Abstract
Background
Neogambogic acid (NGA) is used in traditional Chinese medicine. The aim of this study was to investigate the effects of NGA on gene signaling pathways involved in osteoclastogenesis in mouse bone marrow-derived monocyte/macrophages (BMMs) and on bone resorption in vitro.
Material/Methods
Primary mouse BMMs were cultured with increasing concentrations of NGA. Real-time polymerase chain reaction was used to study the expression of mRNAs corresponding to gene products specific to receptor activator of NF-κB ligand (RANKL)-induced osteoclast differentiation, including tartrate-resistant acid phosphatase (TRAP), calcitonin receptor (CTR), cathepsin K (CTSK), and nuclear factor of activated T cells c1 (NFATc1). A cell counting kit-8 assay was used to evaluate cell proliferation. Western blotting and confocal immunofluorescence microscopy were used to investigate the signaling pathways. A bone resorption model was used to quantify bone resorption.
Results
An NGA dose of ≤0.4 μg/ml had no significant effect on the proliferation of mouse BMMs in vitro (P>0.05); concentrations of between 0.1–0.4 μg/ml significantly inhibited RANKL-induced osteoclastogenesis (P<0.01) in a dose-dependent manner. Compared with the control group, NGA significantly reduced RANKL-induced bone resorption in vitro (P <0.01), and downregulated the expression of osteoclast-related mRNAs of TRAP, CTR, CTSK, and NFATc1. NGA suppressed the activation of JNK but not the p38 signaling pathway and significantly reduced NF-κB p65 phosphorylation and the nuclear transport of NF-κB molecules, which inhibited NFATc1 expression.
Conclusions
NGA suppressed RANKL-induced osteoclastogenesis by inhibiting the JNK and NF-κB pathways in mouse BMMs in vitro and reduced osteoclastic bone resorption.
MeSH Keywords: Garcinia, MAP Kinase Signaling System, NF-kappa B, Osteoclasts, RANK Ligand
Background
Osteoporosis is a systemic metabolic bone disease, characterized by a decrease in bone density and bone mass, destruction of the bone microstructure, and a predisposition to bone fracture [1–3]. Normally, osteoblasts and osteoclasts maintain a dynamic balance between bone formation and bone resorption but failure of this balance can lead to bone disease [4,5]. Currently, the available anti-osteoporosis drugs can be divided into three categories: bone resorption-inhibitor drugs, such as estrogen, calcitonin, and bisphosphonate; drugs that accelerate bone formation, such as fluorine and parathyroid hormone; and strontium, which is provided as a ranelate salt and has both bone forming and anti-resorption effects. However, there are serious adverse reactions associated with some of these drugs, which have led to increasing interest in the use of traditional Chinese medicine [6,7].
Recently published studies have investigated the use of Chinese herbal monomers to treat osteoporosis by regulating critical cytokines and signaling pathways [7]. Neogambogic acid (NGA) is a compound produced by Garcinia hanburyi. Although NGA exhibits immune enhancing, anti-inflammatory, anti-tumor, and pro-apoptotic activities, few studies have investigated the effects of NGA on osteoclast differentiation or the underlying mechanism of action [8–10].
Previous studies have shown that osteoclast activation and differentiation rely on the receptor activator of NF-κB ligand (RANKL)-related signaling pathway, which plays a crucial role in the differentiation of bone marrow-derived monocyte/macrophages (BMMs) into osteoclasts [11]. In a preliminary experimental study, we found that NGA suppressed a few key proteins in the NF-κB and mitogen-activated protein kinase (MAPK) pathways, which are closely related to the RANKL/RANK pathway [12,13].
Therefore, the aim of this study was to investigate the effects of NGA on gene signaling pathways involved in osteoclastogenesis in mouse BMMs and on bone resorption in vitro.
Material and Methods
Animal model and ethical approval
Six-week-old male C57BL/6 mice (n=30) were provided by the Animal Experiment Center of Shanghai Jiao Tong University. All in vivo experiments were performed in accordance with the principles and procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Ethics Committees of Zhejiang Cancer Hospital and the Health Department of Zhejiang Province in Hangzhou, China provided ethical approval and approved the experimental protocol.
Materials
Neogambogic acid (MB5759) was purchased from Dalian Meilun Biotechnology Co. (Dalian, China). Cell culture medium, penicillin, streptomycin, and fetal bovine serum (FBS) were purchased from Gibco-BRL (Gaithersburg, MD, USA). Recombinant mouse macrophage colony-stimulating factor (M-CSF) and mouse receptor activator of NF-κB ligand were purchased from R&D Systems (Minneapolis, MN, USA). The cell counting kit-8 (CCK-8) was purchased from Dojindo Molecular Technology (Kumamoto, Japan). A tartrate-resistant acid phosphatase (TRAP) staining kit was purchased from Sigma-Aldrich (St Louis, MO, USA). Specific antibodies against p38, phospho-p38 (Thr180/Tyr182), JNK, phospho-JNK (Thr183/Tyr185), nuclear factor of activated T cells c1 (NFATc1), p65, phospho-p65, GAPDH, and beta-actin were purchased from Cell Signaling Technology (Cambridge, MA, USA).
Isolation of mouse bone marrow-derived monocyte/macrophages and cell culture
After anesthesia, the mice were euthanized by cervical dislocation. The mouse tibial and femoral bones were removed while maintaining as much of the length of the bone as possible. After removing the epiphysis, the bone marrow cavity was flushed with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. The flushed cell suspension was collected and centrifuged for 5 minutes at 1,000 rpm. The sediment was then removed and cultured in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 30 ng/ml M-CSF in a 5% CO2 incubator at 37°C for at least 96 hours to induce BMMs to develop into osteoclasts. The culture medium was replaced every two days throughout the study.
Cell cytotoxicity and cell proliferation using the cell counting kit-8 assay
BMMs that had been cultured in DMEM containing M-CSF for 96 hours were then added to 96-well plates at a density of 2×104 cells/well with 30 ng/ml M-CSF for 24 hours. The cells were then treated with increasing concentrations of NGA (0, 0.2, 0.4, 0.8, 1.6 μg/ml) in an incubator containing 5% CO2 at 37°C for 48 hours or 96 hours. All experiments and analysis were performed in triplicate. The cytotoxic effects of NGA on BMMs was evaluated using the CCK-8 assay according to the manufacturer’s instructions. The absorbance value in each well at 450 nm was measured using a microplate ELX800 reader (Bio-Tek Instruments, Winooski, VT, USA).
Tartrate-resistant acid phosphatase staining of osteoclasts
BMMs were seeded into a 96-well plate at a density of 8×103 cells/well in the presence of 30 ng/ml M-CSF and 50 ng/ml RANKL, as well as increasing concentrations of NGA (0, 0.1, 0.2, 0.4 μg/ml) for six days. Once cells with more than five nuclei (multinucleated osteoclast cells) were present, the cells were washed three times with phosphate-buffered saline (PBS), fixed with 40 mg/ml paraformaldehyde for 30 minutes, washed again with PBS, and stained with TRAP according to the manufacturer’s instructions. TRAP-positive cells with more than five nuclei were counted under a microscope at high magnification.
Bone resorption assay using bovine bone
BMMs were seeded onto bovine bone slices in a 96-well plate at a density of 1×104 cells/well with three replicates. After 24 hours, the cells were treated with 50 ng/ml RANKL, 30 ng/ml M-CSF, and 0, 0.1, 0.2, or 0.4 μg/ml NGA until mature multinucleated osteoclasts formed. The resorption pits in the bone were photographed under a scanning electron microscope (FEI Instruments, Hillsboro, OR, USA) at ×400 magnification and the bone resorption area was quantified using Image J software (National Institutes of Health, Bethesda, MD, USA).
Quantitative real-time polymerase chain reaction (PCR)
BMMs were seeded in a 6-well plate at a density of 10×104 cells per well and cultured with 30 ng/ml M-CSF, 50 ng/ml RANKL, and increasing concentrations of NGA (0, 0.2, or 0.4 μg/ml) for five days. Total RNA was isolated from the cells using an RNeasy Mini kit (Qiagen, Valencia, CA, USA). Complementary DNA (1 μg) was synthesized from the RNA of each sample according to the recommendations of the manufacturer of the PCR kit. Quantitative real-time PCR was performed using SYBR Premix Ex Taq (Takara Bio, Otsu, Japan) and an ABI Prism 7500 system (Applied Biosystems, Foster City, CA, USA). The specific gene expression results were normalized to those of GAPDH. The equation RQ=2−ΔΔCt was used to analyze relative mRNA expression levels. The sequence of primers was as follows:
-
TRAP:
forward, 5′-CTGGAGTGCACGATGCCAGCGACA-3′ and
reverse, 5′-TCCGTGCTCGGCGATGGACCAGA-3′
-
Calcitonin receptor (CTR):
forward, 5′-TGCAGACAACTCTTGGTTGG-3′ and
reverse, 5′-TCGGTTTCTTCTCCTCTGGA-3′
-
Cathepsin K (CTSK):
forward, 5′-CTTCCAATACGTGCAGCAGA-3′ and
reverse, 5′-TCTTCAGGGCTTTCTCGTTC-3′
-
NFATc1:
forward, 5′-CCGTTGCTTCCAGAAAATAACA-3′ and
reverse, 5′-TGTGGGATGTGAACTCGGAA-3′
-
GAPDH:
forward 5′-ACCCAGAAGACTGTGGATGG-3′ and
reverse 5′-CACATTGGGGGTAGGAACAC-3′
Western blotting
BMMs were seeded in 6-well plates at a density of 5×105 cells per well and pretreated with 0.2 or 0.4 μg/ml of NGA for 2 hours. The cells were then stimulated with 50 ng/ml of RANKL for 5, 10, 20, or 30 minutes to determine the involved signaling pathways. To determine the effect of NGA specifically on NFATc1, BMMs were treated with 50 ng/ml RANKL and 0.2 or 0.4 μg/ml NGA for three days. Subsequently, all cells were rinsed twice with ice-cold PBS and lysed in radioimmunoprecipitation assay lysis buffer (Sigma Aldrich, St Louis, MO, USA), followed by centrifugation at 12,000×g and 4°C for 15 min. The supernatants were collected and subjected to a bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA, USA) to determine the total protein concentrations. Equal amounts of protein per sample were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA).
The PVDF membranes were first blocked for 1 hour in 5% nonfat skimmed milk powder, followed by overnight incubation with primary antibodies (1: 1000) at 4°C. The membranes were then treated with a secondary horseradish peroxidase conjugated goat anti-rabbit immunoglobulin G (1: 5000) (Abcam, Cambridge, MA, USA) at room temperature for 1 hour, after which the labeled protein bands were detected using an electrochemical luminescence reagent (Millipore, Billerica, MA, USA). The protein bands were visualized using a LAS-4000 Science Imaging System (Fuji Photo Film, Tokyo, Japan).
Confocal immunofluorescence
BMMs were cultured with 30 ng/ml M-CSF in the absence or presence of 0.4 μg/mL NGA for 2 hours. After a 30 minute stimulation with 50 ng/ml RANKL, the cells were fixed with 4% paraformaldehyde for 20 minutes, permeated with 0.1% Triton X-100 for 10 minutes, and blocked with 1% bovine serum albumin for 1 hour. The cells were then stained with the red-orange fluorophore, rhodamine phalloidin (Invitrogen) for at least 1 hour at 4°C, washed with PBS, and mounted using Prolong Gold antifade mounting medium (Invitrogen) for confocal microscopy.
Statistical analysis
The data were expressed as the mean ± standard errors of the mean. All experiments were performed in triplicate. The results were analyzed using SPSS for Windows, version 16.0 (SPSS, Chicago, IL, USA). The Student’s t-test was used to compare the two groups. A P-value <0.05 indicated a significant difference between the groups.
Results
NGA suppressed osteoclast differentiation from mouse BMMs in vitro
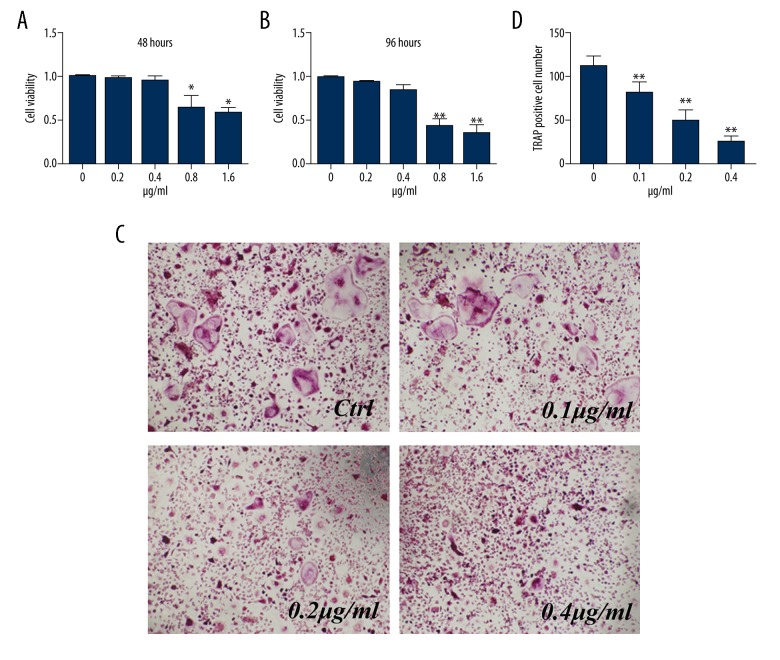
Cytotoxicity analysis showed that concentrations of NGA ≥0.8 μg/ml significantly reduced the viability of mouse BMMs relative to the control (untreated) group, and this difference became more pronounced as the culture time increased. However, no cytotoxic effects of NGA at doses ≤0.4 μg/ml were found (Figure 1A, 1B). Therefore, to determine the effect of NGA on osteoclast differentiation, BMMs were treated with RANKL and M-CSF in the presence of 0, 0.1, 0.2, or 0.4 μg/ml of NGA.
Figure 1.
NGA inhibited osteoclastogenesis in mouse BMMs in vitro. (A, B) The effect of NGA on the proliferation of mouse BMMs after 48 hours (A) or 96 hours (B) of treatment with NGA. BMMs were treated with increasing concentrations of NGA (0, 0.2, 0.4, 0.8, 1.6 μg) for 48 hours or 96 hours. A CCK-8 assay was used to measure the inhibition BMMs following NGA treatment. Significant effects of treatment are shown with doses of NGA at 48 hours and 96 hours compared with the 0 μg/ml treatment group (t-test), * p<0.05 and ** p<0.01, respectively. (C, D) The effect of increasing concentrations of NGA on osteoclast formation shows that BMMs were cultured in the presence of 30 ng/ml M-CSF and 50 ng/ml RANKL, as well as increasing concentrations of NGA (0, 0.1, 0.2, 0.4 μg/ml) for 6 days, and were positively stained for TRAP. (C) The light microscopy appearance of the TRAP-positive cells at high magnification (magnification ×100). (D) The number of osteoclasts that formed after being treated with NGA, compared with the 0 μg/ml treatment group (t-test), ** p<0.01.
Untreated BMMs grew in an adherent manner and were morphologically small, polygonal or round, and mononuclear. Treatment with RANKL and M-CSF led to a transformation in cell morphology, including the presence of multiple nuclei, the formation of nuclear clusters, with a spiculated outline, and positive staining with TRAP, consistent with osteoclasts (Figure 1C). The numbers of TRAP-positive multinucleated osteoclasts decreased significantly in the NGA pretreatment groups relative to the control group, which was dose-dependent (Figure 1D).
NGA inhibited osteoclastic bone resorption in vitro
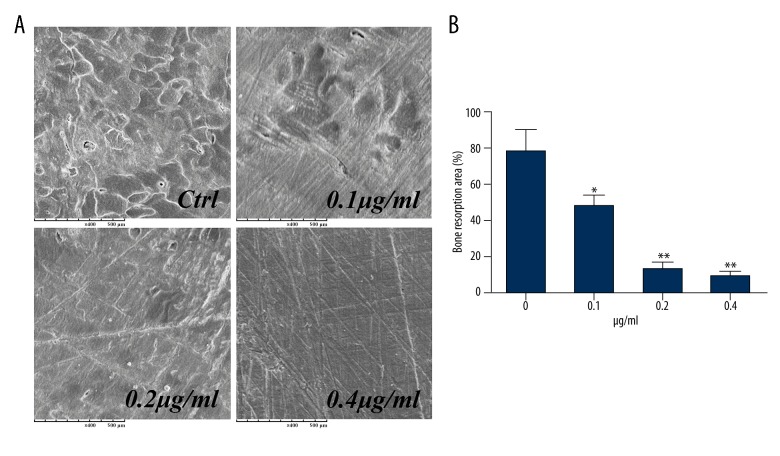
When BMMs were plated on bovine bone slices and treated with M-CSF and RANKL, a large number of irregular, circular, high-density shadows were observed by scanning electron microscopy. These shadows corresponded to three-dimensional structures that were areas of resorption on the bone surface. These areas of bone resorption were significantly reduced in the presence of NGA (Figure 2A). At an NGA concentration of 0.1 μg/ml, the bone resorption area was <61% less than the area in the control group, an increase to 0.4 μg/ml NGA almost completely inhibited the bone resorbing effect of BMMs (Figure 2B). These data suggest that NGA could inhibit BMM-related osteoclastic bone resorption in vitro.
Figure 2.
NGA suppressed osteoclastic bone resorption in mouse BMMs in vitro. Mouse BMMs were seeded onto bovine bone slices and treated in the presence of 30 ng/ml M-CSF and 50 ng/ml RANKL, as well as increasing concentrations of NGA (0, 0.1, 0.2, 0.4 μg/ml) for 6 days, until mature (multinucleated) osteoclasts formed. (A) Representative images of the areas of bone resorption area after being treated with increasing doses of NGA. The resorption pits were photographed under a scanning electron microscope (magnification ×400). (B) The percentage of the resorbed area of bone, after being treated with NGA, compared with the 0 μg/ml treatment group (t-test), * p<0.05 and ** p<0.01, respectively.
NGA inhibited the expression of mRNAs related to osteoclastogenesis
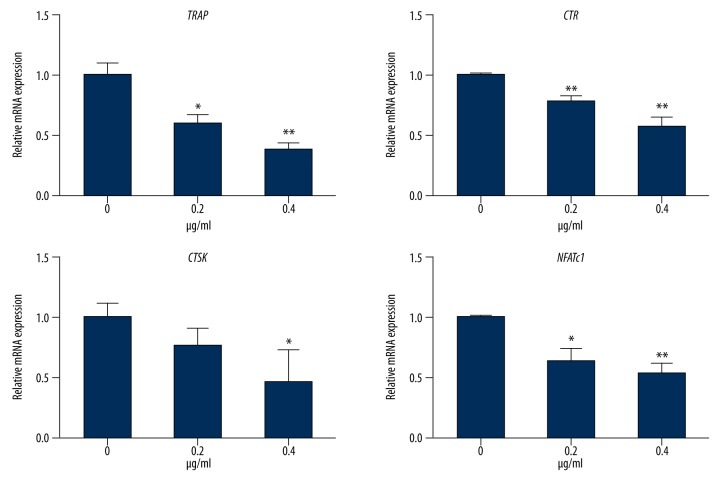
The expression of mRNAs corresponding to several gene products specific to RANKL-induced osteoclast differentiation was evaluated, including TRAP, CTR, CTSK, and NFATc1 genes. There was significant suppression of expression of TRAP, CTR, CTSK, and NFATc1 genes by mouse BMMs after treatment with NGA for five days, compared with the control group, and this effect was enhanced in a dose-dependent manner (Figure 3).
Figure 3.
NGA inhibited osteoclast gene expression in mouse BMMs in vitro. The expression of mRNAs corresponding to gene products specific to RANKL-induced osteoclast differentiation, including TRAP, CTR, CTSK, and NFATc1 in mouse BMMs treated with 30 ng/ml M-CSF, 50 ng/ml RANKL, and increasing concentrations of NGA (0, 0.2 or 0.4 μg/ml) for 5 days, measured by real-time PCR. RNA expression levels were normalized relative to the expression of GAPDH. Gene expression after being treated with NGA, compared with the 0 μg/ml treatment group (t-test), * p<0.05 and ** p<0.01, respectively.
NGA treatment inhibited the JNK and NF-κB signaling pathway
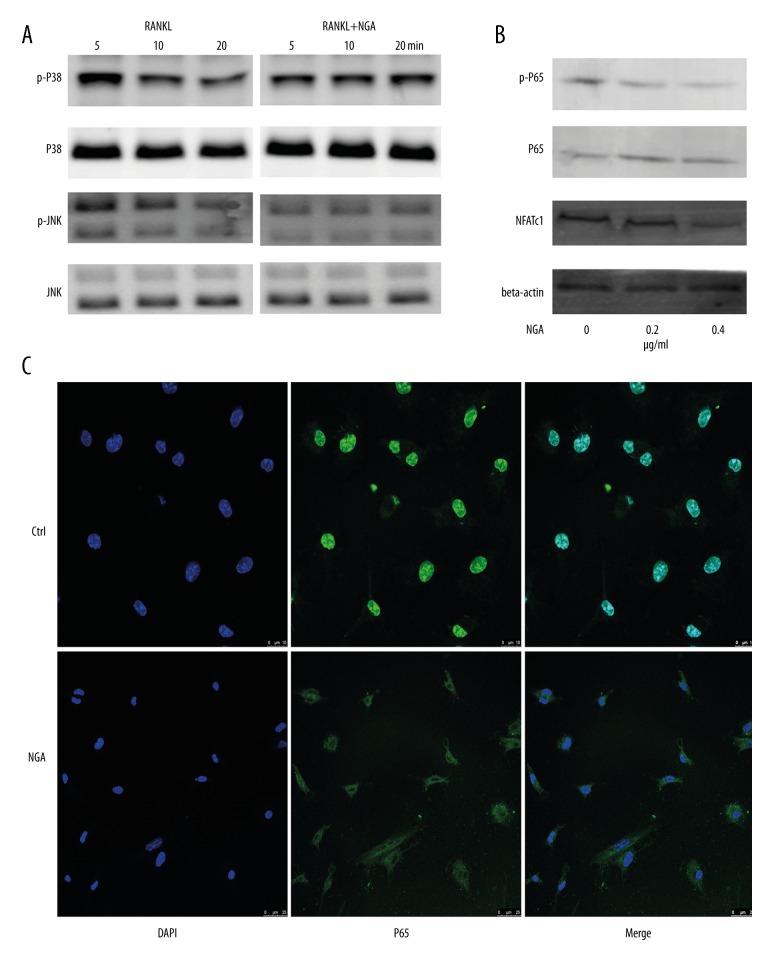
The expression of key proteins in multiple signaling pathways was detected in BMMs, and the expression of their corresponding phosphorylation levels was detected after pretreatment with 0.4 μg/ml NGA and exposure to RANKL for different time intervals. However, differences in the phosphorylation of the MAPK protein JNK were only observed (Figure 4A). The expression of phospho-JNK decreased gradually over time, as demonstrated by the lack of any difference between cells exposed to RANKL for 20 minutes and the control group (Figure 4A). However, the activation of p38 was not affected. These findings suggested the involvement of additional signaling pathways.
Figure 4.
NGA inhibited the JNK and NF-κB signaling pathways in mouse BMMs in vitro. (A) Mouse BMMs were pretreated with 0.4 μg/ml NGA for 2 hours. The cells were then stimulated with 50 ng/ml of RANKL for 5, 10, or 20 minutes to detect the expression of key proteins in multiple BMM signaling pathways and their corresponding phosphorylation levels. (B) The effect of NGA on the p65 signaling pathway and NFATc1 expression. To determine p65 signaling pathway, BMMs were pretreated with 0, 0.2 or 0.4 μg/ml NGA for 2 hours and then stimulated with 50 ng/ml RANKL for 30 minutes. To determine the effect of NGA son NFATc1, BMMs were treated with 50 ng/ml RANKL, and increasing concentrations of NGA (0, 0.2, 0.4 μg/ml) for three days. (C) Confocal fluorescence microscopy shows the effects of NGA on p65 nuclear translocation in BMMs cultured with 30 ng/ml M-CSF in 0 or 0.4 μg/ml NGA for 2 hours and then treated with 50 ng/ml of RANKL for 30 minutes are shown. All cells were fixed and stained with the red-orange fluorophore, rhodamine phalloidin.
Because the transcription factor NF-κB must enter the nucleus to exert its functions [14], in this study p65 was fluorescence labeled in cells to study the relationship between the levels of p65 phosphorylation and intracellular localization. Following costimulation with M-CSF and RANKL, the green fluorescent marker-tagged NF-κB was rapidly transferred into the cell nuclei of control group (Non-treated) cells, but not of cells in the NGA-treated group, which was consistent with the western blotting results (Figure 4). These findings indicated that NGA treatment blocked P65 nuclear translocation and inhibited the NF-κB signaling pathway in mouse BMMs in vitro. Consequently, the NFATc1 expression levels were significantly lower in the NGA-treated mouse BMM cells compared with the untreated control cells and decreased as the drug concentration increased, as supported by the PCR findings (Figures 3, 4B).
Discussion
Osteoclasts, which differentiate from osteoclast precursor cells and can fuse with peripheral mononuclear cells, are cells involved in bone resorption [15]. BMMs are considered to be osteoclast precursor cells and can differentiate into osteoclast-like multinucleated cells in vitro via the synergistic actions of RANKL and M-CSF [11,13]. NGA is an active component of Garcinia, which is used in traditional Chinese medicine and has been shown to have a beneficial clinical effect on bone fractures and hemostasis and has been reported to have anti-tumor and anti-inflammatory activities [10,12]. However, the effects of treatment with NGA on osteoporosis has not been investigated before. Therefore, the aim of this study was to evaluate the effects of increasing concentrations of NGA on RANKL-induced osteoclastogenesis and to investigate the possible associated signaling pathways in mouse BMMs and on bone resorption in vitro.
The findings of this study showed that within a certain dose range, NGA could inhibit osteoclast differentiation in a dose-dependent manner in vitro, both in cell culture and in a bovine bone resorption model. Analysis of the bone resorption areas indicated that NGA had an inhibitory effect on bone resorption. The findings from this preliminary in vitro study indicate that NGA has an inhibitory effect on RANKL-induced osteoclast differentiation.
Previously published studies have shown that a variety of hormones and cytokines regulate BMMs during osteoclast differentiation [16–19]. The type I transmembrane receptor protein, RANK, is distributed on the surface of osteoclasts and osteoclast precursor cells, where it regulates osteoclast activation. The interaction between RANK and RANKL can stimulate differentiation, maturation, and bone resorption in osteoclasts. NFATc1, an important regulator of osteoclast formation, can promote the differentiation and maturation of osteoclasts. NFATc1 is also an important transcription factor in the RANKL/RANK signaling pathway. The increased expression of the NFATc1 gene can induce the activation of a series of proteins associated with the differentiation and activity of osteoclasts, TRAP, CTR, and CTSK. Recent studies on the role of NFATc1 in osteoclast differentiation have identified a novel therapeutic target for titanium particle-induced osteolysis [20,21]. Therefore, this study investigated the expression of mRNAs related to osteoclast differentiation and maturation to determine whether NGA could effectively suppress osteoclastogenesis at the gene transcription level. The results demonstrated NGA downregulated the mRNA expression of the genes, TRAP, CTR, CTSK, and NFATc1, in a dose-dependent manner.
Both the MAPK and NF-κB pathways are closely related to osteoclast differentiation [11–13,16–18]. The MAPK pathway, which includes ERK, JNK, and p38, is important for the regulation of osteoclast differentiation, as MAPK-related gene knockout mice have been shown to develop osteoclast formation disorders and have an increased risk of osteosclerosis [22,23]. The NF-κB signaling pathway is also an important research target in osteoporosis treatment, as many molecular events within this pathway, including protein phosphorylation and nuclear translocation, have been proposed as drug targets [13,14,16,18]. The findings of this study showed that in mouse BMMs in vitro, NGA pretreatment decreased the translocation of p65 and activation of JNK, which is important components in the MAPK signaling system. In particular, the suppression of NF-κB signaling cascade is crucial for the expression of NFATc1. These findings clarify the mechanisms by which NGA inhibits the RANKL-induced osteoclastogenesis of mouse BMMs in vitro.
Conclusions
The findings from this in vitro study using mouse BMMs have shown that the natural product, NGA, which is used in traditional Chinese medicine, suppressed RANKL-induced osteoclast differentiation and reduced RANKL-induced osteoclastic bone resorption by altering JNK expression and activation and by preventing NF-κB from activating the transcription of NFATc1. The differentiation and activity of mouse BMMs were inhibited at NGA concentrations ≥0.8 μg/ml in vitro. NGA, a monomer of a traditional Chinese medicine, might be considered as an alternative treatment for osteoporosis. Future studies should be undertaken to investigate the mechanism of action of NGA and involvement of further pathways involved in its potential effects on osteoclastogenesis and bone resorption.
Acknowledgments
The authors thank Yexin Wang of Shanghai Jiao Tong University School of Medicine for providing technical support. This manuscript was edited by Armstrong-Hilton Limited.
Footnotes
Source of support: This work was supported by the Health Department of Zhejiang Province (grant number 2017KY024)
References
- 1.Wang C, Meng H, Wang X, et al. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med Sci Monit. 2016;22:226–33. doi: 10.12659/MSM.897044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: Now and the future. Lancet. 2011;377(9773):1276–87. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Q, Ni J, Hou Y, et al. Expression of sclerostin scFv and the effect of sclerostin scFv on healing of osteoporotic femur fracture in rats. Cell Biochem Biophys. 2014;69(2):229–35. doi: 10.1007/s12013-013-9787-1. [DOI] [PubMed] [Google Scholar]
- 4.Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8(6):225–35. doi: 10.1177/1759720X16670154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda K, Takeshita S. Factors and mechanisms involved in the coupling from bone resorption to formation: How osteoclasts talk to osteoblasts. J Bone Metab. 2014;21(3):163–67. doi: 10.11005/jbm.2014.21.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milat F, Ebeling PR. Osteoporosis treatment: A missed opportunity. Med J Aust. 2016;205(4):185–90. doi: 10.5694/mja16.00568. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Ma R, Wang L, et al. Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J Ethnopharmacol. 2017;198:351–62. doi: 10.1016/j.jep.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Zhang H, Liu P, et al. Gambogenic acid synergistically potentiates bortezomib-induced apoptosis in multiple myeloma. J Cancer. 2017;8(5):839–51. doi: 10.7150/jca.17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Zhang M, Wang Z, et al. Neogambogic acid prevents silica-induced fibrosis via inhibition of high-mobility group box 1 and MCP-1-induced protein 1. Toxicol Appl Pharmacol. 2016;309:129–40. doi: 10.1016/j.taap.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Yu XJ, Zhao Q, Wang XB, et al. Gambogenic acid induces proteasomal degradation of CIP2A and sensitizes hepatocellular carcinoma to anticancer agents. Oncol Rep. 2016;36(6):3611–18. doi: 10.3892/or.2016.5188. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Sapkota M, Gao M, et al. Macrolactin F inhibits RANKL-mediated osteoclastogenesis by suppressing Akt, MAPK, and NFATc1 pathways and promotes osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway. Eur J Pharmacol. 2017;815:202–9. doi: 10.1016/j.ejphar.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Zhao Q, Zhang H, et al. Gambogenic acid inhibits LPS-simulated inflammatory response by suppressing NF-κB and MAPK in macrophages. Acta Biochim Biophys Sin (Shanghai) 2016;48(5):454–61. doi: 10.1093/abbs/gmw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thummuri D, Naidu VGM, Chaudhari P. Carnosic acid attenuates RANKL-induced oxidative stress and osteoclastogenesis via induction of Nrf2 and suppression of NF-κB and MAPK signaling. J Mol Med (Berl) 2017;95(10):1065–76. doi: 10.1007/s00109-017-1553-1. [DOI] [PubMed] [Google Scholar]
- 14.Sigal LH. Basic science for the clinician 39: NF-kappaB-function, activation, control, and consequences. J Clin Rheumatol. 2006;12(4):207–11. doi: 10.1097/01.rhu.0000231385.94784.e4. [DOI] [PubMed] [Google Scholar]
- 15.Drissi H, Sanjay A. The multifaceted osteoclast; Far and beyond bone resorption. J Cell Biochem. 2016;117(8):1753–56. doi: 10.1002/jcb.25560. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Huang Y, Huang Z, et al. FTY-720P suppresses osteoclast formation by regulating expression of interleukin-6 (IL-6), interleukin-4 (IL-4), and matrix metalloproteinase 2 (MMP-2) Med Sci Monit. 2016;22:2187–94. doi: 10.12659/MSM.896690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X, Mei L, Pei J, et al. Sophoridine from sophora flower attenuates ovariectomy induced osteoporosis through the RANKL-ERK-NFAT pathway. J Agric Food Chem. 2017;65(44):9647–54. doi: 10.1021/acs.jafc.7b03666. [DOI] [PubMed] [Google Scholar]
- 18.Liu YQ, Hong ZL, Zhan LB, et al. Wedelolactone enhances osteoblastogenesis by regulating Wnt/β-catenin signaling pathway but suppresses osteoclastogenesis by NF-κB/c-fos/NFATc1 pathway. Sci Rep. 2016;6:32260. doi: 10.1038/srep32260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu DJ, Gu R, Sarin R, et al. Autophagy-linked FYVE containing protein WDFY3 interacts with TRAF6 and modulates RANKL-induced osteoclastogenesis. J Autoimmun. 2016;73:73–84. doi: 10.1016/j.jaut.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Kim EY, Lee B, et al. The effects of Lycii Radicis Cortex on RANKL-induced osteoclast differentiation and activation in RAW 264.7 cells. Int J Mol Med. 2016;37(3):649–58. doi: 10.3892/ijmm.2016.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek JM, Park SH, Cheon YH, et al. Esculetin attenuates receptor activator of nuclear factor kappa-B ligand-mediated osteoclast differentiation through c-Fos/nuclear factor of activated T-cells c1 signaling pathway. Biochem Biophys Res Commun. 2015;461(2):334–41. doi: 10.1016/j.bbrc.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Vattakuzhi Y, Abraham SM, Freidin A, et al. Dual-specificity phosphatase 1-null mice exhibit spontaneous osteolytic disease and enhanced inflammatory osteolysis in experimental arthritis. Arthritis Rheum. 2012;64(7):2201–10. doi: 10.1002/art.34403. [DOI] [PubMed] [Google Scholar]
- 23.Thouverey C, Caverzasio J. The p38α MAPK positively regulates osteoblast function and postnatal bone acquisition. Cell Mol Life Sci. 2012;69(18):3115–25. doi: 10.1007/s00018-012-0983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]