Summary
Objective
Reproductive dysfunction is a comorbidity that commonly occurs with temporal lobe epilepsy (TLE). Characterization of this comorbidity in various models of TLE in mice will greatly facilitate mechanistic investigations of the relationship between reproductive disorders and seizures initiated in the hippocampus. Here we investigate the impact on female reproductive estrous cyclicity in the intrahippocampal kainic acid mouse model of TLE and demonstrate the utility of using this model for future mechanistic studies.
Methods
Kainic acid (KA) or saline vehicle was stereotaxically injected in the right dorsal hippocampus of adult female C57BL/6J mice. Development of epilepsy was assessed by video monitoring for behavioral seizures. Reproductive function was assessed by daily estrous cycle monitoring and ovarian morphology. Estrous cycles were monitored for up to 2 months after injection. Ovarian morphology was examined by histological staining and assessment of follicular and luteal development.
Results
We observed spontaneous behavioral seizures in 82% of kainic‐acid‐treated mice. Irregular estrous cycles developed within 2 months after kainic acid injection. Sixty‐seven percent of KA‐treated mice showed disrupted estrous cycles, typically characterized by increased estrous cycle length, increased time spent in diestrus (nonfertile stage), and decreased time spent in estrus by 42 days post‐KA injection. The estrous cycle disruption, however, was not accompanied by major changes in ovarian morphology or follicular development. KA‐treated mice also displayed increased weight gain compared to control mice.
Significance
These data indicate that comorbid female irregular estrous cyclicity arises in the intrahippocampal kainic acid mouse model of TLE. This is the first demonstration of disrupted reproductive endocrine function in a mouse model of TLE initially produced by an insult specifically targeted to the hippocampus. This model should thus be useful for basic studies investigating the neural mechanisms driving comorbid reproductive dysfunction in epilepsy in women.
Keywords: Hippocampus, HPG axis, Seizure, Comorbidity
Key Points.
Unilateral right intrahippocampal kainic acid injection alters the estrous cycle in female mice, demonstrating comorbid reproductive dysfunction in a mouse model of epilepsy with a local initial insult to the hippocampus
This estrous cycle disruption develops in the absence of major pathology to the ovaries
Kainic‐acid‐treated mice display increased weight gain compared to controls
The intrahippocampal kainic acid mouse model of temporal lobe epilepsy appears appropriate for further mechanistic studies of the changes underlying the development of comorbid reproductive dysfunction in epilepsy
Reproductive dysfunction is a comorbidity commonly observed in both men and women with epilepsy.1, 2 Temporal lobe epilepsy (TLE) is the most prevalent type of partial epilepsy in adults.3 The rate of developing reproductive disorders in patients with TLE is much higher than the general population, and these changes include irregular menstrual cycles, polycystic ovary syndrome, hypothalamic amenorrhea, and hyperandrogenism in women, and lower levels of free testosterone, hypogonadism, abnormal semen, and erectile dysfunction in men.4, 5, 6, 7 Women with TLE may show higher or lower frequencies of pulsatile release of gonadotropin‐releasing hormone (GnRH) from the hypothalamus, potentially affected by whether the seizures initiate and propagate on the left or right hemisphere of the brain.4
This comorbidity not only affects reproduction but also affects overall health for epilepsy patients because sex steroids have wide‐ranging effects on many physiological systems and tissues, including ovary, breast, prostate, testis, cardiovascular, bone, and the brain.8, 9 The mechanisms underlying this comorbidity remain unclear. In many patients with epilepsy, reproductive dysfunction appears to arise from seizure activity and/or antiepileptic drug treatment,10, 11 but the relative contributions of seizures versus antiepileptic drugs to the development of this comorbidity can be difficult to determine. Therefore, animal models of TLE that give rise to reproductive comorbidities recapitulating some of the effects seen in humans would be extremely valuable for mechanistic studies.
The importance of understanding and treating comorbid reproductive dysfunction is also highlighted by the reciprocal effects on seizure severity and management. About 40% of women with epilepsy display a catamenial pattern, in which the frequency of seizures increases at certain points of the menstrual cycle, typically near ovulation or menstruation. Moreover, in women with impaired menstrual cycles (e.g., anovulation and inadequate luteal phase development) the window for increased seizure frequency is lengthened.4
In female rodents, overall reproductive health can be determined by examination of the estrous cycle. So far, most work has been done using the systemic pilocarpine model, demonstrating disrupted cyclicity in both rats and mice.12, 13 In systemic models, however, it is difficult to distinguish whether the disrupted estrous cyclicity that develops is the specific result of seizures or off‐target effects of pilocarpine on other brain regions critical to controlling reproduction, such as the hypothalamus. In this regard, use of a model with local infusion of a chemoconvulsant in the hippocampus could potentially be advantageous. In a study using the amygdala kindling rat model, estrous cyclicity was arrested.14 In another study examining the effects of epilepsy induced by intrahippocampal injection of the convulsant glutamate receptor agonist kainic acid (KA) in rats, the proportional times spent in proestrus, estrus, and metestrus were significantly altered.15 These findings suggest that seizures originating in the amygdala and/or hippocampus could lead to estrous cycle disruption, but it is not known whether this occurs in mice as well. Because mouse models have the advantage of a larger number of genetic tools enabling direct evaluation and manipulation of specific populations of neurons involved in the neural/hypothalamic control of reproduction,16, 17 confirmation that estrous cyclicity is compromised in the intrahippocampal kainic acid mouse model of TLE would represent a valuable resource for investigation.
In this study, we test the hypothesis that unilateral intrahippocampal KA injection in female mice leads to development of estrous cycle disruption. The intrahippocampal KA injection model was chosen because it is a well‐established model of TLE that recapitulates hippocampal sclerosis and seizures.18 Our findings show that both epilepsy and altered estrous cyclicity are robustly induced, indicating that this model will be useful for further mechanistic studies on comorbid reproductive dysfunction that arises with TLE.
Methods
Animals
C57BL/6J female mice were obtained from the Jackson Laboratory (Bar Harbor, ME, U.S.A.). Mice were housed in a standard environment in a 14:10 h light:dark cycle, with 5 mice per cage. This light cycle was chosen to optimize cyclicity and breeding.19 Food and water were provided ad libitum. All mice were housed near cages holding male mice and exposed to enough light to facilitate maintenance of regular estrous cycles. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana‐Champaign.
Intrahippocampal injection of kainic acid
Mice were anesthetized by isoflurane inhalation (2–3%, vaporized in oxygen). KA (Tocris Bioscience; 50 nl, 20 mm prepared in 0.9% sterile saline) was administered into the right dorsal hippocampal CA1 region by stereotaxic injection (coordinates: 1.8 mm posterior and 1.5 mm lateral to Bregma; 1.5 mm ventral to cortical surface). Control mice were subjected to the same surgical procedure but injected with sterile saline.
Estrous cycle monitoring
A schematic of the experimental design is shown in Fig. 1A. Preinjection estrous cycle monitoring was performed for at least 2 weeks to confirm that mice had regular cycles before any experimentation. All mice tested for the present studies showed at least two consecutive regular cycles prior to injection. After KA/saline injection, mice were allowed to rest for a 2‐week recovery period, and then were monitored daily for estrous cycle stages for an average of 66 days (range from 60 to 75 days) postsurgery. Daily vaginal cytology was performed to determine the estrous cycle stages (proestrus, estrus, metestrus, and diestrus). The vaginal lavage method20 was used for vaginal cytology examination and was performed between 10 am and 12 pm (relative to lights off at 7 pm). Twenty microliters of sterile 1% phosphate‐buffered saline (PBS) was inserted into the vaginal cavity, withdrawn, and smeared on a microscope slide. Estrous cycle stages were determined according to the type, number, and morphology of cells in the smear. Proestrus is indicated by the presence of mostly nucleated epithelial cells, estrus by cornified epithelial cells, metestrus by cornified epithelial cells with leukocytes, and diestrus by leukocytes21 (Fig. 1B). Regularly cycling mice showed cycles with an average 5‐day periodicity (1 day each of proestrus, estrus, metestrus, diestrus day I, and diestrus day II; Fig. 1B).20
Figure 1.
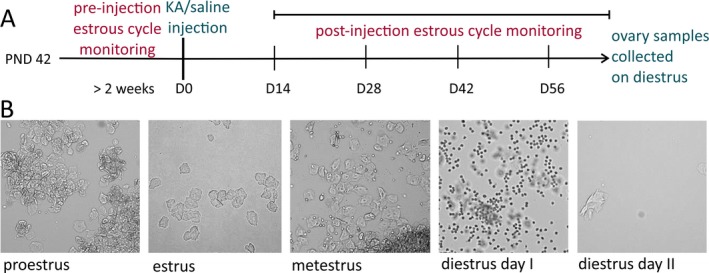
Illustration of experimental design showing times of drug treatment and estrous cycle examination as well as representative vaginal smears. (A) Schematic of experimental design for the time points of surgery, pre‐ and postinjection estrous cycle monitoring, and collection of ovary samples. (B) Brightfield microscopic images for unstained vaginal smears from different estrous cycle stages. Proestrus (mostly nucleated epithelial cells), estrus (cornified epithelial cells), metestrus (cornified epithelial cells with leukocytes), diestrus day I (mostly leukocytes), and diestrus day II (very few cells). PND, postnatal day.
Estrous cyclicity was evaluated from two aspects. The cycle length was calculated by counting the days between two successive estrus stages with both metestrus and diestrus stages occurring in between. The percentage of time spent in each stage was calculated by the number of days spent in each stage divided by the total number of days of monitoring. In order to determine whether each mouse displayed regular or irregular estrous cyclicity, the average cycle length and percentages of time in each stage were calculated from the data collected from 42 days postinjection until the last day of monitoring. If a mouse spent more than 50% of time in one stage, or the average cycle length was 7 days or longer, this mouse was considered to have an irregular estrous cycle. Note that here we use the term “regular” to refer to mice whose cycle parameters fall within a normal range, as assessed through these measurements. For rodents, prolonged diestrus may indicate an acyclic period or pseudopregnancy; these two situations are usually accompanied by heavy mucus secretion.22, 23 The mice in this study, however, did not show this type of secretion. The ages of saline‐treated and KA‐treated mice were not significantly different at the time of injection (saline 11.5 + 0.3 weeks, KA 11.9 + 0.7 weeks, p = 0.45 Mann–Whitney test). The estrous cycle of both groups was monitored for 2 months after injections, and then the mice were euthanized, at which time the ovaries were collected for histology (time postinjection: saline 9.4 ± 0.9 weeks, KA 9.5 ± 0.6 weeks, p = 0.67 two‐sample t test). The body weight of each mouse was measured on the day of surgery and the last day of monitoring, and these values were used to calculate mean weight gain per week and final body weight values.
Seizure monitoring
Mice were video monitored for seizures at both acute and chronic time points. Acute seizures were recorded immediately following the injection, and spontaneous behavioral seizures were monitored from 1 month after injection onward. For acute seizure monitoring, mice were placed in a transparent recovery chamber and were video recorded for 4–5 h to characterize acute seizures that developed after injection. Seizures were rated according to the Racine scale.24 Behavioral seizures of stage 3 and higher were observed through video and defined as forelimb clonus and head bobbing (stage 3); forelimb clonus and rearing (stage 4); forelimb clonus, rearing, and falling (stage 5).
To confirm the development of epilepsy, KA‐treated mice were videotaped for the presence of spontaneous behavioral seizures starting from 30 days after surgery until the last week of estrous cycle monitoring. Acrylic dividers were inserted in glass terrariums lined with bedding to provide separate compartments for individual mice. Simultaneous recordings of up to 10 mice were made by webcams (Logitech) and a PC running Windows 7. Videotaping of each mouse was performed at the frequency of 3–4 days/week, and 6–8 h/day. Seizures rated higher or equal to Racine stage 3 were detected and evaluated by eye by investigators blinded to estrous cycle stage or regularity. To avoid possible influences to the estrous cycle from moving the mice from the colony room to the laboratory for recording, control mice were also brought at the same time and kept in the same room with the recorded KA‐treated mice.
Ovarian morphology
Ovarian morphology was assessed by quantifying ovarian follicles and corpora lutea.25 Ovaries were collected at the end of 2 months after KA/saline injection from diestrous mice, fixed in formalin (10%) overnight at 4°C, and preserved in 70% ethanol. Both left and right ovaries were paraffin‐embedded, and six consecutive sections of 5‐μm thickness were collected from each ovary and stained with hematoxylin and eosin. An investigator blinded to treatment group screened the ovarian sections to quantify the number of the primary, secondary, and antral follicles and corpora lutea. Follicles at different stages and the corpus luteum are readily defined according to their morphology.25 The numbers of each type of ovarian follicle and corpora lutea were counted from both left and right ovaries for each mouse.
Statistics
Statistical comparisons were made using OriginPro (OriginLab, Northampton, MA, U.S.A.). Comparisons of the percentage of time in diestrus, cycle length, and quantification of ovarian follicles between control and KA‐treated mice were made using two‐tailed Student's t tests or nonparametric Mann–Whitney tests as appropriate. For the percentage of time spent in each cycle stage, the comparisons between different time points were performed using two‐way repeated measures ANOVA with Bonferroni post hoc pairwise tests. The comparison of estrous cyclicity pattern (regular/irregular) between control and KA‐treated mice was made using a chi‐square test. The criterion for statistical significance (p) was ≤0.05. Results are reported as means ± SEM.
Results
Efficacy of development of epilepsy
At the acute time point after KA injection, 11 out of 12 mice showed several stage 4 and stage 5 seizures. One mouse was not observed to show acute behavioral seizures within 5 h of injection and this mouse also did not show any spontaneous behavioral seizures during the following seizure monitoring. Of the 12 mice treated with intrahippocampal injection of KA, 9 were confirmed to have developed epilepsy by observation of at least two spontaneous behavioral seizures between 2 weeks and 2 months after injection. Three mice did not show any spontaneous behavioral seizures during this time period in our observations.
Irregular estrous cycle patterns develop within 2 months after intrahippocampal KA injection
The regular mouse estrous cycle pattern is characterized by a 5‐day cycle with the chronological order of proestrus, estrus, metestrus, diestrus I, and diestrus II (Fig. 2A, Saline & KA‐Regular Cycle). After 42 days postinjection, KA‐treated mice showed larger variance in cycle stage percentages; some showed regular estrous cycles, but the large majority showed irregular estrous cycles, often characterized by increased time in diestrus (Fig. 2A, KA‐Increased Diestrus). Four mice showed irregular cycles with increased estrus/metestrus observed during the third to fourth week after injection (Fig. 2A, KA‐Increased Estrus). Nine of 10 control mice showed the typical regular 5‐day cycle, with only one showing an irregular cycle with increased diestrus. By contrast, 9 of 12 KA‐treated mice showed disrupted estrous cycles (Fig. 2B). The irregular estrous cyclicity was typically characterized by longer cycle length (p = 0.03) and increased time spent in diestrus (p = 0.049) (Fig. 2C,D). The estrous cycles of all mice examined in this study are provided in Figs. S1 and S2. KA‐treated mice displayed different patterns of irregular cyclicity. For the 9 mice with irregular estrous cycles, 5 of them showed increased time in diestrus and increased cycle duration. Among those 5, 2 of them showed continuous days (5 or 6 days) of estrus before entering into a long period (>7 days) of continuous diestrus. The other 4 mice showed increased cycle duration without a single dominant stage.
Figure 2.
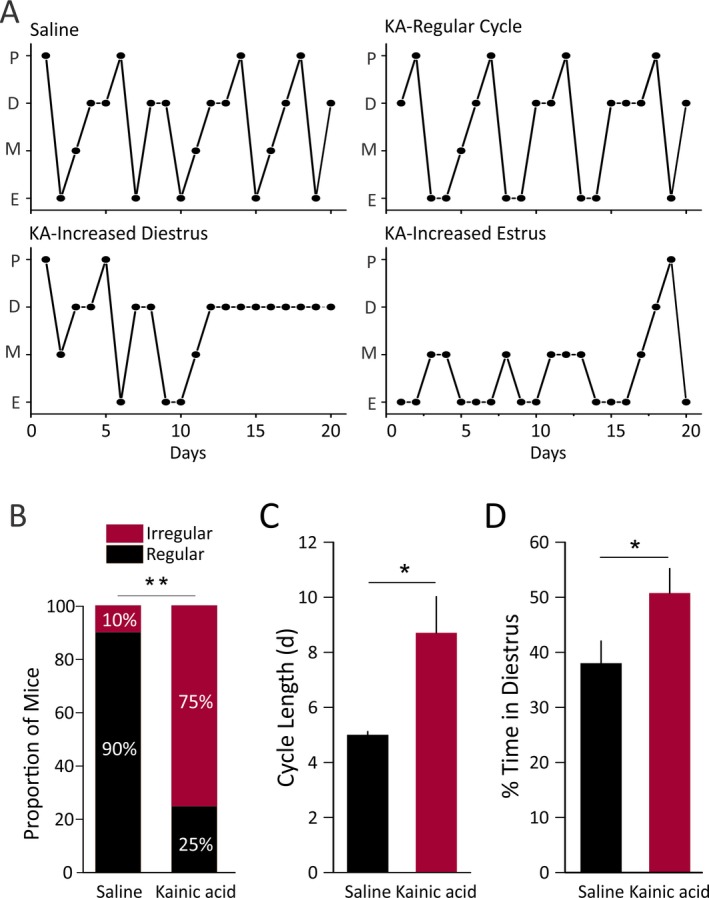
Estrous cycle disruption in KA‐treated mice. (A) Representative estrous cycles for saline‐treated control mice (top left), KA‐treated regularly cycling mice (top right), and KA‐treated irregularly cycling mice (bottom). Control mice showed regular 5‐day cycles. KA‐treated mice showed increased diestrus, increased estrus, or regular estrous cycles. (B) The proportion of mice with irregular cyclicity is significantly increased in KA‐treated mice (chi‐square test, p = 0.008). Prolonged average cycle length (>7 days) or increased percentage of time in one stage (>50%) was considered as irregular. (C, D) The cycle length is longer (C, p = 0.03, Mann–Whitney test) and the percentage of time spent in diestrus is increased (D, p = 0.04, two‐sample t test) in the KA‐treated group compared to saline‐treated controls. Values for B–D are from 42 days postinjection onward.
To quantify the degree and time course of estrous cycle disruption, we graphed the data as percent time spent in each stage of the cycle. In control mice, a regular estrous cycle pattern was observed both during the presurgical time and by the end of 2 months after injection (Fig. 3, Saline). The control mice showed slightly more time spent in estrus and less time spent in metestrus and proestrus during the third to fourth week after injection. This may be a transient response to the surgical procedure and was not statistically significant. During the second month after injection, the estrous cycle pattern of control mice returned to regular. KA‐treated mice, by contrast, did not regain regular estrous cyclicity but instead developed irregular cycles (Fig. 3, Kainic Acid). The mean time spent in diestrus increased during the second month and reached 51% by the end. After 42 days postinjection, the percentage of time spent in diestrus was significantly larger than the percentage of time during the preinjection control period (p = 0.014). By contrast, the time spent in estrus was significantly decreased compared to the preinjection control period (p = 0.006). Notably, proestrus returned to the regular proportion in the control group, but not in the KA‐treated group. Together, these data demonstrate a clear development of estrous cycle irregularity in KA‐treated mice that occurs between 2 weeks and 2 months after injection.
Figure 3.
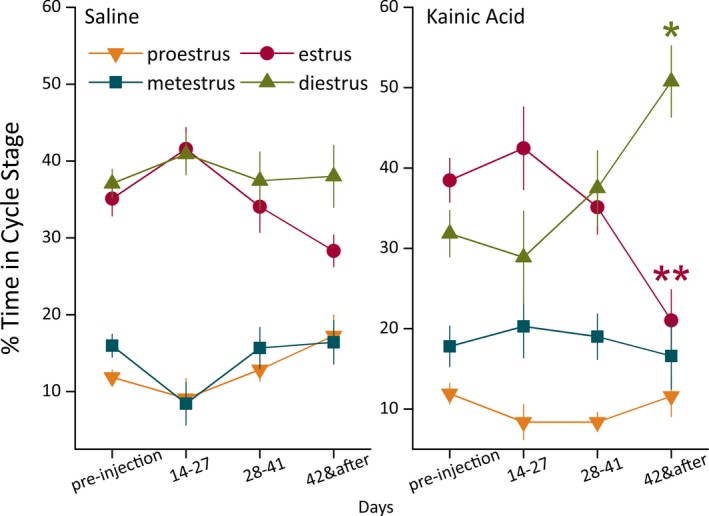
Development of estrous cycle irregularity within 2 months of KA injection. For both groups, the percentage of time spent in each estrous cycle stage is displayed for every 2‐week period, from weeks 3–4 (days 14–27) to week 7 and after (days 42 and after). KA‐treated mice showed significantly increased time in diestrus and decreased time in estrus by 42 days postinjection compared to the preinjection control period (%D p = 0.014, %E p = 0.006, two‐way repeated measures ANOVA, Bonferroni post hoc test, n = 12 mice). By contrast, estrous cycle stage percentages in saline‐treated control mice were not significantly different between any time points. Saline‐injected mice displayed typical regular estrous cycles by 28 days after injection with 5‐day cycle periodicity, including 2 days (40% of time) in diestrus per cycle (n = 10 mice). Orange downward triangle: proestrus; red circle: estrus; teal square: metestrus; green upward triangle: diestrus.
KA‐treated mice display increased weight gain
Increased weight gain has been observed in the systemic pilocarpine mouse and rat models of TLE.12, 26 In one study examining the intrahippocampal KA female rat model, increased food intake and body weight gain were observed only when both ventral and dorsal hippocampus was injected.27 In our study, the KA was injected only to the right dorsal hippocampus of the mice. Therefore, we also examined whether unilateral dorsal hippocampal injection of KA leads to weight gain. KA‐injected mice gained more weight per week than saline control mice (p = 0.04) (Fig. 4A). The average body weight of KA‐injected mice at 2 months after injection was also significantly higher than that of controls (p = 0.01) (Fig. 4B).
Figure 4.
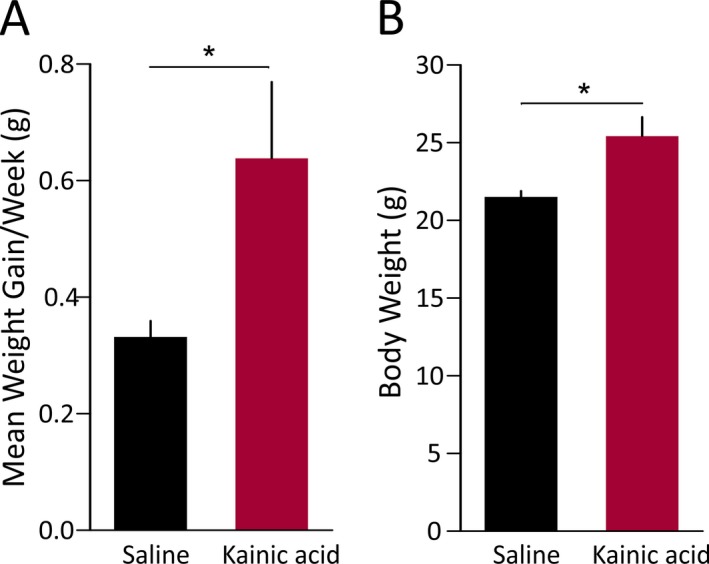
Increased weight gain in KA‐treated female mice. (A) KA‐treated mice showed a higher rate of weight gain per week than saline‐treated control mice (p = 0.04, two‐sample t test). (B) Final body weight at approximately 2 months after injection was also significantly higher for KA‐treated mice than control mice (p = 0.01, two‐sample t test).
Ovarian follicular and luteal development is not altered in epileptic mice
Estrous cycle disruption could be, at least in part, a result of disruption to the ovaries. Therefore, we examined the gross morphology of ovaries from KA‐treated and control mice, as assessed by the number of follicles at each stage of development and presence of corpora lutea. Ovaries obtained on diestrus from KA‐treated mice and control mice showed similar gross morphology, as assessed by the number and types of follicles and presence of corpora lutea (Fig. S3). No significant difference was observed for the numbers of ovarian follicles at each developmental stage or the presence of corpora lutea (Table 1), and no ovaries displayed evidence of cysts. This finding indicates that the altered estrous cyclicity observed in KA‐treated mice is not associated with major pathology of the ovaries.
Table 1.
Quantification of ovarian follicles and corpora lutea in saline‐ and kainic‐acid‐treated mice
| Treatment | Number of follicles per mouse | |||
|---|---|---|---|---|
| Primary follicles | Secondary follicles | Antral follicles | Corpora lutea | |
| Saline | 1.22 ± 0.37 | 3.83 ± 0.80 | 6.78 ± 0.76 | 1.44 ± 0.29 |
| Kainic acid | 0.75 ± 0.31 | 2.58 ± 0.49 | 7.00 ± 1.13 | 1.25 ± 0.40 |
| p Value (t test) | 0.50 | 0.26 | 0.87 | 0.85 |
Discussion
In the present study, we demonstrate that intrahippocampal KA injection in the majority of female mice leads to irregular estrous cycles concomitant with the progression of epileptogenesis. This finding suggests that this model can be used to study mechanisms underlying comorbid reproductive dysfunction with TLE. In this model, the majority of KA‐treated mice showed irregular cycles, characterized by prolonged cycle length and increased time spent in diestrus. The remainder showed similar regular estrous cycle patterns as control mice. Notably, not all epileptic mice developed irregular estrous cycles, and those mice that did show irregular cycles did not display a complete collapse of cyclicity. This recapitulates clinical findings that although women with epilepsy display increased rates of reproductive dysfunction, many women with epilepsy do not exhibit this comorbidity. In addition, various types of clinical symptoms have been reported, including menstrual disorders, polycystic ovaries, and amenorrhea.4, 10 This model thus appears appropriate for further studies of the interrelationships between seizure activity and comorbid reproductive dysfunction.
These findings indicate that intrahippocampal KA injection in mice leads to altered estrous cyclicity. By the end of 2 months after injection, KA‐treated mice displayed longer time spent in diestrus as well as increased cycle length compared to control mice. Prolonged diestrus is a commonly observed pattern in rodent models of disordered fertility, such as hyperandrogenic models of polycystic ovary syndrome,28 Clock mutant mice (which have disrupted circadian rhythms),29 and mice in which kisspeptin neurons have been ablated.30 Besides increased diestrus, other irregular cycle patterns were also observed. Persistent vaginal cornification (estrus) is a sign of reproductive aging31 and has also been observed in a prenatal androgenization model of polycystic ovary syndrome.32 Furthermore, the pilocarpine rat model of TLE is associated with a high rate of development of persistent estrus.12 Although the mechanisms driving altered cyclicity are unclear, failure to generate proper neural control of the hypothalamic‐pituitary‐gonadal axis is a likely mechanism driving epilepsy‐associated irregular estrous cycles. Further studies are thus needed to elucidate the exact neuroendocrine disruption mechanisms underlying irregular cyclicity.
Interestingly, estrous cycle disruption emerged without a change in the gross morphology of ovaries. This indicates that disrupted estrous cycles may arise at least in part from alterations in the neural control for reproduction.33 The hypothalamic GnRH neurons form the final common pathway in the central control of mammalian reproduction. Although we did not examine hypothalamic pathology in these experiments, it is important to note that in other studies using the preclinical animal TLE models, reports conflict as to whether GnRH immunoreactivity is changed. Specifically, in the mouse systemic pilocarpine injection model of TLE, the number of GnRH neurons and intensity of GnRH immunoreactivity are not affected.13 Similarly, in a study using a macaque model of unilateral KA‐induced epilepsy, neither cell damage nor gliosis was observed in the hypothalamus, including the anterior hypothalamus which contains GnRH neurons.34 By contrast, a reduction in GnRH immunoreactivity was observed in both the systemic pilocarpine and intra‐amygdala KA injection rat models.35, 36 These histological studies, however, do not indicate whether GnRH neuron function is altered. Application of the intrahippocampal KA model to transgenic mice that enable identification of GnRH neurons16 should thus be fruitful for investigations of changes in GnRH neuron function under the influence of seizures.
The lack of discernable ovarian cysts in the intrahippocampal KA mouse model contrasts with findings in previous studies using the pilocarpine rat model, in which epileptic rats showed statistically greater numbers of cysts per ovary than control rats.12 The systemic pilocarpine model shows robust behavioral seizure occurrence with around three seizures per week.37 In the intrahippocampal KA mouse model, however, the seizure frequency was variable across different individuals and only 2 of 12 mice were observed to show three or more seizures/week by the end of 2 months after injection. Higher seizure frequency could possibly lead to more severe reproductive dysfunction and changes in ovarian morphology. In addition to higher behavioral seizure frequency, the systemic pilocarpine model affects both hippocampi and both dorsal and ventral hippocampus, while the unilateral intrahippocampal KA model we used here was targeted to the right dorsal hippocampus only. The laterality of epileptic focus may be associated with the type of reproductive disorder in women with TLE, with left‐sided TLE associated with polycystic ovary syndrome and right‐sided TLE associated with hormonal changes resembling hypothalamic amenorrhea.4 Increasingly severe collapse of estrous cycles may develop over time, and the ovarian morphology may undergo pathological changes under the influence of persistently disrupted neuroendocrine control.
Body weight gain is commonly reported in epileptic patients and is often associated with antiepileptic drug treatment or changed lifestyle, or it is potentially the direct result of seizures.38 Long‐term weight gain in women could be both cause and consequence of menstrual irregularity.39 Our results indicate that intrahippocampal KA injection in female mice leads to weight gain. This finding suggests that increased weight gain occurs on a similar timeframe as the development of disrupted estrous cyclicity. In male mice, however, the same KA injection did not induce weight gain (data not shown), indicating a potential sex difference in this response.
One future direction of this study is to correlate the seizure activity with estrous cycle disruption. The intrahippocampal KA model has been shown to display recurrent electrographic seizures that are only occasionally associated with behavioral seizure presentation.18 Electroencephalographic (EEG) recording of these mice could indicate whether disrupted estrous cycles are associated with more severe epilepsy, and whether the severity of epilepsy is correlated with the degree of estrous cycle disruption.
In summary, this study demonstrates that irregular estrous cyclicity is robustly induced in the unilateral intrahippocampal KA mouse model of TLE. By 2 months after injection, most mice treated in this epilepsy model showed prolonged cycle length and increased time spent in diestrus, accompanied by increased body weight. The estrous cycle disruption does not appear to be a consequence of ovarian pathology because ovarian morphology remained unaffected for KA‐treated mice. Overall, these findings suggest that the intrahippocampal KA mouse model is appropriate for investigating the underlying mechanisms of comorbid reproductive neuroendocrine dysfunction in TLE.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Individual estrous cycle graphs for all saline‐injected control mice.
Figure S2. Individual estrous cycle graphs for all KA‐injected mice.
Figure S3. Representative micrographs of sections of H&E‐stained ovarian tissue from saline and KA‐treated mice.
Acknowledgments
This work was supported by start‐up funds from the University of Illinois at Urbana‐Champaign (CAC) and Summer Undergraduate Research Fellowships from the School of Molecular and Cellular Biology (VAA, JBL). We thank Karen Doty for performing ovary sectioning and staining, Mary Laws for guidance on follicle identification, Rao Li and Denise Reynish for assistance in screening videos for behavioral seizures, Amin Ghane for collection of mouse ovaries, and Lori Raetzman for helpful editorial comments.
Biography
Jiang Li is a graduate student in Neuroscience at the University of Illinois at Urbana‐Champaign.

References
- 1. Herzog AG, Seibel MM, Schomer DL, et al. Reproductive endocrine disorders in men with partial seizures of temporal lobe origin. Arch Neurol 1986;43:347–350. [DOI] [PubMed] [Google Scholar]
- 2. Herzog AG, Seibel MM, Schomer DL, et al. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch Neurol 1986;43:341–346. [DOI] [PubMed] [Google Scholar]
- 3. Engel J. Mesial temporal lobe epilepsy: what have we learned? Neuroscientist 2001;7:340–352. [DOI] [PubMed] [Google Scholar]
- 4. Herzog AG. Disorders of reproduction in patients with epilepsy: primary neurological mechanisms. Seizure 2008;17:101–110. [DOI] [PubMed] [Google Scholar]
- 5. Bauer J, Cooper‐Mahkorn D. Reproductive dysfunction in women with epilepsy: menstrual cycle abnormalities, fertility, and polycystic ovary syndrome. Int Rev Neurobiol 2008;83:135–155. [DOI] [PubMed] [Google Scholar]
- 6. Herzog AG. Reproductive endocrine considerations and hormonal therapy for men with epilepsy. Epilepsia 1991;32:S34–S37. [DOI] [PubMed] [Google Scholar]
- 7. Murialdo G, Galimberti CA, Fonzi S, et al. Sex hormones and pituitary function in male epileptic patients with altered or normal sexuality. Epilepsia 1995;36:360–365. [DOI] [PubMed] [Google Scholar]
- 8. Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013;9:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turgeon JL, Carr MC, Maki PM, et al. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev 2006;27:575–605. [DOI] [PubMed] [Google Scholar]
- 10. Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol‐17beta throughout the 4‐day estrous cycle of the rat. Endocrinology 1974;94:1704–1708. [DOI] [PubMed] [Google Scholar]
- 11. Rattya J, Turkka J, Pakarinen AJ, et al. Reproductive effects of valproate, carbamazepine, and oxcarbazepine in men with epilepsy. Neurology 2001;56:31–36. [DOI] [PubMed] [Google Scholar]
- 12. Scharfman HE, Kim M, Hintz TM, et al. Seizures and reproductive function: insights from female rats with epilepsy. Ann Neurol 2008;64:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fawley JA, Pouliot WA, Dudek FE. Pilocarpine‐induced status epilepticus and subsequent spontaneous seizures: lack of effect on the number of gonadotropin‐releasing hormone‐positive neurons in a mouse model of temporal lobe epilepsy. Neuroscience 2012;203:153–159. [DOI] [PubMed] [Google Scholar]
- 14. Edwards HE, Burnham WM, Ng MM, et al. Limbic seizures alter reproductive function in the female rat. Epilepsia 1999;40:1370–1377. [DOI] [PubMed] [Google Scholar]
- 15. Amado D, Verreschi IT, Berzaghi MP, et al. Effects of intrahippocampal injection of kainic acid on estrous cycle in rats. Braz J Med Biol Res 1987;20:829–832. [PubMed] [Google Scholar]
- 16. Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin‐releasing hormone neurons: characterization of whole‐cell electrophysiological properties and morphology. Endocrinology 2000;141:412–419. [DOI] [PubMed] [Google Scholar]
- 17. Spergel DJ, Kruth U, Hanley DF, et al. GABA‐ and glutamate‐activated channels in green fluorescent protein‐tagged gonadotropin‐releasing hormone neurons in transgenic mice. J Neurosci 1999;19:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouilleret V, Ridoux V, Depaulis A, et al. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience 1999;89:717–729. [DOI] [PubMed] [Google Scholar]
- 19. Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, eds. The Mouse in Biomedical Research: Normative biology, husbandry, and models. San Diego: Academic Press; 2006. [Google Scholar]
- 20. Byers SL, Wiles MV, Dunn SL, et al. Mouse estrous cycle identification tool and images. PLoS One 2012;7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 2009;Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ryan KD, Schwartz NB. Grouped female mice: demonstration of pseudopregnancy. Biol Reprod 1977;17:578–583. [DOI] [PubMed] [Google Scholar]
- 23. Richards M. Progesterone and pseudopregnancy in the golden hamster. J Reprod Fertil 1966;11:463–464. [DOI] [PubMed] [Google Scholar]
- 24. Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- 25. Myers M, Britt KL, Wreford NG, et al. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004;127:569–580. [DOI] [PubMed] [Google Scholar]
- 26. Hester MS, Hosford BE, Santos VR, et al. Impact of rapamycin on status epilepticus induced hippocampal pathology and weight gain. Exp Neurol 2016;280:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forloni G, Fisone G, Guaitani A, et al. Role of the hippocampus in the sex‐dependent regulation of eating behavior: studies with kainic acid. Physiol Behav 1986;38:321–326. [DOI] [PubMed] [Google Scholar]
- 28. Caldwell AS, Middleton LJ, Jimenez M, et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014;155:3146–3159. [DOI] [PubMed] [Google Scholar]
- 29. Miller BH, Takahashi JS. Central circadian control of female reproductive function. Front Endocrinol (Lausanne) 2013;4:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci 2011;14:704–710. [DOI] [PubMed] [Google Scholar]
- 31. Nelson JF, Felicio LS, Osterburg HH, et al. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod 1981;24:784–794. [DOI] [PubMed] [Google Scholar]
- 32. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin‐releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A 2004;101:7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herzog AG, Coleman AE, Jacobs AR, et al. Relationship of sexual dysfunction to epilepsy laterality and reproductive hormone levels in women. Epilepsy Behav 2003;4:407–413. [DOI] [PubMed] [Google Scholar]
- 34. Chen N, Liu C, Yan N, et al. A macaque model of mesial temporal lobe epilepsy induced by unilateral intrahippocampal injection of kainic acid. PLoS ONE 2013;8:e72336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waldstreicher J, Santoro NF, Hall JE, et al. Hyperfunction of the hypothalamic‐pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab 1988;66:165–172. [DOI] [PubMed] [Google Scholar]
- 36. Friedman MN, Geula C, Holmes GL, et al. GnRH‐immunoreactive fiber changes with unilateral amygdala‐kindled seizures. Epilepsy Res 2002;52:73–77. [DOI] [PubMed] [Google Scholar]
- 37. Wolf DC, Bueno‐Junior LS, Lopes‐Aguiar C, et al. The frequency of spontaneous seizures in rats correlates with alterations in sensorimotor gating, spatial working memory, and parvalbumin expression throughout limbic regions. Neuroscience 2016;312:86–98. [DOI] [PubMed] [Google Scholar]
- 38. Ben‐Menachem E. Weight issues for people with epilepsy–a review. Epilepsia 2007;48(Suppl. 9):42–45. [DOI] [PubMed] [Google Scholar]
- 39. Michalakis K, Mintziori G, Kaprara A, et al. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism 2013;62:457–478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Individual estrous cycle graphs for all saline‐injected control mice.
Figure S2. Individual estrous cycle graphs for all KA‐injected mice.
Figure S3. Representative micrographs of sections of H&E‐stained ovarian tissue from saline and KA‐treated mice.


