Abstract
This editorial refers to ‘Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction’†, by V.R. Taqueti et al., on page 840.
A variety of observations suggest that coronary microvascular dysfunction (CMD) is related to the pathogenesis of cardiovascular disease, including myocardial ischaemia and heart failure.1,2 CMD is due to changes in the function and structure of the coronary microcirculation and, in the absence of obstructive coronary artery disease (CAD), is more prevalent among women, is poorly understood mechanistically, and therefore represents a major unmet therapeutic need.3 While termed ischaemia with no obstructive CAD (INOCA) and myocardial infarction with no obstructive CAD (MINOCA),3 it is also sometimes called ‘female-pattern’ cardiovascular disease along with heart failure with preserved ejection fraction (HFpEF) because these occur more commonly in women, and the relative paucity of studies of women has resulted in therapeutic deserts for these conditions. Notably, the ‘female-pattern’ terminology may soon be irrelevant, as INOCA, MINOCA, and HFpEF are increasingly being diagnosed in men.3
We have suggested that CMD contributes to signs and symptoms of ischaemia in patients both with and without evidence of obstructive CAD, and to HFpEF, both of which share common pathophysiological pathways involving primary or secondary changes in the coronary microcirculation.3,4 Our work, and that of others, indicates that CMD and HFpEF appear associated along a continuum linked by alterations in the coronary microcirculation.5–7 However, causal mechanistic links and the sequence of causality remain unknown, challenging therapeutic inroads in both conditions. Specifically, does primary CMD lead to ventricular remodelling/diastolic dysfunction and HFpEF or do alterations in myocardial remodelling/diastolic dysfunction observed in HFpEF lead to secondary CMD, i.e. the chicken or the egg?
Previous work has demonstrated that coronary microvascular abnormalities contribute critically to cardiac impairment in hypertrophic cardiomyopathy.8 Contributing to the area, Taqueti and colleagues9 demonstrate that CMD, indicated by low positron emission tomography (PET) coronary flow reserve, combined with evidence of diastolic dysfunction, measured by echo, predicts adverse prognosis including death (7%), with the major adverse cardiac event (MACE) rate dominated by HFpEF hospitalization. Notably, minor elevations in troponins measured prior to the non-invasive testing further elevated MACE risk.
Further, in this issue of the journal, Taqueti et al.9 report a 2- to 3-fold higher mortality rate and a 2- to 5-fold higher MACE rate than previously reported in cohorts with stable angina and no obstructive CAD.2,10 The current study subjects did not undergo coronary angiography and were categorized as without obstructive CAD due to the absence of segmental PET perfusion defects. However, plaque rupture often occurs in non-flow limiting stenoses, and obstructive CAD often has relatively higher MACE rates than non-obstructive CAD, thus raising the possibility that occult obstructive CAD may have contributed to their reported high MACE rate. The lack of validation of obstructive CAD status is a limitation of this current work, although it reflects a single centre highly experienced with PET perfusion imaging. The relatively high MACE rate in this work may also be due to referral bias, as these patients underwent serial troponin measurements prior to their PET scan, suggesting a higher risk cohort.
The major novel finding of this investigation was that CMD-associated diastolic dysfunction predicted a >5-fold increased risk of HFpEF hospitalization. This is consistent with a growing body of work from our group showing that women with CMD often have left ventricular (LV) diastolic dysfunction,5,11,12 and are at increased risk of developing HFpEF.6 Indeed, closer examination of these studies reveals several striking parallels. For example, LV early diastolic strain rate,5,11,12 and early volumetric filling rate,13 are reduced in women with CMD, consistent with the impaired early LV relaxation rate (e') reported by Taqueti et al.9 Likewise, women with CMD often have elevated LV filling pressures,5 supporting the echo-derived E/e' data reported here.
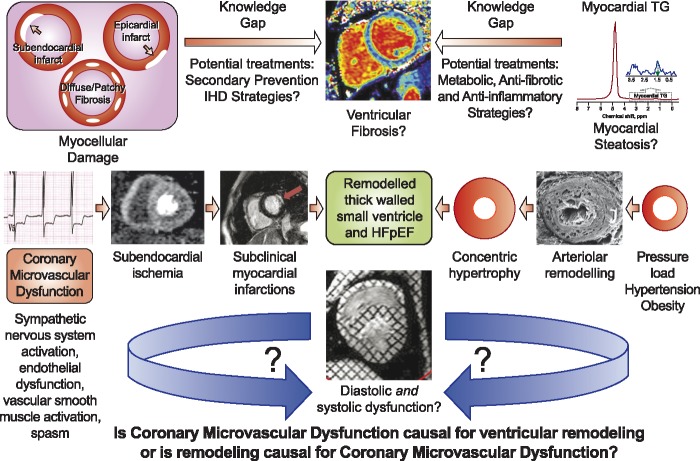
The exact mechanism(s) contributing to CMD-related diastolic dysfunction, however, remains incompletely understood. Diastolic dysfunction is a multifactorial process, and factors such as inflammation, adverse LV remodelling, cardiometabolic dysfunction, myocardial fibrosis, and coronary microvascular ischaemia have all been implicated. Indeed, elevated interleukin-6 was found strongly to predict HF hospitalization and all-cause mortality in women with CMD, suggesting that inflammation plays an important role in the pathogenesis of disease progression.14 The present study did not report LV mass or dimensions, so it is unclear if overt myocardial hypertrophy directly contributed to the diastolic dysfunction; however, our group consistently finds normal LV concentricity in women with CMD, suggesting that the concentric remodelling is not mandatory for diastolic dysfunction. The observation in the present study that CMD was associated with a measurable increase in cardiac troponin (∼25% of patients), however, provides novel mechanistic insight. These new observations support the hypothesis that repeat micro-infarctions caused by coronary microvascular ischaemia lead to diffuse myocardial fibrosis, increased LV stiffness, and impaired ventricular relaxation (Take home figure). These data further suggest that isolated diastolic dysfunction, in the absence of abnormal flow reserve and myocardial injury, may be benign. Consistent with emerging understanding in this area, diastolic dysfunction is neither mandatory nor sufficient for the development of HFpEF.
Take home figure.
Is coronary microvascular dysfunction (CMD) causal for ventricular remodelling and diastolic dysfunction and/or are ventricular remodelling and diastolic dysfunction causal for CMD? Emerging evidence suggest that a progressive clustering of risk factors (hypertension, dyslipidaemia, dysglycaemia, and oestrogen loss) promote a pro-inflammatory and pro-oxidative state, rendering the coronary microvasculature vulnerable to repeat epidsodes of myocardial ischaemia. These repeat episodes of ischaemic insult lead to progressive impairment of energy-dependent cardiomyocyte relaxation and a shift in substrate metabolism, leading to cardiac steatosis, left ventricular diastolic dysfunction, and ultimately the development of heart failure. Several knowledge gaps exist, however, including therapies to treat metabolic and inflammatory pathways, and prevent myocellular damage. Moreover, it remains unclear to what extent adverse remodelling and diastolic dysfunction contribute to CMD. Future work focusing on these important questions is desperately needed to fill key knowledge gaps (indicated by ‘?’).
Of course, myocardial ischaemia itself could directly, and indirectly, contribute to diastolic dysfunction in these patients; however, this hypothesis has not been tested directly. ATP is required for several stages of the myocellular relaxation process, including dissociation of myosin from actin, calcium dissociation from troponin C, sequestration of calcium into the sarcoplasmic reticulum, and calcium extrusion from the cytoplasm. Such ischaemia would be expected to cause a shift in energy metabolism from free fatty acids to glucose, and we have observed an abnormal elevation in myocardial triglyceride content (i.e. myocardial steatosis) in women with CMD compared with a group of matched reference controls; the level of triglyceride was strongly related to the LV relaxation rate.5 Further investigation of myocardial steatosis in CMD and HFpEF is needed to understand relationships to inflammatory, metabolic, and fibrotic mechanistic pathways. This pathway is novel and attractive given existing and developing therapies for other organ steatosis, specifically for non-alcoholic fatty liver and steatohepatitis.
A critical question is: to what extent does CMD cause HFpEF and therefore, could CMD-related ischaemia be a therapeutic target for HFpEF? CMD itself is a heterogenous condition shaped by a variable combination of endothelial dysfunction, vascular smooth muscle cell hyperreactivity, sympathetic nervous system activation, vascular rarefaction and arterial remodelling, and aortic stiffness.4 In addition to its pivotal role in subendocardial ischaemia and subclinical myocardial infarctions, CMD appears to interplay with various factors including systemic inflammation,14 interstitial fibrosis,7 and myocardial steatosis5 (Take home figure). However, how do diastolic dysfunction and ventricular remodelling then contribute to CMD, and/or does CMD cause diastolic dysfunction and ventricular remodelling? Patients with HFpEF and no obstructive CAD have impaired myocardial flow reserve,15 so perhaps CMD and HFpEF are involved in a vicious cycle, initiating and exacerbating each other, as depicted in Take home figure. Intervention trials of ischaemic heart disease secondary prevention agents (statins, angiotensin-converting enzyme inhibitors) found to be effective in pharmacological probe trials in CMD3 should include HFpEF as an outcome to test the hypothesis that CMD is causal for HFpEF.
While the present data provide some of the strongest evidence to date favouring CMD as a causal mechanistic pathway for HFpEF, the opposite may, alternatively or also, be true. Analysis of the troponin level as a predictor of HFpEF in this population may have helped address this question. If elevated troponin levels predicted HFpEF hospitalization in the Taqueti cohort9 with low coronary flow reserve but not in preserved coronary microvascular function, this would suggest that chronic CMD-related ischaemia leads to HFpEF via myocardial injury. On the other hand, the absence of a relationship between the troponin level and future HFpEF hospitalization in patients with CMD would suggest that ventricular processes such as remodelling in response to pressure overload may be the primary process, perhaps indicative of myocardial tissue apoptosis and remodelling cell turnover. Specific study in CMD subjects at rest and during stress testing with serial troponins and cardiac magnetic resonance imaging- (CMRI) measured myocardial ischaemia, late gadolinium, and fibrosis tissue characterization is needed to understand the pattern, chronicity, and specific relevance of low troponin elevations in CMD. Indeed, understanding of the role of CMD-ischaemia-related micro-infarct/damage as an aetiological role in HFpEF is important because putative preventive therapies for this pathophysiology exist (aspirin, statin) and could be tested.
Prospective serial CMRI anatomical, perfusion, and T1 imaging with serial inflammatory and troponin biomarkers over longer periods of time is also needed to answer ‘the chicken or the egg’ question, e.g. to determine the causal sequence of ‘ventricular remodelling causing CMD’ vs. ‘CMD causing ventricular remodelling’, including investigation to understand if and how either or both of the hypothesized pathways lead to HFpEF. These outlined studies, along with the contributions from other advanced imaging modalities, such as PET, coronary Doppler flow, and invasive coronary flow determinations, are needed to understand these hypothesized causal mechanistic pathways. Supportive findings then can advise the design of clinical intervention trials definitively to test causality, and inform evidence-based therapeutic guidelines for CMD and HFpEF. These studies and trials are urgently needed to address the epidemics of CMD and HFpEF, as female-pattern cardiovascular disease that is also increasingly relevant to men and currently lacks therapeutic strategies.
Funding
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos N01-HV-68161, N01-HV-68162, N01-HV-68163, and N01-HV-68164, grants U01 64829, U01 HL649141, U01 HL649241, T32 HL69751, and 1R03 AG032631 from the National Institute on Aging, K12 HD051959 Building Interdisciplinary Research Careers in Women’s Health (Taqueti), GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, CA, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, American Heart Association grant 16SDG27260115, and the Harry S. Moss Heart Trust.
Conflict of interest: none declared.
References
- 1. Gulati M. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN.. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results From the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL.. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG.. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 5. Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LEJ, Berman DS, Li D, Bairey Merz CN, Szczepaniak LS. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol 2016;310:H14–H19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakir M, Nelson MD, Jones E, Li Q, Wei J, Sharif B, Minissian M, Shufelt C, Sopko G, Pepine CJ, Merz CNB.. Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the women’s ischemia syndrome evaluation study. Int J Cardiol 2016;223:936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paulus WJ, Tschope C.. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 8. Camici PG, Crea F.. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 9. Taqueti VR,, Solomon SD,, Shah AM,, Desai AS,, Groarke JD,, Osborne MT,, Hainer J,, Bibbo CF,, Dorbala S,, Blankstein R,, Di Carli MF.. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E.. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 11. Nelson MD, Sharif B, Shaw JL, Cook-Wiens G, Wei J, Shufelt C, Mehta PK, Thomson LE, Berman DS, Thompson RB, Handberg EM, Pepine CJ, Li D, Bairey Merz CN.. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clin Cardiol 2017;40:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, Mehta P, Zhang X, Thomson LE, Berman DS, Li D, Bairey Merz CN.. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging 2014;7:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei J, Mehta PK, Shufelt C, Yang Y, Gill E, Kahlon R, Cook-Wiens G, Minissian M, Kar S, Thomson L, Berman D, Merz CN.. Diastolic dysfunction measured by cardiac magnetic resonance imaging in women with signs and symptoms of ischemia but no obstructive coronary artery disease. Int J Cardiol 2016;220:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AlBadri A, Lai K, Wei J, Landes S, Mehta PK, Li Q, Johnson D, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Bairey Merz CN.. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One 2017;12:e0177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM.. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 2016;9:e002562. [DOI] [PubMed] [Google Scholar]