Abstract
Great variability exists in the response of urinary stones to SWL, and this is true even for stones composed of the same mineral. Efforts have been made to predict stone fragility to shock waves using computed tomography (CT) patient images, but most work to date has focused on the use of stone CT number (i.e., Hounsfield units). This is an easy number to measure on a patient stone, but its value depends on a number of factors, including the relationship of the size of the stone to the resolution (i.e., the slicewidth) of the CT scan. Studies that have shown a relationship between stone CT number and failure in SWL are reviewed, and all are shown to suffer from error due to stone size, which was not accounted for in the use of Hounsfield unit values. Preliminary data are then presented for a study of calcium oxalate monohydrate (COM) stones, in which stone structure—rather than simple CT number values—is shown to correlate with fragility to shock waves. COM stones that were observed to have structure by micro CT (e.g., voids, apatite regions, unusual shapes) broke to completion in about half the number of shock waves required for COM stones that were observed to be homogeneous in structure by CT. This result suggests another direction for the use of CT in predicting success of SWL: the use of CT to view stone structure, rather than simply measuring stone CT number. Viewing stone structure by CT requires the use of different viewing windows than those typically used for examining patient scans, but much research to date indicates that stone structure can be observed in the clinical setting. Future clinical studies will need to be done to verify the relationship between stone structure observed by CT and stone fragility in SWL.
Keywords: shock wave lithotripsy, kidney stones, computed tomography, micro CT, Hounsfield unit
PACS: 43.40.Ng, 81.70.Tx, 87.54.Hk
INTRODUCTION
Since the earliest days of shock wave lithotripsy (SWL), it has been known that some stones respond to lithotripsy more readily than do others, and this quality was correlated to the radiological appearance of the stones. For example, Chaussy has been quoted as saying that stones with x-ray density greater than that of spinal bone were generally more difficult to break [1]. As x-ray computed tomography (CT) began to be used for diagnosis and identification of urinary stones, the power of CT seemed to be ideal for identifying the fragility of stones before treatment [2].
Decisions about treatment of stones include more than just whether the stone is amenable to treatment by SWL. There is now ample evidence to show that shock waves can be injurious to renal tissue [3], and that long-term consequences of lithotripsy can be significant, and are related to the number of shock waves received by the patient [4,5]. Thus, there is a need to be able to judge the number of shock waves required to break a given stone, so that the patient will not receive unnecessary shock waves over what is required to comminute the stone. Ideally, this could be done by monitoring the stone during lithotripsy, and stopping lithotripsy when the stone was sufficiently broken. However, present imaging modalities make it difficult to distinguish large stone fragments from small ones, and techniques to more directly monitor stone breakage are still in their infancy [6].
Two directions have been taken for prediction of stone fragility using CT. The first is to use CT to identify the primary composition of the stone. Then, for example, if a stone was identified as uric acid, it would be predicted to break easily with shock waves, while a stone identified as calcium oxalate monohydrate (COM) would be suspected as a difficult-to-break stone. A number of studies have attempted to use CT for compositional determination of stones [7–17]. These attempts are described as more or less successful by most workers, although the most specific determinations take into account the impact of stone size on the Hounsfield unit value [9], or utilize multiple x-ray energies to distinguish some stone types [7].
An overall problem with this approach is that knowing the composition of the stone does not necessarily allow prediction of the fragility of the stone to shock waves. That is, there is a tremendous variability of stone fragility within all types of stone [18]. So, knowing that a stone is composed primarily of COM indicates that it may be difficult to break, but certainly many COM stones respond quite well to SWL [19]. Thus, knowing that a stone is composed primarily of COM really does not help in making decisions about treatment.
The second approach for using CT to help with this problem has been to use the CT number (Hounsfield unit) values directly for predicting stone fragility in SWL. This has been done by a number of groups [20–25], but there are real problems with this sort of approach, as the Hounsfield unit values in clinical CT are profoundly affected by stone size [26,27]. In general, the results show a correlation between high values of Hounsfield unit in stones and failure of lithotripsy, but this correlation is entirely attributable to artifact of stone size on Hounsfield unit values. CT has tremendous potential to help guide treatment planning in SWL, but this can only be realized if the inherent limitations are thoroughly understood.
THE EFFECT OF STONE SIZE ON HOUNSFIELD UNIT VALUES IN HELICAL CT
CT number values in clinical CT are measured in Hounsfield units (HU), on a scale in which air has a value of −1000, water a value of 0, and bone a value of +1000. These values are calculated from the x-ray absorptions measured as the scan is taken, and are used for display of the reconstructed images of the body, with a specific range of Hounsfield unit values being assigned levels of gray according to the ‘windows’ and ‘levels’ selected by the viewer.
The computation of these Hounsfield unit values is affected by the resolution of the scanning system and by the x-ray attenuation coefficients of both the material surrounding the objects of interest, and that of the objects themselves. In the case of urinary stones, the x-ray attenuation of the surrounding material (urine and tissue), is much lower than that of the stone, and the CT system ‘confuses’ the edge of the stone with the surrounding urine and tissue, introducing error in the apparent x-ray attenuation of the stone’s edge [26,28]. The distance over which this error extends depends on the spatial resolution of the CT scan. The spatial resolution of scanners continues to improve, but for now, a true ‘error zone’ of about 2 mm is about the best that can be expected from an abdominal patient scan [29].
This 2 mm error is large in comparison to the size of urinary stones. The error is even more significant when it is realized that the error zone extends over the entire surface of the stone; that is, CT is a three-dimensional imaging method, so that errors must be considered in three dimensions, and not just in two.
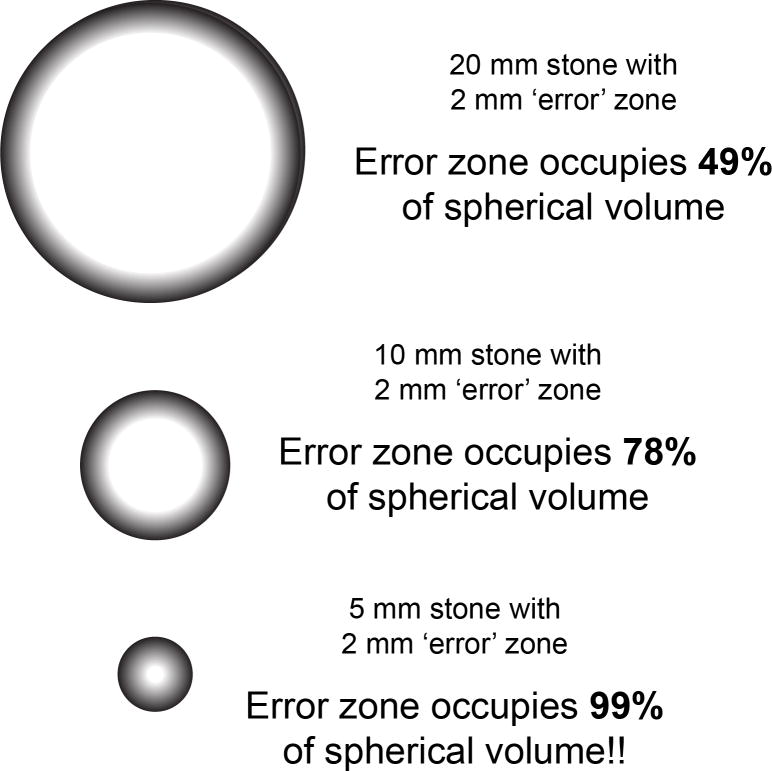
The relative volume occupied by such an error zone is surprisingly large. For example, in a sphere of 20 mm diameter, fully half of its volume resides in an outer layer just little more than 2 mm in thickness (Fig. 1). Thus, for a 20 mm stone, scanned with helical CT using a high-resolution protocol, half of the stone volume will be displayed with Hounsfield unit values confused by volume averaging error from the surrounding urine and tissue.
FIGURE 1.
The impact of volume averaging error with presently available high-resolution abdominal CT scans. Examples show the portion of a spherical stone affected by CT volume averaging error when spatial uncertainty of x-ray attenuation values extends 2 mm into the stone. Note that the volume of the stone represented in the outermost 2 mm region is substantial, even for larger stones.
The fraction of the stone image affected by this error will, of course, be larger as the stone size decreases. For stones between 5 and 10 mm in diameter, 80–99% of the image volume will be affected by the volume averaging error. If the stone is non-spherical (which is likely), the fraction of the stone image affected by volume averaging with the surround will be even greater.
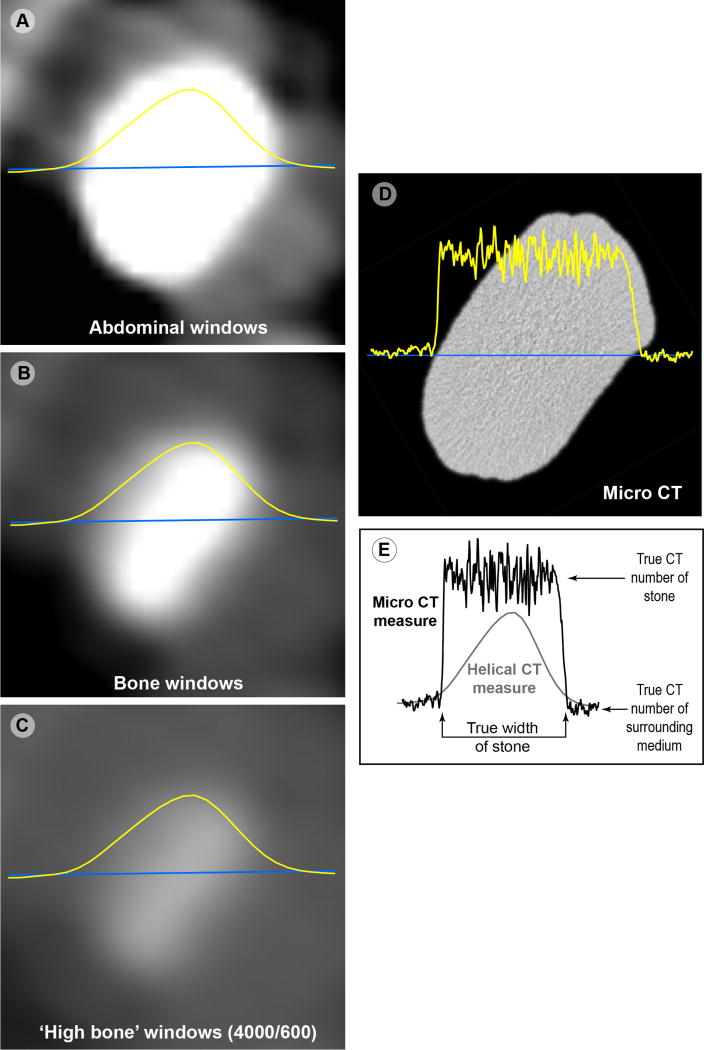
Images of a stone are shown in Fig. 2, with the measured Hounsfield unit values shown along a line through the center of the stone. The images on the left are from the same helical CT scan, and demonstrate how different the stone can look using different viewing windows. The curve shown over each image is a graph of the measured Hounsfield unit values, which are, of course, the same for all viewing windows, as these are basic to the scan, and not to the viewing windows. As can be seen by the micro CT image of the same stone (panel D), the mineral in the stone is actually quite uniform, and thus would ideally yield uniform measures of Hounsfield units throughout the stone. Such is not the case, however, and the clinical CT scan of this stone shows the sort of error in CT number illustrated schematically in Fig. 1. The curve for Hounsfield units measured by helical CT in Fig. 2 shows a peak value of about 1400 HU, for a scan using 2.5 mm slicewidth; the same stone scanned at 0.7 mm yielded a consistently higher value through the center of the stone (mean of 1938±43 HU), demonstrating that all of the Hounsfield unit values in this 6 mm COM stone were artifactually reduced in the 2.5 mm scan—which is likely the smallest slicewidth that would be used for an abdominal scan in a patient setting. Indeed, most abdominal scans are done at a slicewidth of 3 mm or more, as shown in the cases explored below.
FIGURE 2.
Images of a calcium oxalate monohydrate (COM) stone, scanned using 64 detector-row clinical CT with 2.5mm slicewidth (panels A–C) and micro CT (panel D).
So, we see from Figs. 1 and 2 that error in CT measurement of Hounsfield units in kidney stones can be significant, and that this error will be worse for smaller stones than for larger ones. Putting these two ideas together, one can see that, for stones made of similar material, small stones will yield lower values for Hounsfield units than will large stones, as has been shown previously [26]. How does this affect interpretation of published studies? Six published studies are reviewed here to show the variety of effects.
Specific case studies
Case 1
Joseph et al., Computerized tomography attenuation value of renal calculus: can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J Urol 167: 1968, 2002 [20]
This study was performed using 30 patients having solitary renal stones, with a range of stone size of 5–20 mm. The CT scans were reconstructed with 2 mm slices, but the setting for patient scans was not given. Regions of interest were chosen while viewing stones using abdominal windows, and Hounsfield unit values were recorded. Patients were treated with as many as three sessions of SWL, with each session limited to a maximum of 3500 shock waves. In 6 cases lithotripsy failed, as judged by detection of residual fragments larger than 3 mm after 6 weeks. These 6 stones were large (15±5 mm), and the 4 for which analysis was done were composed of calcium oxalate. For stones successfully treated, composition was determined in 16 cases, with 7 of those stones being made of uric acid or calcium oxalate dihydrate, both of which are known to respond well to SWL.
The study showed a significant relationship between the number of shock waves required for treatment (or administered without successful stone comminution) and the Hounsfield unit values of the stones, pre-treatment, as reproduced in Fig. 3. However, this relationship of shock wave dose to Hounsfield units can be accounted for by stone size effects, with a probable additional effect of stone composition, and does not support the principle that x-ray density of stones is in itself a predictor of lithotripsy success.
FIGURE 3.
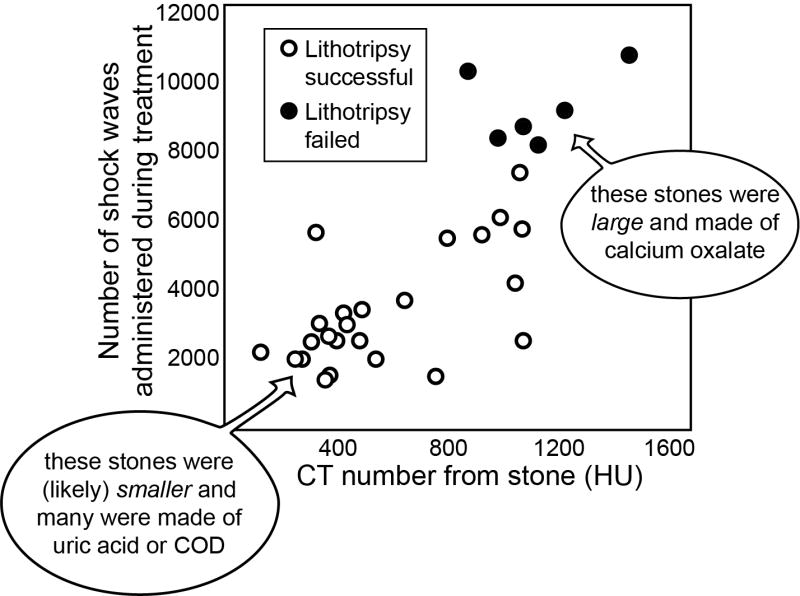
Results from Joseph et al. [20] showing a relationship between the CT number for kidney stones (in Hounsfield units, HU) and the resistance of the stones to treatment by SWL. However, this relationship is not fundamental to the Hounsfield unit values of the stones, being a combination of size artifact on CT and the effects of different stone compositions.
Case 2
Pareek et al., Hounsfield units on computerized tomography predict stone-free rates after extracorporeal shock wave lithotripsy. J Urol 169: 1679, 2003 [21]
50 patients with solitary renal or ureteral stones were studied, first being scanned with CT at 5 mm collimation, and images examined following reconstruction using 2.5 mm slices. All stones were 5–10 mm in size, and Hounsfield unit values were recorded for each. Patients were treated with a single session of lithotripsy, and stone fragments were recovered for analysis. Success was evaluated by plain x-ray at 6 weeks, and fragments of 3 mm were taken as indication of failure of lithotripsy treatment.
Although stone size was measured in this study, no test of size effect was reported. The authors suggest that using patients with stones in between 5 and 10 mm represent a ‘homogeneous group,’ but this is exactly the range in which one sees a dramatic effect of stone size on Hounsfield unit values [26]. The results showed that stones successfully treated had a lower value for Hounsfield units than those stones that left residual fragments, and this is easily explained by the successfully treated stones being smaller, and—in the case of 7 stones that broke well—being composed of uric acid. Thus, like Case 1, the correlation of lithotripsy success with Hounsfield unit values in this study resulted from a fortuitous combination of compositional and size effects, and does not represent a general principle that can be applied to lithotripsy treatment.
Case 3
Gupta et al., Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU Int 95: 1285, 2005 [22]
108 patients, each having a solitary calculus of 5–20 mm (5–10 mm in the lower pole or ureter), were imaged using helical CT with 3 mm image sections (scan parameters were not reported). Stones were viewed using abdominal windows, and longest dimension and average Hounsfield units were recorded for each stone. Note that the dimensions of stones were also measured in the longitudinal direction by counting the slices in which the stone was apparent, a measure that is typically less accurate than in lateral directions [30].
Lithotripsy was done in as many as 7 sessions, with 3000 shock waves per session, each 14 days apart. X-rays were taken before and after each lithotripsy session, and also 12 weeks after the last session. Stone fragments were recovered and analyzed from 72 of the patients.
Results showed that lithotripsy success—defined either by complete clearance of fragments or by treatment requiring fewer than 4 lithotripsy sessions—was significantly related both to smaller stone size and lower Hounsfield unit values. However, data were shown only in terms of two size domains (≤1.1 cm or >1.1 cm) and two Hounsfield unit domains (≤750 HU or >750 HU), and complete regression analyses were apparently not done. Despite this, the authors concluded that Hounsfield unit values are the more important predictor of lithotripsy success.
Criticism of this work came in the form of letters to the editor, and a quote from one of these sums up the problems with the study:
…the concept of isolating stone density alone as a predictive factor for fragmentation will result in misleading conclusions in the practical setting. Size and density are not mutually exclusive as predictors but linked in the context of CT attenuation values…. [31]
Again, as with the previous two cases, the relationship reported here between Hounsfield unit values of stones and success in lithotripsy was almost certainly due to a combination of stone size and stone compositional variation, and does not represent a principle that can be universally applied to predicting lithotripsy outcomes.
Case 4
Magnuson et al., Hounsfield unit density accurately predicts ESWL success. Alaska Med 47: 6, 2005. [23]
This study included 101 patients with renal or ureteral stones ranging from 5–15 mm in size. Patients were treated with a single lithotripsy session, and stone-free status was determined either by CT or plain x-ray during the period of 6 to 12 weeks following treatment. No details were given on the pre-lithotripsy CT scanning or analysis parameters used, but Hounsfield unit values were normalized to stone diameter.
The results showed a highly significant difference between the stone-free and residual fragment groups, averaging 94 and 123 HU/mm, respectively. No data were given on stone composition (even though all patients were said to have brought in stone fragments for analysis post-lithotripsy).
In one sense, this paper is an improvement over those previously described, as the authors attempted to account for stone size effects by dividing the measured Hounsfield unit values by the stone diameter. However, this measure does not yield a number that is consistent for stones of same composition but of different sizes. As shown in Fig. 4, if the Hounsfield unit value for a stone is accurately measured, then dividing that value by the stone diameter creates a measure that declines with increasing stone size. If one calculates the predicted effect of volume averaging on smaller stones (shown in the gray region in Fig. 4), the measured Hounsfield units divided by the stone diameter still varies with stone size, though in a manner that is somewhat flat for smaller stones, as division by the stone diameter corrects in a rough way for the decrease in apparent Hounsfield units when stone size is less than about twice the slicewidth of the CT scan [26].
FIGURE 4.
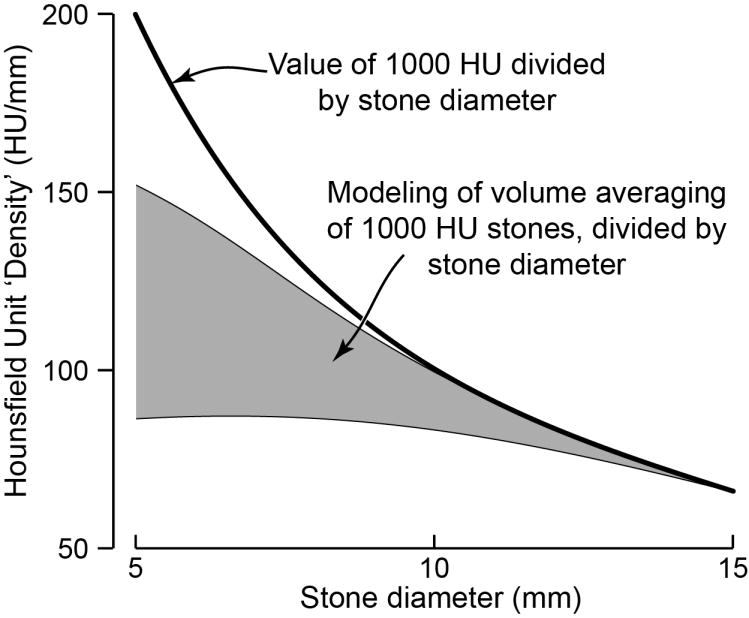
Variation of Hounsfield unit ‘density’ with stone diameter. (Hounsfield unit ‘density’ is calculated as the measured value of average Hounsfield units for a stone, divided by the stone’s diameter.) Note that if the true CT number for the stone is known—shown here for a true value of 1000 Hounsfield units—then division by the diameter yields a hyperbolic relationship. However, at smaller stone sizes, apparent CT number will be reduced by volume averaging. This effect was modeled as per Hu and Fox [28] for maximum and minimum values expected for stones of each size [26], assuming a true CT number of 1000 HU, and the range of results is shown in gray.
Thus, the use of a ‘Hounsfield density’ value helps in correcting for volume averaging effects in smaller stones, but it artificially depresses this value for larger stones, and this two-fold effect results in data that are difficult to interpret. So, for the results of Magnuson et al., we are left with the possibility that many of the stones that broke more easily were composed of uric acid, and thus yielded a low CT density [9], and there is no indication that the use of this density measure would be valuable for general prediction of success in SWL.
Case 5
Wang et al., Predictions of outcomes of renal stones after extracorporeal shock wave lithotripsy from stone characteristics determined by unenhanced helical computed tomography: a multivariate analysis. Eur Radiol 15: 2238, 2005 [24]
This study included 80 patients, many of whom had multiple stones (mean of 2.3 stones per patient). Each patient was scanned by multi-detector helical CT, using 3.75 mm slicewidth, and reconstructions at 2.5 mm. Images were viewed (apparently using only abdominal windows) for measure of stone dimensions, and average Hounsfield unit values for each stone were taken using the largest possible region of interest. Patients were treated using a single session of SWL, and re-scanned by CT for follow-up at 3 months. The presence of any fragments measuring 4 mm or greater was considered a mark of failure of the lithotripsy treatment, with a total failure rate of 48%.
The results showed highly significant correlations between the outcome of lithotripsy and all of the following measures: stone number, stone size, total stone burden, stone shape being non-oval, and Hounsfield unit measure. The authors used multivariate analysis to suggest that stone burden, stone shape, and the Hounsfield unit measure of the brightest stone were all statistically significant predictors of lithotripsy success or failure. The dependence of Hounsfield unit measure on stone size was not acknowledged in this, and the two were treated as independent measures.
Although the authors place a great deal of emphasis on the usefulness of Hounsfield unit values, their results are much more supportive of the relationship between stone size, stone number, and total stone burden as predictors of failure in SWL. The authors may have been unaware of the impact of stone size on apparent values of Hounsfield units. Similarly, their use of regions-of-interest just smaller than the stone image does not acknowledge the probable effects of volume averaging.
The paper also shows no information about the structure of stones, which could have been obtained by using alternative viewing windows [32]. Although the radiologist took time to view the stones in both cross-sectional and coronal views, there was no mention of the use of windows and levels other than those for typical abdominal viewing.
Case 6
Yoshida et al., Role of volume and attenuation value histogram of urinary stone on noncontrast helical computed tomography as predictor of fragility by extracorporeal shock wave lithotripsy. Urology 68: 33, 2006 [25]
This study used 56 stones in 49 patients, with all stones evaluated before lithotripsy using an 8-detector row scanner, with 1.25 mm beam collimation, a pitch of 5.0, and 1.25 mm slices on reconstruction. All stones were between 5 and 20 mm in maximum dimension (by plain x-ray). The volume of each stone was selected using a threshold of 100 HU, and histograms created for each stone, as described further below.
Each patient was treated using a Siemens Lithostar, with a maximum of 3500 shock waves per session. Plain x-ray was done within 3 weeks after the session to look for residual fragments, which were the indication for another treatment session. A maximum of 3 sessions were done for each patient, and fragments greater than 3 mm visible after that indicated failure of lithotripsy treatment.
The results showed 17 failures and 39 successes. The stones on which lithotripsy failed were significantly larger (both by maximal dimension and by volume) and higher in measured CT number (in Hounsfield units). The failed stones also showed a higher incidence of a ‘hump’ in the histogram, as illustrated in Fig. 5.
FIGURE 5.
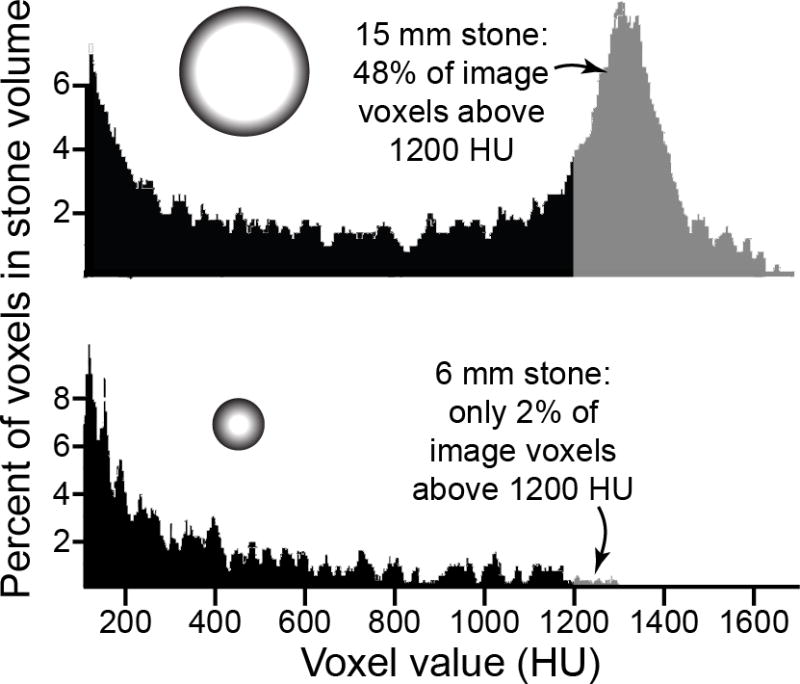
Histograms of helical CT Hounsfield unit values for two stones, based upon data from Yoshida et al. [25] (In the paper, histograms were shown, but stone size was mentioned only in the figure legend.) The larger stone apparently had a significant quantity of calcium oxalate (or perhaps calcium phosphate), as shown by the number of voxels above 1200 HU [13]. The scan of the smaller stone shows few voxels above 1200 HU, but this can be explained entirely by volume averaging. That is, the smaller stone could have been pure calcium oxalate, but still show only a few percent of voxels with high HU values, as described above in Fig. 1. The histogram of the larger stone was referred to by Yoshida et al. as having a ‘hump’ in the region greater than 1200 HU. If all stones were made of calcium oxalate, then the ‘hump’ would be an indicator of a stone being large enough that a significant portion of its volume was reported with accurately high Hounsfield units.
Although the idea of identifying a hump in the histogram provides a tantalizing quantitative measure in CT analysis of stones, this ends up being just another measure of stone size. Only stones made of materials that absorb x-rays well (calcium minerals) and also are greater than about 10 mm in diameter, would be expected to show a hump in this way. The hump shows the portion of the calcium stone measured without significant volume averaging error, and calcium stones above 10 mm in diameter would be expected to have at least 20% of their volume measured correctly (calculated as per Fig. 1) and thus showing a hump in the histogram of Hounsfield unit values. Smaller stones, regardless of their composition, would have such significant volume averaging error so as to not have many voxels of high value, and thus would be less likely to show a ‘hump,’ even if they were made of calcium oxalate. Thus, the Yoshida data use Hounsfield units in a new way, but still primarily as an indicator of stone size, which was not acknowledged directly in the paper, and which also can be measured more directly, and more simply, by other methods.
ANOTHER APPROACH: VIEWING STONE STRUCTURE BY CT TO PREDICT FRAGILITY OF COM CALCULI
We have taken a different approach in recent work, described in preliminary form here. Briefly, the idea was to examine the fragility of COM stones with the hypothesis that stones that looked homogeneous by CT would break differently from those that were not homogeneous. The kind of structure that was observed as indicating inhomogeneity included the presence of significant amounts of calcium phosphate (which shows up brighter than COM on CT), the presence of voids in the stone, and irregular shapes, such as in stones having significant protuberances from their surfaces.
This study was done on COM stones, with the rationale that calcium oxalate stones are the most common stones treated using SWL, and that the monohydrate form of these stones can be especially resistant to shock wave treatment.
Methods
Urinary stones were obtained as discards from a stone lab, with no patient identification, but with the mineral analysis obtained from each stone (either using part of that stone, or another stone from that patient event). Stones were selected that had been determined to be ≥96% COM (whewellite). Stones were scanned by micro CT and the micro CT scans judged by independent observers as indicating a COM stone that was homogeneous in structure, or one that had visible, internal structural features, as indicated in Fig. 6. Only those stones that were consistently judged by four independent observers as being either homogeneous or heterogeneous in structure by micro CT were included in this study.
FIGURE 6.

Representative examples of COM stones used for in vitro lithotripsy experiment. 16 stones were found that were judged by all four observers, independently, as homogeneous in structure by micro CT. 25 stones were consistently judged to be heterogeneous by micro CT.
Stones were hydrated for 4 days, and broken in vitro in a Dornier Doli-50 Lithotripter, power level 4, 120 SW/min) over 2 mm mesh, and shock waves to comminution counted for each stone. Order of stone breakage was randomized, and personnel carrying out the lithotripsy had no knowledge of the structural classification of stones by micro CT. Data were analyzed using Student’s t-test.
Results and Discussion
Stones judged to be homogeneous showed micro CT profiles that were characterized by evenness of x-ray attenuation, suggesting a uniform distribution of mineral within each stone. Stones judged to ‘show structure’ exhibited regions of low x-ray attenuation (voids [33]), regions of high attenuation (indicating the presence of calcium phosphate [34]), and sometimes irregularity of surface contour. Examples of all of these structural forms can be seen in Fig. 6.
Stones that appeared homogeneous in structure by micro CT required, on average, twice as many shock waves for comminution as those showing observable structure, as shown in Table 1.
TABLE 1.
Results of in vitro lithotripsy breakage of COM stones.
| Structure classification | Shock waves per gram stone (±S.D.) |
|---|---|
| Homogeneous | 1874±821 (n=16) |
| Heterogeneous | 912±678 (n=25) |
| t-test | p=0.0005 |
These preliminary results show a significant relationship between structure visible by CT in COM stones and stone susceptibility to shock waves. The difference in fragility of stones in the two groups was quite dramatic, with the homogenous stones requiring an average of nearly 1900 shock waves per gram, while the stones that were identified as having structure by CT required half as many shock waves.
In this study, micro CT was used, which provides better resolution of stone structure than does helical CT [13]. However, stone structure is quite visible by helical CT in vitro [32], and stone structure can be seen in patient scans, as well [35,36]. Moreover, the viewing of stone structure, rather than measuring Hounsfield unit value, avoids most of the problems caused by the interaction of stone size with CT resolution. Thus, it is possible that patient scans could be used to view stone structure (using bone windows [27,32]) for identifying COM stones that would be susceptible or resistant to SWL.
CONCLUSIONS
Efforts to predict stone fragility to shock waves using CT number have fallen short because they have not recognized the effect of stone size on CT number in the clinical setting. The preliminary data presented here for COM stones shows that stone structure—rather than simple CT number values—correlates with fragility to shock waves. COM stones that were observed to have structure by micro CT (e.g., voids, apatite regions, unusual shapes) broke to completion in about half the number of shock waves required for COM stones that were observed to be homogeneous in structure by CT. This result suggests another direction for the use of CT in predicting success of SWL: the use of CT to view stone structure, rather than measuring stone CT number alone. Viewing stone structure by CT requires the use of different viewing windows than those typically used for examining patient scans, but studies have clearly demonstrated the ability of CT to show the internal structural features of stones in patient scans. We believe that these data provide a sound rationale for moving ahead with clinical studies to determine the relationship between stone structure observed by CT and stone fragility in shock wave lithotripsy.
Acknowledgments
This work was funded by NIH R01DK59933.
References
- 1.Dretler SP. Stone fragility—a new therapeutic distinction. J Urol. 1988;139:1124. doi: 10.1016/s0022-5347(17)42801-1. [DOI] [PubMed] [Google Scholar]
- 2.Dretler SP, Spencer BA. CT and stone fragility. J Endourol. 2001;15(1):31. doi: 10.1089/08927790150500926. [DOI] [PubMed] [Google Scholar]
- 3.Evan AP, Willis LR, Lingeman JE, et al. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron. 1998;78:1. doi: 10.1159/000044874. [DOI] [PubMed] [Google Scholar]
- 4.Janetschek G, Frauscher F, Knapp R, et al. New onset hypertension after extracorporeal shock wave lithotripsy: age related incidence and prediction by intrarenal resistive index, Journal Of. Urology. 1997;158(2):346. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 5.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes Mellitus and Hypertension Associated with Shock Wave Lithotripsy of Renal and Proximal Ureteral Stones at 19 Years Follow-up. J Urol. 2006 May;175:1742. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 6.Owen NR, Bailey MR, Crum LA. Characterization of a vibro-acoustography system designed to detect kidney stones during lithotripsy. J Acoust Soc Am. 2005;117:2588. [Google Scholar]
- 7.Mostafavi MR, Ernst RD, Saltzman B. Accurate determination of chemical composition of urinary calculi by spiral computerized tomography. J Urol. 1998;159:673. [PubMed] [Google Scholar]
- 8.Saw KC, McAteer JA, Monga AG, et al. Helical CT of urinary calculi: Effect of stone composition, stone size, and scan collimation. AJR Am J Roentgenol. 2000 Aug;175:329. doi: 10.2214/ajr.175.2.1750329. [DOI] [PubMed] [Google Scholar]
- 9.Nakada SY, Hoff DG, Attai S, et al. Determination of stone composition by noncontrast spiral computed tomography in the clinical setting. Urology. 2000;55:816. doi: 10.1016/s0090-4295(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 10.Motley G, Dalrymple N, Keesling C, et al. Hounsfield unit density in the determination of urinary stone composition. Urology. 2001;58(2):170. doi: 10.1016/s0090-4295(01)01115-3. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez Enguita C, Rodriguez Minon-Cifuentes JL, Cabrera Perez J, et al. La litiasis resistente a LEOC [ESWL-resistant lithiasis] Actas Urologicas Espanolas. 1999;23(3):247. [PubMed] [Google Scholar]
- 12.Demirel A, Suma S. The efficacy of non-contrast helical computed tomography in the prediction of urinary stone composition in vivo. J Int Med Res. 2003;31:1. doi: 10.1177/147323000303100101. [DOI] [PubMed] [Google Scholar]
- 13.Zarse CA, McAteer JA, Tann M, et al. Helical CT accurately reports urinary stone composition using attenuation values: In vitro verification using high resolution micro CT calibrated to FT-IR microspectroscopy. Urology. 2004;63:828. doi: 10.1016/j.urology.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Bellin MF, Renard-Penna R, Conort P, et al. Helical CT evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of CT-attenuation values and density. European Radiology. 2004;14:2134. doi: 10.1007/s00330-004-2365-6. [DOI] [PubMed] [Google Scholar]
- 15.Deveci S, Coskun M, Tekin MI, et al. Spiral computed tomography: role in determination of chemical compositions of pure and mixed urinary stones—an in vitro study. Urology. 2004;64:237. doi: 10.1016/j.urology.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Sheir KZ, Mansour O, Madbouly K, et al. Determination of the chemical composition of urinary calculi by noncontrast spiral computerized tomography. Urol Res. 2005;33:99. doi: 10.1007/s00240-004-0454-2. [DOI] [PubMed] [Google Scholar]
- 17.Burgos FJ, Sanchez J, Avila S, et al. The usefulness of computerized axial tomography (CT) in establishing the composition of calculi. Arch Esp Urol. 1993;46(5):383. [PubMed] [Google Scholar]
- 18.Williams JC, Jr, Saw KC, Paterson RF, et al. Variability of renal stone fragility in shock wave lithotripsy. Urology. 2003;61(6):1092. doi: 10.1016/s0090-4295(03)00349-2. [DOI] [PubMed] [Google Scholar]
- 19.Chaussy CG, Fuchs GJ. Extracorporeal shock wave lithotripsy. Monographs in Urology. 1987;8:80. doi: 10.1111/j.1464-410x.1987.tb09122.x. [DOI] [PubMed] [Google Scholar]
- 20.Joseph P, Mandal AK, Singh SK, et al. Computerized tomography attenuation value of renal calculus: can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J Urol. 2002 May;167:1968. doi: 10.1016/s0022-5347(05)65064-1. [DOI] [PubMed] [Google Scholar]
- 21.Pareek G, Armenakas NA, Fracchia JA. Hounsfield units on computerized tomography predict stone-free rates after extracorporeal shock wave lithotripsy. J Urol. 2003 May;169:1679. doi: 10.1097/01.ju.0000055608.92069.3a. [DOI] [PubMed] [Google Scholar]
- 22.Gupta NP, Ansari MS, Kesarvani P, et al. Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU Int. 2005;95(9):1285. doi: 10.1111/j.1464-410X.2005.05520.x. [DOI] [PubMed] [Google Scholar]
- 23.Magnuson WJ, Tomera KM, Lance RS. Hounsfield unit density accurately predicts ESWL success. Alaska Med. 2005;47(2):6. [PubMed] [Google Scholar]
- 24.Wang LJ, Wong YC, Chuang CK, et al. Predictions of outcomes of renal stones after extracorporeal shock wave lithotripsy from stone characteristics determined by unenhanced helical computed tomography: a multivariate analysis. European Radiology. 2005;15(11):2238. doi: 10.1007/s00330-005-2742-9. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, Hayashi T, Ikeda J, et al. Role of volume and attenuation value histogram of urinary stone on noncontrast helical computed tomography as predictor of fragility by extracorporeal shock wave lithotripsy. Urology. 2006;68(1):33. doi: 10.1016/j.urology.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 26.Williams JC, Jr, Saw KC, Monga AG, et al. Correction of helical CT attenuation values with wide beam collimation. Academic Radiology. 2001;8:478. doi: 10.1016/S1076-6332(03)80619-0. [DOI] [PubMed] [Google Scholar]
- 27.Williams JC, Jr, Kim SC, Zarse CA, et al. Progress in the use of helical CT for imaging urinary calculi. J Endourol. 2004;18(10):937. doi: 10.1089/end.2004.18.937. [DOI] [PubMed] [Google Scholar]
- 28.Hu H, Fox SH. The effect of helical pitch and beam collimation on the lesion contrast and slice profile in helical CT imaging. Med Phys. 1996;23:1943. doi: 10.1118/1.597774. [DOI] [PubMed] [Google Scholar]
- 29.Rollano-Hijarrubia E, Stokking R, van der Meer F, et al. Imaging of Small High-Density Structures in CT: A Phantom Study. Academic Radiology. 2006;13(7):893. doi: 10.1016/j.acra.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Narepalem N, Sundaram CP, Boridy IC, et al. Comparison of helical computerized tomography and plain radiography for estimating urinary stone size. J Urol. 2002 Mar;167:1235. [PubMed] [Google Scholar]
- 31.Zaman ZR, Kommu SS, Watkin NA. Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU International. 2005;96(4):685. doi: 10.1111/j.1464-410x.2005.05788_2.x. [DOI] [PubMed] [Google Scholar]
- 32.Williams JC, Jr, Paterson RF, Kopecky KK, et al. High resolution detection of internal structure in renal calculi by helical computerized tomography. J Urol. 2002;167:322. [PubMed] [Google Scholar]
- 33.Kim SC, Hatt EK, Lingeman JE, et al. Cystine: helical computerized tomography characterization of rough and smooth calculi in vitro. J Urol. 2005 Oct;174:1468. doi: 10.1097/01.ju.0000173636.19741.24. [DOI] [PubMed] [Google Scholar]
- 34.Zarse CA, McAteer JA, Sommer AJ, et al. Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urology. 2004;4:15. doi: 10.1186/1471-2490-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobsen N, Ng D, Varma V, et al. Does internal structure of renal calculi on high resolution CT predict SWL treatment outcome? Can J Urol. 2004;11(3):2274. (abstract) [Google Scholar]
- 36.Kim SC, Zarse CA, McAteer JA, et al. Stone internal structure is visible in patients by helical CT. J Urol. 2004;171(4suppl):509. [Google Scholar]