Abstract
Introduction
Contrast-induced nephropathy (CIN) is a common clinical problem that is growing in importance as an increasing number of tests and procedures which utilize contrast media (CM) are performed.
Aim
To evaluate the efficacy of intravenous N-acetylcysteine (NAC) for prevention of CIN after diagnostic and/or interventional procedures requiring CM administration.
Material and methods
In a prospective, single-center, randomized, placebo-controlled trial the preventive effects of N-acetylcysteine were evaluated in 222 patients undergoing elective angiography and/or angioplasty. Patients were randomly assigned to receive either NAC or placebo. All patients received intravenous hydration with normal saline before and after catheterization. Serum creatinine (SCr) and estimated glomerular filtration rate were assessed at baseline, at 48–72 h and 10–15 days after CM administration. Contrast-induced nephropathy was defined as an increase in SCr of at least 44 µmol/l (0.5 mg/dl) or an increase of ≥ 25% of the baseline value 48–72 h after CM administration.
Results
Contrast-induced nephropathy occurred in 30 of 222 patients (13.5%): 9 of 108 patients in NAC (8.3%) and 21 of 114 patients in the control group (18.4%; p = 0.0281). The multivariate Cox analysis revealed that elevated SCr at 10–15 days (HR = 2.69; p = 0.018) and baseline SCr level (HR = 1.009; p = 0.015) were independent prognostic variables for adverse events during follow-up.
Conclusions
Our findings suggest that intravenous NAC along with intravenous hydration may help prevent declining renal function after CM exposure. Elevated SCr level 10–15 days after CM administration was associated with increased risk of adverse events in long-term observation, while elevated SCr within 72 h was not. Measuring SCr at least 10 days after exposure to CM may provide a better outcome measure.
Keywords: N-acetylcysteine, contrast-induced nephropathy, contrast agent, contrast-induced acute kidney injury, contrast medium
Introduction
Contrast-induced nephropathy (CIN) is a serious complication of procedures requiring contrast media and is associated with rising costs, prolonged hospitalization, and increased mortality [1, 2]. It is a growing clinical issue due to large quantities of contrast being used with rapid development of medical imaging technology and the rise of interventional cardiology for the treatment of cardiovascular and cerebrovascular diseases.
The most important contrast-induced nephropathy risk factors are impaired renal function, heart failure, diabetes mellitus, advanced age and procedure-dependent factors such as volume and type of contrast media (CM) used [3–5].
The effect of contrast media on renal function is complex and not fully understood. There are several proposed mechanisms of the pathogenesis of CIN. The major theories include renal vasoconstriction (mediated by alterations in nitric oxide (NO), endothelin, or adenosine) leading to acute tubular necrosis, and the direct cytotoxic effects of CM [6]. Administration of CM initially causes transient dilation, which can be followed by a period of sustained vasoconstriction that lasts for several hours, causing a reduction in renal blood flow. This may lead to impaired oxygenation to the outer medulla, resulting in ischemia to the proximal and distal tubules. With high concentrations of contrast within and surrounding renal tubular cells, there is direct cellular toxicity. The combination of both ischemic and chemotoxic injury to the proximal tubules triggers a process called tubuloglomerular feedback, which signals the glomerulus to reduce filtration.
Many strategies have been investigated trying to prevent this complication. Some of them include hydration with sodium bicarbonate [7, 8], hydration with 0.9% and 0.045% NaCl solution, administration of N-acetylcysteine [9–11], vitamin C [8], statins [12], hemofiltration, hemodialysis or short-term controlled tissue ischaemia [13], withdrawal of certain drugs such as metformin, angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARB), and nonsteroidal anti-inflammatory drugs (NSAIDs). Taking into account the pathophysiology of CIN, the main focus is put on preventing hemodynamic changes by adequate hydration and the use of antioxidants. N-acetylcysteine (NAC), which is a potent antioxidant, is believed to have a positive impact on renal haemodynamics through the vasodilating effect of nitric oxide (NO) and reduction of direct renal oxidative injury [14]. N-acetylcysteine has been studied in various clinical trials without uniform results [8, 15]. Outcomes of several meta-analyses on the effectiveness of N-acetylcysteine are also inconsistent [16–18].
In this study we aimed to assess whether administration of intravenous NAC prior to and after contrast administration in well-hydrated patients was efficient in reducing the incidence of contrast-induced nephropathy.
Aim
The aim of this study was to evaluate the efficacy of intravenous N-acetylcysteine for the prevention of CIN after diagnostic and/or interventional procedures requiring administration of contrast agent.
Material and methods
This randomized controlled trial was a single-center study on angiography and/or angioplasty patients, who were randomized to single-blind treatment with intravenous N-acetylcysteine or placebo, with parallel design and an allocation ratio of 1 : 1. The study has been accepted by local Ethic Committee of Jagiellonian University Medical College. All patients gave written, informed consent.
Patient population and randomization
We studied prospectively 222 patients with normal and impaired renal function referred to our institution to undergo elective coronary and/or peripheral angiography and/or angioplasty. Patients were considered eligible for enrolment if they were over 18 years of age with stable creatinine levels in the last 3 months with serum creatinine (SCr) < 400 µmol/l and estimated glomerular filtration rate (eGFR) > 10 ml/min/1.72 m² (if non-diabetic) and > 15 ml/min/1.72 m² (if diabetic) and LVEF > 20%. Exclusion criteria were: haemodynamic instability (systolic blood pressure ≤ 90 mm Hg or diastolic ≤ 50 mm Hg); acute coronary syndrome; NYHA functional class IV; dialysis; percutaneous renal artery angioplasty procedure, active gastric or duodenal ulcer, exacerbated asthma; known sensitivity to NAC and/or to contrast agents and inability to provide written informed consent. Additional exclusion criteria during the study included cardiogenic shock, pulmonary edema, and active gastrointestinal bleeding. Diagnosis and staging of impaired renal function was based on K/DIGO clinical practice guidelines for chronic kidney disease. None of the patients received theophylline, dopamine, or NSAIDs during the study. Metformin and ACEIs were withheld 1 day before the contrast administration and reinstituted 2 days after the procedure.
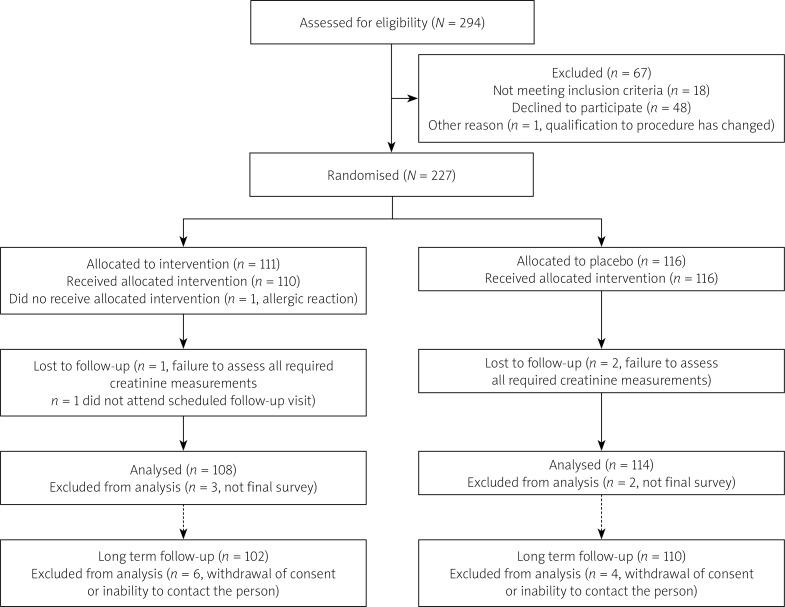
Patients were randomly assigned to receive either antioxidant N-acetylcysteine or placebo. Randomization was performed based on computer generated tables. One hundred and eight patients were randomized to the NAC group and 114 patients to the placebo group. One patient did not receive the full allocated intervention. Ten patients were lost to long-term follow-up due to inability to contact the person or withdrawal of consent (6 participants from the NAC group and 4 participants from the placebo group). For details check the Consort Diagram (Figure 1) [19, 20].
Figure 1.
Consort flow diagram
Procedural protocol and follow-up
Both groups received intravenous hydration with 0.9% saline before and after administration of the contrast agent. N-acetylcysteine was given intravenously at a dose of 600 mg, diluted in 100 ml of 0.9% NaCl, on the day before the procedure (about 12 h before), on the day of administration of the CM twice daily, and on the day after administration of the contrast agent twice daily (5 doses, of which 2 were given before and 3 were given after exposure to CM). Patients in the placebo group received 100 ml of 0.9% NaCl solution. Patients did not know whether N-acetylcysteine was diluted in 100 ml of 0.9% NaCl or whether they received 0.9% NaCl solution alone. Physicians and nurses however were not blinded (single-blinded study). Hydration with 0.9% NaCl solution – the same in both groups – was given intravenously at a rate of 1–1.5 ml/kg body weight per hour for 12 h before and 12 h after administration of the contrast agent. Patients were not allowed to drink extra fluids for 12 h before and after the procedure, after which there were no oral fluid restrictions. All patients received a nonionic contrast agent (one of the following: iomeprol, iohexol, iopromide, iodixanol). The dose of contrast agent was left to the discretion of the attending cardiologist.
Serum creatinine level was measured immediately before, 48–72 h after administration of the contrast agent and 10–15 days after exposure to CM. In all patients estimated glomerular filtration rate (eGFR) was calculated by applying the mdification of diet in renal disease (MDRD) formula to the baseline serum creatinine level: eGFR [ml/min/1.72 m²] = 186 × SCr–1.154 × age [years]–0.203 × (0.743 if female) × 1.210, where: eGFR – estimated glomerular filtration rate, SCr – serum creatinine level [µmol/l].
Contrast-induced nephropathy was defined as an increase in the serum creatinine concentration of at least 44 µmol/l (0.5 mg/dl) or an increase in the serum creatinine concentration of at least 25% of the baseline value at 48–72 h after administration of contrast agent [6].
Patients underwent long-term clinical follow-up including occurrence of major adverse cardiac events (MACE) such as cardiovascular death, heart infarct, as well as dialysis and recurrent hospitalization (due to cardiac or renal causes). The first follow-up visit was performed 10–15 days after CM exposure and included history taking and physical examination. Blood was also drawn to measure the serum creatinine level. The second follow-up contact was obtained at least 6 months after CM exposure via telephone, at the end of the study. If an adverse event occurred the patient was asked to attend a clinical visit, where medical records were analyzed and again history was taken and physical examination performed.
Statistical analysis
Results are presented as mean SD or a percentage of the total. Continuous data were compared by means of Student’s t test. Categorical variables were analyzed by Fisher’s exact test and the χ2 test. Differences between the groups in creatinine concentration were analyzed by the nonparametric Mann-Whitney test. Non-paired variables were analyzed by the unpaired t test. A p-value 0.05 was considered statistically significant.
Overall survival was calculated using the Kaplan-Meier method. Independent variables were first analyzed with univariate analysis. Variables with p < 0.10 in the univariate model were entered into a Cox proportional hazards regression model for multivariate analysis. Statistical significance was defined as p < 0.05.
Data were analyzed using SPSS 24.0 for Windows (SPSS Inc., Chicago, IL) and Statistica 13.0 for Windows by StatSoft.
Results
Clinical characteristics
The clinical and biochemical characteristics of the patients in the two groups are shown in Table I.
Table I.
Baseline demographic, clinical and procedural characteristics
| Parameter | NAC (+)N = 108 | NAC (–)N = 114 | P-value |
|---|---|---|---|
| Age | 66.0 ±8.9 | 64.3 ±9.5 | NS |
| Male, n (%) | 88 (81.5) | 83 (72.8) | NS |
| Hypertension, n (%) | 98 (90.7) | 103 (90.4) | NS |
| DM, n (%) | 34 (31.5) | 36 (31.6) | NS |
| CAD, n (%) | 71 (65.7) | 81 (71.1) | NS |
| PAD, n (%) | 33 (30.6) | 25 (21.9) | NS |
| VHD, n (%) | 4 (3.7) | 8 (7.0) | NS |
| Prior MI, n (%) | 38 (35.2) | 40 (35.1) | NS |
| Prior stroke, n (%) | 20 (18.5) | 16 (14.0) | NS |
| RBC [× 106/mm3] | 4.8 ±0.5 | 4.7 ±0.5 | NS |
| HGL [g/dl] | 14.5 ±1.4 | 14.3 ±1.4 | NS |
| HCT (%) | 42.4 ±3.8 | 41.8 ±3.8 | NS |
| SCr [µmol/l] | 98.5 ±42.0 | 94.3 ±36.3 | NS |
| eGFR [ml/min/1.72 m²] | 76.2 ±24.5 | 77.2 ±27.2 | NS |
| ACR [mg/g] | 40.4 ±111.1 | 38.2 ±101.9 | NS |
| LVEF (%) | 58.4 (9.6) | 56.2 (10.9) | NS |
| NYHA class, n (%): | |||
| I | 18 (16.7) | 18 (15.8) | NS |
| II | 71 (65.7) | 75 (65.8) | NS |
| III | 19 (17.6) | 21 (18.4) | NS |
| IV (exclusion criteria) | 0 (0.0) | 0 (0.0) | |
| BMI [kg/m2] | 27.8 ±3.6 | 28.3 ±4.1 | NS |
| Total contrast volume [ml] | 196.7 ±85.9 | 204.0 ±84.2 | NS |
| Intravenous hydration volume [ml] | 993.4 ±154.1 | 983.8 ±146.1 | NS |
DM – diabetes mellitus, CAD – coronary artery disease, PAD – peripheral artery disease, VHD – valvular heart disease, ACR – urine albumin/creatinine ratio, LVEF – left ventricular ejection fraction, BMI – body mass index.
Among 222 patients, 210 underwent a diagnostic procedure (coronary angiography or peripheral artery angiography), with an eventual (ad-hoc) therapeutic procedure in 111 of the patients (percutaneous coronary intervention (PCI) or percutaneous transluminal angioplasty (PTA) of a peripheral artery). Twelve patients underwent elective PCI or PTA.
The amount of contrast agent administered was similar between the two groups (196.7 ±85.9 ml in the NAC group vs. 204.0 ±84.2 ml in the placebo group; p = NS). There was no significant difference in the intravenous hydration volume between the NAC group (993.4 ±154.1 ml) and the placebo group (983.8 ±146.1 ml).
None of the patients required dialysis. In 1 patient from the NAC group during the infusion of N-acetylcysteine a rash on the forearm was noted, and the infusion was stopped; no other systemic manifestations were present. No other adverse effects were observed.
Changes in renal function
The mean serum creatinine concentration for all patients was 96.4 ±39.1 µmol/l and the mean eGFR (using MDRD formula) was 76.7 ±25.9 ml/min/1.72 m². In the N-acetylcysteine group, the mean serum creatinine concentration increased from 98.5 ±42.0 to 103.9 ±46.7 µmol/l 48–72 h after administration of the contrast agent and eGFR decreased from 76.2 ±24.5 to 72.6 ±23.9 ml/min/1.72 m². In the placebo group, the mean serum creatinine concentration increased from 94.3 ±36.3 to 105.7 ±48.0 µmol/l 48–72 h after administration of the contrast agent and eGFR decreased from 77.2 ±27.2 to 69.7 ±25.6 ml/min/1.72 m². The absolute change in serum creatinine concentration was significantly greater in the placebo group than in the N-acetylcysteine group (11.4 ±21.7 vs. 5.4 ±15.1 µmol/l; p < 0.05). Likewise, the absolute change in eGFR was significantly greater in the control group as compared to the NAC group (7.5 ±11.9 vs. 3.6 ±11.8 µmol/l; p < 0.05).
Contrast-induced nephropathy occurred in 30 of the 222 (13.5%) patients: 9 of the 108 patients in the N-acetylcysteine group (8.3%) and 21 of the 114 patients in the control group (18.4%; p < 0.05).
During hospitalization there were no heart infarcts, no need of dialysis and no cardiovascular deaths. Mean hospitalization time in patients with CIN was significantly longer than in patients without CIN (5.9 ±3.2 days vs. 4.9 ±2.0 days; p < 0.05).
At the 6-month follow-up (542 ±222 days) among 212 patients there were 6 deaths (2 of them were cardiovascular deaths), 5 heart infarcts and 1 dialysis. Thirty-six patients required at least 1 recurrent hospitalization due to cardiac causes and 5 patients required at least 1 recurrent hospitalization due to renal causes (Table II).
Table II.
Adverse events at 6-month follow-up
| Adverse events | Placebo group N = 110 | NAC group N = 102 | P-value |
|---|---|---|---|
| Death (all causes): | 2 (1.8%) | 4 (3.9%) | NS |
| CV | 1 (0.9%) | 1 (0.9%) | NS |
| Other | 1 (0.9%) | 3 (2.6%) | NS |
| MI | 2 (1.8%) | 3 (2.6%) | NS |
| Dialysis | 1 (0.9%) | 0 (0%) | NS |
| Rehospitalization (cardiac causes) | 20 (18.1%) | 16 (15.7%) | NS |
| Rehospitalization (renal causes) | 3 (2.7%) | 2 (2.0%) | NS |
NAC – N-acetylcysteine, CV – cardiovascular, MI – myocardial infarction.
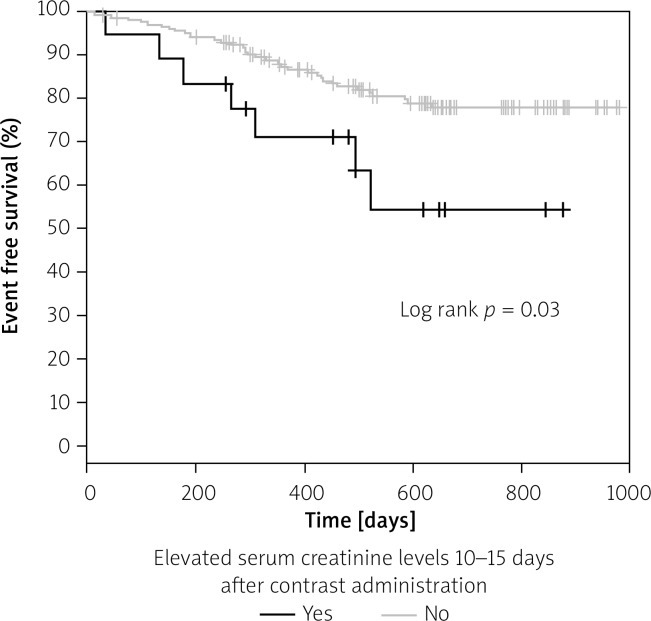
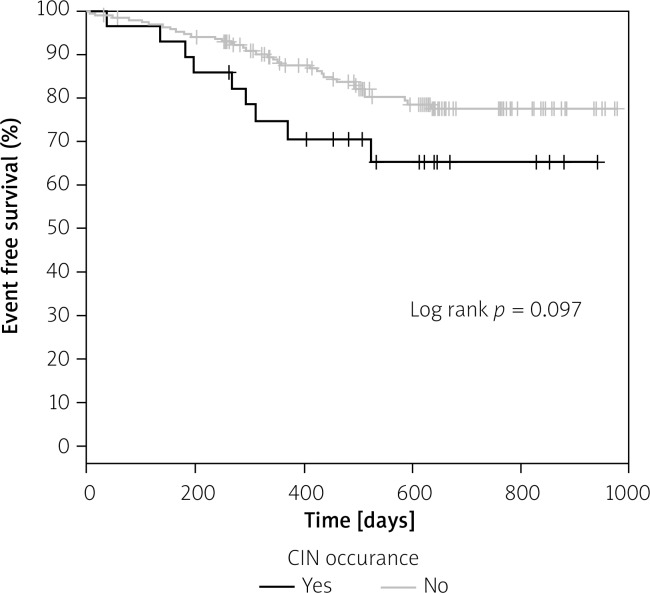
In long-term follow-up probability of adverse event-free survival was significantly greater in patients without elevated creatinine levels (defined as an increase in SCr of at least 44 µmol/l (0.5 mg/dl) or an increase of ≥ 25% of the SCr baseline value) 10–15 days after CM administration, as compared to those with elevated creatinine levels (Figure 2). Probability of adverse event-free survival in patients with CIN after 48–72 h did not differ significantly from the patients without CIN (Figure 3).
Figure 2.
Kaplan-Meier adverse event free survival in patients with elevated SCr after 10–15 days
Figure 3.
Kaplan-Meier adverse event free survival in patients with CIN
Univariate analysis showed that age (p = 0.028), elevated baseline serum creatinine level (p = 0.002), left ventricle ejection fraction (LVEF) (p = 0.021) and elevated serum creatinine level at 10–15 days after CM administration meeting criteria described above (p = 0.035) were significantly associated with increased risk of adverse events including death, myocardial infarction, dialysis, and rehospitalization due to renal or cardiac causes.
The multivariate Cox analysis revealed that elevated baseline serum creatinine level (HR = 1.009, 95% CI: 1.003–1.016, p = 0.015), and elevated serum creatinine level at 10–15 days (HR = 2.69, 95% CI: 0.168–1.812, p = 0.018) remained independent prognostic variables for adverse events during follow-up (Table III).
Table III.
Multivariate Cox regression model of predictors associated with long-term mortality
| Variables | HR | –95% CI | +95% CI | P-value |
|---|---|---|---|---|
| Age | 1.027985 | 0.006404 | 0.061606 | 0.111638 |
| Baseline SCr | 1.008612 | 0.003183 | 0.016278 | 0.015249 |
| Elevated SCr after 10–15 days | 2.692329 | 0.168428 | 1.812385 | 0.018198 |
| LVEF | 0.975952 | 0.002435 | 0.051119 | 0.074793 |
SCr – serum creatinine concentration, LVEF – left ventricular ejection fraction.
Discussion
Many interventions to reduce the risk of CIN have been studied, but to date, the evidence has been inconclusive.
In our study the use of intravenous N-acetylcysteine and hydration with 0.9% saline reduced the incidence of CIN in patients receiving intra-arterial contrast media as compared with standard hydration alone.
Tepel et al. were the first to evaluate the role of preventing CIN in patients with impaired renal function with antioxidant N-acetylcysteine [9]. Patients undergoing computed tomography were randomly treated with hydration plus placebo or hydration plus N-acetylcysteine (600 mg orally twice daily) before and after administration of the contrast agent. A difference between our study and that of Tepel was in the protocol for N-acetylcysteine administration. Tepel et al. [9] gave the drug at 600 mg orally twice daily, the day before and on the day of contrast infusion, while in our study protocol the drug was applied at the same dosage but intravenously and for 3 days rather than 2 days (the day before, on the day of contrast infusion and on the day after the procedure). We used intravenous N-acetylcysteine because of a high first-pass effect, resulting in very low bioavailability of < 10% after oral administration [21].
Some authors have shown a dose-dependent effect of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy in patients receiving contrast media [10, 22].
However, other trials showed lack of benefit of N-acetylcysteine in the prevention of contrast-induced nephropathy [23].
The largest randomized controlled trial to date, the Acetylcysteine for Contrast-induced nephropathy Trial (ACT) [11], found neutral effects of N-acetylcysteine for the prevention of contrast-induced acute kidney injury. However, there are some important differences between this and our study that are worth noting. First of all, in the ACT study patients were administered 0.9% NaCl, 1 ml/kg/h for 6 h before and after the procedure, whereas in our study it was 1–1.5 ml/kg for 12 h before and after contrast administration. That is a slight difference. Volume and timing and route of hydration may be important. Some studies have demonstrated that the adoption of hydration may influence efficacy of NAC in preventing CIN [18]. Secondly, the mean volume of contrast medium administered in the ACT trial was significantly smaller (circa 100 ml), while in our study it was 200 ±85.0 ml. Thirdly, N-acetylcysteine in the ACT trial was administered orally, while in our study NAC was given intravenously. Taking into consideration the low bioavailability of oral NAC due to first-pass hepatic metabolism, this might have also contributed to the different results of our study and the ACT trial as only a small proportion of the administered dose is available for renal protection.
The results of our study are consistent with recent meta-analyses, which supported the use of NAC in the prevention of CIN in patients exposed to contrast media [16–18] (although the strength of evidence favoring the use of low-dose NAC was low). That may explain why low-dose NAC is not used more often and helps to explain differing recommendations on its use to prevent CIN.
The 2014 ESC/EACTS Guidelines on myocardial revascularization recommend against the use of NAC for patients receiving intra-arterial contrast media in cardiac procedures [24], whereas the 2012 Kidney Disease: Improving Global Outcomes Clinical Practice Guideline for Acute Kidney Injury suggests using oral N-acetylcysteine with intravenous fluids in patients with increased CIN risk, acknowledging low strength of evidence [25].
To conclude, research on intravenous N-acetylcysteine and the incidence of CIN is very inconsistent. It is difficult to warrant a definite conclusion at present on the efficacy of NAC.
A large, well-designed trial that incorporates the evaluation of clinically relevant outcomes in participants with different underlying risks of CIN is required to more adequately assess the role for intravenous NAC in CIN prevention.
Taking into consideration the pathomechanism of CIN development, perhaps there are some factors that influence the efficacy of CIN, such as insufficient hydration in trials failing to show NAC usefulness.
Contrast-induced nephropathy is associated with significantly increased in-hospital morbidity, mortality, and costs. It is believed that CIN occurring within 72 h after administration of CM is associated with increased in-hospital and long-term mortality [26].
In our study there were no in-hospital deaths and no need for dialysis during hospitalization, but mean hospitalization time in patients with CIN was significantly longer than in patients without CIN, which is consistent with some other studies [26]. In our study we found that elevated creatinine level 10–15 days after contrast administration was associated with increased risk of adverse events in long-term follow-up. Interestingly, there was no such association with CIN occurring within 72 h after CM exposure in our study. Similar findings were reported by Holscher et al. [27]. In a study reported by Holscher et al. [27] elevated creatinine levels at 30 days after catheterization, but not within 72 h, had a significant effect on the long-term outcome of the patients. Taking into consideration the pathophysiology of acute kidney injury caused by contrast media and the fact that recovery and regeneration of tubular cells from within the tubule structure may take 8 to 10 days [28], assessing kidney function within 72 h may be too early, as the process of renal function recovery may take longer. In healthy subjects there is a great tubular repair capability and exposure to contrast media may have no significant clinical consequences [29]. However, patients with CKD and diabetes mellitus may develop clinically important CIN more easily, as they have a reduced number of functioning nephrons and impaired ability to regenerate tubular epithelial cells. Some of the nephrons may thus never fully recover, leading to loss of the functional unit and finally fibrosis [28]. In study reported by Abaci et al. [30], exposure to CM was an independent predictor of the composite outcome measure of death or renal failure requiring dialysis in 3-year long-term observation. Interestingly, exposure to CM was an independent predictor of long-term adverse events, even when if it did not cause acute kidney injury (creatinine was measured 48–96 h after administration of CM).
So far this subject has not been extensively investigated. A large, well-designed trial is required to give more insight into this topic.
Conclusions
In our study intravenous administration of N-acetylcysteine at a dose of 600 mg and hydration with 0.9% NaCl saline solution reduced the incidence of contrast-induced nephropathy in patients undergoing cardiac or peripheral angiography and/or angioplasty. The investigated strategy was also safe and inexpensive. Elevated creatinine level 10–15 days after contrast administration was associated with increased risk of adverse events in long-term observation, while elevated creatinine level within 72 h was not. Measuring creatinine level at least 10 days after exposure to contrast media may provide a better outcome measure.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–9. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 2.Kitajima K, Maeda T, Watanabe S, Sugimura K. Recent issues in contrast-induced nephropathy. Int J Urol. 2011;18:686–90. doi: 10.1111/j.1442-2042.2011.02830.x. [DOI] [PubMed] [Google Scholar]
- 3.Pieniążek P. Prevention of adverse reactions to contrast media. In: Brzezińska-Rajszys G, Dąbrowski M, editors. Interventional Cardiology. Warsaw: PZWL; 2009. pp. 591–8. [Google Scholar]
- 4.Silver SA, Shah PM, Chertow GM, et al. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. doi: 10.1136/bmj.h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wybraniec MT, Mizia-Stec K, Więcek A. Contrast-induced acute kidney injury: the dark side of cardiac catheterization. Pol Arch Med Wewn. 2015;125:938–49. doi: 10.20452/pamw.3218. [DOI] [PubMed] [Google Scholar]
- 6.Detrenis S, Meschi M, Musini S, et al. Lights and shadows on the pathogenesis of contrast-induced nephropathy: state of the art. Nephrol Dial Transpl. 2005;20:1542–50. doi: 10.1093/ndt/gfh868. [DOI] [PubMed] [Google Scholar]
- 7.Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–34. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 8.Briguori C, Airoldi F, D’Andrea D, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–7. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 9.Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 10.Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–82. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 11.Berwanger O. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized acetylcysteine for contrast-induced nephropathy trial (ACT) Circulation. 2011;124:1250–9. doi: 10.1161/CIRCULATIONAHA.111.038943. [DOI] [PubMed] [Google Scholar]
- 12.Patti G, Nusca A, Chello M, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008;101:279–85. doi: 10.1016/j.amjcard.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Dybich P, Pietrzyk E. Short-term controlled tissue ischaemia in the prevention of contrast-induced nephropathy before coronary angiography. Folia Cardiol. 2014;9:355–7. [Google Scholar]
- 14.Drager LF, Andrade L, Barros de Toledo JF, et al. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803–7. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Shechter M, Matetzky S, et al. Oral acetylcysteine as an adjunct to saline hydration for the prevention of contrast-induced nephropathy following coronary angiography: a randomized controlled trial and review of the current literature. Eur Heart J. 2004;25:212–8. doi: 10.1016/j.ehj.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam RM, Suarez-Cuervo C, Wilson RF, et al. Effectiveness of prevention strategies for contrast-induced nephropathy a systematic review and meta-analysis. Ann Intern Med. 2016;164:406–16. doi: 10.7326/M15-1456. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Qian P, Kumar S, et al. The effect of N-acetylcysteine on the incidence of contrast-induced kidney injury: a systematic review and trial sequential analysis. Int J Cardiol. 2016;209:319–27. doi: 10.1016/j.ijcard.2016.02.083. [DOI] [PubMed] [Google Scholar]
- 18.Sun Z, Fu Q, Cao L, et al. Intravenous N-acetylcysteine for prevention of contrast-induced nephropathy: a meta-analysis of randomized, controlled trials. PLoS One. 2013;8:e55124. doi: 10.1371/journal.pone.0055124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 20.Briguori C, Manganelli F, Scarpato P, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002;40:298–303. doi: 10.1016/s0735-1097(02)01958-7. [DOI] [PubMed] [Google Scholar]
- 21.Borgström L, Kågedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31:217–22. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- 22.Briguori C, Colombo A, Violante A, et al. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J. 2004;25:206–11. doi: 10.1016/j.ehj.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Webb JG, Pate GE, Humphries KH, et al. A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: lack of effect. Am Heart J. 2004;148:422–9. doi: 10.1016/j.ahj.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2014;35:2541–619. [Google Scholar]
- 25.Kellum JA, Lameire N, Aspelin P, et al. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 26.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 27.Holscher B, Heitmeyer C, Fobker M, et al. Predictors for contrast media-induced nephropathy and long-term survival: prospectively assessed data from the randomized controlled dialysis-versus-diuresis (DVD) trial. Can J Cardiol. 2008;24:845–50. doi: 10.1016/s0828-282x(08)70193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough P, Choi JP, Feghali GA, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2016;68:1465–73. doi: 10.1016/j.jacc.2016.05.099. [DOI] [PubMed] [Google Scholar]
- 29.Akrawinthawong K, Ricci J, Cannon L, et al. Subclinical and clinical contrast-induced acute kidney injury: data from a novel blood marker for determining the risk of developing contrast-induced nephropathy (ENCINO): a prospective study. Ren Fail. 2015;37:187–91. doi: 10.3109/0886022X.2014.991994. [DOI] [PubMed] [Google Scholar]
- 30.Abaci O, Hermankaya O, Kocas B, et al. Long-term follow-up of patients at high risk for nephropathy after contrast exposure. Angiology. 2015;66:514–8. doi: 10.1177/0003319714546527. [DOI] [PubMed] [Google Scholar]