 mg BD, phenytoin 100 mg three times a day (TDS), levetiracetam 1,500 mg BD, and VPA 600 mg TDS. Additionally, intravenous midazolam 10 mg/h and intermittent intravenous propofol and sodium thiopental administration were also required to control her seizures. She was initially treated with a combination of vancomycin, piperacillin/tazobactam, and caspofungin for her respiratory and central line sepsis. Because of persistent sepsis, meropenem was introduced, which was changed 3 days later to aztreonam on the basis of microbial sensitivity results. Despite low‐satisfactory serum VPA levels prior to treatment (45 mg/L), after 3 days of meropenem therapy, serum VPA levels dropped to 7 mg/L. In spite of the multiple supplemental i.v. VPA boluses and the increased daily VPA dose, serum VPA levels remained subtherapeutic and never reached pre‐meropenem serum levels. Because of persistent sepsis, multiorgan failure, and refractory status epilepticus, palliative comfort measures were introduced, and the patient died.
Case 4
A 42‐year‐old male patient with a background of type 2 diabetes and hypertension was admitted to our center for the resection of a large olfactory groove meningioma with significant mass effect. He developed postsurgical complications: cerebritis, subdural hematoma, right lower lobe pneumonia, and refractory seizures. His postoperative AEDs included levetiracetam 2,000 mg BD, phenytoin 200 mg BD, clobazam 10 mg TDS, and lamotrigine 100 mg BD. Owing to ongoing respiratory sepsis, he received two courses of i.v. meropenem therapy, first for 24 days and 2 weeks later for another 7 days. Because of refractory seizures, VPA was added to his AEDs during his second course of meropenem, with an initial dose of 625 mg BD that was later increased to 500 mg TDS. Because of persistently low VPA levels, VPA was further increased to 800 mg TDS, then to 1,000 mg TDS, and finally to 1,400 mg TDS. Eventually, 4 weeks after the discontinuation of meropenem, serum VPA levels reached 47 mg/L, the patient became seizure free, and VPA was decreased to 1,250 TDS.
Case 5
A 78‐year‐old male patient was admitted with respiratory sepsis due to recurrent aspiration pneumonias. His medical background included LRE secondary to a large right frontal meningioma, hypertension, adrenal insufficiency, hypercholesterolemia, bronchial asthma, and recurrent lower respiratory tract infections. He took VPA 600 mg BD prior to admission. Soon after his admission, he developed type I respiratory failure. Owing to progressive respiratory failure, he was admitted to the ICU, where he developed acute respiratory distress syndrome (ARDS) and was intubated. Bronchoalveolar lavage identified Klebsiella pneumoniae, and polymerase chain reactions (PCRs) were also positive for Pneumocystis pneumonia (PCP). Antimicrobial therapy was adjusted to include meropenem, vancomycin, and co‐trimoxazole. Though meropenem therapy was given for only 3 days, serum VPA levels dropped to 9 mg/L and only increased to 26 mg/L—close to preadmission levels—10 days after the discontinuation of meropenem therapy. Despite aggressive ICU management, ARDS did not resolve; the patient developed renal, hepatic, and cardiac insufficiency; became anuric, and died.
Case 6
A 25‐year‐old male patient with a background of LRE, tuberous sclerosis complex, and osteoporosis was admitted with severe respiratory sepsis. His seizures were well controlled prior to his admission on VPA 1,300 mg mane 1,200 nocte, lacosamide 100 mg BD, phenobarbitone 45 mg OD, clobazam 10 mg OD, and phenytoin 150 mg BD. His chest radiograph was suggestive of bilateral pneumonia, and his CT thorax confirmed evidence of consolidation within the right upper lobe and the left lower lobe. Despite empirical antibiotic therapy with piperacillin/tazobactam and clarithromycin, the patient clinically deteriorated, and antibiotic therapy was escalated to aztreonam and vancomycin. On the basis of sputum and bronchoalveolar lavage cultures and the persistent sepsis, the antimicrobial therapy was adjusted to meropenem and vancomycin. On the day of his admission, his VPA levels were 106 mg/L. Seven days after the initiation of intravenous meropenem therapy, his VPA levels dropped to 11 mg/L. He had a cluster of seizures on day 7 of his antibiotic therapy that was treated acutely with i.v. lorazepam, and meropenem was then discontinued. VPA dose was not changed, and his levels normalized when checked at his follow‐up outpatient appointment.
Case 7
A 69‐year‐old female patient with longstanding diagnosis of bipolar disorder established on PO VPA 300 mg BD became septic secondary to a severe urinary tract infection with markedly raised inflammatory markers. Group‐2 extended‐spectrum beta‐lactamase‐producing Escherichia coli was isolated from the blood cultures sensitive to meropenem. She received 10 days of intravenous meropenem therapy. On day 3 of her antibiotic therapy, VPA levels became undetectable. The patient became restless, overfamiliar, and distractible and developed pressured speech and insomnia suggestive of hypomania. Her VPA dose was increased to 600 mg BD, and no bolus supplementation was administered. Her VPA levels rose to 56 mg/L 8 days after the meropenem was discontinued.
Discussion
Our cases illustrate the clinical challenges of coadministering VPA and meropenem. The presented cases also highlight the rapid depletion of VPA levels following carbapenem therapy and the relatively slow normalization of VPA levels after discontinuation of the antibiotic therapy. In two patients, we detected significantly subtherapeutic levels within 24 h (cases 1 and 2), and three patients had depleted VPA levels within 72 h on meropenem (cases 3, 5, and 7). The majority of our patients became acutely symptomatic secondary to their low VPA levels, either developing seizures or becoming hypomanic (case 7). Relatively short courses of meropenem, such as 3 days (cases 3 and 5), may have a similar effect as longer courses. Our cases also suggest that meropenem therapy has a relatively long‐lasting effect on VPA levels. In one of our patients, it took 8 days for the levels to normalize (case 7) after meropenem therapy was discontinued; in another patient, it took 4 weeks for the levels to normalize despite supplemental doses and dose escalation (case 4). In our patient to whom no supplemental doses were given (case 2), it took 4 weeks for VPA levels to normalize. On the basis of our case series, we believe that once the patient is on meropenem therapy, the introduction of an alternative AED is advisable because increasing VPA doses is likely to be ineffective (cases 1, 3, 4, and 7).
Case 4 demonstrates the difficulty of initiating sodium VPA therapy in patients on meropenem antibiotics. This patient has not been on VPA therapy previously but needed the addition of an intravenously administered AED as a result of refractory seizures. This case supports the view that VPA should not be initiated in patients on meropenem.
Our cases suggest that the interaction cannot be primarily absorption‐mediated because all of our patients received intravenous VPA administration. A number of mechanisms have been proposed in the literature underpinning the VPA‐carbapenem interaction. Increased renal clearance,1 reduced intestinal VPA absorption,2 increased VPA glucuronidation,1 increased distribution of VPA into erythrocytes, decreased hydrolysis of VPA glucuronide (VPA‐G) to VPA,3 and inhibition of plasma protein binding of VPA4 were implicated. One of the most widely accepted explanations, however,3 is that carbapenems inhibit the enzymatic breakdown of VPA‐G to VPA, resulting in swift hepatic clearance of VPA‐G and subsequent drop in plasma VPA concentrations. It is hypothesized that acylpeptide hydrolase (APEH) is the enzyme responsible for VPA‐G hydrolysis;5 it has been shown to be inhibited by both panipenem and meropenem in vitro.
Most of the reported clinical cases in the literature are adult patients,6 many of whom became symptomatic of subtherapeutic VPA levels secondary to carbapenem therapy.7 , 8 The majority of these cases are either haematological9 or neurosurgical10 patients. The youngest published cases include a 14‐month‐old boy treated for pyelonephritis and a 7‐month‐old girl treated with meropenem for pneumonia.11
Although in our case series all patients received meropenem therapy, we note that all carbapenem antibiotics, including panipenem, tebipenem, ertapenem and imipenem, have a similar depleting effect on serum VPA levels.12, 13, 14, 15
Conclusion
Coadministration of meropenem and VPA is typically undertaken in a vulnerable patient cohort, frequently in an intensive care or high‐dependency unit setting. The consideration, prompt recognition, and adequate management of this interaction are paramount to prevent life‐threatening complications. There is an urgent need to raise awareness of this interaction among critical care physicians, neurosurgeons, neurologists, and pharmacists because carbapenems are increasingly used in critically ill patients worldwide.
Disclosure of Conflict of Interest
Peter Bede is supported by the Irish Institute of Clinical Neuroscience (IICN)–Novartis Ireland Research Grant and the Perrigo Clinician‐Scientist Research Fellowship. The remaining authors have no conflicts of interests. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Biography
Dr. Peter Bede is a clinical lecturer at Trinity College Dublin and the leader of the Quantitative Neuroimaging Group of the Academic Unit of Neurology.

References
- 1. Yamamura N, Imura K, Naganuma H, et al. Panipenem, a carbapenem antibiotic, enhances the glucuronidation of intravenously administered valproic acid in rats. Drug Metab Dispos 1999;27:724–730. [PubMed] [Google Scholar]
- 2. Torii M, Takiguchi Y, Izumi M, et al. Carbapenem antibiotics inhibit valproic acid transport in Caco‐2 cell monolayers. Int J Pharm 2002;233:253–256. [DOI] [PubMed] [Google Scholar]
- 3. Nakajima Y, Mizobuchi M, Nakamura M, et al. Mechanism of the drug interaction between valproic acid and carbapenem antibiotics in monkeys and rats. Drug Metab Dispos 2004;32:1383–1391. [DOI] [PubMed] [Google Scholar]
- 4. Hobara N, Hokama N, Ohshiro S, et al. Possible mechanisms of low levels of plasma valproate concentration following simultaneous administration of sodium valproate and meropenem. Biog Amines 2003;17:409–420. [Google Scholar]
- 5. Suzuki E, Yamamura N, Ogura Y, et al. Identification of valproic acid glucuronide hydrolase as a key enzyme for the interaction of valproic acid with carbapenem antibiotics. Drug Metab Dispos 2010;38:1538–1544. [DOI] [PubMed] [Google Scholar]
- 6. Clause D, Decleire PY, Vanbinst R, et al. Pharmacokinetic interaction between valproic acid and meropenem. Intensive Care Med 2005;31:1293–1294. [DOI] [PubMed] [Google Scholar]
- 7. Fudio S, Carcas A, Pinana E, et al. Epileptic seizures caused by low valproic acid levels from an interaction with meropenem. J Clin Pharm Ther 2006;31:393–396. [DOI] [PubMed] [Google Scholar]
- 8. Coves‐Orts FJ, Borras‐Blasco J, Navarro‐Ruiz A, et al. Acute seizures due to a probable interaction between valproic acid and meropenem. Ann Pharmacother 2005;39:533–537. [DOI] [PubMed] [Google Scholar]
- 9. Spriet I, Meersseman W, De Troy E, et al. Meropenem‐valproic acid interaction in patients with cefepime‐associated status epilepticus. Am J Health Syst Pharm 2007;64:54–58. [DOI] [PubMed] [Google Scholar]
- 10. De Turck BJ, Diltoer MW, Cornelis PJ, et al. Lowering of plasma valproic acid concentrations during concomitant therapy with meropenem and amikacin. J Antimicrob Chemother 1998;42:563–564. [DOI] [PubMed] [Google Scholar]
- 11. Nacarkucuk E, Saglam H, Okan M. Meropenem decreases serum level of valproic acid. Pediatr Neurol 2004;31:232–234. [DOI] [PubMed] [Google Scholar]
- 12. Wu CC, Pai TY, Hsiao FY, et al. The effect of different carbapenem antibiotics (ertapenem, imipenem/cilastatin, and meropenem) on serum valproic acid concentrations. Ther Drug Monit 2016;38:587–592. [DOI] [PubMed] [Google Scholar]
- 13. Paulzen M, Eap CB, Grunder G, et al. Pharmacokinetic interaction between valproic acid, meropenem, and risperidone. J Clin Psychopharmacol 2016;36:90–92. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki E, Nakai D, Ikenaga H, et al. In vivo inhibition of acylpeptide hydrolase by carbapenem antibiotics causes the decrease of plasma concentration of valproic acid in dogs. Xenobiotica 2016;46:126–131. [DOI] [PubMed] [Google Scholar]
- 15. Mori H, Takahashi K, Mizutani T. Interaction between valproic acid and carbapenem antibiotics. Drug Metab Rev 2007;39:647–657. [DOI] [PubMed] [Google Scholar]


