Abstract
Aims
Lamin A/C (LMNA) mutations cause familial dilated cardiomyopathy (DCM) with frequent conduction blocks and arrhythmias. We explored the prevalence, cardiac penetrance, and expressivity of LMNA mutations among familial DCM in Norway. Furthermore, we explored the risk factors and the outcomes in LMNA patients.
Methods and results
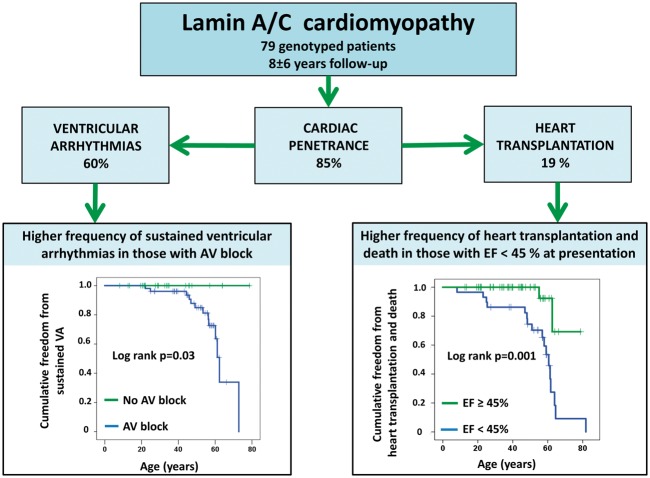
During 2003–15, genetic testing was performed in patients referred for familial DCM. LMNA genotype-positive subjects were examined by electrocardiography, Holter monitoring, cardiac magnetic resonance imaging, and echocardiography. A positive cardiac phenotype was defined as the presence of atrioventricular (AV) block, atrial fibrillation/flutter (AF), ventricular tachycardia (VT), and/or echocardiographic DCM. Heart transplantation was recorded and compared with non-ischaemic DCM of other origin. Of 561 unrelated familial DCM probands, 35 (6.2%) had an LMNA mutation. Family screening diagnosed an additional 93 LMNA genotype-positive family members. We clinically followed up 79 LMNA genotype-positive [age 42 ± 16 years, ejection fraction (EF) 45 ± 13%], including 44 (56%) with VT. Asymptomatic LMNA genotype-positive family members (age 31 ± 15 years) had a 9% annual incidence of a newly documented cardiac phenotype and 61% (19/31) of cardiac penetrance during 4.4 ± 2.9 years of follow-up. Ten (32%) had AV block, 7 (23%) AF, and 12 (39%) non-sustained VT. Heart transplantation was performed in 15 of 79 (19%) LMNA patients during 7.8 ± 6.3 years of follow-up.
Conclusion
LMNA mutation prevalence was 6.2% of familial DCM in Norway. Cardiac penetrance was high in young asymptomatic LMNA genotype-positive family members with frequent AV block and VT, highlighting the importance of early family screening and cardiological follow-up. Nearly 20% of the LMNA patients required heart transplantation.
Keywords: Lamin A/C, Familial dilated cardiomyopathy, Prevalence, Cardiac penetrance, Heart transplantation
Introduction
Lamin A/C (LMNA) mutations cause familial dilated cardiomyopathy (DCM) with autosomal dominant inheritance and variable expressivity of symptoms,1 such as early-onset atrioventricular (AV) block, supraventricular and ventricular arrhythmia (VA), and progressive DCM. Sudden cardiac death due to VA occurs frequently, often before the development of DCM.2–7 The natural history of LMNA cardiomyopathy has been described in a few epidemiological and clinical studies,5,7,8 but recommendations for the management of these patients are sparse. Family members of LMNA patients are offered genetic counselling and testing as part of family screening. This enables early identification of LMNA genotype-positive family members, enabling risk stratification and a determination of timing for therapy. Cardiac penetrance is high in LMNA cardiomyopathy, but the optimal age for starting genetic and clinical follow-up of family members, intervals of follow-up, and risk stratification for sudden cardiac death are still not well defined. Furthermore, no studies have reported the frequency of cardiac transplantation in LMNA patients.
In Norway, all genetic testing for cardiomyopathies have been performed at Oslo University Hospital since 2003, and the majority of LMNA genotype-positive patients have been clinically followed up at our centre. Our centre has also performed all heart transplantations in Norway.
We investigated the prevalence of LMNA mutations among patients with familial DCM in Norway from the start of genetic testing. We aimed to explore the cardiac penetrance and expressivity in LMNA genotype-positive patients and asymptomatic LMNA genotype-positive family members. Furthermore, we wanted to determine the frequency of heart transplantations in LMNA genotype-positive patients and compare it with the prevalence of LMNA mutations among non-ischaemic DCM patients. From this data, we aimed to create an overview of the onset of cardiac symptoms related to age, the time span of development of a cardiac phenotype, and the risk factors for unfavourable outcome in LMNA genotype-positive patients.
Methods
The study was a single-centre observational follow-up study including genotyped LMNA patients and genotype-positive family members followed up at the Unit for Genetic Cardiac Diseases, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Norway. All patients provided written informed consent. The study complied with the Declaration of Helsinki and was approved by the Regional Committees for Medical Research Ethics.
Familial dilated cardiomyopathy, genetic testing, and family screening
Since 2003, Department of Medical Genetics, Oslo University Hospital, has provided a national service for genetic testing of familial DCM in Norway, including LMNA, MYH7, MYBPC3, TNNT2, TNNI3, ACTC1, MYL2, and MYL3. We recorded the number of patients referred for genetic testing for phenotypic familial DCM and their subsequent genotypes. Familial DCM was defined and separated from idiopathic DCM according to the Mestroni criteria9 when other known aetiologies of DCM were excluded. Genetic counselling and cascade genetic screening were performed in the family members of LMNA genotype-positive probands.
Molecular testing
For molecular testing, individual exons with flanking intron sequences of the LMNA gene were amplified from DNA extracted from the whole blood collected in ethylenediaminetetraacetic acid. DNA sequencing was performed as described previously.2 A pathogenic mutation was considered based on a combination of co-segregation, absence of variant in the Exome Aggregation Consortium data set, and in silico analyses. Allele frequencies of mutations in the LMNA gene were reported among patients referred to genetic testing for familial DCM in Norway. Each mutation was cross checked for novelty in The Human Gene Mutation Database (http://www.hgmd.cf.ac.uk).
Clinical characteristics
Probands were defined as the first patients in a family referred for genetic testing due to a diagnosis of phenotypic familial DCM based on the Mestroni criteria for familial DCM.9
The age at the start of symptoms or documented first traits of the disease was recorded. Family members who underwent genetic testing as part of family screening and had no reported cardiac symptoms at the time of this genetic testing were defined as asymptomatic genotype-positive family members. Time from genotype diagnosis to phenotypic penetrance defined by the start of symptoms or documented cardiac traits was recorded.
Conduction disease and arrhythmias
Atrioventricular block block by PR interval was assessed from a resting 12-lead electrocardiography (ECG). Arrhythmias (atrial and ventricular) were collected from a resting 12-lead ECG, exercise ECG, Holter monitoring, and pacemaker and implantable cardioverter defibrillator (ICD) monitoring. Ventricular arrhythmias were classified as non-sustained ventricular tachycardia (VT), defined as ≥3 consecutive ventricular beats with a rate ≥120/min lasting <30 s, or sustained VA, defined as VT with a rate ≥120/min lasting >30 s, ventricular fibrillation (VF), appropriate antitachycardia pacing (ATP) therapy, appropriate defibrillator shock therapy, and aborted cardiac arrest. Implantable cardioverter defibrillator and cardiac resynchronization therapy (CRT) interrogations were retrospectively reviewed and eventual therapies (ATP or defibrillator shock) recorded.
A positive cardiac phenotype was defined as LMNA genotype-positive patients with AV block Grade I, II, or III; paroxysmal or permanent atrial fibrillation/flutter (AF), VA, and/or echocardiographic DCM.
Outcome by mortality and heart transplantation
All LMNA subjects followed up at our institution were cross-checked for outcome in June 2016. Outcome data included death, status with ventricular assist device, and heart transplantation during follow-up. Heart transplantation data were collected from our department’s heart transplant registry, including the aetiology of heart disease leading to transplantation. The proportion of heart transplantations in LMNA genotype-positive patients among non-ischaemic DCM was compared with the prevalence of the LMNA genotype among non-ischaemic DCM patients in general.
Echocardiography
Two-dimensional echocardiography was performed at the subject’s first visit using the Vivid 7 or Vivid E9 system (GE Healthcare, Horten, Norway) and analysed using commercially available software (EchoPAC®, GE). Left ventricular (LV) ejection fraction (EF) and LV volumes were calculated from apical views using Simpson’s biplane method.10 Left ventricular diameters were obtained from the parasternal long-axis view.10 Dilated cardiomyopathy was defined as LVEF < 45% or LV end-diastolic diameter ≥ 60 mm (men) or ≥54 mm (women).10 Echocardiographic recordings were analysed while blinded to clinical outcome data.
Cardiac magnetic resonance
Due to the high frequency of implantable devices at LMNA diagnosis, cardiac magnetic resonance imaging (CMR) was performed in only a subset of subjects (n = 28) using a 1.5 T Magnetom Sonata or Magnetom (Avanto; Siemens, Erlangen, Germany) as described previously.2
Statistical analysis
Continuous data were presented as mean ± standard deviation. Comparisons of means were analysed using the Student’s unpaired t-test. Proportions were compared using the χ2 test, Fisher’s exact test, and McNemar test for paired analyses (SPSS 21, SPSS, Inc., Chicago, IL, USA). We calculated the positive predictive values (PPVs) and negative predictive values (NPVs) for AV block, AF, and LVEF < 45% in relation to sustained VA. Cox regression analyses were performed to explore markers of age at severe outcome (i.e. heart transplantation and mortality). Survival analyses were performed for age at penetrant phenotype, the outcome of sustained VA, and severe outcome (i.e. heart transplantation and mortality) and compared using the log-rank test. Time from genotype diagnosis to onset of a cardiac phenotype was analysed separately in asymptomatic genotype-positive family members. Two-sided P-values < 0.05 were considered significant.
Results
Lamin A/C mutations among patients with familial dilated cardiomyopathy
From 2003 to December 2015, 561 unrelated DCM probands were referred for genetic testing for familial DCM and 35 (6.2%) had a pathogenic LMNA mutation, comprising 18 different LMNA mutations (Table 1). Family genetic screening diagnosed further 93 LMNA mutation-positive family members, giving a total population of 128 LMNA genotype-positive probands and family members identified in Norway during these years. No probands were homozygous or compound heterozygous for LMNA mutations, and no pathogenic mutations were found in the other genes tested.
Table 1.
Lamin A/C mutations diagnosed in Norway (2003–15) and the mutations in our clinical cohort
| cDNA | Amino acid | Number of unrelated probands/different families (n = 35) | Allel frequency among probands referred to genetic testing for familial DCM | Subjects followed clinically (n = 79), n (%) |
|---|---|---|---|---|
| c.43C>T | p.Q15X | 1 | 1/1122 | 1 (1.3) |
| c.322A>G | p.K108Ea | 1 | 1/1122 | |
| c.427T>C | p.S143P | 1 | 1/1122 | 1 (1.3) |
| c.642delG | p.E214DfsX266a | 2 | 1/561 | 13 (17) |
| c.730G>A | p.A244Ta | 1 | 1/1122 | 1 (1.3) |
| c.868G>A | p.E290K | 2 | 1/561 | 1 (1.3) |
| c.886_887insA | p.R296QfsX35a | 11 | 1/102 | 18 (23) |
| c.961C>T | p.R321X | 6 | 1/187 | 35 (44) |
| c.976T>A | p.S326T | 1 | 1/1122 | |
| c.986G>A | p.R329Ha | 1 | 1/1122 | |
| c.992G>A | p.R331Q | 1 | 1/1122 | 2 (3) |
| c.1016C>A | p.A339Ea | 1 | 1/1122 | |
| c.1063C>T | p.Q355X | 1 | 1/1122 | 4 (5) |
| c.1064_1066del | p.Gln355dela | 1 | 1/1122 | |
| c.1129C>T | p.R377C | 1 | 1/1122 | 2 (3) |
| c.1381-1G>A | «-»a,b | 1 | 1/1122 | 1 (1.3) |
| c.1412G>A | p.R471H | 1 | 1/1122 | |
| c.1622G>A | p.R541H | 1 | 1/1122 |
cDNA, complementary DNA; DCM, dilated cardiomyopathy.
Novel mutation.
Mutations in an acceptor site may cause more than one mutant transcript with different effects at the protein level.
Penetrance and cardiac phenotype in lamin A/C probands and genotype-positive family members
We clinically followed up 79 (61%) of the 128 diagnosed LMNA-genotype positive subjects in Norway at our centre, comprising 11 different mutations (Table 1). Age at first visit was 42 ± 16 years and 36 (46%) of the subjects were female (Table 2). Forty-eight (61%) of the subjects were probands and 31 (39%) were LMNA genotype-positive family members who were asymptomatic at genetic testing.
Table 2.
Clinical characteristics and cardiac phenotype in 79 lamin A/C genotype-positive subjects at our centre divided into asymptomatic family members referred after genotyping as part of family screening and symptomatic patients referred as probands
| All LMNA subjects (n = 79) | Asymptomatic family members (n = 31) | Symptomatic patients (n = 48) | P-value | |
|---|---|---|---|---|
| Age (years) | 42 ± 16 | 31 ± 15 | 49 ± 12 | <0.001 |
| Female/male | 36/43 (46/54) | 16/15 (50/50) | 20/28 (43/57) | 0.51 |
| Atrial fibrillation | 48 (61) | 7 (23) | 41 (85) | <0.001 |
| Atrioventricular block | 51 (65) | 10 (32) | 41 (85) | <0.001 |
| Ventricular arrhythmias | 47 (60) | 12 (39) | 35 (73) | 0.001 |
| Non-sustained VT only | 33 (42) | 12 (39) | 21 (44) | 0.14 |
| Sustained ventricular arrhythmias | 14 (18) | 0 (0) | 14 (30) | 0.001 |
| ICD/CRT-Da | 26/23 (33/29) | 7/0 (23/0) | 19/23 (40/48) | <0.001 |
| Echocardiographic and CMR parameters at first clinical visit | ||||
| Ejection fraction (%) | 45 ± 13 | 54 ± 7 | 39 ± 12 | <0.001 |
| End diastolic diameter (mm) | 55 ± 8 | 50 ± 5 | 59 ± 7 | <0.001 |
| LGE on CMR (n=28) | 13 (46) | 6 (30) | 7 (88) | 0.01 |
| Outcome | ||||
| Heart transplantation | 15 (19) | 0 (0) | 15 (32) | <0.001 |
| LVAD | 1 (1) | 0 (0) | 1 (2) | 1.0 |
Data are given as n (%) or mean ± SD.
CMR, cardiac magnetic resonance; CRT-D, cardiac resynchronization therapy with ICD; ICD, implantable cardiac defibrillator; LMNA, lamin A/C gene; LGE, late gadolinium enhancement; LVAD, left ventricular assist device; VT, ventricular tachycardia.
Received implantable device during follow-up.
As expected, the probands had a high frequency of AV block, AF, and VA, and the LVEF was reduced (39 ± 12%; Table 2). The probands were older than the asymptomatic family members (P < 0.001) at the time of their first visit to our centre. Cardiac magnetic resonance revealed late gadolinium enhancement as a sign of fibrosis in the basal and mid-interventricular septum in 13 of 28 subjects (46%), 6 of which were asymptomatic family members (Table 2).
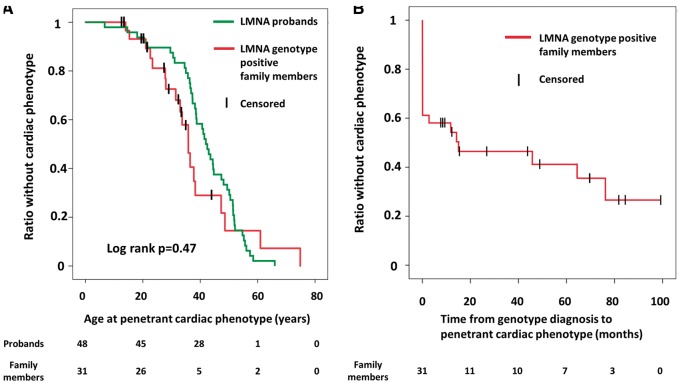
The age at which a cardiac phenotype was penetrant did not differ between probands and family members (log-rank P = 0.47), and cardiac penetrance approached 100% at 60 years of age in both groups (Figure 1A). A positive cardiac phenotype was present in 12 (39%) initially asymptomatic family members at first examination, and another 7 developed a positive cardiac phenotype during follow-up. Cardiac penetrance was 50% only 15 months after first visit and 61% (19/31) at 4.4 ± 2.9 years of follow-up at a mean age of 35 ± 15 years (Figure 1B). Cardiac penetrance occurred with 9% annual incidence in LMNA genotype-positive family members (Figure 1B). Among the 19 family members with a cardiac phenotype, 10 (32%) had AV block, 7 (23%) had AF, 12 (39%) had non-sustained VT documented during follow-up, and 1 had DCM (LVEF 41%; Table 2). Therefore, 18 of 19 (95%) family members with cardiac phenotypes had only conduction or arrhythmic disease without DCM.
Figure 1.
(A) Comparison of ages of documented penetrant cardiac phenotype between 48 lamin A/C genotype-positive probands and in 31 asymptomatic lamin A/C genotype-positive family members. Censored individuals still lacked the phenotype at last follow-up. (B) Kaplan–Meier plot showing time from genotype diagnosis to a documented penetrant cardiac phenotype in 31 asymptomatic lamin A/C genotype-positive family members. Censored individuals still lacked the phenotype at last follow-up.
Conduction disease
In the total population, 51 (72%) presented with AV block or developed AV block during follow-up. Thirteen patients presented with 3rd-degree AV block, and 16 with 1st- or 2nd-degree AV block progressed to 3rd-degree AV block during follow-up (P < 0.001). Therefore, 29 (37%) patients were pacemaker dependent due to 3rd-degree AV block at the end of follow-up.
Ventricular arrhythmia
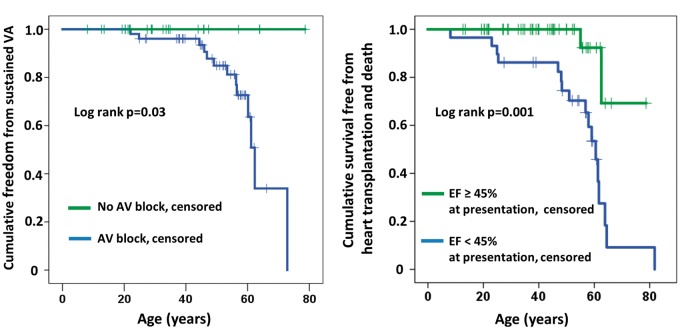
A total of 14 patients had documented sustained VA during follow-up. Patients with sustained VA more frequently had AV block (P = 0.003), AF (P = 0.007), and reduced LVEF at presentation (P < 0.001) than those without VA (Table 3). However, in survival analyses, only AV block (log-rank P = 0.03) and LVEF < 45% (log-rank P < 0.05) remained significant markers of sustained VA (Figure 2A). All patients with VA had AV block preceding the arrhythmia, giving an NPV for AV block and sustained VA of 100% and a PPV of 26%. The PPV and NPV for AF and sustained VA was 27% (13/48) and 97% (30/31), respectively, and 38% (11/29) and 94% (45/48), respectively, for LVEF < 45% and sustained VA (Table 3).
Table 3.
Clinical characteristics in 79 lamin A/C genotype-positive subjects in relation to sustained ventricular arrhythmia during follow-up and survival analyses of predictors of sustained ventricular arrhythmia
| No sustained ventricular arrhythmia (n = 65) | Sustained ventricular arrhythmia (n = 14) |
P-value |
||
|---|---|---|---|---|
| Student’s t-test | Log-rank P-value | |||
| Female/male | 30/35 (46/54) | 6/8 (43/57) | 0.82 | 0.61 |
| Missense/non-missense mutation | 6/59 (9/91) | 1/13 (7/93) | 0.99 | 0.54 |
| Atrial fibrillation | 35 (54) | 13 (93) | 0.007 | 0.73 |
| Atrioventricular block | 38 (59) | 13 (93) | 0.003 | 0.03 |
| Ejection fraction <45% | 18 (28) | 11 (79) | <0.001 | <0.05 |
| End-diastolic diameter ≥54 mm (women), ≥60 mm (men) | 19 (29) | 11 (79) | 0.001 | 0.06 |
Data are represented as n (%).
Figure 2.
(A) Kaplan–Meier plot showing better freedom from sustained ventricular arrhythmia (VA) in patients without atrioventricular (AV) block. (B) Kaplan–Meier plot showing better survival free from heart transplantation and death in patients with left ventricular ejection fraction (LVEF) ≥45% at presentation.
Forty-nine patients had or received an ICD during follow-up [23 ICD only and 26 CRT with ICD (CRT-D)]. Of these, seven were family members (age 44 ± 10 years, 3.5 ± 2.7 years after the start of follow-up). Over 2.9 ± 2.0 years after ICD implantation, 7 (14%) patients experienced successful appropriate defibrillator shocks and ATP for VT/VF, and another 7 (14%) patients had successful ATP therapy for sustained VT. Two (4%) patients had sustained slow VT with a heart rate under the therapy detection limit. No inappropriate shocks were reported.
Mortality and heart transplantation
Mortality over a mean 7.8 ± 6.3 years was 8% (6/79) at a mean age of 66 ± 8 years. Three patients died from end-stage heart failure, two patients died after heart transplantation due to intracerebral haemorrhage or infection (n = 1 each), and one patient died 4 days after a left ventricular assist device (LVAD) was implanted. Fifteen of our 79 LMNA patients (19%) needed heart transplantation, all of whom initially presented as probands. Therefore, the combined mortality and transplantation rate was 24% (19/79). Reduced LVEF at presentation was the only predictor of severe outcome (i.e. mortality and heart transplantation) in univariate Cox regression (log-rank P = 0.001, Table 4 and Figure 2B) and remained significant in multivariate Cox analyses in models with each of the other parameters.
Table 4.
Clinical characteristics in 79 lamin A/C genotype-positive subjects in relation to severe outcome consisting of death or heart transplantation during follow-up
| No severe outcome (n = 60) | Heart transplantation or death (n = 19) | P-value | Univariate Cox regression |
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | ||||
| Age at penetrant phenotype (phenotype pos only; n=67, years) | 40 ± 13 | 40 ± 15 | 0.93 | ||
| Age at start of follow-up (years) | 36 ± 15 | 40 ± 5 | 0.31 | ||
| Follow-up time (years) | 6.9 ± 6.3 | 10.5 ± 5.6 | 0.03 | ||
| Female/male | 28/32 (47/53) | 8/11 (42/58) | 0.73 | 0.62 (0.23–1.69) | 0.35 |
| Missense/non-missense | 4/56 (7/93) | 3/16 (16/84) | 0.35 | 1.17 (0.33–4.15) | 0.81 |
| Atrial fibrillation | 31 (52) | 17 (90) | <0.01 | 1.02 (0.21–5.08) | 0.98 |
| Atrioventricular block | 36 (60) | 15 (88) | 0.03 | 2.33 (0.51–10.60) | 0.27 |
| Ventricular arrhythmias | 31 (52) | 16 (84) | 0.01 | 1.96 (0.56–6.82) | 0.29 |
| ICD | 33 (55) | 16 (84) | 0.02 | 0.76 (0.20–2.86) | 0.69 |
| Ejection fraction (%) | 50 ± 9 | 31 ± 12 | <0.001 | 0.94 (0.90–0.97) | 0.001a |
| End-diastolic diameter (mm) | 54 ± 7 | 61 ± 7 | <0.001 | 1.07 (0.99–1.15) | 0.06 |
Data are given as mean ± SD or n (%). P-value from Student’s t-test (left column) and from Cox univariate regression analysis of predictors of severe outcome (right column).
CI, confidence interval; HR, hazard ratio; ICD, implantable cardiac defibrillator.
All parameters were adjusted for ejection fraction in multivariate Cox regression without reaching significance (P > 0.05). Ejection fraction remained a significant predictor in all multivariate models.
From the start of genetic testing for cardiomyopathies in 2003, 199 patients underwent heart transplantation due to non-ischaemic DCM, among others including familial, toxic, infectious, and idiopathic DCM, in Norway. LMNA cardiomyopathy patients constituted 6.5% (13/199) of the non-ischaemic DCM patients requiring transplantation from the start of genetic testing in 2003 (two LMNA cardiomyopathy patients underwent heart transplantation before the start of genetic testing in 2003 and were not included in this calculation). The age at the time of heart transplantation in patients with non-ischaemic DCM (n = 199) was 46 ± 17 years, compared with 47 ± 17 years (P = 0.71) in LMNA genotype-positive patients (n = 13).
Discussion
This study found a 6.2% prevalence of LMNA mutation in patients referred for genetic testing due to familial DCM in Norway from 2003 to 2015. Cardiac penetrance was high, and the cardiac phenotype was severe, with a high incidence of heart transplantation. Importantly, cardiac penetrance was higher than expected (61%) in asymptomatic LMNA genotype-positive family members at a mean age of 35 years. These young, reportedly asymptomatic family members demonstrated frequent AV block, AF, reduced ventricular function, and myocardial fibrosis, and a substantial proportion also had non-sustained VT. The annual incidence of the newly documented cardiac phenotype was 9% in the first years following genetic diagnosis, indicating rapid development of cardiac disease. Almost a quarter of LMNA patients died or needed heart transplantation during 8 years of follow-up. Our findings emphasize the importance of family genetic screening and close cardiac follow-up of young family members. LMNA probands and family members had similar and rapid disease progression requiring close follow-up to enable optimal timing for therapy, including medication, device therapy, and heart transplantation.
Prevalence of lamin A/C mutations among familial dilated cardiomyopathy in Norway
Approximately half of the cases of idiopathic DCM are familial with a genetic cause.11 The LMNA prevalence of 6.2% among patients with familial DCM in our study is in line with previous reports describing a 5–8% LMNA prevalence of familial DCM.12 Our findings confirm that, although clinically important, LMNA mutation is a rare underlying cause of DCM; therefore, considering the specific features of the LMNA-related disease, patient follow-up should be centralized to experienced centres.
Penetrance and cardiac phenotype in young asymptomatic family members
The cardiac penetrance in our total LMNA population was 85%, and almost 100% at age 60, which was also reported by others.13 By definition, the LMNA probands have 100% penetrance. The high cardiac penetrance among young asymptomatic family members has not been previously reported. At the first cardiac evaluation, cardiac penetrance among family members was almost 40% and progressed to 61% penetrance after 4.4 years of follow-up at a mean age of 35 years. Also the cardiac phenotype progressed during a relatively short follow-up period as indicated by the progression of AV conduction delay. Our data highlight the similar onset of disease, disease severity, and disease progression in family members and in probands.
Ventricular arrhythmia
As expected, patients with sustained VA had more symptoms and disease traits than patients without sustained VA. Atrioventricular block and reduced LVEF at presentation were risk factors from survival analyses, which is in line with previous studies.2,7 In contrast to previous studies,1,7 neither genotype nor gender was a marker of sustained VA, which may be explained by fewer events in our study.
Appropriate ICD therapy was delivered in more than a quarter of our ICD patients during a relatively short follow-up. Our study was not designed to evaluate the efficacy of ICD therapy and the additional effect on survival in LMNA cardiomyopathy patients, but our results support previous studies7,14 that ICD is an efficient therapy and should be considered at the presence of risk factors and at a lower threshold than DCM of other origin.4
Mortality and heart transplantation in lamin A/C-related disease
We reported an 8% mortality during follow-up. The diagnosed LMNA population in this study was closely followed up and received medical and device treatment. In contrast, undiagnosed LMNA patients are rarely under medical surveillance and unprotected from the risk of dying suddenly at an early age.5,6 Therefore, we think that the mortality rate in our study may be lower, whereas the age at mortality may be higher compared with the true LMNA population.
The number of heart transplant procedures in our population was high (19%). From 2003 to 2015, LMNA genotype-positive patients constituted at least 6.5% of the non-ischaemic DCM patients requiring heart transplantation in Norway. Given that familial DCM constitutes 25% of non-ischaemic DCM and 6% of these are due to LMNA mutations, the prevalence of LMNA genotype-positive patients in the non-ischaemic DCM population in general can be estimated to be 1.5%.12,15 Thus, LMNA genotype-positive patients were over-represented (6.5%) in the non-ischaemic DCM population undergoing heart transplantation compared with the LMNA genotype-positive proportion in the non-ischaemic DCM population in general (1.5%). This indicates a more severe natural history in LMNA disease than non-ischaemic DCM of other origin. The high frequency of heart transplantation in LMNA genotype-positive patients has not been reported previously and emphasizes the severe and progressive phenotype and poor prognosis in LMNA-related disease. The combined incidence of mortality or heart transplantation during almost 8 years of follow-up was 24%, underscoring the poor prognosis of LMNA-related disease.
Clinical implications
Our study highlights the importance of early family screening in young family members and close cardiological follow-up of LMNA genotype-positive subjects. The majority of family members presented with conduction disease or arrhythmia without DCM. Screening for LMNA mutations should also be considered in young patients presenting with new AV block or atypical atrial arrhythmias, even in the absence of LV dysfunction, particularly in cases with a family history.
The high annual incidence of new symptoms and traits and the rapid development of a cardiac phenotype in LMNA genotype-positive family members suggest a need for at least yearly follow-up to enable prophylactic measures. Furthermore, LMNA genotype-positive subjects should be educated to alert their centre of any new symptoms, and ICD implantation must be continuously evaluated according to the presence of risk factors.4 The timing of ICD implantation in LMNA patients is still challenging, though the risk markers for VA were reported previously.2,3,5,7 Our study supported most of these markers, and we found high NPVs for the absence of AV block, AF, and reduced LVEF, which may indicate that patients without these or other risk markers are not in the immediate need for ICD implantation.7,13 Reduced LVEF at presentation was an independent marker of unfavourable prognosis, indicating the need for echocardiographic examinations, which should be repeated during follow-up.
Limitations
This study was an observational follow-up study. The difference in the duration of follow-up between study subjects may be an important confounding variable. Furthermore, lifelong follow-up would be needed to assess the true number of LMNA genotype-positive patients requiring heart transplantation during their lifetime. Prospective cohort studies are needed to further explore and document disease progression and the natural course of LMNA disease. We reported the LMNA prevalence among familial DCM referred for genetic testing in Norway. The number of patients with primary DCM who were not referred for genetic testing is unknown. Genetic diseases are regionally clustered, and the prevalence of LMNA may differ in other regions.
Conclusions
LMNA mutation prevalence was 6.2% in familial DCM patients referred for genetic testing in Norway. Early onset of cardiac symptoms was observed in both LMNA genotype-positive probands and family members. Disease progression was rapid, with a 9% annual incidence of new symptoms in family members, and the phenotype was severe, with more than a quarter of patients with ICD receiving appropriate ICD therapy and a quarter of our population dying or needing heart transplantation during follow-up. Our findings suggest a need for family genetic screening at an early age and close follow-up of genotype-positive subjects to provide preventive treatment.
Take home figure.
Illustration showing cardiac penetrance and outcome in 79 genotype-postitive patients with lamin A/C cardiomyopathy. AV, atrioventricular; EF, ejection fraction; VA, ventricular arrhythmia.
Funding
This work was supported by the Research Council of Norway funding the Center for Cardiological Innovation (203489 to N.E.H., T.F.H., J.S., T.E., and K.H.H.) and the South-Eastern Norway Regional Health Authority (2011094 to J.S. and K.H.H.).
Conflict of interest: none declared.
References
- 1. van Rijsingen IA, Nannenberg EA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, van Tintelen JP, van den Berg MP, Grasso M, Serio A, Jenkins S, Rowland C, Richard P, Wilde AA, Perrot A, Pankuweit S, Zwinderman AH, Charron P, Christiaans I, Pinto YM.. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail 2013;15:376–384. [DOI] [PubMed] [Google Scholar]
- 2. Hasselberg NE, Edvardsen T, Petri H, Berge KE, Leren TP, Bundgaard H, Haugaa KH.. Risk prediction of ventricular arrhythmias and myocardial function in lamin A/C mutation positive subjects. Europace 2014;16:563–571. [DOI] [PubMed] [Google Scholar]
- 3. Haugaa KH, Hasselberg NE, Edvardsen T.. Mechanical dispersion by strain echocardiography: a predictor of ventricular arrhythmias in subjects with lamin A/C mutations. JACC Cardiovasc Imaging 2015;8:104–106. [DOI] [PubMed] [Google Scholar]
- 4. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 5. van Berlo JH, de Voogt WG, van der Kooi AJ, van Tintelen JP, Bonne G, Yaou RB, Duboc D, Rossenbacker T, Heidbüchel H, de Visser M, Crijns HJGM, Pinto YM.. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med (Berl) 2005;83:79–83. [DOI] [PubMed] [Google Scholar]
- 6. van Berlo JH, Duboc D, Pinto YM.. Often seen but rarely recognised: cardiac complications of lamin A/C mutations. Eur Heart J 2004;25:812–814. [DOI] [PubMed] [Google Scholar]
- 7. van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, van Tintelen JP, van den Berg MP, Pilotto A, Pasotti M, Jenkins S, Rowland C, Aslam U, Wilde AA, Perrot A, Pankuweit S, Zwinderman AH, Charron P, Pinto YM.. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European Cohort Study . J Am Coll Cardiol 2012;59:493–500. [DOI] [PubMed] [Google Scholar]
- 8. Kumar S, Baldinger SH, Gandjbakhch E, Maury P, Sellal JM, Androulakis AF, Waintraub X, Charron P, Rollin A, Richard P, Stevenson WG, Macintyre CJ, Ho CY, Thompson T, Vohra JK, Kalman JM, Zeppenfeld K, Sacher F, Tedrow UB, Lakdawala NK.. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol 2016;68:2299–2307. [DOI] [PubMed] [Google Scholar]
- 9. Mestroni L, Maisch B, McKenna WJ, Schwartz K, Charron P, Rocco C, Tesson F, Richter A, Wilke A, Komajda M.. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. Eur Heart J 1999;20:93–102. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU.. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 11. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP.. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 2011;13:1077–1109. [DOI] [PubMed] [Google Scholar]
- 12. Jacoby D, McKenna WJ.. Genetics of inherited cardiomyopathy. Eur Heart J 2012;33:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, Mannarino S, Gambarin F, Favalli V, Grasso M, Agozzino M, Campana C, Gavazzi A, Febo O, Marini M, Landolina M, Mortara A, Piccolo G, Vigano M, Tavazzi L, Arbustini E.. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol 2008;52:1250–1260. [DOI] [PubMed] [Google Scholar]
- 14. Meune C, Van Berlo JH, Anselme F, Bonne G, Pinto YM, Duboc D.. Primary prevention of sudden death in patients with lamin A/C gene mutations. N Engl J Med 2006;354:209–210. [DOI] [PubMed] [Google Scholar]
- 15. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A.. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2007;29:270–276. [DOI] [PubMed] [Google Scholar]