Abstract
Bisphenol A (BPA) is a base chemical used extensively in numerous consumer products, and human exposure to BPA is ubiquitous. Higher BPA exposure has been associated with an increased risk of atherosclerosis and cardiovascular disease (CVD) in multiple human population-based studies. However, the underlying mechanisms responsible for the associations remain elusive. We previously reported that BPA activates the xenobiotic receptor pregnane X receptor (PXR), which has proatherogenic effects in animal models. Because BPA is a potent agonist for human PXR but does not affect rodent PXR activity, a suitable PXR-humanized apolipoprotein E–deficient (huPXR•ApoE−/−) mouse model was developed to study BPA’s atherogenic effects. Chronic BPA exposure increased atherosclerosis in the huPXR•ApoE−/− mice. We report that BPA exposure can also activate human PXR signaling in the heart tubes of huPXR•ApoE−/− embryos, and perinatal BPA exposure exacerbated atherosclerosis in adult male huPXR•ApoE−/− offspring. However, atherosclerosis development in female offspring was not affected by perinatal BPA exposure. Perinatal BPA exposure did not affect plasma lipid levels but increased aortic and atherosclerotic lesional fatty acid transporter CD36 expression in male huPXR•ApoE−/− offspring. Mechanistically, PXR epigenetically regulated CD36 expression by increasing H3K4me3 levels and decreasing H3K27me3 levels in the CD36 promoter in response to perinatal BPA exposure. The findings from the present study contribute to our understanding of the association between BPA exposure and increased atherosclerosis or CVD risk in humans, and activation of human PXR should be considered for future BPA risk assessment.
Plastic base chemical BPA is an agonist for human xenobiotic receptor PXR but not for rodent PXR. Perinatal BPA exposure increases atherosclerosis in male offspring in a human PXR-dependent manner.
Despite major advances in diagnosis and treatment, atherosclerotic cardiovascular disease (CVD) is still the leading cause of mortality and morbidity worldwide (1). Atherosclerosis is a complex chronic disease involving the interaction of genetic and environmental factors over multiple years. In addition to the obvious contributions of diet and lifestyle, the chemical environment to which we are exposed has significantly changed in the past few decades and has recently been implicated in the etiology of CVD (2–6). Mounting evidence has suggested that many chemicals such as endocrine-disrupting chemicals (EDCs) can interfere with organisms’ complex endocrine signaling and result in adverse consequences. Thus, the influences of EDC exposure on human health have become the subject of intense interest (6–11). However, the mechanisms of how exposure to EDCs contributes to the development of various chronic diseases, including atherosclerosis, are still poorly understood.
One EDC in particular, bisphenol A (BPA), has attracted considerable attention and controversy. BPA is a base chemical used extensively in polycarbonate plastics in many consumer products. Numerous biomonitoring studies have indicated that human exposure to BPA is ubiquitous and >95% of the U.S. population has been exposed to BPA (12, 13). Although research on the adverse effects of BPA initially focused primarily on reproduction and development, recent findings have linked BPA exposure to CVD (2–5). Several large and well-conducted cross-sectional and longitudinal studies have found that greater BPA exposure is consistently associated with increased CVD risk in the general population (2–4). Lang et al. (2) first reported that higher urinary BPA levels were significantly associated with an increased incidence of CVD, including coronary heart disease, myocardial infarction, and angina, all of which can be caused by atherosclerosis. Melzer et al. (3) replicated the early association between urinary BPA concentrations and coronary heart disease using a separate database. The association between BPA exposure and incident coronary artery disease has also been confirmed in a longitudinal study (4). These associations are independent of traditional CVD risk factors, including body mass index, blood pressure, lipid concentrations, and levels of physical activity (2, 4). Several independent studies have also directly associated BPA exposure with coronary atherosclerosis (14), carotid atherosclerosis (5), and peripheral arterial disease (15), suggesting that BPA exposure might directly affect atherosclerosis development.
BPA is a weak agonist of the estrogen receptor (ER) (16, 17), and studies have found similar adverse effects of exposure to BPA or estrogen on cardiac functions in isolated rodent hearts (18, 19). Chronic or lifelong BPA exposure has also been demonstrated to affect cardiac functions and cardiac remodeling after myocardial infarction in mice (20–22). However, the estrogenic effects of BPA might not be sufficient to explain the link between BPA exposure and increased atherosclerosis in humans, because numerous animal and human studies have identified protective effects of estrogen against atherosclerosis (23–26).
In addition to ER, we previously reported that BPA can activate another nuclear receptor, the pregnane X receptor (PXR; also known as steroid and xenobiotic receptoror SXR) (27, 28). PXR functions as a xenobiotic sensor that regulates many genes involved in xenobiotic metabolism (29–31). Although the function of PXR in the regulation of xenobiotic metabolism has been extensively studied, we recently reported PXR’s proatherogenic effects in animal models and found that activation of PXR can increase atherosclerosis in atherosclerosis-prone apolipoprotein E–deficient (ApoE−/−) mice (32–34). Therefore, BPA-mediated PXR activation could potentially accelerate atherosclerosis development and increase CVD risk in humans.
Unlike many other nuclear receptors, PXR’s ligand-binding domain is remarkably divergent, and PXR exhibits substantial differences in its pharmacology across species (29, 31, 34). BPA is a potent agonist for human PXR (hPXR) but does not activate mouse PXR (mPXR) or rat PXR (27). Because BPA is an hPXR-selective ligand, one of the key challenges to studying the effects of BPA-mediated hPXR activation on atherosclerosis is the development of a mouse model that recapitulates the human response to PXR ligands. To address this issue, we developed a PXR-humanized ApoE−/− mouse model (huPXR•ApoE−/−) to investigate BPA’s atherogenic effects (35). Chronic exposure to BPA increased atherosclerosis in huPXR•ApoE−/− mice but not in their control littermates (35). BPA exposure did not affect plasma lipid levels but increased fatty acid transporter CD36 expression and foam cell formation in huPXR•ApoE−/− mice (35). After we first demonstrated that chronic BPA exposure can increase atherosclerosis development in the huPXR•ApoE−/− mouse model, other studies have also found that BPA can increase atherosclerosis in hyperlipidemic rabbit models (36, 37). Although the differences between human and rodent PXR pharmacology are clear, the activation profiles of the human and rabbit PXR are very similar (31, 38). Therefore, it is plausible that PXR signaling also contributes to BPA’s atherogenic effects in those rabbit models.
The progression of atherosclerosis is considered to begin during adolescence in humans; however, atherosclerotic lesions can be detected in young children and, even, in fetuses (39, 40). The prenatal period is known to be a susceptible period for adverse health effects of environmental exposure, and studies have demonstrated that environmental factors can contribute to the CVD risk of the exposed individuals’ offspring (41–43). BPA can cross the placenta (44), and the associations between prenatal exposure to BPA and adverse health effects in early childhood have been reported (45–47). Animal studies have also demonstrated that in utero or perinatal BPA exposure can increase metabolic disorders in the offspring (48–51). To the best of our knowledge, however, no studies have reported on the effect of perinatal BPA exposure on atherosclerosis development in the offspring. We report that perinatal BPA exposure exacerbated atherosclerosis in adult male huPXR•ApoE−/− offspring but had no effects on their control littermates. Perinatal BPA exposure did not alter plasma lipid levels but did increase aortic and atherosclerotic lesional CD36 expression, potentially through PXR-dependent epigenetic regulation.
Materials and Methods
Animals and treatment
huPXR•ApoE−/− mice were generated as previously described (35). In brief, PXR-humanized mice (mPXR knockout/hPXR transgenic) (52) were crossed with ApoE−/− mice to generate huPXR•ApoE−/− (hPXRtg-PXR−/−ApoE−/−) and PXR−/−ApoE−/− mice (35). All the mice used in the present study had the same background (PXR and ApoE null alleles), except for one allele of huPXR•ApoE−/− mice carrying the hPXR gene.
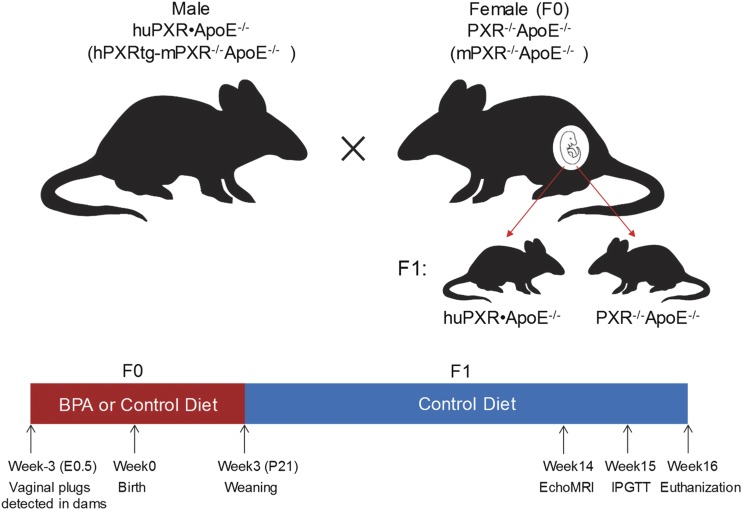
For the perinatal BPA exposure study, male huPXR•ApoE−/− mice were mated with female PXR−/−ApoE−/− mice (Fig. 1). The day of vaginal plug detection was designated as embryonic day (E)0.5. Pregnant PXR−/−ApoE−/− females were then housed separately and fed a modified semisynthetic diet containing 4.2% fat and 0.02% cholesterol (control diet) or the same diet supplemented with BPA (Sigma-Aldrich, St. Louis, MO) at a dose of 50 mg/kg (BPA diet) by Harland Laboratories, Inc. (Cumberland, IN) (35). Dams continued consuming the control or BPA diet until their pups were weaned on postnatal day 21 (P21). The offspring were then fed with the control diet for 13 weeks until euthanization at 16 weeks of age (Fig. 1). The mice were housed in a specific pathogen-free room with a 12-hour light/dark cycle in the University of Kentucky Division of Laboratory Animal Resources under a protocol approved by the institutional animal care and use committee.
Figure 1.
Scheme of animal models and experimental design. Female PXR−/−ApoE−/− mice (F0) were mated with male huPXR•ApoE−/− mice. The pregnant female PXR−/−ApoE−/− mice were fed a control diet or BPA diet until the pups were weaned on P21. The weaned offspring were fed a control diet until euthanization at 16 weeks of age. IPGTT, intraperitoneal glucose tolerance test.
Embryo culture
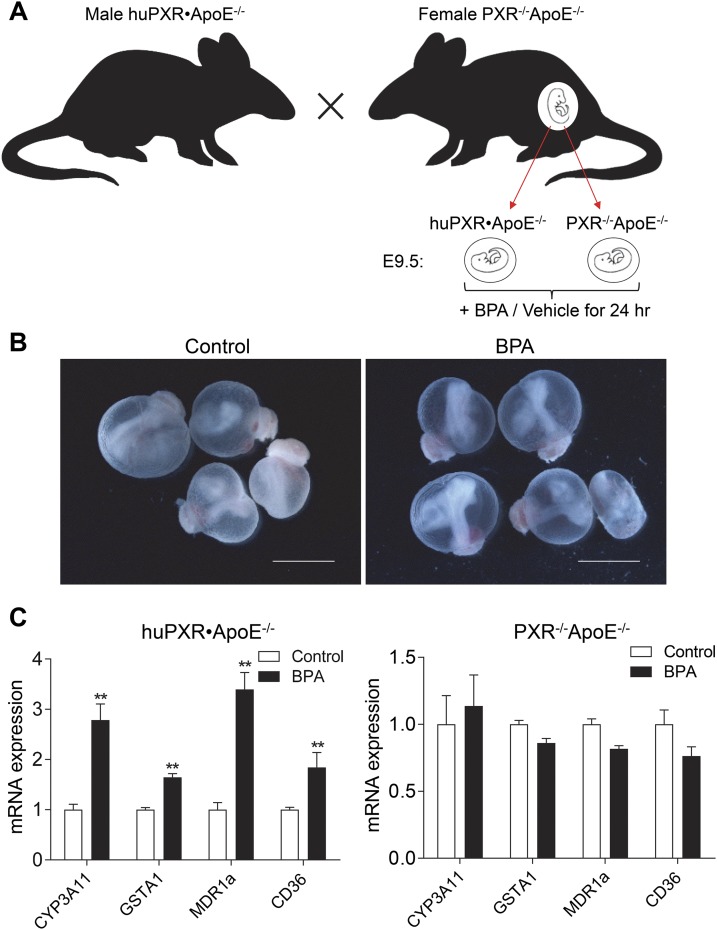
Whole-embryo culture was performed as previously described (53, 54). In brief, male huPXR•ApoE−/− mice were mated with female PXR−/−ApoE−/− mice. Mouse embryos at E9.5 were dissected out of the uteri in Tyrode salt (Sigma-Aldrich) (Fig. 2A). The parietal yolk sac was removed using forceps, and the visceral yolk sac was left intact. Embryos (two per bottle) were cultured in 25% Tyrode salt solution and 75% rat serum that was freshly prepared from male rats. The embryos were cultured at 37°C in 30 revolutions per1 minute of rotation in the roller bottles, which were gassed with 5% oxygen/5% carbon dioxide/90% nitrogen for the first 12 hours and 20% oxygen/5% carbon dioxide/75% nitrogen for the last 12 hours. Embryos were cultured for a total of 24 hours in the presence or absence of 20 μM of BPA. At the end of culture, the embryos were dissected from the yolk sac, and embryonic heart tubes were collected for analysis. The yolk sac was washed with phosphate-buffered saline (PBS) three times and used for genotyping. Images of the embryos were captured using a DFC420 5 MPix digital camera (Leica Microsystems, Buffalo Grove, IL).
Figure 2.
BPA ex vivo treatment activates PXR in the heart tubes of PXR-humanized embryos. (A) Scheme of animal models and embryo genotypes. Female PXR−/−ApoE−/− mice were mated with male huPXR•ApoE−/− mice. The embryos at E9.5 were isolated and cultured in control media or media supplemented with 20 μM BPA for 24 hours. (B) Representative images of cultured embryos. Scale bar = 2.5 mm. (C) The heart tubes of the embryos were dissected for RNA extraction, and messenger RNA (mRNA) levels of PXR target genes were analyzed using qPCR (n = 4 to 7; **P < 0.01).
Metabolic measurement
Body weight was measured weekly, and body composition was measured using nuclear magnetic resonance spectroscopy (EchoMRI) (55). An intraperitoneal glucose tolerance test was performed as previously described (56).
Plasma analysis and atherosclerosis quantification
Plasma total cholesterol and triglyceride concentrations were determined enzymatically using colorimetric methods (Wako, Mountain View, CA) as described previously (35). Lipoprotein fractions were also isolated by centrifuging at 70,000 rpm for 3 hours in a Beckman Optima TL-100 tabletop ultracentrifuge at its own density (1.006 g/mL). Next, the infranatant was adjusted to a density of 1.063 g/mL with solid potassium bromide to harvest the high-density lipoprotein (HDL) fraction by spinning at 70,000 rpm for 18 hours. The cholesterol content of HDL infranatant was measured enzymatically (Wako). To quantify the lesion areas at the aortic root, optimal cutting temperature compound–embedded hearts were sectioned at a 12-μm thickness, keeping all three valves of the aortic root in the same plane, and stained with Oil Red O, as described previously (32, 35).
Immunostaining
Immunohistochemical staining of atherosclerotic lesions was performed on 12-μm sections of aortic roots freshly embedded in optimal cutting temperature compound, as previously described (56). The sections were first fixed in 100% ice-cold acetone for 15 minutes and then washed with PBS for 20 minutes. The sections were permeabilized with PBS plus 0.1% Triton X-100 (PBST) for 10 minutes. Nonspecific binding was reduced by incubating slides in 10% rabbit sera diluted in PBST for 20 minutes at room temperature. The sections were then incubated with rat anti-mouse CD36 antibodies (1:100; AbD Serotec, Hercules, CA) at 4°C overnight. The detailed information of the antibodies used in the present study is listed in Table 1. The next day, the slides were rinsed with PBST and incubated with Texas Red-labeled goat anti-rat secondary antibodies (1:500; Vector Laboratories, Burlingame, CA). The nuclei were stained by mounting the slides with 4′,6-diamidino-2-phenylindole medium (Vector Laboratories). Images were acquired with a Nikon fluorescence microscope (Nikon, Melville, NY).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Histone 3 lysine 4 trimethyl | Proprietary | Anti-H3K4me3 | Abcam, ab8580 | Rabbit; polyclonal | 2.5 μg/5 μg DNA (ChIP) | AB_306649 |
| Histone 3 lysine 27 trimethyl | Proprietary | Anti-H3K27me3 | Abcam, ab6002 | Mouse; monoclonal | 2.5 μg/5 μg DNA (ChIP) | AB_305237 |
| CD36 | Proprietary | Anti-mouse CD36 | Bio-Rad/AbD Serotec, MCA2748 | Rat; monoclonal | 1:100 | AB_2072640 |
| Rat IgG | Whole IgG | Texas Red goat anti-rat IgG (H+L) | Vector Laboratories, TI-9400 | Goat antibody | 1:500 | AB_2336204 |
Abbreviations: AB, antibody; H, heavy (chain); IgG, immunoglobulin G; L, light (chain); RRID, Research Resource Identifier.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) analysis was performed via a SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling Technology, Danvers, MA), as previously described (57). In brief, the tissues were cut into small pieces and washed with cold PBS. The minced tissues were crosslinked with 1% (volume-to-volume ratio) formaldehyde for 10 minutes and then stopped by glycine for 5 minutes at room temperature. The samples containing chromatin were digested with micrococcal nuclease for 20 minutes at 37°C and stopped by EDTA. The nuclei pellet was collected by centrifugation at 16,000g for 1 minute at 4°C and sonicated to release the digested and cross-linked chromatin. For ChIP, the antibodies of H3K4me3 (Abcam, Cambridge, MA) or H3K27me3 (Abcam) were incubated with the cross-linked chromatin overnight at 4°C with shaking. The next day, the protein G magnetic beads were added into the immunoprecipitation mixture and incubated for 4 hours at 4°C. Chromatin binding to the beads was then washed and reverse cross-linked by adding NaCl and proteinase K for 2 hours of incubation at 65°C. The DNA was purified using a spin column purification kit (Cell Signaling Technology), followed by quantitative polymerase chain reaction (qPCR) analysis with the primers targeting the PXR response element (−438 to −424 nt) in the CD36 promoter (58). The forward primer (−487 to −465 nt; 5′-CTGAAAGTCTTCAGGTTCATGC-3′) and reverse primer (−370 to −349 nt; 5′-ATGCTGGAGTGTAGCAGCAAG-3′) were designed for ChIP-qPCR analysis. H3K4me3 or H3K27me3 enrichment in the CD36 promoter was calculated as percentage of chromatin input.
RNA isolation and qPCR analysis
Total RNA was isolated from mouse tissues or cells using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA), and real-time qPCR was performed using gene-specific primers and the SYBR green PCR kit (Bio-Rad, Hercules, CA), as described previously (59).
Statistical analysis
All data are presented as the mean ± standard error of the mean. Individual pairwise comparisons were analyzed using the two-sample, two-tailed Student t test, unless otherwise noted, with P < 0.05 regarded as significant.
Results
BPA ex vivo exposure activates hPXR signaling in the heart tubes of huPXR•ApoE−/− embryos
We previously reported that BPA can increase atherosclerosis in ApoE−/− mice in an hPXR-dependent manner (35). To investigate whether exposure to BPA can also affect PXR signaling during the embryonic period, we adapted a whole-embryo culture system (53, 54) and cultured the E9.5 embryos from the PXR−/−ApoE−/− female dams that were mated with male huPXR•ApoE−/− mice (Fig. 2A). Valvulogenesis during embryonic development is the formation of endocardial cushions in the atrioventricular canal and outflow tract of the primitive looped heart tubes, and cardiac valves form between E9.5 and E14.5 (maturation of leaflets) in mice (60, 61). All the embryos we collected carried the same mPXR and mApoE null alleles, and approximately one half of them carried the hPXR gene. The embryos were incubated with control media or media supplemented with BPA for 24 hours (Fig. 2B). As expected, BPA treatment can stimulate the expression of known PXR target genes, including CYP3A11, GSTA1, MDR1a, and CD36, in the heart tube of huPXR•ApoE−/− embryos but not in PXR−/−ApoE−/− embryos (Fig. 2C). These results suggest that BPA exposure can activate hPXR signaling in PXR-humanized embryos.
Perinatal BPA exposure does not affect the metabolic phenotypes of offspring
The main route of human exposure to BPA is oral and pharmacokinetic studies have demonstrated that exposure via diet is a more natural exposure route than other methods commonly used in exposed animals (62). To determine the effect of perinatal BPA exposure on atherosclerosis development of offspring, female PXR−/−ApoE−/− mice were mated with male huPXR•ApoE−/− mice, and the pregnant dams were then fed a control diet or a diet supplemented with BPA at a dose of 50 mg/kg until the pups were weaned on P21 (Fig. 1). Previous studies have demonstrated that exposure to 50 mg BPA/kg feed weight can induce epigenetic changes in laboratory rodents, and this dose is also well below the diet-administered maximum nontoxic dose for rodents (200 mg/kg body weight daily) (62–65). BPA pharmacokinetic studies have indicated that this dose will likely provide circulating serum concentrations similar to that observed in humans (62). We also reported that exposure of mice to 50 mg/kg BPA feed weight provided urinary BPA concentrations similar to that observed in humans (35). Furthermore, BPA exposure also stimulated expression of the prototypic PXR target genes in huPXR•ApoE−/− mice but not in PXR−/−ApoE−/− mice (35), indicating that exposure huPXR•ApoE−/− mice to 50 mg BPA/kg feed weight can efficiently activate hPXR in vivo.
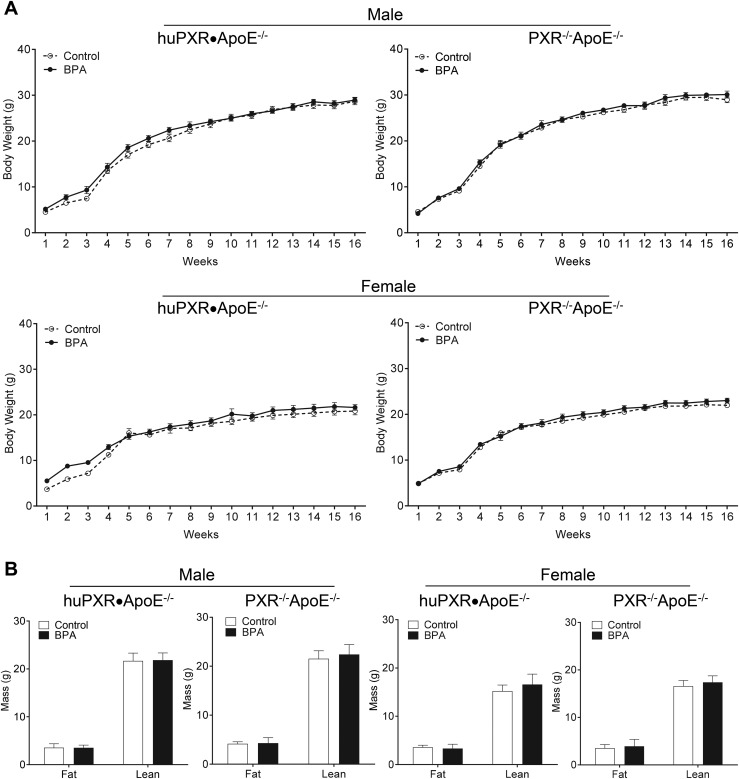
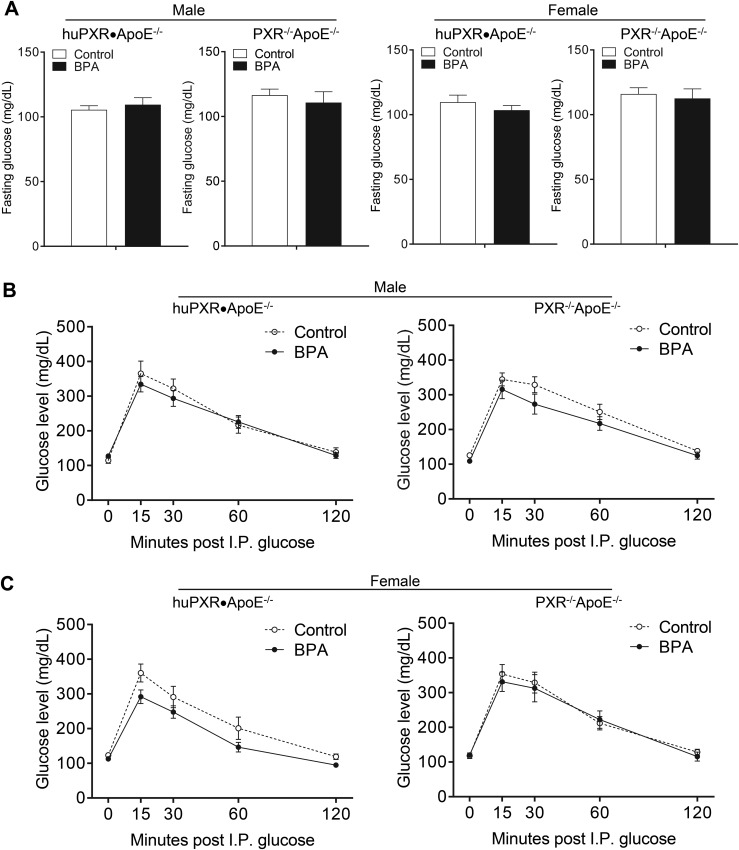
To determine whether perinatal BPA exposure affects weight gain and body composition of the offspring, the body weight was measured weekly and the body composition was measured by EchoMRI. Perinatal BPA exposure did not affect the growth curve (Fig. 3A) or the body composition, including fat and lean mass of both huPXR•ApoE−/− and PXR−/−ApoE−/− offspring (Fig. 3B). In addition, plasma glucose levels were not affected by perinatal BPA exposure in those mice (Fig. 4A). Glucose tolerance tests also demonstrated that perinatal BPA exposure did not alter the glucose tolerance in either genotype (Fig. 4B).
Figure 3.
Perinatal BPA exposure did not affect body weight and body composition of offspring. (A) Growth curves of huPXR•ApoE−/− and PXR−/−ApoE−/− mice from control or BPA-exposed dams (n = 12 to 16). (B) Fat mass and lean mass of 14-week-old huPXR•ApoE−/− and PXR−/−ApoE−/− mice from control or BPA-exposed dams (n = 9 to 14).
Figure 4.
Perinatal BPA exposure did not affect the plasma glucose levels or glucose tolerance of offspring. (A) The fasting glucose levels of huPXR•ApoE−/− and PXR−/−ApoE−/− mice from control or BPA-exposed dams (n = 8 to 14). (B) Intraperitoneal (I.P.) glucose tolerance test of huPXR•ApoE−/− and PXR−/−ApoE−/− mice from control or BPA-exposed dams (n = 6 to 14).
Perinatal exposure to BPA increases atherosclerosis in adult male huPXR•ApoE−/− mice without altering plasma lipid levels
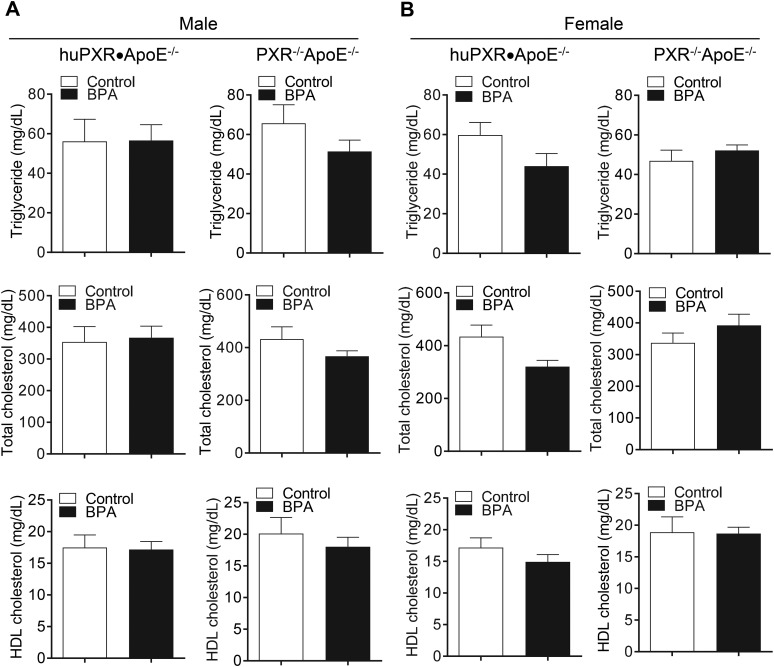
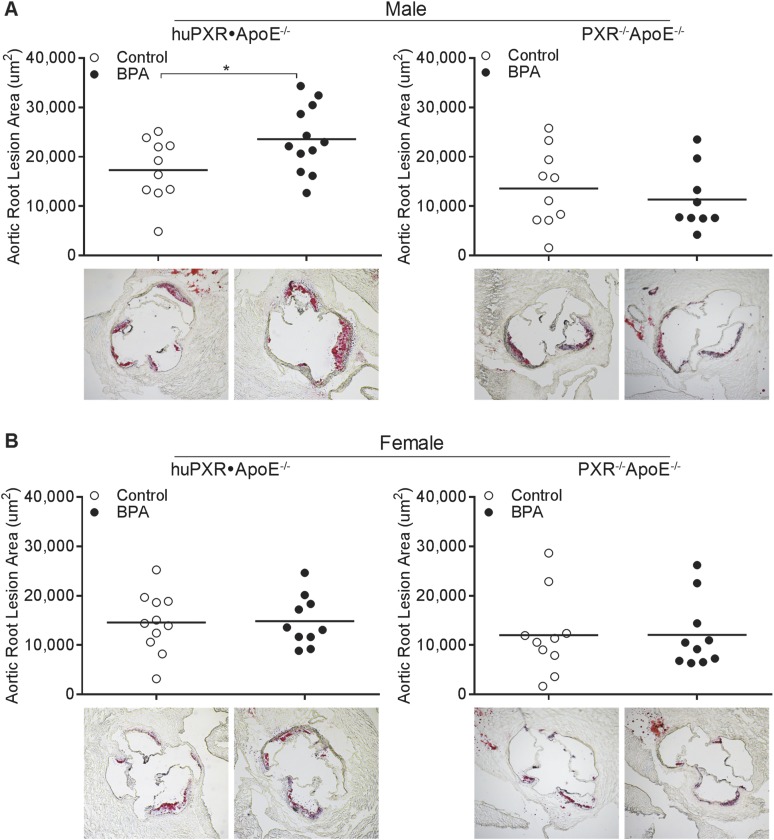
The effects of perinatal BPA exposure on plasma lipid and lipoprotein levels were next determined, and we found that perinatal BPA exposure did not alter the plasma triglyceride, total cholesterol, or HDL cholesterol levels in either huPXR•ApoE−/− or PXR−/−ApoE−/− offspring (Fig. 5). The atherosclerotic lesion areas were then determined in the aortic root (Fig. 6). Adult male huPXR•ApoE−/− offspring from BPA-exposed dams had lesion areas that were increased by 36% compared with huPXR•ApoE−/− mice from control dams (Fig. 6A). However, perinatal BPA exposure did not affect atherosclerosis in male PXR−/−ApoE−/− mice (Fig. 6A), suggesting that perinatal BPA exposure increased atherosclerosis in male mice in an hPXR-dependent manner. Furthermore, perinatal BPA exposure did not alter the atherosclerotic lesion areas in female huPXR•ApoE−/− and PXR−/−ApoE−/− mice (Fig. 6B). Collectively, these results have demonstrated the hPXR- and sex-dependent effect of perinatal BPA exposure on atherosclerosis development in the offspring.
Figure 5.
Perinatal BPA exposure had no effects on the plasma lipid levels of offspring. The plasma triglyceride, total cholesterol, and HDL cholesterol levels were measured in (A) male and (B) female huPXR•ApoE−/− and PXR−/−ApoE−/− mice from control or BPA-exposed dams (n = 10).
Figure 6.
Perinatal BPA exposure increased atherosclerosis in aortic roots of adult male huPXR•ApoE−/− mice. Quantitative analysis of atherosclerotic lesion sizes in the aortic root of (A) male and (B) female huPXR•ApoE−/− and PXR−/−ApoE−/− mice from control or BPA-exposed dams (n = 9 to 12; *P < 0.05). The representative Oil red O-stained sections are shown as indicated.
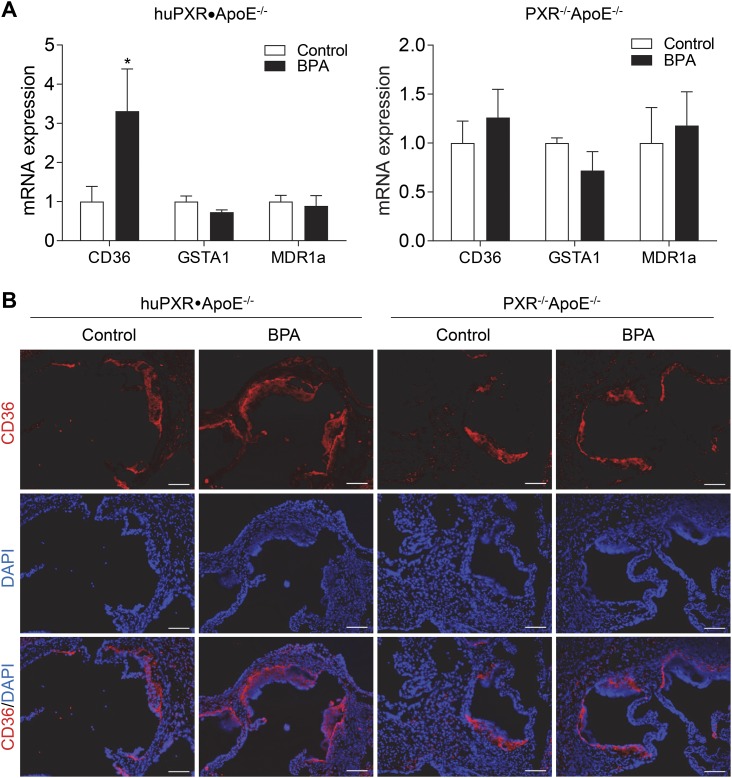
Perinatal BPA exposure increases aortic and atherosclerotic lesional CD36 expression in adult male huPXR•ApoE−/− mice
We previously reported that chronic BPA exposure activated hPXR to increase the expression of the fatty acid transporter CD36, leading to increased atherosclerosis in huPXR•ApoE−/− mice (35). CD36 is a scavenger receptor that plays an important role in mediating lipid uptake and foam cell formation (34, 66, 67). We next measured the messenger RNA levels of CD36 in the aorta of male offspring and found that perinatal BPA exposure increased CD36 expression in huPXR•ApoE−/− mice but not in PXR−/−ApoE−/− mice (Fig. 7A). Furthermore, immunofluorescence staining also showed increased CD36 protein levels in atherosclerotic lesions of huPXR•ApoE−/− mice from BPA-exposed dams compared with mice from control dams (Fig. 7B). Consistently, perinatal BPA exposure did not affect lesional CD36 expression in PXR−/−ApoE−/− mice.
Figure 7.
Perinatal BPA exposure increases aortic and atherosclerotic lesional CD36 expression in adult male huPXR•ApoE−/− mice. (A) Messenger RNA (mRNA) levels of PXR target genes were measured in aorta of male huPXR•ApoE−/− and PXR−/−ApoE−/− offspring by qPCR (n = 5 to 6; *P < 0.05). (B) Sections of atherosclerotic lesions in the aortic root of male huPXR•ApoE−/− and PXR−/−ApoE−/− offspring were stained with anti-CD36 primary antibodies, followed by fluorescein-labeled secondary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar = 100 μm.
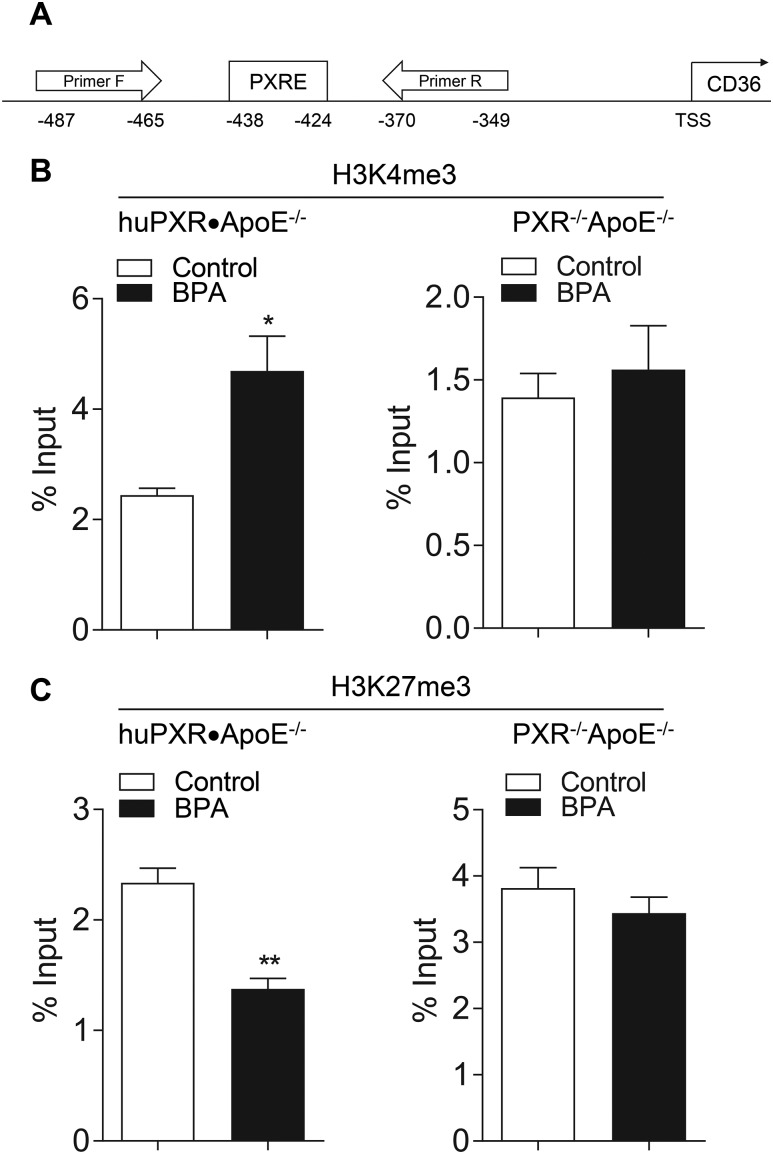
Perinatal BPA exposure leads to PXR-mediated epigenetic regulation of CD36 expression
Although CD36 is a prototypic PXR-activated gene (58), it is unlikely that the increased CD36 expression resulted from the BPA-mediated hPXR transactivation, because BPA has been withdrawn from the food after weaning, and those mice had not been subsequently exposed to BPA. Furthermore, the expression levels of the other PXR target genes, including GSTA1 and MDR1a, were similar in mice from control and BPA-exposed dams (Fig. 7A). In addition to direct transactivation of the target genes, PXR has been demonstrated to epigenetically modulate gene expression by altering histone methylation, and PXR ligands can increase the levels of H3K4me3, an epigenetic mark for gene activation, but decrease the levels of H3K27me3, an epigenetic mark for gene suppression, in the promoter of its target genes (6, 68, 69). To reveal the influence of perinatal BPA exposure on histone modifications of the CD36 promoter, the enriched levels of H3K4me3 and H3K27me3 around the PXR response element of CD36 promoter were analyzed by ChIP assays using the aortas from male huPXR•ApoE−/− and PXR−/−ApoE−/− mice (Fig. 8A). Perinatal BPA exposure significantly increased the levels of H3K4me3 in the CD36 promoter of huPXR•ApoE−/− mice (Fig. 8B). In contrast, the levels of H3K27me3 were found to be significantly decreased in CD36 promoter in huPXR•ApoE−/− mice from BPA-exposed dams (Fig. 8C). Deficiency of hPXR abolished the effect of perinatal BPA exposure on the changes in H3K4me3 and H3K27me3 levels in the CD36 promoter of PXR−/−ApoE−/− mice. Taken together, these results suggest an important role of PXR in the epigenetic regulation of genes that influence CVD risk.
Figure 8.
Perinatal BPA exposure alters histone modification in CD36 promoter. (A) Scheme of ChIP-qPCR primers that cover the PXR element (PXRE) in CD36 promoter. ChIP-qPCR analysis of (B) H3K4me3 and (C) H3K27me3 enrichment of CD36 promoter in aorta of male huPXR•ApoE−/− and PXR−/−ApoE−/− offspring (n = 3; *P < 0.05 and **P < 0.01).
Discussion
Although considerable progress has been achieved in identifying the risk factors that contribute to atherosclerotic CVD, the role played by “gene–environment interactions” in predisposing individuals to atherosclerosis remains relatively unexplored. To sense and respond to environmental chemicals, mammals have evolved a defensive network governed by xenobiotic receptors such as PXR (31, 34). The role of PXR as a xenobiotic sensor has been well-studied, and PXR has been considered a master regulator of xenobiotic metabolism (31, 34). The identification of PXR as a xenobiotic sensor has also provided an important tool for the study of new mechanisms through which chemical exposure affects diseases (34). Numerous studies have reported that exposure to the ubiquitous EDC BPA could cause adverse health effects in humans (10–12, 70–72), and recent studies have also associated BPA exposure with the increased CVD risk (2–5, 14). However, the underlying mechanisms for these associations are still poorly understood and contribute to the hampered risk assessment of BPA. We previously reported that BPA is an hPXR-selective ligand, and chronic BPA exposure can increase atherosclerosis development in huPXR•ApoE−/− mice (27, 35). In the present study, we found that perinatal BPA exposure also exacerbated atherosclerosis in adult male huPXR•ApoE−/− offspring. Perinatal BPA exposure led to increased expression of CD36 in these mice, likely through PXR-dependent epigenetic regulation. CD36 plays a key role in foam cell formation and atherosclerosis development, which could contribute to the increased atherosclerosis in huPXR•ApoE−/− mice from BPA-exposed dams. To the best of our knowledge, ours is the first study to demonstrate the effect of perinatal BPA exposure on atherosclerosis development in adult offspring.
In addition to activating ER and hPXR (27, 73), BPA has been shown to activate another nuclear receptor, constitutive androstane receptor (CAR) (74). However, it is unlikely that BPA-mediated CAR activation contributed to the increased atherosclerosis in our model, because studies have demonstrated that activation of CAR can decrease the plasma lipid levels and atherosclerotic lesions in animal models (75). Furthermore, BPA exposure should have similar effects on CAR activity in both huPXR•ApoE−/− and PXR−/−ApoE−/− mice, which have the same background except for one allele of huPXR•ApoE−/− mice carrying the hPXR gene. In addition to nuclear receptor signaling, BPA has been shown to cause epigenetic changes that might increase the incidence of metabolic disorders in both humans and animal models (10, 48, 63, 73). Higher urinary concentrations of BPA in prepubescent girls have been associated with decreased global methylation, which has been corresponded to changes in expression of genes involved in inflammation and metabolism (76). In utero BPA exposure altered methylation within the carnitine palmitoyltransferase 1a gene, leading to hepatic deposition of triglycerides in rats fed a high-fat diet (77). Several well-controlled animal studies were conducted to investigate the effect of maternal exposure to BPA during pregnancy on body weight, glucose homeostasis, and gene expression in adult offspring (49–51). The investigators found that in utero exposure to BPA led to hyperglycemia, glucose intolerance, increased plasma nonesterified fatty acids and hepatic triglyceride levels in adult male offspring (50). In utero BPA exposure influenced the expression of genes involved in β-cell development, which might contribute to the impaired glucose tolerance observed in adult offspring (51). They also found that in utero BPA exposure affected the expression of several key genes involved in lipid and fatty acid metabolism, including SREBP-1c and PPARα in the offspring (50). Although the epigenetic status such as DNA methylation of those genes was not investigated, it is plausible that the effect of in utero BPA exposure on the expression of those genes was through epigenetic regulation.
In the present study, we found that perinatal BPA exposure increased the expression of the fatty acid transporter CD36 in adult male offspring in an hPXR-dependent manner. CD36 is a direct PXR target gene (58) that plays an important role in atherosclerosis development (32, 34, 66, 67). We previously demonstrated that activation of PXR increases CD36 levels and foam cell formation in ApoE−/− mice (32). Chronic BPA exposure-mediated hPXR activation can also increase CD36 expression and exacerbate atherosclerosis in huPXR•ApoE−/− mice (35). In the present study, however, the adult offspring had not been exposed to BPA after P21; thus, the increased CD36 expression could not be explained by direct PXR transactivation. If the increased CD36 expression was due to the BPA-mediated direct PXR transactivation, the expression levels of other PXR target genes should also have been elevated. However, the expression of GSTA1 and MDR1a was not affected by perinatal BPA exposure. In addition to binding to its responsive motifs and transcriptionally regulating target gene expression, recent studies have indicated a potential role of PXR in epigenetic regulation of downstream targets. For example, PXR can directly interact with arginine methyltransferase 1, the primary methyltransferase that deposits the asymmetric dimethylarginine mark (69). ChIP analysis revealed a ligand-dependent recruitment of arginine methyltransferase 1 to PXR regulatory regions within the CYP3A4 promoter (69). A more recent study demonstrated that hPXR ligand rifampicin treatment can increase levels of H3K4me3 and decrease levels of H3K27me3 in the CYP3A4 promoter in human intestinal cells, and knockdown of PXR abolished those epigenetic changes in the promoter (68). The levels of H3K4me3 and H3K27me3 in the CD36 promoter were respectively increased and decreased by perinatal BPA exposure in huPXR•ApoE−/− mice. These changes were apparently mediated by hPXR, because deficiency of hPXR abolished these effects elicited by perinatal BPA exposure. Our findings have not only confirmed the role of PXR in the epigenetic regulation of gene expression but have also demonstrated that EDC-mediated modulation of PXR activity during early life can lead to adverse health effects in late life.
In addition to the hPXR-dependent impact on atherosclerosis, perinatal BPA exposure also had sex-dependent atherogenic effects in the offspring. Perinatal BPA exposure only increased atherosclerosis in male PXR-humanized mice but not in female mice. Human and animal studies have also demonstrated that BPA exposure has sex-dependent effects on metabolic diseases and cardiac functions. For example, higher urinary BPA exposure might be associated with an elevated lean body mass in boys but not in girls (78). In contrast, higher BPA exposure might be associated with an elevated fat mass in girls but not in boys (78). Bhandari et al. (79) also found a significantly positive association between urinary BPA levels and obesity in boys but not in girls. Another study of Chinese school-age children found that girls with higher urinary BPA levels had twice the risk of becoming overweight than did those with lower BPA levels; however, a similar association was not observed in boys (80). Animal studies also showed the sex-dependent effect of BPA exposure on metabolic diseases and cardiac functions in rodents (18–20, 49). Although BPA is a weak activator of nuclear ER, studies have demonstrated that BPA can activate membrane ER signaling at relatively low concentrations (73, 81, 82). However, the effect of BPA-mediated membrane ER activation on atherosclerosis development have not been investigated. It is plausible that membrane ER signaling-dependent mechanisms might contribute to the sex-dependent effects between males and females. Future studies are required to investigate the potential contribution of membrane ER signaling to BPA’s atherogenic effects in both animals and humans. In addition, many animal BPA exposure studies only use either male or female animals, and our findings suggest that it would be important to include both sexes for future studies to investigate the adverse health effects of BPA and the underlying mechanisms.
In conclusion, we found that perinatal BPA exposure increased atherosclerosis in adult male ApoE−/− mice in an hPXR-dependent manner. Perinatal BPA exposure led to hPXR-mediated epigenetic regulation of aortic CD36 expression, which might contribute to the increased atherosclerosis in those mice. These findings demonstrate that perinatal BPA exposure can affect atherosclerosis development in a suitable laboratory animal model. The findings from our study will contribute to our understanding of the association between BPA exposure and increased atherosclerosis and CVD risk in humans. Because PXR can be activated by numerous EDCs in addition to BPA, activation of hPXR should be considered for future EDC risk assessment.
Acknowledgments
We thank Dr. Frank Gonzalez at the National Cancer Institute for the huPXR mice and Jennifer Rios-Pilier and Murong Ma for technical support.
Financial Support: This work was supported by Grants R01ES023470, R21ES022745, R01HL123358, and R01HL131925 from the National Institutes of Health (to C.Z.). Y.S. was supported by a Postdoctoral Fellowship 14POST18740064 from the American Heart Association. The authors also acknowledge the core services (supported by National Institutes of Health Grant P20GM103527).
Author Contributions: C.Z. conceptualized and designed the research. Y.S., S.-H.P., and F.W. performed the experiments and analyzed the data. C.Z., Y.S., and F.W. wrote the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ApoE−/−
apolipoprotein E–deficient
- BPA
bisphenol A
- CAR
constitutive androstane receptor
- ChIP
chromatin immunoprecipitation
- CVD
cardiovascular disease
- EDC
endocrine-disrupting chemical
- ER
estrogen receptor
- E
embryonic day
- HDL
high-density lipoprotein
- hPXR
human pregnane X receptor
- huPXR•ApoE−/−
pregnane X receptor–humanized apolipoprotein E–deficient
- P21
postnatal day 21
- mPXR
mouse pregnane X receptor
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline plus 0.1% Triton X-100
- PXR
pregnane X receptor
- qPCR
quantitative polymerase chain reaction
References
- 1. Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. [DOI] [PubMed] [Google Scholar]
- 3. Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1):e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, Galloway TS. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125(12):1482–1490. [DOI] [PubMed] [Google Scholar]
- 5. Lind PM, Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218(1):207–213. [DOI] [PubMed] [Google Scholar]
- 6. Helsley RN, Zhou C. Epigenetic impact of endocrine disrupting chemicals on lipid homeostasis and atherosclerosis: a pregnane X receptor-centric view. Environ Epigenet. 2017;3(4):dvx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996;104(Suppl 4):715–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Colborn T, Dumanoski D, Meyers JP. Our Stolen Future. New York: Dutton; 1996. [Google Scholar]
- 9. Gross L. The toxic origins of disease. PLoS Biol. 2007;5(7):e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melzer D, Gates P, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Schofield P, Mosedale D, Grainger D, Galloway TS. Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PLoS One. 2012;7(8):e43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120(9):1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24(2):178–198. [DOI] [PubMed] [Google Scholar]
- 17. Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belcher SM, Chen Y, Yan S, Wang HS. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17β-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153(2):712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. 2011;6(9):e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel BB, Raad M, Sebag IA, Chalifour LE. Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci. 2013;133(1):174–185. [DOI] [PubMed] [Google Scholar]
- 21. Patel BB, Kasneci A, Bolt AM, Di Lalla V, Di Iorio MR, Raad M, Mann KK, Chalifour LE. Chronic exposure to bisphenol A reduces successful cardiac remodeling after an experimental myocardial infarction in male C57bl/6n mice. Toxicol Sci. 2015;146(1):101–115. [DOI] [PubMed] [Google Scholar]
- 22. Kasneci A, Lee JS, Yun TJ, Shang J, Lampen S, Gomolin T, Cheong CC, Chalifour LE. From the Cover: lifelong exposure of C57bl/6n male mice to bisphenol A or bisphenol S reduces recovery from a myocardial infarction. Toxicol Sci. 2017;159(1):189–202. [DOI] [PubMed] [Google Scholar]
- 23. Nathan L, Chaudhuri G. Estrogens and atherosclerosis. Annu Rev Pharmacol Toxicol. 1997;37(1):477–515. [DOI] [PubMed] [Google Scholar]
- 24. Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in ApoE−/− mice. J Clin Invest. 2001;107(3):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Billon-Galés A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci USA. 2011;108(32):13311–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arnal JF, Fontaine C, Billon-Galés A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30(8):1506–1512. [DOI] [PubMed] [Google Scholar]
- 27. Sui Y, Ai N, Park SH, Rios-Pilier J, Perkins JT, Welsh WJ, Zhou C. Bisphenol A and its analogues activate human pregnane X receptor. Environ Health Perspect. 2012;120(3):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabb MM, Kholodovych V, Grün F, Zhou C, Welsh WJ, Blumberg B. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR). Environ Health Perspect. 2004;112(2):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blumberg B, Sabbagh W Jr, Juguilon H, Bolado J Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12(20):3195–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23(5):687–702. [DOI] [PubMed] [Google Scholar]
- 31. Zhou C, Verma S, Blumberg B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal. 2009;7:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou C, King N, Chen KY, Breslow JL. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J Lipid Res. 2009;50(10):2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sui Y, Xu J, Rios-Pilier J, Zhou C. Deficiency of PXR decreases atherosclerosis in apoE-deficient mice. J Lipid Res. 2011;52(9):1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou C. Novel functions of PXR in cardiometabolic disease. Biochim Biophys Acta. 2016;1859(9):1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sui Y, Park SH, Helsley RN, Sunkara M, Gonzalez FJ, Morris AJ, Zhou C. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J Am Heart Assoc. 2014;3(2):e000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fang C, Ning B, Waqar AB, Niimi M, Li S, Satoh K, Shiomi M, Ye T, Dong S, Fan J. Bisphenol A exposure enhances atherosclerosis in WHHL rabbits. PLoS One. 2014;9(10):e110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fang C, Ning B, Waqar AB, Niimi M, Li S, Satoh K, Shiomi M, Ye T, Dong S, Fan J. Bisphenol A exposure induces metabolic disorders and enhances atherosclerosis in hyperlipidemic rabbits. J Appl Toxicol. 2015;35(9):1058–1070. [DOI] [PubMed] [Google Scholar]
- 38. Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, Kliewer SA, Moore JT. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14(1):27–39. [DOI] [PubMed] [Google Scholar]
- 39. Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354(9186):1234–1241. [DOI] [PubMed] [Google Scholar]
- 40. Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia: intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100(11):2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Power C, Atherton K, Thomas C. Maternal smoking in pregnancy, adult adiposity and other risk factors for cardiovascular disease. Atherosclerosis. 2010;211(2):643–648. [DOI] [PubMed] [Google Scholar]
- 42. Jaddoe VW, de Ridder MA, van den Elzen AP, Hofman A, Uiterwaal CS, Witteman JC. Maternal smoking in pregnancy is associated with cholesterol development in the offspring: a 27-years follow-up study. Atherosclerosis. 2008;196(1):42–48. [DOI] [PubMed] [Google Scholar]
- 43. Mone SM, Gillman MW, Miller TL, Herman EH, Lipshultz SE. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004;113(4Suppl):1058–1069. [PubMed] [Google Scholar]
- 44. Mørck TJ, Sorda G, Bechi N, Rasmussen BS, Nielsen JB, Ietta F, Rytting E, Mathiesen L, Paulesu L, Knudsen LE. Placental transport and in vitro effects of bisphenol A. Reprod Toxicol. 2010;30(1):131–137. [DOI] [PubMed] [Google Scholar]
- 45. Harley KG, Aguilar Schall R, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121(4):514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, Morisset AS, Taback S, Bouchard MF, Monnier P, Dallaire R, Fraser WD. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health. 2014;13(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spanier AJ, Kahn RS, Kunselman AR, Schaefer EW, Hornung R, Xu Y, Calafat AM, Lanphear BP. Bisphenol A exposure and the development of wheeze and lung function in children through age 5 years. JAMA Pediatr. 2014;168(12):1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alonso-Magdalena P, Rivera FJ, Guerrero-Bosagna C. Bisphenol-A and metabolic diseases: epigenetic, developmental and transgenerational basis. Environ Epigenet. 2016;2(3):dvw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. García-Arevalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, Nadal A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One. 2014;9(6):e100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. García-Arévalo M, Alonso-Magdalena P, Servitja JM, Boronat-Belda T, Merino B, Villar-Pazos S, Medina-Gómez G, Novials A, Quesada I, Nadal A. Maternal exposure to bisphenol-A during pregnancy increases pancreatic β-cell growth during early life in male mice offspring. Endocrinology. 2016;157(11):4158–4171. [DOI] [PubMed] [Google Scholar]
- 52. Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. The PREgnane X receptor gene-humanized mouse: a model for investigating drug–drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos. 2007;35(2):194–200. [DOI] [PubMed] [Google Scholar]
- 53. Piliszek A, Kwon GS, Hadjantonakis AK. Ex utero culture and live imaging of mouse embryos. Methods Mol Biol. 2011;770:243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu Y, Wang F, Reece EA, Yang P. Curcumin ameliorates high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Am J Obstet Gynecol. 2015;212(6):802.e1–802.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Helsley RN, Sui Y, Park SH, Liu Z, Lee RG, Zhu B, Kern PA, Zhou C. Targeting IκB kinase β in adipocyte lineage cells for treatment of obesity and metabolic dysfunctions. Stem Cells. 2016;34(7):1883–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sui Y, Park SH, Xu J, Monette S, Helsley RN, Han SS, Zhou C. IKKβ links vascular inflammation to obesity and atherosclerosis. J Exp Med. 2014;211(5):869–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sui Y, Helsley RN, Park SH, Song X, Liu Z, Zhou C. Intestinal pregnane X receptor links xenobiotic exposure and hypercholesterolemia. Mol Endocrinol. 2015;29(5):765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281(21):15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sui Y, Liu Z, Park S-H, Thatcher SE, Zhu B, Fernandez JP, Molina H, Kern PA, Zhou C. IKKβ is a β-catenin kinase that regulates mesenchymal stem cell differentiation. JCI Insight. 2018;3(2):e96660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pucéat M. Embryological origin of the endocardium and derived valve progenitor cells: from developmental biology to stem cell-based valve repair. Biochim Biophys Acta. 2013;1833(4):917–922. [DOI] [PubMed] [Google Scholar]
- 61. Savolainen SM, Foley JF, Elmore SA. Histology atlas of the developing mouse heart with emphasis on E11.5 to E18.5. Toxicol Pathol. 2009;37(4):395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sieli PT, Jašarevic E, Warzak DA, Mao J, Ellersieck MR, Liao C, Kannan K, Collet SH, Toutain PL, Vom Saal FS, Rosenfeld CS. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect. 2011;119(9):1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104(32):13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jašarević E, Sieli PT, Twellman EE, Welsh TH Jr, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci USA. 2011;108(28):11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rosenfeld CS, Sieli PT, Warzak DA, Ellersieck MR, Pennington KA, Roberts RM. Maternal exposure to bisphenol A and genistein has minimal effect on A(vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proc Natl Acad Sci USA. 2013;110(2):537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RLA. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4(3):211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kennedy DJ, Kuchibhotla SD, Guy E, Park YM, Nimako G, Vanegas D, Morton RE, Febbraio M. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arterioscler Thromb Vasc Biol. 2009;29(10):1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yan L, Wang Y, Liu J, Nie Y, Zhong XB, Kan Q, Zhang L. Alterations of histone modifications contribute to pregnane X receptor-mediated induction of CYP3A4 by rifampicin. Mol Pharmacol. 2017;92(2):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xie Y, Ke S, Ouyang N, He J, Xie W, Bedford MT, Tian Y. Epigenetic regulation of transcriptional activity of pregnane X receptor by protein arginine methyltransferase 1. J Biol Chem. 2009;284(14):9199–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ Jr, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30(1):75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environ Health Perspect. 2010;118(8):1051–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127(1-2):27–34. [DOI] [PubMed] [Google Scholar]
- 74. DeKeyser JG, Laurenzana EM, Peterson EC, Chen T, Omiecinski CJ. Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol Sci. 2011;120(2):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sberna AL, Assem M, Xiao R, Ayers S, Gautier T, Guiu B, Deckert V, Chevriaux A, Grober J, Le Guern N, Pais de Barros JP, Moore DD, Lagrost L, Masson D. Constitutive androstane receptor activation decreases plasma apolipoprotein B-containing lipoproteins and atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(10):2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim JH, Rozek LS, Soliman AS, Sartor MA, Hablas A, Seifeldin IA, Colacino JA, Weinhouse C, Nahar MS, Dolinoy DC. Bisphenol A-associated epigenomic changes in prepubescent girls: a cross-sectional study in Gharbiah, Egypt. Environ Health. 2013;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX, Lezmi S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol Appl Pharmacol. 2015;284(2):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li J, Lai H, Chen S, Zhu H, Lai S. Gender differences in the associations between urinary bisphenol A and body composition among American children: the National Health and Nutrition Examination Survey, 2003-2006. J Epidemiol. 2017;27(5):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. Am J Epidemiol. 2013;177(11):1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li DK, Miao M, Zhou Z, Wu C, Shi H, Liu X, Wang S, Yuan W. Urine bisphenol-A level in relation to obesity and overweight in school-age children. PLoS One. 2013;8(6):e65399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113(8):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bouskine A, Nebout M, Brücker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect. 2009;117(7):1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]