Abstract
Alcohol operant self-administration paradigms are critical tools for studying the neural circuits implicated in both alcohol-seeking and consummatory behaviors and for understanding the neural basis underlying alcohol-use disorders. In this study, we investigate the predictive value of two operant models of oral alcohol self-administration in mice, one in which alcohol is delivered into a cup following nose-poke responses with no accurate measurement of consumed alcohol solution, and another paradigm that provides access to alcohol via a sipper tube following lever presses and where lick rate and consumed alcohol volume can be measured. The goal was to identify a paradigm where operant behaviors such as lever presses and nose pokes, as well as other tracked behavior such as licks and head entries, can be used to reliably predict blood alcohol concentration (BAC). All mice were first exposed to alcohol in the home cage using the “drinking in the dark” (DID) procedure for 3 weeks and then were trained in alcohol self-administration using either of the operant paradigms for several weeks. Even without sucrose fading or food pre-training, mice acquired alcohol self-administration with both paradigms. However, neither lever press nor nose-poke rates were good predictors of alcohol intake or BAC. Only the lick rate and consumed alcohol were consistently and significantly correlated with BAC. Using this paradigm that accurately measures alcohol intake, unsupervised cluster analysis revealed three groups of mice: high-drinking (43%), low-drinking (37%), and non-drinking mice (20%). High-drinking mice showed faster acquisition of operant responding and achieved higher BACs than low-drinking mice. Lick rate and volume consumed varied with the alcohol concentration made available only for high- and low-drinking mice, but not for non-drinking mice. In addition, high- and low-drinking mice showed similar patterns during extinction and significant cue-induced reinstatement of seeking. Only high-drinking mice showed insensitivity to quinine adulteration, indicating a willingness to drink alcohol despite pairing with aversive stimuli. Thus, this study shows that relying on active presses is not an accurate determination of drinking behavior in mice. Only paradigms that allow for accurate measurements of consumed alcohol and/or lick rate are valid models of operant alcohol self-administration, where compulsive-like drinking could be accurately determined based on changes in alcohol intake when paired with bitter-tasting stimuli.
Keywords: Blood alcohol concentration, Breakpoints, Quinine adulteration, Alcohol drinking, Ethanol
Introduction
Alcohol-use disorders (AUDs) cause significant health and societal problems, affecting nearly 17 million American adults (SAMHSA, 2013). Enhancing our understanding of the neural circuitry that drives and regulates alcohol drinking is a critically needed advancement. Animal models that test the reinforcing properties of alcohol and the motivation to obtain alcohol are invaluable tools for capturing the endophenotypes associated with AUD and identifying the circuits and neuroanatomical substrates underlying the disease. Operant self-administration of alcohol is well suited for this purpose, in part because of the flexibility in the reinforcement schedule that allows measurement of the effort exerted to obtain alcohol and because seeking and drinking behaviors can be dissociated and measured within a session, in addition to throughout sessions (Lopez & Becker, 2014; Samson & Czachowski, 2003). Further, operant models of oral alcohol self-administration allow for testing the effect of alcohol dose and taste on seeking, which are important to understand the reinforcing and aversive properties of alcohol and for measuring compulsive-like alcohol seeking and drinking when paired with an aversive stimulus (Tabakoff & Hoffman, 2000). The goal of this study is to identify an operant model of oral alcohol consumption in mice by which the operant parameters/measures could be used to estimate the pharmacological reinforcing properties of alcohol in individual animals, and to be able to distinguish between mice that consumed alcohol to intoxication from those with low-alcohol drinking behavior. In other words, this would be a mouse operant model in which the measured operant behaviors could be used to estimate alcohol consumption, blood alcohol concentration (BAC), and seeking behavior to measure the reinforcing and motivational properties of alcohol without relying on food restriction to enhance operant behaviors.
Numerous procedures of operant alcohol drinking have been developed for pre-clinical research in animal models (reviewed in Lopez & Becker, 2014; Samson & Czachowski, 2003); however, several limitations are apparent. First, few studies use mice, limiting the potential to test transgenic mice to manipulate gene expression or neural activity using chemogenetic or optogenetic approaches. Second, most studies rely solely on the operant responding, often assuming alcohol is consumed and estimating intoxication levels without measuring alcohol intake or sampling blood for BAC. However, this is assuming consumption is only warranted in cases of intravenous and intra-gastric alcohol self-administration experiments during which responding is followed by contingent infusion of alcohol solution in the bloodstream and digestive system, respectively (Fidler, Clews, & Cunningham, 2006; Grahame & Cunningham, 2002). In all other procedures of oral self-administration in which operant responding is dissociated from consumption, alcohol consumption is not guaranteed and should be measured rather than assumed. If there is no strong correlation between responding and alcohol drinking, it is then very difficult to identify subjects that consume alcohol to intoxication based on the level of responding in the operant task (i.e., lever pressing, head entries, etc.). If this is the case, alcohol consumption and/or intoxication must be measured to validate any changes in responding seen during manipulation/treatment.
An accurate measurement of alcohol consumption is particularly relevant for alcohol oral self-administration given the animals’ aversion to alcohol’s taste, which varies between different mouse strains (Cunningham, 2014; Phillips et al., 2005; Risinger & Cunningham, 2000). Some strategies have been used to minimize alcohol taste aversion to encourage consumption in mice. One involves selective breeding to isolate the genetic component that drives low taste aversion and high alcohol drinking (Barkley-Levenson, Cunningham, Smitasin, & Crabbe, 2015; Phillips, Belknap, Buck, & Cunningham, 1998; Phillips et al., 2005). Another popular approach is the addition of sucrose or another sweetener to the alcohol solution, which certainly increases alcohol consumption, but it also confounds the reinforcing properties of alcohol and complicates the study of acquisition of the alcohol self-administration behavior (Stafford, Anderson, Shelton, & Brunzell, 2015; Tolliver, Sadeghi, & Samson, 1988). Other approaches have been successful in using a habituation period in which the alcohol solution is offered intermittently for oral consumption in the home cage before the start of operant self-administration training (Carnicella, Yowell, & Ron, 2011; McCool & Chappell, 2009; Tsiang & Janak, 2006; Wang et al., 2010).
In this study, we sought to identify a model of operant alcohol self-administration where operant responding and other behaviors measured during the task can be used to reliably predict a mouse’s intoxication level and discriminate between high- and low-alcohol drinking mice. To accomplish this, we tested several cohorts of mice on two different operant paradigms of oral alcohol self-administration. On one operant paradigm, a small amount of alcohol solution is dispensed into a cup within the food receptacle every time the animal completes a reinforcing schedule through active nose-poke. Once delivered, the alcohol solution is available at all times and the alcohol accumulates with subsequent active responding, if not consumed. Indeed, the solution can and will overflow from the cup if responding continues without drinking and as such, it very difficult to obtain accurate measurements of consumed alcohol and to accurately record licks to the cup because a short circuit develops after there is liquid spillage. On the other operant paradigm, animals earn access to a sipper containing an alcohol solution for a limited time following an active lever press schedule ratio, and alcohol consumption can be reliably measured through a graduated cylinder connected to the sipper. Multiple intrinsic and procedural differences prevent direct comparisons between the models and limit the comparative conclusions to be made. However, the study’s goal is not to compare the models but rather to identify a good mouse operant model to accurately measure the reinforcing and motivational properties of alcohol without pairing with sweetener and food restriction. As described below in detail, operant responding was only weakly correlated with BAC and intoxication in mice under both paradigms. However, the model using the sipper was successful at measuring other behavioral parameters to predict intoxication. Here, we conclude that the paradigm using the sipper tube for delivery was shown to be effective in measuring consumed alcohol and drinking behaviors, and it is suitable for testing the reinforcing properties of alcohol, dose dependence, alcohol seeking, and drinking resilience to quinine adulteration and other aversive consequences.
Materials and methods
Animals
Adult (7–13 weeks old at the start of the experiment) male and female C57BL/6J mice (n = 57) were housed 1–3 per cage during all experiments and given food ad libitum. Water was ad libitum except during DID or SA alcohol drinking sessions. All mice were acclimated to a reversed light cycle (lights on from 6:30 p.m. to 6:30 a.m.) at least 5 days before drinking experiments began. Protocols were approved and performed in accordance with the National Institute on Alcohol Abuse and Alcoholism’s Animal Care and Use Committee.
Outline of the experimental design
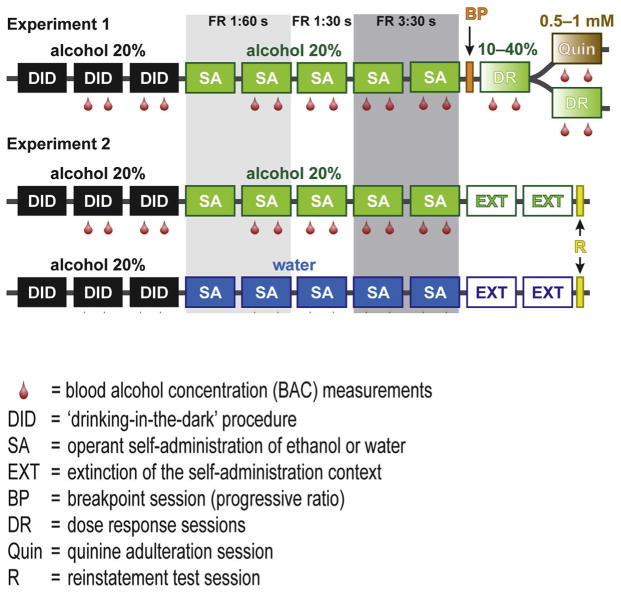
As shown in Fig. 1, all mice included in the two experiments had access to a 20% alcohol solution for 3 weeks under the “drinking in the dark” (DID) paradigm (Wilcox et al., 2014). In Experiment 1, DID was followed by 4 weeks of alcohol self-administration (SA) in operant chambers under either the CUP or SIPPER paradigm. Dose response (DR) sessions for alcohol and quinine adulteration tests (QUIN) were performed for the following 2 weeks. In the case of the SIPPER paradigm, a breakpoint (BP) for each mouse was measured during a single progressive responding session performed between SA and DR sessions. Experiments 2 and 3 were run only under the SIPPER paradigm. Experiment 2 consisted of 4 weeks of alcohol self-administration followed by 2 weeks of extinction (EXT) sessions and one session of cue-induced reinstatement (R). Experiment 3 consisted of 4 weeks of water self-administration using the same protocol as the alcohol in Experiment 2, followed by 2 weeks of EXT sessions and one RI session. Operant water self-administration was performed under the SIPPER paradigm to evaluate how mice acquire responding, licking, and drinking behavior for a natural reward without previous restriction and to compare it to alcohol self-administration under the same paradigm. Blood alcohol concentrations (BAC) were measured at the end of the SA session twice a week throughout the experiments as marked with red drops.
Fig. 1. Experimental outline.
Each box represents a week of treatment/testing and the red drop drawings represent the blood samples extracted for BAC measurements. Shaded areas defined the different schedules and access times provided. Abbreviations: DID: ‘drinking-in-the-dark’ procedure; SA: operant self-administration of alcohol or water; EXT: extinction of the self-administration context; BP: breakpoint session (progressive ratio); DR: dose response sessions; Quin: quinine adulteration session; R: reinstatement test session.
Modified drinking-in-the-dark (DID) procedure
Three hours into the dark cycle (9:30 a.m.), water bottles were replaced with bottles containing 20% alcohol. Four hours later (1:30 p.m.), alcohol bottles were removed and water bottles were returned to the cage. This procedure was repeated 5 days per week, for 3 weeks. Alcohol bottles were glass (25 × 100 mm, Pyrex®) and were fitted with straight, open-tipped metal sippers. The alcohol solution was prepared daily by diluting 95% alcohol (190 proof, stored in glass, Deacon Labs) with tap water to 20% (v/v). Because the animals were group-housed, we did not measure individual alcohol intake during the DID phase. The blood alcohol concentrations (BAC) were collected twice a week, starting from the second week of the DID procedure. The overall average BAC was 77.71 ± 7 mg/dl in males and 85.35 ± 5.8 mg/dl in females.
Blood alcohol concentration (BAC) measurements
Blood samples for BACs were collected twice weekly, immediately after drinking sessions. The tail vein was nicked with a razor blade and 15–50 μL of blood was collected into heparinized capillary tubes, which was then centrifuged for 5 min to separate the plasma. An alcohol assay was run on the plasma samples using the Analox analyzer model GM7 MicroStat (Analox Instruments, Lunenburg, MA). Each individual blood sample was measured twice and the average of the two readings was used. To estimate the intra-assay variability, we calculated the percent variation of each duplicate from the mean, which was 9 ± 1.5%, and the difference between the two measurements, which was 4.9 ± 0.6 mg/dl (n = 78 duplicates). BACs were also measured in negative control samples of serum from water-drinking control mice. Baseline readings for negative control were on average 13.04 ± 0.79 mg/dl, which is in agreement with manufacturer’s specifications.
Operant alcohol self-administration (SA)
CUP paradigm
Following 3 weeks of DID, mice (n = 27) were trained to self-administer alcohol (20% v/v) in size-modified operant chambers (internal dimensions: 11 × 18 × 13 cm) in sound-attenuating boxes that delivered alcohol from a syringe into a food magazine fitted with a liquid cup. The magazine was equipped with a sensor to detect head entries. The session began with the vivarium white light turning off and a drug availability light above the active nose-poke hole turning on. An active nose-poke caused 10 μL of alcohol to be dispensed into the liquid cup and a cue light above the nose-poke hole to illuminate, while an inactive nose-poke produced no response. Training and maintenance sessions were 2 h with a fixed poke:reward ratio (FR) of 1. Alcohol intake was determined by subtracting the liquid remaining in the cup at the end of the session from the volume dispensed into the cup. In some cases, more alcohol was dispensed than the volume of the cup, and overflow occurred, which skewed the intake data and prevented the accurate use of a lickometer in the CUP paradigm because the overflow will cause an electrical short circuit. Therefore, to exclude extremely high and inaccurate values that do not reflect what an animal could actually drink in 2 h, we excluded intake (g/kg) that was greater than 2 SEM from the mean.
SIPPER paradigm
The SIPPER training procedure employed in this work is different from that introduced by Samson, Sharpe and Denning in 1999. Following 3–4 weeks of DID, mice (n = 30) were trained to self-administer alcohol (20% v/v) in operant mouse chambers (internal dimensions: 15.9 × 40 × 12.7 cm, see Supplementary Fig. 1 for details) with a retractable lixit sipper (ENV-352-2W) in sound-attenuating boxes (ENV-307A-CT, Med Associates). The retractable sipper was connected to a glass serological pipette containing the alcohol solution, which allowed for precise measurements of the consumed volume (Vc = volume consumed of 20% alcohol/mouse/session). A lickometer (ENV-250) was attached to the sipper, which recorded every contact. A modified guillotine door (CT-ENV-340A-X1) was installed to prevent the mice accessing the sipper in the retracted state (Supplementary Fig. 1). The session began when the red vivarium light turned off and the cue light above the active lever turned on. An active lever press resulted in sipper extension into the chamber for 30–60-s access to the alcohol solution. The alcohol availability cue light was turned off while the sipper was extended and for the duration of the access time, because the sipper presentation is the only cue paired with the reinforcer. Inactive lever presses were recorded but had no effect. Because of the longer session duration (3.5 h), a food pellet was made available in the chamber only in the SIPPER paradigm. Training began with 6-h sessions on FR1, in which each active press resulted in a 60-s access time to alcohol for 5–6 sessions. Following this acquisition phase, the session length was reduced to 3.5 h, the access time to the sipper was reduced to 30 s, and the FR increased to 3 at different times during the procedure and as marked in the figures and experimental design. Data are presented as responding or intake per 3.5 h. When sessions were longer (e.g., 6-h training sessions), data from only the first 3.5 h is presented. A group of control animals was allowed to self-administer water under this same protocol, and glass pipettes were then filled with tap water alone while maintaining the same parameters for the operant training. There was no significant sex difference in the operant behaviors, so the data from males and females were combined.
Progressive responding ratio for alcohol
Progressive responding was measured in one session (maximum 5 h long) in order to test the motivation to seek alcohol. The number of lever presses required to gain access to the sipper was increased exponentially with each consecutive earned access according to the equation ratio = [5 × e(infusion number × 0.2)] − 5 (Richardson & Roberts, 1996). The starting ratio was matched to the fixed ratio of the last SA session (i.e., FR3). The breakpoint achieved by each mouse was defined as the last successful fixed ratio (FR) achieved during that single session (see Bock et al., 2013).
Alcohol dose-response
Dose-response dependence of the responding was measured only using the SIPPER paradigm over 2–6 consecutive sessions (3.5 h long, FR3) in order to determine the dependence of the operant responding with concentration of alcohol made available. The dose of alcohol (10, 30, and 40%) was varied daily using a Latin square design. In most cohorts, animals were given access to each dose for two consecutive sessions, and BACs were taken on the second session of each dose. In one cohort, animals were given access only to 10% and 30% alcohol during single sessions.
Quinine adulteration sessions
Over two sessions, the 20% alcohol solution was adulterated with increasing concentrations of quinine (0.5 and 1.0 mM), and responding was measured. Mice had a session of non-adulterated alcohol solution between the two quinine adulteration sessions. Quinine adulteration sessions were otherwise similar to training sessions using the SIPPER paradigm (3.5 h long, FR3).
Extinction sessions and reinstatement
Fourteen extinction sessions were performed using the SIPPER paradigm after training was completed and the responding had plateaued. Extinction (EXT) sessions were 3.5 h long. No sipper extension occurred after lever press in the active or inactive lever and the alcohol availability light was kept off. A single reinstatement (R) session was performed after extinction. The reinstatement session was identical to an FR3 self-administration in which the alcohol availability light was on and the sipper was extended after three active presses but no solution was made available via the sipper. Despite the absence of solution via the sipper tube, the number of contacts to the sipper (licks) was quantified during the reinstatement session and interpreted as an expression of alcohol-seeking behavior.
Analysis and classification criteria
The Med-PC® acquisition software recorded the counts and time stamps of the responses and the time stamp of sipper extensions. The data files were analyzed using COBAI, a custom written software in Igor Pro (Wavemetrics). The consumed volume of 20% alcohol solution per each mouse (Vc) was measured by reading the graduated pipette before and after each session in the SIPPER paradigm. Vc was used to calculate intake (I) in grams of alcohol per kilogram of body weight (g/kg) according to the following formula: I = (Vc × δa) × 0.2, where δa is the density of alcohol (0.789 g/mL). Mice were classified into three groups: high, low, and non-drinkers/non-responders, based on the average alcohol intake per session throughout all training sessions. A hierarchical clustering analysis was also performed using the weekly average alcohol intake (g/kg/week) for each mouse during the self-administration paradigm using the hclust function from the basic stats package of the R software (version 3.2.0). Clusters were identified using the Ward hierarchical agglomerative cluster algorithm based on the Euclidean distance between subject’s intakes.
Statistical and data analysis
Data were analyzed using Igor Pro 6.37 (WaveMetrics, Tigard, OR) using Microsoft Excel and a custom-written software (COBAI) for behavior analysis. Statistical analysis was performed using Igor Pro, Prism 5 (GraphPad, La Jolla, CA) and R (The R Project for Statistical Computing, http://www.r-project.org). Two-way ANOVA (2W-ANOVA), repeated-measures 2W-ANOVA (RM2W-ANOVA), 1-way ANOVA, and paired or unpaired t tests were used and corrected for unequal variance using Welch’s correction when appropriate. All tests used were 2-tailed and results were considered significant at an alpha of 0.05. Experimental design determined the statistical test used and data met the assumptions for each test. Significant interactions were followed up with pairwise t tests and corrected for multiple comparisons. Data are shown as mean ± standard error of the mean.
Drugs
Alcohol (190 proof, in glass container) were dissolved in tap water at 20% v/v. Quinine (Sigma-Aldrich) was dissolved in the 20% alcohol solution or in tap water, accordingly to the experiment conditions.
Results
Mice acquire operant alcohol self-administration under both paradigms
We tested for the acquisition of operant alcohol self-administration using two different behavioral chambers: one having a pair of nose-poke holes and a food magazine where 10 μL of alcohol solution was dispensed into a cup upon active pokes (referred to as CUP paradigm for simplicity), another having a pair of levers and a retractable sipper that provided access to the alcohol solution over 30–60 s upon active lever presses (referred here as SIPPER for simplicity, Supplementary Fig. 1). Over the first week of training in the CUP paradigm, the number of active pokes increased nearly 2-fold to 49 ± 6.7 pokes/2 h for session 7 and plateaued for the following sessions (Fig. 2). Nose-pokes in the inactive hole decreased over the first two sessions and remained low at 12.8 ± 1.2 pokes/2 h across sessions (n = 27). Using the SIPPER paradigm, lever pressing behavior also increased progressively during the first week of training from 4.2 ± 1 on session 1 to an average of 18.2 ± 3.4 presses/3.5 h by session 7 and 26.5 ± 2.3 presses/3.5 h by sessions 9–14 (Fig. 2d, n = 30). The rate of responding on the inactive manipulanda was lower than for the active in both the SIPPER (inactive: 12.3 ± 2.6 presses/3.5 h; active: 25 ± 3.7 presses/3.5 h; t = 5.39, p < 0.0001) and the CUP paradigm (inactive: 13.5 ± 2 pokes/2 h; active: 47.8 ± 8.5 pokes/2 h), suggesting selective responding on the active lever/hole across both paradigms.
Fig. 2. Similar rates of operant responding for alcohol oral self-administration in mice under the CUP and SIPPER paradigm.
(a, b) Schematic representation of the operant panel showing (a) two nose poke holes (active and inactive) with corresponding cue lights flanking a magazine with a cup in which the alcohol solution is delivered (CUP paradigm) and (b) two levers (active and inactive) with corresponding cue lights and sipper tube in the middle panel from where alcohol solution can be consumed while licks are recorded (SIPPER paradigm). (c, d) Rate of operant responding in the active (filled) and inactive (open) poke hole/2 h and lever presses/3.5 h during the first 10–15 sessions. (d, f, h) Shaded areas mark self-administration sessions with FR1 and 60-s access time/active press. Access time was reduced to 30 s/active press after that. (e, f) Rate of seeking responses measured as head entries in the food magazine (e, CUP) or licks to the sipper (f, SIPPER) over sessions. (g, h) Estimated (g, CUP) and measured (h, SIPPER) alcohol intake in g/kg body weight of mice over the weeks of operant self-administration. (i) Overall average blood alcohol concentrations (BAC) achieved at the end of the session for mice under the CUP (black) and SIPPER (blue) paradigm. (j) Weekly average blood alcohol concentrations (BAC) achieved at the end of the session for mice under the CUP (black) and SIPPER (blue) paradigm. All data are mean ± SEM (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article).
Indicators of alcohol seeking and intake differ
We also tracked other behaviors that could be associated with alcohol seeking and intake. For the CUP paradigm, head entries into the food magazine where the cup is located were recorded. For the SIPPER paradigm, licks during the time that the sipper was extended were also measured. More than 200 ± 34.3 head entries were performed per 2-h session, and this number was maintained from the first operant session and throughout the experiment (Fig. 2e, n = 27). In the SIPPER paradigm, on the other hand, mice performed fewer licks at the onset (26.6 ± 9.3/3.5 h), but the number of licks increased with the sessions and the number plateaued near 283 ± 35 licks/3.5 h session (Fig. 2f, n = 30).
Throughout the 10 sessions of operant self-administration, mice increased their alcohol intake from 2.9 ± 0.6 g/kg to nearly 4.1 ± 1.3 g/kg alcohol in the 2-h session under the CUP paradigm (Fig. 2g, n = 27). However, note that the measure of consumption in the CUP paradigm must be calculated from the number of earned rewards, with no means of verifying the consumption other than measuring BAC. In contrast, under the SIPPER paradigm, alcohol consumption is calculated from the graduated pipette connected to the sipper, and thus the volume consumed can be directly and accurately measured. Under the SIPPER paradigm, mice also escalated alcohol consumption but reached a lower average of 2.8 ± 0.4 g/kg alcohol in a 3.5-h session (Fig. 2h, n = 30). When comparing both paradigms, please note the large variability (large SEM) in the intake for the CUP compared to the more stable intake (lower SEM) under the SIPPER paradigm (Fig. 2g and h). BACs were measured twice a week at the end of the SA sessions, and were comparable between the paradigms despite the apparently lower alcohol consumption for the SIPPER paradigm (Fig. 2i and j).
Operant behaviors under SIPPER paradigm are better predictors of alcohol drinking
To determine how well the tracked behaviors under each paradigm predict alcohol drinking and BAC, we measured the degree of correlation between these parameters. We used the data from every session with BAC measurements under both paradigms. Despite the apparent high levels of alcohol drinking (~4 g/kg) under the CUP paradigm, active pokes were not correlated with intake, suggesting that the operant/seeking behavior is not a good predictor of BAC (R2 = 0.009, n.s.; Fig. 3a). There was also no significant correlation between the rate of head entries and the volume of alcohol consumed (R2 = 0.001, n.s.; Fig. 3c). Importantly, alcohol intake (in g/kg) was correlated with BAC (R2 = 0.2; p < 0.0001 Fig. 3e), indicating that mice can train to self-administer alcohol under this operant task and some do reach intoxicating levels of alcohol in the blood. However, this correlation analysis also shows that neither the operant nor other tracked behaviors under the CUP paradigm are good predictors of alcohol intake and BAC, so the behaviors cannot distinguish the high- from the low-drinking mice.
Fig. 3. Licks and responding are better predictors of intake and BAC in the SIPPER paradigm.
(a, b) Correlations between BAC achieved after the session and the rate of responses in the active manipulanda are significant in the SIPPER paradigm but not the CUP paradigm. (c, d) Total dispensed volume correlates with licks in the SIPPER (blue) but not with head entries in the cup magazine under the CUP (black). (e, f) Correlation between BACs and alcohol intake for individual mice and session under the CUP (e) and SIPPER (f) paradigm. Each symbol represents data from individual sessions from individual mice. *p < 0.05; (n.s.), non-significant (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article).
In contrast, we found that operant seeking behaviors consistently and significantly predicted alcohol drinking under the SIPPER paradigm. Sessions in which mice made more presses in the active lever corresponded to sessions in which mice achieved higher BACs as measured at the end of the session (R2 = 0.1, p < 0.0001; Fig. 3b). Some correlations were also found between active presses and rate of licking and intake (Supplementary Fig. 2a and b). Similarly, when mice licked at higher rates, alcohol consumption increased as well (R2 = 0.47, p < 0.0001; Fig. 3d). High alcohol intake (g/kg) also predicted high BAC for mice in the SIPPER paradigm (R2 = 0.45, p < 0.0001; Fig. 3f). The slope of this plot shows that there is a 14.77 mg/dl rise in the BAC for every 1 g/kg alcohol consumed. Altogether, these data show that operant and other tracked behaviors in the SIPPER paradigm, such as active presses and licks, better predict intake and BAC, suggesting that this paradigm more accurately identifies high- and low-drinking mice.
High-alcohol drinking mice acquire operant self-administration faster
Once we established that the SIPPER model is a reliable paradigm for capturing alcohol seeking and drinking behaviors, it was used in further investigations. Individual mice displayed a range of alcohol consumption under the SIPPER paradigm, and hierarchical cluster analysis distinguished three distinct groups of mice based on their intake (Supplementary Fig. 2c). We found that 13 out of 30 mice (43%) consumed more than 3 g/kg of alcohol on average per session (“high-drinking mice”), versus 11 (37%) who drank between 1 and 3 g/kg alcohol on average (“low-drinking mice”) (Fig. 4a and b). Those high-drinking mice who averaged more than 3 g/kg alcohol per session also showed higher BACs compared to the lower-drinking group (Fig. 4b). There was also a portion of the mice (20%) who drank less than 0.3 g/kg alcohol and failed to achieve BACs above the levels seen in water-drinking control mice, which classified them as “non-responders”. The high-drinking group consistently drank more alcohol across every session compared to the other two groups (RM2W-ANOVA: significant group × session interaction F(42,588) = 1.46, p = 0.035 and significant group and sessions p < 0.003; Fig. 4c), and the BACs measured at the ends of the sessions were significantly higher across the phases of the SA experiment, averaging >80 mg/dl, which is higher than 0.08%, the level defined as the legal limit for intoxication in humans (RM2W-ANOVA: significant difference between groups F(2,100) = 42.89, p = 0.001 and no effect of time or interaction [F’s < 0.63, p’s > 0.59; Fig. 4d]). The high-drinking mice also learned the operant drinking task at a faster rate than the low-drinking mice, with more active presses in the first seven sessions (main effect of group: F(2,27) = 3.48, p = 0.045 and interaction: F(12,162) = 1.87, p = 0.041; Fig. 4e). Inactive presses were not different between high-drinking mice, low-drinking mice, and non-responders (Fgroup(2,27) = 1.98, p = 0.16; Fig. 4f).
Fig. 4. Mice with low and high alcohol intake differ in BAC and responding rates in SIPPER paradigm.
(a) Frequency histogram of the overall mean alcohol intake for individual mice reveals three groups: non-responders (black), low- (red) and high- (green) drinking mice. (b) Overall BAC as a function of overall mean intake for all mice showing three groups color-coded. Inset: Pie chart shows the frequency distribution of non-, low-, and high-drinking mice. (c, e, f) Alcohol intake per session (c) and rate of active (e) and inactive (f) lever presses per 3.5-h self-administration session for non-, low-, and high-drinking mice (black, red, and green, respectively). Light-shaded areas mark self-administration sessions with FR1 schedule and 60-s access time/active press. Access time was then reduced to 30 s/active press (white area) and schedule changed to FR3 during sessions marked in dark gray (access time kept at 30 s/earned reward). (d) Weekly BAC achieved for mice in each group during the DID phase and the operant self-administration phase. All data are mean ± SEM. *p < 0.05 in 2WRM-ANOVA comparison between Low vs. High drinking mice (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article).
High-drinking mice lick more despite equal access time
We analyzed the pattern of licking behavior for high- and low-drinking mice. Representative examples from two mice are shown in Fig. 5a, and highlight the difference in the rate of responding and licks between high-drinking mice and low-drinking mice during sessions 4 and 5. The examples show that the high-drinking mice earns more access time to the sipper (purple) and makes more licks (red tick) than the low-drinking mice. In part, the difference is also explained by the fact that the low-drinking mice have a higher percentage than the high-drinking mice of access time in which there are no contacts to the sipper. Because mice can only lick while the sipper is extended, the total access time to the sipper was calculated and plotted. High-drinking mice earned more access time to the sipper than low-drinking mice during the first nine sessions of training (access time = 1 min/active press) (mean access time [1–9]: 48.4 ± 5.6 min for high, n = 13; 25.7 ± 6 min for low, n = 11). In fact, the earned access time increased dramatically during the first three sessions for the high-drinking mice, in agreement with their higher rate of responding, which further supported their faster acquisition. Although low-drinking mice reached a similar maximum access time of ~60 min/session, it took them longer, about nine sessions (Fig. 5b, n = 13 [high], 11 [low], and 6 [non-drinking]). Starting at session 10, access time was reduced to 0.5 min/active press and consequently, the total access time dropped by half for both groups of mice, and active responding was unchanged. High-drinking mice also licked at faster rates than low-drinking and non-drinking mice (licks/3.5 h = 1066 ± 107.1 [high] vs. 587.9 ± 111.6 [low] vs. 41.2 ± 18.1 [non]; RM2W-ANOVA significant effect of groups, sessions and interaction: F’s > 2.9, p’s < 0.004; post hoc Tukey: differences between high- and low-drinking mice for session 2 through 5, p < 0.049). Lick rates were similar between high- and low-drinking mice during the last sessions (RM2W-ANOVA [session 18–24]: main effect of groups driven by differences with the non-drinkers F(2,27) = 11.93, post hoc Tukey: high vs. low n.s.) toward the end of the experiment. Thus, high-drinking mice showed enhanced licking rates compared to low-drinking mice under the SIPPER paradigm, especially for the first sessions. Furthermore, due to the close correlation between licks and alcohol intake, and between intake and BAC (Fig. 3), we conclude that higher rates of licking correspond to higher consumption and intoxication.
Fig. 5. Pattern of alcohol drinking under the operant SIPPER paradigm.
(a) Example raster plots of the operant responding for two mice belonging to (top) the high and (bottom) the low drinking group through two consecutive sessions (sessions 4 and 5). Black ticks denote active presses, purple is the earned access time and a recorded lick to the sipper is red. (b) Total access time earned per session for non-responders (black), low (red), and high (green) drinking mice. (c) Lick rate per session for each group. Light shaded areas mark self-administration sessions with FR1 schedule and 60-s access time/active press. Access time was then reduced to 30 s/active press (white area) and schedule changed to FR3 during sessions marked in dark gray (access time kept at 30 s/press). All data are mean ± SEM. *p < 0.05 in 2WRM-ANOVA comparison between Low vs. High drinking mice (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article).
Alcohol concentration affects the rate of licking but not responding
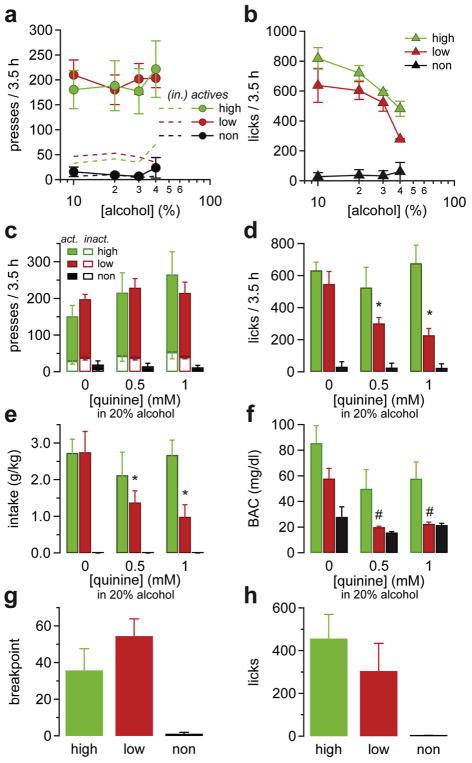
To investigate the dose dependence of the behaviors measured, we varied the concentration of alcohol in the solution between 10, 20, 30, and 40% during individual sessions. The active lever pressing behavior was unchanged by altering the alcohol concentration in either high- or low-drinking mice (2W-ANOVA Fgroup(1,31) = 0.05331, p = 0.8; Fig. 6a). However, the rate of licks significantly varied with the alcohol concentration. High- and low-drinking mice licked less as the concentration of alcohol rose, suggesting that mice titrated their alcohol intake (2W-ANOVA: Fdose(3,44) = 5.24, p = 0.035; Fig. 6b). Mice also consumed less volume of solution and high-drinking mice maintained the intake and BAC quite constant when varying the alcohol concentration available, although total alcohol intake dropped with 10% (Supplementary Fig. 3). At the highest concentration of alcohol, 40%, the high-drinking mice showed a trend to higher rates of licking than low-drinking mice (482.3 licks/3.5 h for high-drinking mice; 278.5 licks/3.5 h for low-drinking mice, Tukey’s post hoc n.s.), though they were not significantly different. Note that non-responding mice show a very slow rate of level pressing and licking at all concentrations.
Fig. 6. High- and low-drinking mice have different sensitivity to alcohol dose and quinine adulteration.
(a, b) Dose-response curve showing (a) rate of active presses and (b) rate of licks as a function of the alcohol concentrations in the delivered solution for each group. (c–f) Effect of quinine adulteration on (c) the rate of lever presses, (d) the rate of licks, (e) the mean intake per session, and (f) the BAC after the session for each group of mice. Note that alcohol seeking (licks) and intake, but not active presses, are decreased in low-drinking mice when alcohol is mixed with 0.5–1 mM quinine but remain constant in high-alcohol drinking mice. (g) Breakpoints and (h) licks performed by mice in the low-, high-, and non-drinking groups during a progressive responding session. All data are mean ± SEM. *p < 0.05 in 2WRM-ANOVA in dose comparison within groups, #p < 0.05 in 2WRM-ANOVA comparison between Low vs. Higher drinking mice.
High-drinking mice are less sensitive to quinine adulteration
We also tested whether pairing alcohol with an unpleasant taste would alter alcohol seeking or drinking behaviors. The 20% alcohol solution was adulterated with increasing concentrations of quinine (0.5 or 1.0 mM), a bitter tasting compound, in two sessions separated by an unadulterated session. Quinine addition did not significantly change the rate of lever responding in high- or low-drinking mice, but slightly enhanced responding in some high-drinking mice, likely an extinction-like response (Fig. 6c). Quinine adulteration reduced the rate of licking (Fig. 6d) by low-drinking mice in a dose-dependent manner (low: 548.4 [0 mM] vs. 303 [0.5 mM] vs. 228.5 [1 mM] licks/3.5 h; RM2W-ANOVA: Fdose(2,26) –7.204, p = 0.0032; Fgroup(2,13) = 23.53, p < 0.0001), but did not impact licks in high-drinking mice, who continued to lick at similar rates (high: 633.5 [0 mM] vs. 527 [0.5 mM] vs. 677.6 [1 mM] licks/3.5 h). The same pattern was seen for alcohol intake and BAC, where high-drinking mice continued to achieve higher alcohol intakes and BACs, even at 1 mM of quinine (2W-ANOVA: significant interaction between groups and quinine dose F(4,26) = 4.887, p = 0.0045 for intake; Fig. 6e). There is a significant difference in BACs between groups (F(2,13) = 14.7, p = 0.0005), and BACs in high-drinking mice are different at 1 mM quinine from the BACs in low-and non-drinking mice (p < 0.05; Fig. 6f). Thus, high-drinking mice were more insensitive to the quinine adulteration and continued to lick and drink despite the addition of the bitter taste to the alcohol solution. Once again, the licking behavior proved to be a reliable measurement of the alcohol intake.
Performance during progressive ratio schedule and extinction-reinstatement
High-, low-, and non-drinking mice were also tested on a single progressive ratio session. Similar to the poor correlation between active lever responding and alcohol intake, we found no difference in the breakpoint nor the rate of licking between the high- and low-drinking mice during the progressive responding session (BP = 35.8 ± 11.85, 54.5 ± 9.43, and 1.2 ± 0.73; licks = 457 ± 113, 304.7 ± 129.2, and 1.8 ± 1.8, for high-, low-, and non-drinking mice, respectively, n = 5/5/6, Tukey’s multiple comparison high vs. low p > 0.05 for BP and licks; 1-way ANOVA FBP(2,13) = 9.299, p = 0.0031, Flicks(2,13) = 4.635, p = 0.0302; Fig. 6g and h).
Next, we evaluated the acquisition of the SIPPER operant task for a natural reward such as water, which is also consumed orally and for which mice are accustomed to consuming voluntarily in their home cage. A plenitude of studies have used a model of operant responding for water as reinforcer, and most included some degree of water restriction (Ljungberg, 1989; McSweeney, Kowal, Murphy, & Wiediger, 2005; Robinson & Carelli, 2008). Although no water restriction was imposed here, water-reinforced mice showed discrimination of active and inactive levers (2W-ANOVA: Flever-presses(1,16) = 5.09; p = 0.038, Fig. 7). The operant behaviors of water-reinforced mice were compared to those of alcohol-reinforced mice, both low- and high-alcohol drinking combined, and no statistical difference was found in the patterns of responding, licks, and drinking access time (Supp. Fig. 5). However, when comparing the high-alcohol drinking mice with water-reinforced mice separately, the licking rate was now significantly higher during sessions at FR1 (RM2W-ANOVA: Fgroup(1,20) = 13.38, p = 0.0016, Fig. 7c), suggesting that this increase in the high-alcohol drinking group was generating the trend observed in the alcohol group as a whole. No difference was seen when comparing the low-alcohol drinking groups with the water-reinforced mice (RM2W-ANOVA: Fgroup(1,18) = 0.015, p = 0.9). There was no difference in the patterns of operant responding between water-drinking and high-alcohol drinking mice (Fgroup(1,20) = 1.33, p = 0.26), and a tendency was observed for longer earned access time during FR1:60 s than water-drinking mice (Fgroup(1,20) = 4.05, p = 0.058; Fig. 7a and d).
Fig. 7. Operant responding and lick rates for high-alcohol drinking and water-drinking mice.
(a–c) Rate of (a) active presses, (b) inactive presses, and (c) licks for high-alcohol drinking (green) and water-drinking (blue) mice. (d) Total access time earned per session for high-alcohol drinking (green) and water-drinking (blue) mice. The light gray area shows the FR1 schedule and 60-s access/press. Later, access time to alcohol was reduced to 30 s/press (white area) and schedule changed to FR3 during the dark gray area sessions (30 s/access time). (e) Rate of responding during extinction sessions (no solution delivered) for high- (green) and low- (red) alcohol drinking and also water-drinking (blue) mice. (f) Seeking behavior measured as rate of operant responding in the active lever pressing during a single cue-induced reinstatement session (hatched bars) compared to baseline after extinction (filled bars) for mice in the high- (green) and low- (red) alcohol drinking group, alcohol-reinforced group, and also for water-drinking mice (blue). Alcohol-reinforced group showed a trend. All data are mean ± SEM. Data from the alcohol-reinforced group corresponds to the low- and high-drinking mice data (same as Figs. 4 and 5) combined as one different group. Data from the high-drinking mice shown in A–D are the same as data from high-drinking mice shown in Figs. 4 and 5 (For interpretation of the references to color/colour in this figure legend, the reader is referred to the Web version of this article).
During the extinction sessions, all groups of mice showed a time-dependent decrease in the rate of responding in the active lever (Fig. 7e). There was no significant difference between groups in the rate of pressing at day 1 of extinction; with high-drinking mice reaching 187 ± 32 presses/3.5 h, low-drinking mice reaching 166 ± 18 presses/3.5 h, and water-drinking mice reaching 130 ± 20 presses/3.5 h (2W-ANOVA F(2,19) = 1.437, p = 0.26). In addition, there was no difference between the groups when comparing the first hour of FR3 and EXT (Supplementary Fig. 4a). Cues previously paired with alcohol or water (cue light and sipper presentation) were reintroduced during a single cue-induced reinstatement session performed after 15 extinction sessions. There was a significant effect of reinstatement across the groups, but there was no interaction between the alcohol groups or water (RM2W-ANOVA main effect of reinstatement Ftest(1,19) = 5.77, p = 0.02; no interaction F(2,19) = 0.84, p = 0.45; Fig. 7f). When the low- and high-drinking mice were pooled together in a single alcohol-reinforced group, similar effects were found (RM2W-ANOVA main effect of reinstatement Ftest(1,20) = 5.59, p = 0.03; no interaction F(1,20) = 0.69, p = 0.41; Fig. 7f), and licking rates during the reinstatement sessions reached similar values as during the FR3 self-administration sessions for all groups (Supplementary Fig. 3). These data suggest that the sipper presentation and cue light have some reinforcing value and can act to promote alcohol and water seeking in mice previously trained.
Discussion
This study evaluates two operant models of alcohol self-administration in mice that differ in the alcohol delivery method. In one model, active responding is followed by delivery of a fixed volume of alcohol solution for an unlimited time into a cup where the volume consumed can only be estimated. In the other model, referred to as SIPPER, responding is followed by presentation of a sipper tube for a limited time from which alcohol solution can be consumed and the ethanol intake can be reliably measured at the end of the session. We found that mice acquired operant responding under both paradigms, but this responding was only poorly correlated with alcohol intake and intoxication levels. The SIPPER paradigm can accurately measure consummatory behaviors such as volume consumed and licks to the sipper tube, two reliable predictors of intoxication. In contrast, the utility of the CUP paradigm is limited because although it measures active lever responding and earned rewards (both weakly correlated with intoxication and predicted intake), it fails to measure consumption of those rewards. Previous works have explored the abilities of mice (Finn et al., 2008; Ford, Fretwell, Mark, & Finn, 2007; Ford et al., 2011) and rats (Bertholomey, Verplaetse, & Czachowski, 2013; Czachowski, Legg, & Samson, 2001; McCool & Chappell, 2009; Samson, Sharpe, & Denning, 1999) in acquiring, maintaining, extinguishing, and reinstating oral alcohol self-administration over both CUP and SIPPER chambers. Also, the study by Samson and Czachowski (2003) pre-exposed rats to alcohol in the home cage and reached similar conclusions about the poor correlation between alcohol intake in the home cage and the operant paradigm (Samson & Czachowski, 2003). Despite the similarities in the operational procedures and some conclusions, the majority of these studies have relied on either sucrose fading or water deprivation to pre-habituate the animals with the lever pressing. Furthermore, the SIPPER procedure applied in those previous works differs substantially from the SIPPER procedure used in the present work. Recent approaches have used similar methods to initiate and maintain oral alcohol self-administration in rats with no need of water or food deprivation nor sucrose fading (Augier, Dulman, Singley, & Heilig, 2017; Augier et al., 2014; Simms, Bito-Onon, Chatterjee, & Bartlett, 2010). Samson, Czachowski, and Slawecki (2000) explored a similar issue comparing the “DIPPER” and the “SIPPER” procedures using a different approach where they measured the “reinforcing strength” of alcohol in rats relying on the alcohol-taking behavior alone (Samson et al., 2000). Here, we measure the natural alcohol acquisition and assess the validity or strength in estimating the pharmacological reinforcing properties of alcohol in individual animals through the measurement of the consummatory (taking) and appetitive (seeking) behaviors simultaneously. It is also noteworthy that the SIPPER model proposed by Samson uses a temporal separation between consummatory and appetitive behaviors, where the number of required responses increases from one day to the next, rather than within a single session, which is different from that used in this work.
Rodent models of operant ethanol self-administration are useful to study the factors that control the reinforcing properties of alcohol drinking and the motivation to seek alcohol (Corbit & Janak, 2016b; Grahame & Cunningham, 2002). These models have been used in mice and rats to determine signaling pathways activated by alcohol, investigate the effects of alcohol-paired cues, and identify the brain circuits that control seeking and taking (Barak et al., 2013; Corbit & Janak, 2016a; Corbit, Fischbach, & Janak, 2016; Sciascia, Reese, Janak, & Chaudhri, 2015; Stafford et al., 2015; Wang et al., 2010). In this study, we further show that when using quinine adulteration as an aversive stimulus, these operant tasks of alcohol self-administration can be used to study the compulsive aspects of the alcohol-seeking behavior, as previously done with foot shock (Halladay, Kocharian, & Holmes, 2017; Radke et al., 2015).
It is important to note that a subset of mice consumed significant amounts of alcohol under both operant paradigms, and mice reached similar average BACs overall in the CUP and SIPPER model. The fact that a significant proportion of mice acquired the behavior under both paradigms also provides strong evidence that sucrose fading, although it is still used, is not required. Our results here appear to indicate that sucrose fading is not required for mice to acquire operant responding, because 80% of all mice trained using the SIPPER paradigm acquired and escalated their ethanol intake. The main goal of adding a sweetener to alcohol is to foster drinking, accomplished in part by masking the bitter taste of alcohol. To habituate mice to the taste and other properties of the alcohol solution, mice were pre-exposed to the solution in the home cage. This strategy proved successful and is a similar approach used for operant training for food rewards when food pellets, instead of chow, are delivered, with mice pre-exposed to the new pellets in the home cage before operant training begins.
A subset of mice consumed significant amounts of alcohol under both operant paradigms, and mice reached similar average BACs in the CUP and SIPPER models. Despite this, the responding rate on the manipulanda was poorly correlated with BAC under both paradigms, demonstrating the limitation of using active responding to determine alcohol intake and BAC. Accurate intake data and/or measurement of drinking behavior (licking) are needed to separate high-drinking mice, low-drinking mice, and non-drinking mice. If consumed volume cannot be accurately measured, BAC should be used instead. Relying on cluster analysis of alcohol intake data, three groups of C57BL/6J mice were identified in the SIPPER paradigm: high-, low-, and non-drinking. Mice in each group reached consistently different BACs throughout the weeks of self-administration testing. The rate of responding alone failed to distinguish between high- and low-drinking mice during the last weeks of testing. Only during the first 7–10 sessions did high-drinking mice show higher rates of active responding than low-drinking mice, suggesting a faster rate of acquisition of operant alcohol drinking behavior. In addition, high-drinking mice have an enhanced rate of pressing not just in the active but also in the inactive lever. These observations could reflect some level of hyperactivity in the high-drinking mice. On the other hand, the increased rate of pressing could reflect heightened sensitivity to the stimulant effect of alcohol for the high-drinking mice. These explanations are just mere speculations at this point because we lack any other direct measurements of activity level for each mouse in baseline and after alcohol administration. Further, high-drinking mice show higher rates of licking during the first weeks and earned more access time to the alcohol sipper. The difference in responding during the first sessions may represent a generalized deficit in learning for the low-drinking mice or a decrease in the reinforcing properties of alcohol. It is noteworthy that mice from the three groups drank similar amounts of alcohol during the early phase of modified DID, reaching average BACs above intoxication. The mismatch with the early DID phase could also indicate a difference in the overall ability to maintain high levels of drinking over time by each group.
A heightened willingness to exert effort to obtain alcohol could underlie the difference between high- and low-drinking mice. However, this is not supported by the results of the progressive responding session, which showed no difference in breakpoints between the groups. Note that the effectiveness of these progressive-responding sessions still needs to be validated. Another consideration is that low-drinking mice showed lower lick rates but not lower responding rates than high-drinking mice, indicating the main difference is in consumption and not in responding. Then, higher intake could be simply driven by thirst. This is a valid interpretation; however, note that similar intake levels were seen during the modified DID phase in the home cage, arguing against inherent individual differences in thirst, but rather that these differences are related to the operant paradigm itself and/or the reinforcing properties and acquisition of the behavior rather than inherent difference in the alcohol preference or taste preference.
Lick rate and consumption varied as a function of the alcohol dose delivered for both groups. Interestingly, there was also a difference in consummatory behaviors during quinine adulteration testing. Licking rates and intake remained elevated in high-drinking mice despite addition of the aversive-tasting quinine, suggesting enhanced drive to continue drinking. Both low- and high-drinking mice showed a reduction in BACs after quinine adulteration, but only the low-drinking mice showed a significant reduction compared with the baseline intake. Again, responding rates during quinine adulteration and breakpoint sessions were unchanged, indicating that responding rates lack sensitivity for identifying high- and low-drinking mice.
Compared to water-drinking mice, high-alcohol drinking mice also showed a higher rate of licking during the first weeks. However, when all alcohol-reinforced mice (low- and high-drinking mice pooled together) or the low-drinking mice were compared to water-drinking mice, the difference was lost. Together, these data suggest that the main effects in the alcohol groups are driven by the high-drinking group. During extinction, all groups of mice behaved similarly and showed similar decreased responding to the active lever and a modest increase during cue-induced reinstatement when compared with extinction baseline.
Altogether, our data suggest that tracking licking behavior and alcohol intake is crucial in an operant model of alcohol drinking because it is the most reliable predictor of intoxication across individual mice and across experiments. Simply measuring changes in responding at the manipulanda, as is often done, is not sufficient to determine the actual alcohol consumption. Although improvements on the CUP paradigm can be made to try to get more reliable correlations with the intake and intoxication data, the appetitive behaviors recorded on the SIPPER procedure in the way used in the present study was able to capture a close correlation between intake and alcohol blood levels. Thus, this well-characterized SIPPER paradigm appears to accurately capture alcohol intake via licking behavior, making it a valuable model to be applied to future studies investigating the neural mechanisms underlying alcohol seeking and drinking.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of NIAAA and NINDS (ZIA-AA000421) to VAA; U01AA023489 to VAA and DR;P50AA017072 and R37 AA01684 to DR; and by CAPES Foundation, Ministry of Education of Brazil (PDSE) grant no. 99999.009920/2014-05 to DSES. We are grateful to the members of the Alvarez lab for helpful comments and discussion.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.alcohol.2017.08.008.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- Augier E, Dulman RS, Singley E, Heilig M. A method for evaluating the reinforcing properties of ethanol in rats without water deprivation, saccharin fading or extended access training. Journal of Visualized Experiments. 2017;119 doi: 10.3791/53305. https://doi.org/10.3791/53305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augier E, Flanigan M, Dulman RS, Pincus A, Schank JR, Rice KC, et al. Wistar rats acquire and maintain self-administration of 20% ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology (Berl) 2014;231:4561–4568. doi: 10.1007/s00213-014-3605-3. https://doi.org/10.1007/s00213-014-3605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, et al. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nature Neuroscience. 2013;16:1111–1117. doi: 10.1038/nn.3439. https://doi.org/10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Cunningham CL, Smitasin PJ, Crabbe JC. Rewarding and aversive effects of ethanol in High Drinking in the Dark selectively bred mice. Addiction Biology. 2015;20:80–90. doi: 10.1111/adb.12079. https://doi.org/10.1111/adb.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Verplaetse TL, Czachowski CL. Alterations in ethanol seeking and self-administration following yohimbine in selectively bred alcohol-preferring (P) and high alcohol drinking (HAD-2) rats. Behavioural Brain Research. 2013;238:252–258. doi: 10.1016/j.bbr.2012.10.030. https://doi.org/10.1016/j.bbr.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature Neuroscience. 2013;16:632–638. doi: 10.1038/nn.3369. https://doi.org/10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Yowell QV, Ron D. Regulation of operant oral ethanol self-administration: A dose-response curve study in rats. Alcoholism: Clinical and Experimental Research. 2011;35:116–125. doi: 10.1111/j.1530-0277.2010.01328.x. https://doi.org/10.1111/j.1530-0277.2010.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Fischbach SC, Janak PH. Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. European Journal of Neuroscience. 2016;43:1229–1236. doi: 10.1111/ejn.13235. https://doi.org/10.1111/ejn.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Changes in the influence of alcohol-paired stimuli on alcohol seeking across extended training. Frontiers in Psychiatry. 2016a;7:169. doi: 10.3389/fpsyt.2016.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Habitual alcohol seeking: Neural bases and possible relations to alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2016b;40:1380–1389. doi: 10.1111/acer.13094. https://doi.org/10.1111/acer.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behavioral Neuroscience. 2014;128:430–445. doi: 10.1037/a0036459. https://doi.org/10.1037/a0036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcoholism: Clinical and Experimental Research. 2001;25:344–350. [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcoholism: Clinical and Experimental Research. 2006;30:414–428. doi: 10.1111/j.1530-0277.2006.00046.x. https://doi.org/10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Mark GP, Fretwell AM, Gililland-Kaufman KR, Strong MN, Ford MM. Reinstatement of ethanol and sucrose seeking by the neurosteroid allopregnanolone in C57BL/6 mice. Psychopharmacology. 2008;201:423–433. doi: 10.1007/s00213-008-1303-8. https://doi.org/10.1007/s00213-008-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Anacker AM, Crabbe JC, Mark GP, Finn DA. The influence of selection for ethanol withdrawal severity on traits associated with ethanol self-administration and reinforcement. Alcoholism: Clinical and Experimental Research. 2011;35:326–337. doi: 10.1111/j.1530-0277.2010.01348.x. https://doi.org/10.1111/j.1530-0277.2010.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Mark GP, Finn DA. Influence of reinforcement schedule on ethanol consumption patterns in non-food restricted male C57BL/6J mice. Alcohol. 2007;41:21–29. doi: 10.1016/j.alcohol.2007.02.003. https://doi.org/10.1016/j.alcohol.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Intravenous self-administration of ethanol in mice. Current Protocols in Neuroscience. 2002;19(9.11):9.11.1–9.11.11. doi: 10.1002/0471142301.ns0911s19. [DOI] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, Holmes A. Mouse strain differences in punished ethanol self-administration. Alcohol. 2017;58:83–92. doi: 10.1016/j.alcohol.2016.05.008. https://doi.org/10.1016/j.alcohol.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T. Attenuation of water intake and operant responding by dopamine D2 antagonists: Raclopride provides important cues for understanding the functional mechanism of action. Pharmacology & Toxicology. 1989;65:9–12. doi: 10.1111/j.1600-0773.1989.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Operant ethanol self-administration in ethanol dependent mice. Alcohol. 2014;48:295–299. doi: 10.1016/j.alcohol.2014.02.002. https://doi.org/10.1016/j.alcohol.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcoholism: Clinical and Experimental Research. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. https://doi.org/10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Kowal BP, Murphy ES, Wiediger RS. Stimulus change dis-habituates operant responding supported by water reinforcers. Behavioural Processes. 2005;70:235–246. doi: 10.1016/j.beproc.2005.07.004. https://doi.org/10.1016/j.beproc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Belknap JK, Buck KJ, Cunningham CL. Genes on mouse chromosomes 2 and 9 determine variation in ethanol consumption. Mammalian Genome. 1998;9:936–941. doi: 10.1007/s003359900903. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, et al. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behavioral Neuroscience. 2005;119:892–910. doi: 10.1037/0735-7044.119.4.892. https://doi.org/10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, et al. Chronic EtOH effects on putative measures of compulsive behavior in mice. Addiction Biology. 2015;22:423–434. doi: 10.1111/adb.12342. https://doi.org/10.1111/adb.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. DBA/2J mice develop stronger lithium chloride-induced conditioned taste and place aversions than C57BL/6J mice. Pharmacology Biochemistry and Behavior. 2000;67:17–24. doi: 10.1016/s0091-3057(00)00310-5. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. European Journal of Neuroscience. 2008;28:1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x. https://doi.org/10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Behavioral health barometer United States, 2013. 2013 Retrieved from http://store.samhsa.gov/shin/content//SMA13-4796/SMA13-4796National.pdf. [PubMed]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: Rodent models. International Review of Neurobiology. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcoholism: Clinical and Experimental Research. 2000;24:766–773. [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology. 1999;147:274–279. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Sciascia JM, Reese RM, Janak PH, Chaudhri N. Alcohol-seeking triggered by discrete pavlovian cues is invigorated by alcohol contexts and mediated by glutamate signaling in the basolateral amygdala. Neuropsychopharmacology. 2015;40:2801–2812. doi: 10.1038/npp.2015.130. https://doi.org/10.1038/npp.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. https://doi.org/10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford AM, Anderson SM, Shelton KL, Brunzell DH. Oral operant ethanol self-administration in the absence of explicit cues, food restriction, water restriction and ethanol fading in C57BL/6J mice. Psychopharmacology. 2015;232:3783–3795. doi: 10.1007/s00213-015-4040-9. https://doi.org/10.1007/s00213-015-4040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Research & Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH. Ethanol preference following the sucrose-fading initiation procedure. Alcohol. 1988;5:9–13. doi: 10.1016/0741-8329(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH. Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol. 2006;38:81–88. doi: 10.1016/j.alcohol.2006.05.004. https://doi.org/10.1016/j.alcohol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. Journal of Neuroscience. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. https://doi.org/10.1523/JNEUR-OSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, et al. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology. 2014;39:579–594. doi: 10.1038/npp.2013.230. https://doi.org/10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.