Abstract
Background
Salvianolic acid B (SB) is a major active phyto-component of the plant Radix Salvia miltiorrhiza, which is traditionally used to treat joint pain and arthritis. The present study examined the anti-rheumatoid arthritis efficacy of SB on collagen-induced rheumatoid arthritis (CIA) in a rat model.
Material/Methods
Forty-eight rats were divided into 4 groups: Control rats treated with saline (Group I), rats subjected to CIA induction by intradermal injection of bovine collagen II type at the tail (Group II), and rats subjected to CIA and supplemented with either 20 or 40 mg/kg of SB for 28 days (group III or IV).
Results
Paw swelling, edema, arthritis score, thymus and spleen indexes, and neutrophil infiltration were significantly decreased (p<0.01) by treatment with 20 or 40 mg/kg of SB. The levels of inflammatory cytokines (interleukin-1β, -6, and -17, and TNF-α) and anti-collagen II-specific immunoglobulins (IgG1 and IgG2a) were markedly decreased (p<0.01), and those of antioxidant enzymes (SOD, CAT, and GSH) were significantly increased (p<0.01) in SB-treated rats. Administration with SB (20 or 40 mg/kg) resulted in lower phosphorylated IκB-α and NF-κB p65 protein levels and markedly downregulated IκB-α expression. Furthermore, CIA rats revealed the presence of highly diffused polymorphonuclear cells (PMNs) infiltration with eroded cartilage; however, these phenomena were considerably ameliorated by SB.
Conclusions
SB alleviates oxidative stress and inflammation in CIA rats, thus verifying its anti-rheumatoid arthritis property.
MeSH Keywords: Arthritis, Juvenile; Immunoglobulins; Inflammation; Oxidative Stress
Background
Rheumatoid arthritis (RA), a common inflammatory disease, is characterized by increased infiltration of inflammatory cells, particularly polymorphonuclear cells (PMNs), into synovial fluid, which leads to degeneration of the synovial membrane, articular cartilage, and joint bones [1,2]. The prevalence of RA in China (especially in women) is increasing owing to a sedentary lifestyle and insufficient awareness of RA [3]. Moreover, RA reduces the quality of life due to severe pain, deformity, and disability, as well as its strong association with cardiovascular and cerebrovascular diseases. Thus, RA imposes an enormous social and economic burden in China [4].
In RA patients, activation of nuclear factor κB (NF-κB) leads to an inflammatory response [5–7]. Moreover, the levels of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and IL-17, are considerably elevated in RA patients, whereas those of anti-inflammatory cytokines, such as IL-10, are reduced (due to excessive activation of NF-κB), which worsens inflammation [8,9]. The enhanced inflammatory response in RA triggers a respiratory burst, resulting in production of high levels of free radicals (reactive oxygen species [ROS] and reactive nitrogen species) and thereby contributing to oxidative stress and synovium damage [10]. Thus, inflammation and oxidative stress play crucial roles in RA. RA is typically treated with non-steroidal anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (DMARDs), and in advanced cases, knee replacement surgery, either individually or in combination [11]. However, these drugs can result in various adverse events such as gastric dysfunction and hepatic and renal failure [12], and they are costly. Therefore, cost-effective anti-inflammatory and antioxidant drugs for RA with few or no adverse effects are needed.
Salvianolic acid B (SAB; CAS: 121521-90-2) is a major phytoconstituent of the plant Radix Salvia miltiorrhiza. Radix S. miltiorrhiza (Red sage) is a perennial herb, and its dried roots (Danshen) are commonly used in traditional Chinese medicine to cure joint pain, RA, and cerebrovascular, cardiovascular, and skin disorders [13]. SB exerts antioxidant, anti-diabetic, anti-inflammatory, immunomodulatory, cardioprotective, hepatoprotective, and neuroprotective effects in animal models [14–16]. Watzke et al. [17] reported that lithospermic acid B and SB have identical structures with different spatial configurations and show similar activity in experimental models. Moreover, Liu et al. [13] reported that S. miltiorrhiza with a higher SB content exerts a therapeutic effect in RA patients by suppressing synovial hyperplasia. In addition, SAB has shown a positive impact on various experimental RA models [13,14]. Indeed, a myriad of natural products with potent anti-inflammatory and antioxidant activities show potential as anti-RA agents [8,18]. Hence, we evaluated the anti-RA activity of SB by examining paw swelling (edema), the arthritis score, spleen and thymus indexes, antioxidant status, inflammatory marker levels, and histological parameters of synovial joint tissue in rats with collagen-induced arthritis (CIA).
Material and Methods
Chemicals and reagents
Salvianolic acid B (SB), bovine type II collagen (CII), complete Freund’s adjuvant (CFA), sodium pentobarbitone, and formalin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glycerol, sodium hydroxide, hematoxylin and eosin (H & E) stain, and hydrogen peroxide were procured from Kangchen Biotechnology (Shanghai, China). Phosphate-buffered saline (PBS), ready-made buffer xylene, and ketamine were supplied by Thermo Fisher Scientific (MA, USA). All reagents and chemicals were of analytical grade.
Experimental animals
Forty-eight healthy male Sprague-Dawley albino rats weighing 220–250 g were housed in the Animal Center at the First People’s Hospital of Huzhou, Zhejiang, China. Rats were maintained in steel cages under a 12/12 h light/dark cycle at room temperature with ad libitum (free) access to food and water. All animal procedures were approved by the Bioethics Committee of the First People’s Hospital of Huzhou (FPHH-902400) and were performed in accordance with the National Institutes of Health guidelines for handling and care of laboratory animals.
Immunization/CIA induction
CIA was carried out using the method of Liu et al. [8]. In brief, CII was mixed with 0.05 M acetic acid and emulsified by adding an equal amount of CFA (with heat-killed Mycobacterium tuberculosis H37Ra). The rats were immunized intradermally with the CFA mixture into the base of the tail on day 0 and boosted with CII and incomplete Freund’s adjuvant (IFA) on day 21. Control rats were not immunized with the CFA mixture.
Animal groups
Forty-eight male healthy Sprague-Dawley rats were arbitrarily divided into 4 treatment groups of 12 rats each. After assimilation in the animal house for 2 weeks, rats were divided into the following groups: (1) control rats that received saline for 28 days; (2) CIA rats that underwent induction of arthritis for 28 days without treatment (CIA); (3) CIA rats treated with 20 mg/kg body weight SB i.p. for 28 days (SB 20 mg/kg); and (4) CIA rats treated with 40 mg/kg body weight SB i.p. for 28 days (SB 40 mg/kg). On days 0, 14, and 28, the paw swelling (edema) volume was evaluated using a plethysmometer. The arthritis score was calculated by the method of Zhang et al. [19]. Swelling and erythema of the paws (hind and fore) were graded using a 5-point scale: 0, no sign of swelling or erythema; 1, signs of swelling or erythema in the ankle or wrist; 2, signs of swelling or erythema in the ankle and tarsal or wrist and carpal; 3, signs of swelling or erythema extending to the metatarsals or metacarpals; and 4, signs of swelling or erythema involving the entire hind or fore paw. Hence, the maximum score was 8 (4×2 hind/fore paws). The body weight of the rats was monitored throughout the study.
Sample preparation
On day 29, rats were euthanized by cervical decapitation under 40 mg/kg sodium pentobarbitone anesthesia (i.p.), and blood samples were collected and the serum separated. Peripheral blood mononuclear cells (PBMCs) were separated using the method of Lin et al. [20]. The spleen and thymus were excised immediately, washed with saline, and weighed. Synovial joint tissue was removed from the rats and fixed in 10% formalin for histological analysis. A portion of joint tissue was homogenized (10%) in PBS and used for biochemical and molecular analyses.
Thymus and spleen index assays
The thymus and spleen indexes were assayed by the method of Zhang et al. [21] and are expressed as ratios (mg/g).
Antioxidant enzymes and lipid peroxidation products
Glutathione (GSH) content, and catalase (CAT) and superoxide dismutase (SOD) activities, in joint-tissue homogenates were assayed using commercial kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Similarly, malondialdehyde (MDA) levels in joint tissue were measured using a commercial kit from Shanghai Yantuo Biotechnology Ltd., (Shanghai, China).
Myeloperoxidase activity assay
The myeloperoxidase (MPO) activity of PBMCs was evaluated using an MPO assay kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions.
Anti-collagen (C) II Ig and inflammatory marker levels
Serum anti-CII IgG1 and G2a levels were quantified as arbitrary units using enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher Scientific). Serum IL-1β, IL-6, IL-10, IL-17, and TNF-α levels were determined using ELISA kits according to the manufacturer’s instructions (Thermo Fisher Scientific).
Western blotting
Homogenized synovial joint tissue was separated into nuclear and cytosolic fractions using the Nuclear/Cytosolic Fractionation Kit (Bio Vision Inc., CA, USA) to quantify cytosolic IκB-α and phosphorylated IκB-α (pIκB-α) and nuclear NF-κB p65 levels. Protein levels were determined using a BCA protein assay kit (Thermo Fisher Scientific). Protein extract (50 μg) was resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrotransferred onto a nitrocellulose membrane. The membrane was blocked using TrisPBS (TPBS) with Tween 20 and 5% skimmed milk and probed with primary antibodies: mouse monoclonal anti-IκB-α, -phospho-IκB-α (1: 1200 dilution), -NF-κB p65 (1: 1000; Cell Signaling Technology, MA, USA), -β-actin (1: 800; Zhongshan Biotechnology, Beijing, China), or -histone H3 (1: 1,000; Zhongshan Biotechnology). Next, an anti-rabbit secondary antibody conjugated to horseradish peroxidase (1: 10 000; Abcam/Cell Signaling Technology) in TPBS was added and incubated at 37°C for 1 h. Bands were visualized using an enhanced chemiluminescence image analyzer, and protein levels were quantified using Image-Pro Plus software (Media Cybernetics, Inc., MD, USA).
Histomorphological evaluation
Synovial joint tissue was obtained from the rats, fixed in 10% buffered formalin for 24 h, dehydrated, and processed using acetone and xylene. The tissues were embedded in liquid paraffin wax for sectioning. Sections (4 μm) of joint tissues were prepared using a microtome, mounted onto microscope slides, covered with cover slips, and stained with H & E. Histological parameters were evaluated under a compound microscope (Leica Microsystems DM 6000 B, Wetzlar, Germany).
Statistical analysis
Data are shown as means ± standard deviation (SD) of 12 rats per group. Differences were evaluated by one-way analysis of variance using the Statistical Package for the Social Sciences (SPSS, ver. 23; IBM, NY, USA) and least significant difference (LSD) multiple comparison tests. Values of p<0.05 were considered indicative of statistical significance.
Results
Effect of SB on paw swelling and arthritis score
The effect of SB on paw swelling and the arthritis score in control and experimental rats is shown in Figures 1 and 2. The edema (water content) and arthritis score of CIA rats were significantly increased (p<0.01) on days 14 and 28. Treatment with 20 mg/kg (p<0.05) and 40 mg/kg (p<0.01) SB reduced the edema and arthritis score as compared with those of untreated rats.
Figure 1.
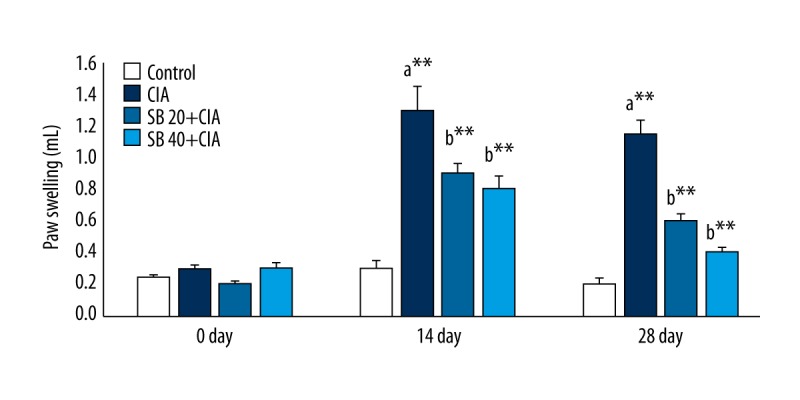
Effect of SB on paw swelling in control and experimental rats. Data are means ±SD of 12 rats per group. ** p<0.01, * p<0.05. a CIA vs. control group, b SB treatment (20 and 40 mg/kg) vs. CIA group.
Figure 2.
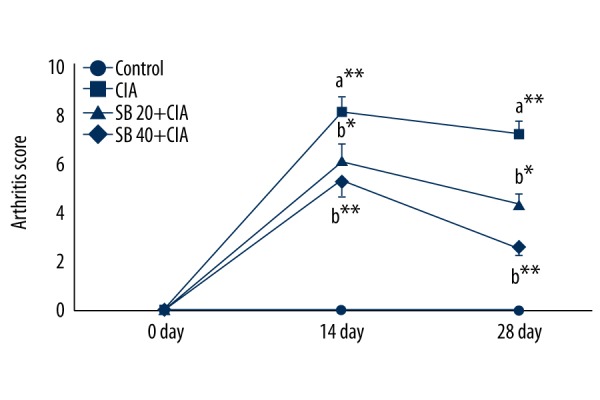
Effect of SB on the arthritis score in control and experimental rats. Data are means ±SD of 12 rats per group. ** p<0.01, * p<0.05. a CIA vs. control group, b SB treatment (20 and 40 mg/kg) vs. CIA group.
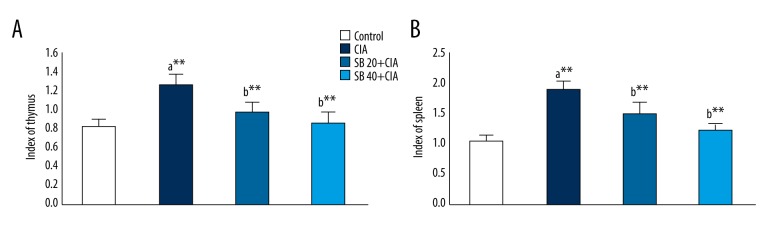
Effect of SB on the thymus and spleen indexes
The effect of SB on the thymus and spleen indexes in control and experimental rats are shown in Figures 3A and 3B, respectively. The thymus and spleen indexes were markedly increased (p<0.01) in the CIA group. However, administration of 20 and 40 mg/kg SB substantially lowered (p<0.01) the thymus and spleen indexes.
Figure 3.
Effect of SB on the thymus (A) and spleen (B) indexes in control and experimental rats. Data are means ±SD of 12 rats per group. ** p<0.01, * p<0.05 a CIA vs. control group, b SB treatment (20 and 40 mg/kg) vs. CIA group.
Effect of SB on joint-tissue antioxidant activities and lipid peroxidation products
The effects of SB on joint-tissue antioxidant activities and MDA levels in control and experimental rats are shown in Table 1. Compared with control rats, the GSH, CAT, and SOD activities in joint tissue were significantly attenuated (p<0.01), and MDA levels were significantly increased (p<0.01), in CIA rats. Treatment with 20 and 40 mg/kg SB (p<0.05 and <0.01, respectively) significantly increased the GSH, CAT, and SOD activities and decreased the MDA levels to near-normal levels.
Table 1.
Effect of SB on the activities of joint tissue antioxidants and lipid peroxidation products in control and experimental rats.
| Group | GSH (μg/mg protein) | CAT (U/mg protein) | SOD (U/mg protein) | MDA (nmols/mg protein) |
|---|---|---|---|---|
| Control | 9.72±0.10 | 69.34±5.86 | 3.21±0.44 | 0.87±0.11 |
| CIA | 7.56±0.74a** | 48.45±7.30a** | 1.53±0.15a** | 1.57±0.15a** |
| SB 20+CIA | 8.73±0.67b* | 59.68±5.10b* | 2.25±0.32b** | 1.08±0.08b* |
| SB 40+CIA | 9.16±0.56b* | 64.67±7.00b** | 2.76±0.18b** | 0.96±0.08b** |
Data are means ± SD of 12 rats per group.
p<0.01
p<0.05.
CIA vs. control group;
SB treatment (20 and 40 mg/kg) vs. CIA group.
One unit (U) of SOD activity was defined as the amount of enzyme required for 1 mg tissue proteins in 1 ml of a reaction mixture SOD inhibition rates to 50% as monitored at 550 nm. One unit (U) of CAT activity was defined as 1 mg tissue proteins consumed 1 μmol H2O2 at 405 nm for 1 second.
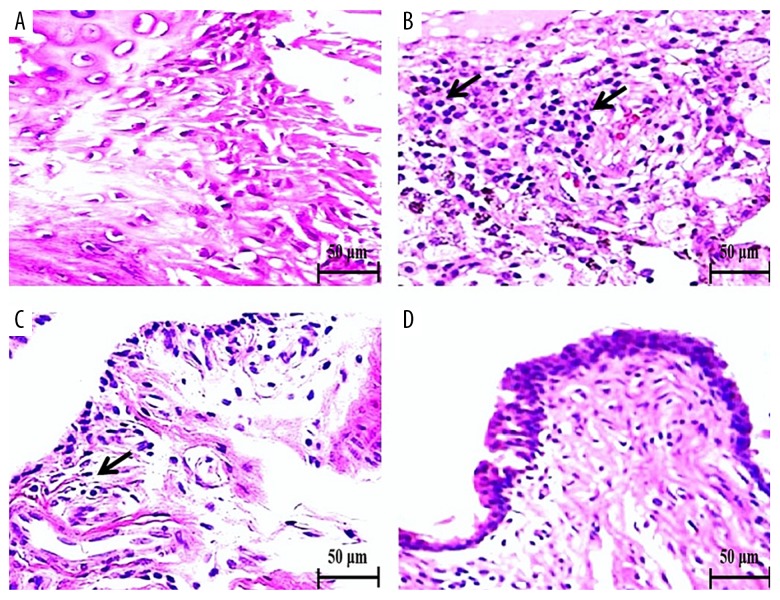
Effect of SB on joint-tissue morphology
The effects of SB on the joint-tissue morphology of control and experimental rats are shown in Figure 4. Control rats showed a normal articular cartilage architecture without mononuclear cell infiltration (Figure 4A). CIA rats exhibited diffuse PMN infiltration with articulated cartilage erosion and synovitis (arrow, Figure 4B). In contrast, CIA rats treated with 20 mg/kg SB displayed moderate PMN infiltration and inflammation with less cartilage erosion and synovitis (Figure 4C). CIA rats treated with 40 mg/kg SB showed no cartilage erosion, mild PMN infiltration, and mild inflammation (Figure 4D).
Figure 4.
Effect of SB on joint tissue in control and experimental rats as determined by H & E staining (400× magnification). Control rats exhibited a normal articular cartilage architecture without mononuclear cell or PMN infiltration (A). CIA rats showed diffuse PMN infiltration with articulated cartilage erosion and synovitis (arrow, B). Rats treated with 20 mg/kg SB showed mild PMN infiltration with slight cartilage erosion and synovitis (C). Rats treated with 40 mg/kg SB showed almost normal articular cartilage without erosion but with infiltration by a few PMNs and inflammation (synovitis) (D). Scale bar, 50 μm.
Effect of SB on PBMC MPO levels
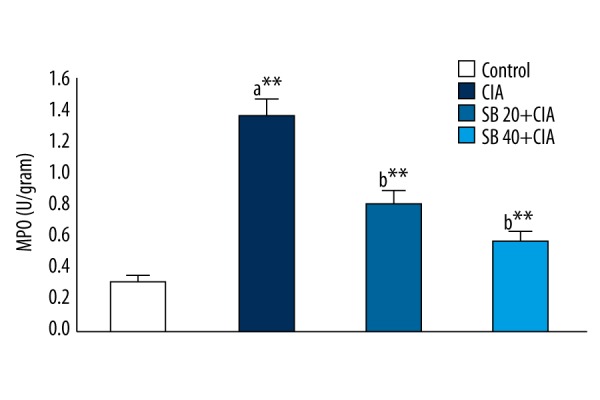
The effect of SB on PBMC MPO levels in control and experimental rats is shown in Figure 5. CIA rats exhibited significantly (p<0.01) higher MPO levels than those of control rats. CIA rats treated with 20 and 40 mg/kg SB showed significantly (p<0.01) lower MPO levels than those of CIA rats.
Figure 5.
Effect of SB on PBMC MPO levels in control and experimental rats. Data are means ±SD of 12 rats per group. ** p<0.01, * p<0.05. a CIA vs. control group, b SB treatment (20 and 40 mg/kg) vs. CIA group.
Effect of SB on anti-CII IgG levels
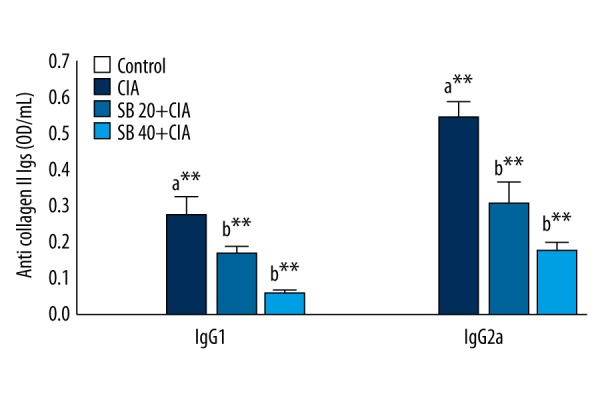
The effects of SB on anti-CII IgG levels in control and experimental rats are shown in Figure 6. IgG1 and IgG2a levels were significantly increased (p<0.01) in CIA rats, and were restored to normal levels by treatment with 20 or 40 mg/kg SB.
Figure 6.
Effect of SB on serum anti-collagen II Ig levels in control and experimental rats. Data are means ±SD of 12 rats per group. ** p<0.01, * p<0.05. a CIA vs. control group, b SB treatment (20 and 40 mg/kg) vs. CIA group.
Effect of SB on inflammatory marker levels
The effects of SB on serum inflammatory marker levels in control and experimental rats are shown in Table 2. IL-1β, IL-6, IL-17, and TNF-α levels increased significantly (p<0.01), and those of IL-10 decreased significantly (p<0.01), in CIA rats. Treatment with 20 and 40 mg/kg SB significantly decreased the IL-1β, IL-6, IL-17, and TNF-α levels, with increased IL-10 level, compared with those of CIA rats.
Table 2.
Effect of SB on the concentration of serum inflammatory markers in control and experimental rats.
| Group | IL-1β (pg/mL) | IL-6 (pg/mL) | IL-10 (pg/mL) | IL-17 (ng/mL) | TNF-α (ng/mL) |
|---|---|---|---|---|---|
| Control | 51.54±5.76 | 22.45±8.67 | 131.56±16.63 | 49.87±6.64 | 88.28±12.45 |
| CIA | 167.34±21.35a** | 108.57±15.66a** | 51.74±7.54a** | 112.58±15.02a** | 177.35±19.62a** |
| SB 20+CIA | 81.45±10.97b** | 66.68±11.77b** | 101.67±11.78b** | 63.74±4.67b** | 120.46±10.24b** |
| SB 40+CIA | 66.87±8.24b** | 41.56±10.75b** | 122.59±10.72b** | 56.80±7.83b** | 100.13±8.94b** |
Data are means ± SD of 12 rats per group.
p<0.01
p<0.05.
CIA vs. control group;
SB treatment (20 and 40 mg/kg) vs. CIA group.
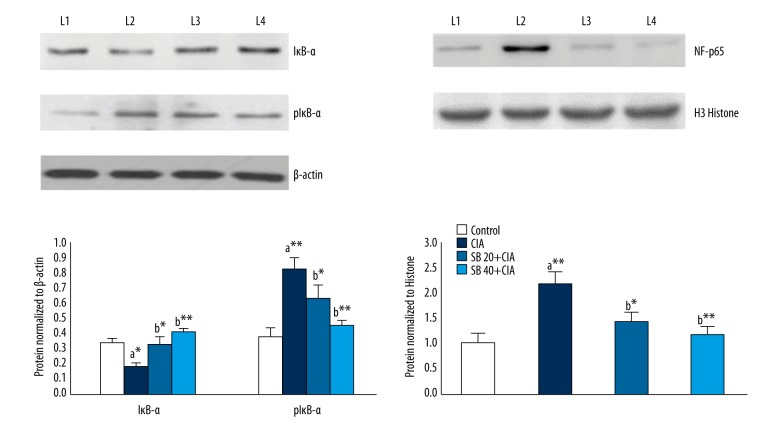
Effect of SB on IκB-α, pIκB-α, and NF-κB p65 subunit levels
The effects of SB on IκB-α, pIκB-α, and NF-κB p65 subunit protein levels in the joint tissue of control and experimental rats are shown in Figure 7. pIκB-α and NF-κB p65 protein levels in joint tissues were markedly upregulated (p<0.01), while that of IκB-α was notably downregulated, in CIA compared with control rats. Treatment with 20 and 40 mg/kg SB for 28 days resulted in significant (p<0.01) decreases in pIκB-α and NF-κB p65 subunit protein expression levels, with a marked increase in the IκB-α protein level as compared with those in CIA rats.
Figure 7.
Effects of SB on IκB-α, p IκB-α, and NF-κB p65 protein levels in the joint tissues of control and experimental rats. Data are means ±SD of 12 rats per group. ** p<0.01, * p<0.05. a CIA vs. control group, b SB treatment (20 and 40 mg/kg) vs. CIA group. β-actin and histone H3 were used as internal standards. Lane 1, control; lane 2, CIA group; lane 3, SB 20 mg/kg group, lane 4, SB 40 mg/kg group.
Discussion
This is the first experimental study of the anti-RA effect of SB in an animal model. A rat model of CIA was used because it is the preferred pre-clinical model of RA [22]. Treatment with 40 mg/kg SB resulted in a greater anti-RA effect than that with 20 mg/kg SB in terms of the arthritis score, paw swelling, PMN activation, oxidative stress and inflammation marker levels, synovitis, and cartilage erosion.
Paw swelling and arthritis scores are used to evaluate the anti-arthritic effects of drugs [20]. The edema and arthritis score of CIA rats were increased on days 14 and 28. Administration of SB markedly reduced the edema, swelling, and erythema compared with those in the CIA rats. Previously, SB has been reported to reduces the edema and swelling of brain tissue by suppressing the inflammatory response [23].
Thymus and spleen indexes are used to evaluate immunoregulatory activity [24,25]. The thymus and spleen indexes were significantly increased in CIA rats; in contrast, 20 and 40 mg/kg SB substantially reduced the thymus and spleen indexes as compared with CIA rats, suggesting the immunomodulatory activity. Danshen (S. miltiorrhiza), the source of SB, exerts immunomodulatory and anti-inflammatory effects in BALB/c mice [26].
Oxidative stress and inflammation are interlinked and play a central role in the pathogenesis of RA [27]. Because the inflammatory response and increased infiltration of macrophages/neutrophils trigger ROS generation, excessive ROS can induce proinflammatory cytokine production and macrophage/neutrophil infiltration, thus triggering inflammation in synovial tissues [28,29]. The activities of endogenous antioxidant enzymes and levels of oxidative markers were measured to assess the protective effects of SB. In CIA rats, GSH, CAT, and SOD activities in joint tissue were considerably decreased, while MDA levels were significantly increased due to oxidation of membrane lipids of chondrocytes and fibrocytes. Similarly, Mateen et al. [30] reported that in RA, MDA levels are significantly increased with decreased activity of GSH, SOD, and CAT owing to the free radical-scavenging and Nrf2-modulatory activities of SB [31], and SB reduced oxidative stress by increasing GSH, CAT, and SOD activities and normalizing MDA levels. Therefore, SB can protect joint tissue against the deleterious effects of free radicals by elevating endogenous antioxidant levels, thereby maintaining the integrity of synovial or joint tissue and cartilage.
The joint tissue of control rats exhibited a normal articular cartilage architecture without mononuclear or PMN infiltration. In contrast, CIA rats exhibited diffuse PMN infiltration with articulated cartilage erosion and synovitis. During CIA induction, the levels of free radicals and proinflammatory cytokines are substantially increased, which results in damage to articular cartilage or joint tissue, leading to synovitis and cartilage erosion. CIA rats treated with 20 mg/kg SB showed mild mononuclear cell or PMN infiltration and only slight cartilage erosion and synovitis. CIA rats treated with 20 mg/kg SB showed almost normal articular cartilage without erosion, together with reduced PMN infiltration and synovitis. Similarly, SB protected against hepatic fibrosis due to its antioxidant and anti-inflammatory properties [32].
Persistent inflammation via excessive infiltration of PMNs contributes to the pathophysiology of RA [28,33]. Therefore, the effect of SB on PMN infiltration in control and experimental rats was quantified by measuring MPO levels. MPO is a heme-containing enzyme that is unique to neutrophils, is involved in free radical production, and is considerably elevated during inflammation [10]. MPO levels were significantly increased in CIA rats (by triggering a CD4 T-cell response), which led to excessive activation of PMNs and neutrophil/macrophage infiltration into damaged synovial joint tissue; in turn, this increases ROS and proinflammatory cytokine levels, resulting in joint-tissue damage [34,35]. MPO levels were normalized by treatment with 20 and 40 mg/kg SB, likely due to the antioxidant and anti-inflammatory activities of SB. Yang et al. [36] reported that SB treatment suppresses neutrophil infiltration and thus ameliorates inflammation.
Anti-CII IgG1 and IgG2a levels were significantly elevated in CIA animal models [37,38] and in CIA-induced rats due to increased activation of Th2 CD cells by CFA and IFA. The increased anti-CII IgG levels were normalized by treatment with 20 or 40 mg/kg SB, likely due to increased activation of B cells or possibly due to regulation of the activation of other immune cells (e.g., PMNs), but the underlying mechanism is unclear.
Proinflammatory cytokines, such as IL-1β, IL-6, IL-17, and TNF-α, play a key role in the physiopathology of RA [39,40]. In contrast, IL-10 ameliorates RA by downregulating proinflammatory cytokine expression [41]. Stimulation of PMNs enhances production of free radicals and proinflammatory cytokines in inflamed synovial tissue, possibly leading to elevated serum levels of proinflammatory cytokines [42]. IL-17 (Th 17) promotes recruitment of neutrophils/macrophages (infiltration) and thus results in synovial joint tissue damage and, subsequently, RA [40]. Indeed, serum IL-1β, IL-6, IL-17, and TNF-α levels were significantly increased, and that of IL-10 was markedly reduced, in CIA rats. However, treatment with 20 and 40 mg/kg SB reduced the serum IL-1β, IL-6, IL-17, and TNF-α levels with increased IL-10 levels. Similarly, Endale et al. [39] reported that suppression of proinflammatory cytokines attenuated further inflammatory response and subsequently lower cartilage destruction in a CIA animal model. To reveal the mechanism underlying the anti-inflammatory activity of SB, we quantified IκB-α, pIκB-α, and NF-κB p65 levels. NF-κB plays a central role in regulating the expression of inflammatory cytokines in RA [6,7].
The NF-κB family comprises homo- or heterodimeric inducible transcription factors that consist of several subunits/transcription factors (p50, p52, p65-Rel A, c-Rel, and RelB). NF-κB resides in the cytosol in its inactive form bound to inhibitory IκB [43, 44]. Upon stimulation, IκB-α is activated by phosphorylation by IκB kinase (IKK) α. pIκB-α undergoes proteasomal degradation to cleave the major subunits of NF-κB (p50 and p65), which is thereby activated [45]. Free NF-κB p50 and p65 translocate into the nucleus and bind to the promoter regions of target genes, such as those encoding inflammatory enzymes (iNOS and COX-2), chemokines, and proinflammatory cytokines (e.g., IL-1β, IL-6, IL-17, and TNF-α) [46]. The pIκB-α and NF-κB p65 protein levels in joint tissues were markedly upregulated, while that of IκB-α was downregulated, in CIA rats. Treatment with 20 and 40 mg/kg SB for 28 days markedly decreased the pIκB-α and NF-κB p65 protein expression levels and increased that of IκB-α, compared with those of CIA rats and thus endorsing its anti-inflammatory activity.
SB prevents NF-κB activation by decreasing the cytoplasmic pIκB protein level and thus dampens inflammation in an animal model [32]. Also, Yang et al. [36] reported that SB significantly inhibits translocation of activated NF-κB p65 subunits into the nucleus in animal model. Our findings are in agreement with the results of Xu et al. [7], who reported that any drug that inhibits NF-κB activation has potential as an anti-RA agent. SB attenuates inflammation by suppressing NF-κB activation, thereby exerting its anti-RA property. The present study has several limitations, including the lack of evaluation of factors related to apoptosis and hyperplasia (synoviocytes), and NF-κB p50 and IKKα protein levels.
Conclusions
SB exerts a concentration-dependent effect on the arthritis score, edema, paw swelling, and oxidative stress and inflammatory marker levels, and ameliorates synovitis and cartilage erosion due to its antioxidant, anti-inflammatory, and immunomodulatory activities. Therefore, SB (especially 40 mg/kg) has potential as an adjuvant therapy for RA together with standard drugs. In the future, we aim to assess the effect of SB on signaling pathways related to its antioxidant and anti-inflammatory properties, which will facilitate development of novel anti-RA drugs.
Footnotes
Conflict of interest
None.
Source of support: This study was financially supported by the National Natural Science Fund Project (No: 81202339)
References
- 1.Jia N, Chu W, Li Y, et al. Iridoid glycosides from the flowers of Gentiana macrophylla Pall. ameliorate collagen-induced arthritis in rats. J Ethnopharmacol. 2016;189:1–9. doi: 10.1016/j.jep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Liu L, Zhang Q, Li M, Wang J. Effect of vitamin D on the recurrence rate of rheumatoid arthritis. Exp Ther Med. 2015;10(5):1812–16. doi: 10.3892/etm.2015.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li ZG. A new look at rheumatology in China – opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):313–17. doi: 10.1038/nrrheum.2014.218. [DOI] [PubMed] [Google Scholar]
- 4.Wang GY, Zhang SL, Wang XR, et al. Remission of rheumatoid arthritis and potential determinants: A national multi-center cross-sectional survey. Clin Rheumatol. 2015;34(2):221–30. doi: 10.1007/s10067-014-2828-3. [DOI] [PubMed] [Google Scholar]
- 5.Jia Z, He J. Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp Ther Med. 2016;11(2):655–59. doi: 10.3892/etm.2015.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Y, Ding CZ, Fang Y. Combination of MTX and LEF attenuates inflammatory bone erosion by down-regulation of receptor activator of NF-κB ligand and interleukin-17 in type II collagen-induced arthritis rats. Rheumatol Int. 2013;33(7):1845–53. doi: 10.1007/s00296-013-2674-7. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Huang M, Zhang Y, et al. Extracts of Bauhinia championii (Benth.) Benth. inhibit NF-B-signaling in a rat model of collagen-induced arthritis and primary synovial cells. J Ethnopharmacol. 2016;185:140–46. doi: 10.1016/j.jep.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Zhu XZ, Feng RB, et al. Crude triterpenoid saponins from Anemone flaccida (Di Wu) exert anti-arthritic effects on type II collagen-induced arthritis in rats. Chin Med. 2015;10(1):1–9. doi: 10.1186/s13020-015-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umar S, Sarwar AH, Umar K, et al. Piperine ameliorates oxidative stress, inflammation and histological outcome in collagen-induced arthritis. Cell Immunol. 2013;284(1):51–59. doi: 10.1016/j.cellimm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Stamp LK, Khalilova I, Tarr JM, et al. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatol. 2012;51(10):1796–803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- 11.Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guidelines for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 12.Singh JA, Furst DE, Bharat A, et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64(5):625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu QS, Luo XY, Jiang H, et al. Salvia miltiorrhiza injection restores apoptosis of fibroblast-like synoviocytes cultured with serum from patients with rheumatoid arthritis. Mol Med Rep. 2015;11(2):1476–82. doi: 10.3892/mmr.2014.2779. [DOI] [PubMed] [Google Scholar]
- 14.Xue L, Wu Z, Ji XP, et al. Effect and mechanism of salvianolic acid B on myocardial ischemia-reperfusion injury in rats. Asian Pac J Trop Med. 2014;7(4):280–84. doi: 10.1016/S1995-7645(14)60038-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang YC, Jin QM, Kong WZ, Chen J. Protective effect of salvianolic acid B on NASH rat liver through restoring intestinal mucosal barrier function. Int J Clin Exp Pathol. 2015;8(5):5203–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Tang M, Feng W, Zhang Y, et al. Salvianolic acid B improves motor function after cerebral ischemia in rats. Behav Pharmacol. 2006;17:493–98. doi: 10.1097/00008877-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Watzke A, O’Malley SJ, Bergman RG, Ellman JA. Reassignment of the configuration of salvianolic acid B and establishment of its identity with lithospermic acid B. J Nat Prod. 2006;69(8):1231–33. doi: 10.1021/np060136w. [DOI] [PubMed] [Google Scholar]
- 18.Umar S, Umar K, Sarwar AH, et al. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomed. 2014;21(6):847–56. doi: 10.1016/j.phymed.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang RX, Fan AY, Zhou AN, et al. Extract of the Chinese herbal formula Huo Luo Xiao LingDan inhibited adjuvant arthritis in rats. J Ethnopharmacol. 2009;121(3):366–71. doi: 10.1016/j.jep.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin B, Zhao Y, Han P, et al. Anti-arthritic activity of Xanthium strumarium L. extract on complete Freund’s adjuvant-induced arthritis in rats. J Ethnopharmacol. 2014;155(1):248–55. doi: 10.1016/j.jep.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LL, Wei W, Yan SX, et al. Therapeutic effects of glucosides of Cheanomeles speciosa on collagen-induced arthritis in mice. Acta Pharmacol Sinica. 2004;25:1495–501. [PubMed] [Google Scholar]
- 22.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Prod. 2007;2(5):1269–75. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 23.Chen T, Liu W, Chao X, et al. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res Bull. 2011;84(2):163–68. doi: 10.1016/j.brainresbull.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Zhao CB, Li XY, Wu N, et al. Effect of Arisaema erubescens (Wall) Schott rhizome extract on rheumatoid arthritis. Trop J Pharm Res. 2016;15(4):805–13. [Google Scholar]
- 25.Niu X, He D, Deng S, et al. Regulatory immune responses induced by IL-1 receptor antagonist in rheumatoid arthritis. Mol Immunol. 2011;49(1):290–96. doi: 10.1016/j.molimm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Gao D, Mendoza A, Lu S, Lawrence DA. Immunomodulatory effects of danshen (Salvia miltiorrhiza) in BALB/c mice. ISRN Inflam. 2012;2012:e954032. doi: 10.5402/2012/954032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–78. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatol. 2012;51(5):3–11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 29.Arii K, Kumon Y, Sugahara K, et al. Edaravone inhibits collagen-induced arthritis possibly through suppression of nuclear factor-kappa B. Mol Immunol. 2008;45(2):463–69. doi: 10.1016/j.molimm.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Mateen S, Moin S, Khan AQ, et al. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. 2016;11(4):e0152925. doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin M, Zhai X, Wang G, et al. Salvianolic acid B protects against acetaminophen hepatotoxicity by inducing Nrf2 and phase II detoxification gene expression via activation of the PI3K and PKC signaling pathways. J Pharmacol Sci. 2015;127(2):203–10. doi: 10.1016/j.jphs.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Yu XY, Guo ZY, et al. Inhibitory effects of salvianolic acid B on CCl4-induced hepatic fibrosis through regulating NF-κB/IκBα signaling. J Ethnopharmacol. 2012;144(3):592–98. doi: 10.1016/j.jep.2012.09.048. [DOI] [PubMed] [Google Scholar]
- 33.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 34.Umar S, Zargan J, Umar K, et al. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in collagen-induced arthritis in Wistar rats. Chem Biol Interact. 2012;197(1):40–46. doi: 10.1016/j.cbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Di Paola R, Mazzon E, Galuppo M, et al. Ethyl pyruvate therapy attenuates experimental severe arthritis caused by type II collagen (CII) in the mouse (CIA) Int J Immunopathol Pharmacol. 2010;23(4):1087–98. doi: 10.1177/039463201002300413. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Chen Z, Lian Z, et al. Inhibitory effects of salvianolic acid B on myocardial cellular nuclear transfer of NF-κB p65 and the expression of TNF-α during ischemia-reperfusion in rabbit hearts in vivo. China J Mod Med. 2009;19:672–79. [Google Scholar]
- 37.Choi JH, Lee JH, Roh KH, et al. Gallium nitrate ameliorates type II collagen-induced arthritis in mice. Int Immunopharmacol. 2014;20(1):269–75. doi: 10.1016/j.intimp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Zhou JX, Ke P, Huan G, et al. Combined treatment with anisodamine and neostigmine inhibits joint inflammation in collagen-induced arthritis mice. CNS Neurosci Ther. 2014;20(2):186–87. doi: 10.1111/cns.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endale M, Lee WM, Kwak YS, et al. Torilin ameliorates type II collagen-induced arthritis in a mouse model of rheumatoid arthritis. Int Immunopharmacol. 2013;16(2):232–42. doi: 10.1016/j.intimp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Liu J, Zeng J, et al. Inhibition of GSK-3β alleviates collagen II-induced rheumatoid arthritis in rats. Med Sci Monit. 2016;22:1047–52. doi: 10.12659/MSM.897739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keystone E, Wherry J, Grint P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 1998;24(3):629–39. doi: 10.1016/s0889-857x(05)70030-2. [DOI] [PubMed] [Google Scholar]
- 42.Adhikary R, Majhi A, Mahanti S, Bishayi B. Protective effects of methanolic extract of Adhatoda vasica Nees leaf in collagen-induced arthritis by modulation of synovial Toll-like receptor-2 expression and release of pro-inflammatory mediators. J Nutr Intermed Metab. 2016;3:1–11. [Google Scholar]
- 43.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-κB signaling module. Oncogene. 2006;25:6706–16. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 44.Moynagh PN. The NF-κB pathway. J Cell Sci. 2005;118:4389–92. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- 45.Carlson CG, Dole E, Stefanski C, Bayless D. The effect of specific IKKβ inhibitors on the cytosolic expression of IκB-α and the nuclear expression of p65 in dystrophic (MDX) muscle. Am J Trans Res. 2014;7(4):670–82. [PMC free article] [PubMed] [Google Scholar]
- 46.Valovka T, Hottiger MO. p65 controls NF-κB activity by regulating cellular localization of IκBβ. Biochem J. 2011;434(2):253–63. doi: 10.1042/BJ20101220. [DOI] [PubMed] [Google Scholar]