Abstract
Transforming growth factor-β (TGF-β) 1 plays a critical role in regulating follicular development, and its dysregulation has been shown to be involved in the pathogenesis of ovulation dysfunction. SNAIL is a well-known transcriptional repressor that mediates TGF-β1–induced cellular functions. Pentraxin 3 (PTX3) is a key enzyme for the assembly and stabilization of the cumulus oophorus extracellular matrix, which is essential for cumulus expansion during the periovulatory stage. The purpose of the present study was to investigate the roles of TGF-β1 and SNAIL in the regulation of PTX3 expression and to examine the underlying mechanism. An established immortalized human granulosa cell (GC) line (SVOG), a GC tumor cell line (KGN), and primary human granulosa-lutein cells were used as study models. We demonstrated that TGF-β1 treatment substantially decreased the messenger RNA and protein levels of PTX3. This suppressive effect was abolished by cotreatment with the soluble TGF-β type II receptor (TβRII) or the ALK4/5/7 inhibitor SB431542. Knockdown of ALK5, SMAD2/3, or SMAD4 reversed the effects of TGF-β1–induced SNAIL upregulation and PTX3 suppression. These results indicate that TGF-β1 upregulates SNAIL and downregulates PTX3 expression via a TβRII-ALK5–mediated SMAD-dependent signaling pathway in human GCs. Additionally, TGF-β1–induced PTX3 suppression was mediated by upregulation of the SNAIL transcription factor, as knockdown of SNAIL completely reversed the suppression of PTX3 in response to TGF-β1. These findings could inform the roles of TGF-β1 and SNAIL in the regulation of follicular function and might provide therapeutic targets for the treatment of ovulation dysfunction.
TGF-β1 is a critical intraovarian growth factor that potentially regulates the expression of pentraxin 3, a key enzyme for the event of human ovulation.
Pentraxin 3 (PTX3) is a prototype of the long pentraxin family produced by diverse cell types and tissues, including human granulosa cells (GCs) (1–3). This long pentraxin is involved in the regulation of a variety of cellular functions, including cell proliferation, angiogenesis, migration, and invasion. Additionally, PTX3 plays a critical role in female fertility by modulating the formation and stabilization of cumulus expansion (4). Depletion of PTX3 in mice results in a phenotype of disturbed cumulus oophorus complex associated with unstable extracellular matrix, which results in a severe defect in female fertility (4, 5). The expression of PTX3 in cumulus cells is closely correlated with both the quality and fertilization rate of the corresponding oocytes (6). Taken together, these studies suggest that dysregulated PTX3 might be involved in the pathogenesis of ovulation dysfunction.
Transforming growth factors-β (TGFs-β) are multifunctional polypeptides that belong to the TGF-β superfamily. At present, three TGF-β isoforms (TGF-β1, TGF-β2, and TGF-β3) have been identified in humans (7). Previous studies have shown that TGF-β1 and its functional receptors are expressed in human ovarian tissues (8, 9). In many cell types, TGF-β1 initiates its cellular action by binding to the TGF-β type II receptor (TβRII) and then recruiting the TGF-β type I receptor (TβRI). The binding of a ligand to these receptors leads to activation and phosphorylation of the downstream molecules SMAD2/3. In association with SMAD4, phosphorylated SMAD2/3 then translocate to the nucleus and regulate the expression of target genes. TGF-β1 acts through this SMAD-dependent signaling pathway to modulate numerous biological and pathological processes, including ovarian function (10, 11).
SNAIL and SLUG belong to the SNAIL superfamily of zinc-finger-type transcription factors that regulate mesoderm formation, cell survival, and cell division (12). Additionally, SNAIL and SLUG are important epithelial-to-mesenchymal transition mediators that have been reported to be overexpressed in gynecological cancers (13). Although the spatial distribution and function of SNAIL and SLUG have been investigated in embryogenesis and organogenesis (14, 15), little is known about the roles of these two transcription factors in regulating human ovarian function. In mice, both SNAIL and SLUG are expressed in epithelial cells, oocytes, follicle cells, and the corpus luteum, indicating that they might play critical roles during follicular development, luteinization, and early embryonic development (16).
Women with polycystic ovary syndrome (PCOS) frequently encounter ovulation dysfunction and poor fertilization rates of retrieved oocytes during in vitro fertilization treatment (17). Although the pathophysiology of PCOS is still not well-characterized, emerging evidence has suggested a possible role for dysregulation of TGF-β1 in this endocrine disease (18–21). In particular, PCOS ovaries display all the characteristics of TGF-β hyperactivity, such as increases in vascularity and deposition of collagen at the stroma and theca (22). Genetic analysis studies have shown that TGF-β dysregulation caused by a mutation of fibrillin-3 is associated with increased insulin resistance and PCOS (23, 24). Moreover, TGF-β1 bioavailability is increased in women with PCOS owing to an increase in serum TGF-β1 levels (20, 21). Collectively, these studies led us to propose a functional role of TGF-β1 in downregulating the expression of PTX3 in human cumulus/GCs and inhibiting the formation of cumulus expansion. Similarly, in normal biological situations, GC-secreted TGF-β1 might act as an inhibitor of cumulus expansion, maintaining the cumulus oophorus in meiotic arrest before ovulation. To test this hypothesis, we investigated the effects of TGF-β1 on the expression of PTX3 to uncover the underlying molecular mechanisms in human GCs.
Materials and Methods
Immortalized human GC (SVOG cell) and granulosa tumor cell (KGN cell) culture
In the present study, we used a nontumorigenic immortalized human GC line (SVOG) previously produced by transfecting primary human granulosa–lutein cells with the SV40 large T antigen and a GC tumor-derived cell line (KGN) (25). These two cell lines have been recently used as cell models to study human ovarian physiology and pathology in several studies (26–30). SVOG and KGN cells were seeded at a density of 5 × 105 cells per well in six-well plates and were cultured in Dulbecco’s modified Eagle medium/nutrient mixture F-12 Ham (Sigma-Aldrich, Oakville, ON) supplemented with 10% charcoal/dextran-treated fetal bovine serum (HyClone, Logan, UT), 100 U/mL penicillin (Invitrogen, Life Technologies, Grand Island, NY), 100 μg/mL streptomycin sulfate (Invitrogen), and 1× GlutaMAX (Invitrogen) in a humidified atmosphere containing 5% carbon dioxide at 37°C. The culture medium was changed every 48 hours for all experiments, and cells were starved in serum-free medium for 24 hours before treatment. For concentration-dependent studies, different concentrations (1, 5, or 10 ng/mL) of TGF-β1 were used to treat the cells for 24 hours. For the time-course studies, the cells were treated with 10 ng/mL TGF-β1 for 1, 3, 6, 12, or 24 hours. After specific treatment, cells were harvested for examining messenger RNA (mRNA) and protein levels using reverse transcription quantitative polymerase chain reaction (RT-qPCR) and western blot analysis, respectively. Culture medium was collected for to examine the secreted PTX3 using an enzyme immunoassay.
Preparation and culture of primary human granulosa–lutein cells
Primary human granulosa–lutein (hGL) cells were obtained with informed patient consent after approval from the University of British Columbia Research Ethics Board. The samples were anonymized immediately after collection. The controlled ovarian stimulation protocol for patients undergoing in vitro fertilization consisted of either luteal-phase nafarelin acetate (Synarel; Pfizer, Kirkland, QC, Canada) or follicular phase gonadotropin-releasing hormone antagonist (Ganirelix; Merck Canada), acting by competitively blocking the gonadotropin-releasing hormone receptors on the pituitary gonadotroph and subsequent transduction pathway. Gonadotropin stimulation began on menstrual cycle day 2 with human menopausal gonadotropin (Menopur; Ferring Canada) and recombinant follicle-stimulating hormone (Puregon; Merck Canada), followed by human chorionic gonadotropin administration 34 to 36 hours before oocyte retrieval, dependent on the follicle size. hGL cells were purified by density centrifugation from follicular aspirates collected from women undergoing oocyte retrieval as previously described (31).
Antibodies and reagents
Anti-SMAD2, anti–phospho-SMAD2, anti-SMAD3, anti–phospho-SMAD3, anti-SMAD4, anti-SNAIL, and anti-SLUG antibodies were obtained from Cell Signaling Technology (Beverly, MA). Anti–α-tubulin (sc-23948) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse IgGs were obtained from Bio-Rad Laboratories (Hercules, CA) and Santa Cruz Biotechnology, respectively. Mouse myeloma cell-derived recombinant human TGF-β1 and recombinant human TGF-β RII Fc chimera protein (rTβRII) were obtained from R&D Systems (Minneapolis, MN). Dorsomorphin dihydrochloride and SB431542 were obtained from R&D Systems and Sigma-Aldrich, respectively.
Reverse transcription quantitative real-time PCR
After treatment, the cells were harvested and total RNA extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. For each reaction, 2.5 μg of RNA was reverse transcribed into first-strand complementary DNA (cDNA) using Moloney murine leukemia virus reverse transcription and random primers (Promega, Madison, WI). Each 20 μL of qPCR contained 10 μL of 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 100 ng of cDNA, and 5 nM of each specific primer. The primers used in the present study were as follows: SNAIL, 5′-CCCCAATCGGAAGCCTAACT-3′ (forward) and 5′-GCTGGAAGGTAAACTCTGGATTAGA-3′ (reverse); SLUG, 5′-TTCGGACCCACACATTACCT-3′ (forward) and 5′-GCAGTGAGGGCAAGAAAAAG-3′ (reverse); SMAD2, 5′-GCCTTTACAGCTTCTCTGAACAA-3′ (forward) and 5′-ATGTGGCAATCCTTTTCGAT-3′ (reverse); SMAD3, 5′-CCCCAGCACATAATAACTTGG-3′ (forward) and 5′-AGGAGATGGAGCACCAGAAG-3′ (reverse); SMAD4, 5′-TGGCCCAGGATCAGTAGGT-3′ (forward) and 5′-CATCAACACCAATTCCAGCA-3′ (reverse); steroidogenic acute regulatory protein (StAR), 5′-AAACTTACGTGG CTACTCAGCATC-3′ (forward) and 5′-GACCTGGTTGATGCTCTTG-3′ (reverse); CYP11A1, 5′-CAGGAGGGGTGGACACGA C-3′ (forward) and 5′-AGGTTGCGT GCCATCTCATAC-3′ (reverse); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-GAGTCAACGGATTTGGTCGT-3′ (forward) and 5′-GACAAGCTTCC CGTTCTCAG-3′ (reverse). Alternatively, TaqMan gene expression assays for PTX3 (Hs00173615_m1), ACVR1B (ALK4, Hs00244715_m1), TβRI (ALK5, Hs00610320_m1), and GAPDH (Hs02758991_g1) (Applied Biosystems) were performed in triplicate on corresponding cDNA samples. For each 20-µL TaqMan reaction, 100 ng of cDNA was mixed with 10 µL of 2× TaqMan gene expression master mix (Applied Biosystems) and 1 µL of 20× TaqMan gene expression probe. RT-qPCR was performed using an Applied Biosystems 7300 Real-Time PCR System. Relative quantification of the mRNA levels was performed with different cultures, and each sample was assayed in triplicate. Mean values were used to determine the mRNA levels using the comparative Ct (2–∆∆Ct) method, with GAPDH as the internal control gene.
Western blot analysis
After treatment, the cells were thoroughly washed with cold phosphate-buffered saline and lysed in lysis buffer (Cell Signaling Technology) containing protease inhibitor cocktail (Sigma-Aldrich). The extracts were centrifuged at 20,000g for 15 minutes at 4°C to remove undissolved cellular debris, and protein concentrations were quantified using the DC Protein Assay (Bio-Rad Laboratories). Equal amounts of protein (30 µg) were loaded and separated by 12% Tris-glycine sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked for 2 hours in Tris-buffered saline containing 0.05% Tween-20 and 5% nonfat milk and incubated overnight at 4°C with the relevant primary antibodies. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Immunoreactive bands were detected using enhanced chemiluminescence reagents or a Super Signal West Femto Chemiluminescence Substrate (Pierce, Rockford, IL), followed by exposure to CL-XPosure film (Thermo Fisher Scientific, Waltham, MA). The membranes were stripped with stripping buffer (50 mM Tris-HCl [pH 7.6], 10 mmol/L β-mercaptoethanol, and 1% sodium dodecyl sulfate) at 50°C for 30 minutes and reprobed with mouse anti–α-tubulin antibody as a loading control. The films were scanned using Scion imaging software (Scion Corp., Frederick, MD).
Small interfering RNA transfection
Transient knockdown assays were performed with ON-TARGET plus nontargeting control pool small interfering RNA (siRNA) or ON-TARGET plus SMART pools targeting ALK4, ALK5, SNAIL, SMAD2, SMAD3, or SMAD4 (Dharmacon; GE Healthcare Life Sciences, Lafayette, CO). The cells were precultured to 50% confluence in antibiotic-free Dulbecco’s modified Eagle/F12 medium containing 10% charcoal/dextran-treated fetal bovine serum and then transfected with 25 nM siRNA using Lipofectamine RNAi MAX in Opti-MEM (Life Technologies) according to the manufacturer’s instructions. The knockdown efficiency for each target was analyzed using RT-qPCR or western blotting.
Measurement of secreted PTX3
After the specified treatment, the culture medium was isolated and stored at −80°C until analysis. PTX3 accumulation in conditioned medium was measured according to the manufacturer’s instructions using a quantitative sandwich enzyme immunoassay Quantikine kit (R&D Systems). The inter- and intra-assay coefficient of variation for these assays was <6%, and the detection limit of PTX3 ranged from 7 to 116 pg/mL. Each sample was measured in triplicate, and the secreted PTX levels were normalized to the total cellular protein content.
Statistical analysis
PRISM software (GraphPad Software, Inc., San Diego, CA) was used to perform one-way analysis of variance, followed by Tukey multiple comparison tests. The results are presented as the mean ± standard error of the mean of at least three separate experiments performed on different cultures. Differences were considered statistically significant at P < 0.05.
Results
TGF-β1 downregulates PTX3 gene expression in SVOG, KGN, and primary hGL cells
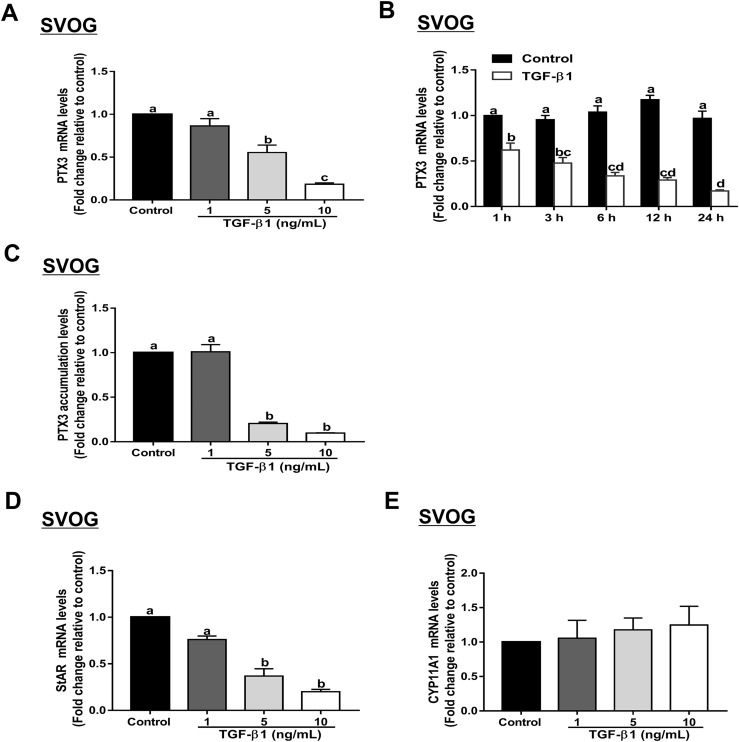
To investigate the effect of TGF-β1 on the expression of PTX3 in human GCs, we used recombinant human TGF-β1 to treat the cells. As shown in Fig. 1A, PTX3 mRNA levels were substantially decreased after treatment with the increasing concentrations (1, 5, or 10 ng/mL) of TGF-β1 in SVOG cells. In addition, the time-course study showed that treatment with 10 ng/mL TGF-β1 decreased the PTX3 mRNA levels starting at 1 hour, and the suppressive effect persisted until 24 hours (Fig. 1B). The suppressive effect of TGF-β1 on PTX3 protein accumulation was examined using an enzyme immunoassay. The results showed that treatment with increasing concentrations (1, 5, or 10 ng/mL) of TGF-β1 for 24 hours decreased PTX3 accumulation levels in SVOG cells in a concentration-dependent manner (Fig. 1C). To further confirm these results, we used a positive control gene (StAR) and a negative control gene (CYP11A1), as previously reported (32). The results showed that StAR mRNA levels were substantially decreased after treatment with increasing concentrations (1, 5, or 10 ng/mL) of TGF-β1 in SVOG cells (Fig. 1D). However, treatment with increasing concentrations (1, 5, or 10 ng/mL) of TGF-β1 did not affect CYP11A1 mRNA levels (Fig. 1E).
Figure 1.
TGF-β1 downregulates PTX3 expression in SVOG cells. (A) SVOG cells were treated for 24 hours with vehicle control or different concentrations (1, 5, or 10 ng/mL) of TGF-β1, and PTX3 mRNA levels were examined using RT-qPCR. (B) SVOG cells were treated with 10 ng/mL TGF-β1 for 1, 3, 6, 12, or 24 hours, and PTX3 mRNA levels were examined using RT-qPCR. (C) SVOG cells were treated with vehicle control or different concentrations (1, 5, or 10 ng/mL) of TGF-β1, and PTX3 accumulation in conditioned medium was measured using an enzyme immunoassay. SVOG cells were treated for 24 hours with vehicle control or different concentrations (1, 5, or 10 ng/mL) of TGF-β1, and (D) mRNA levels of StAR (positive control) and (E) CYP11A1 (negative control) were examined using RT-qPCR. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05).
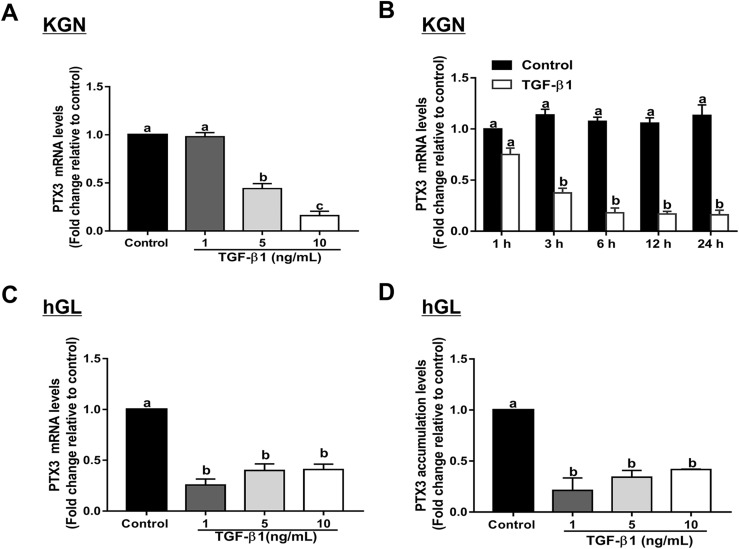
Likewise, the suppressive effects of TGF-β1 on the expression of PTX3 mRNA levels were confirmed in KGN granulosa tumor cells. As shown in Fig. 2A, treatment with increasing concentrations (1, 5, or 10 ng/mL) of TGF-β1 for 24 hours decreased PTX3 mRNA levels in KGN cells in a concentration-dependent manner. The time-course study showed that treatment of KGN cells with 10 ng/mL TGF-β1 substantially decreased PTX3 mRNA levels starting at 3 hours, and the suppressive effect persisted until 24 hours (Fig. 2B). Nonimmortalized primary hGL cells were used to further confirm the regulation of PTX3 by TGF-β1. Treatment of primary hGL cells for 24 hours with TGF-β1 (1, 10, or 100 ng/mL) substantially decreased PTX3 mRNA and PTX3 accumulation levels (Fig. 2C and 2D).
Figure 2.
TGF-β1 downregulates PTX3 expression in KGN and primary hGL cells. (A) KGN cells were treated for 24 hours with vehicle control or different concentrations (1, 5, or 10 ng/mL) of TGF-β1, and PTX3 mRNA levels were examined using RT-qPCR. (B) KGN cells were treated with 10 ng/mL TGF-β1 for 1, 3, 6, 12, or 24 hours, and PTX3 mRNA levels were examined using RT-qPCR. Primary hGL cells were treated for 24 hours with vehicle control or different concentrations (1, 5, or 10 ng/mL) of TGF-β1, and (C) PTX3 mRNA and (D) PTX3 accumulation levels were examined using RT-qPCR and an enzyme immunoassay, respectively. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05).
TGF-β1 downregulates expression of PTX3 via a TβRII/ALK5-mediated signaling pathway
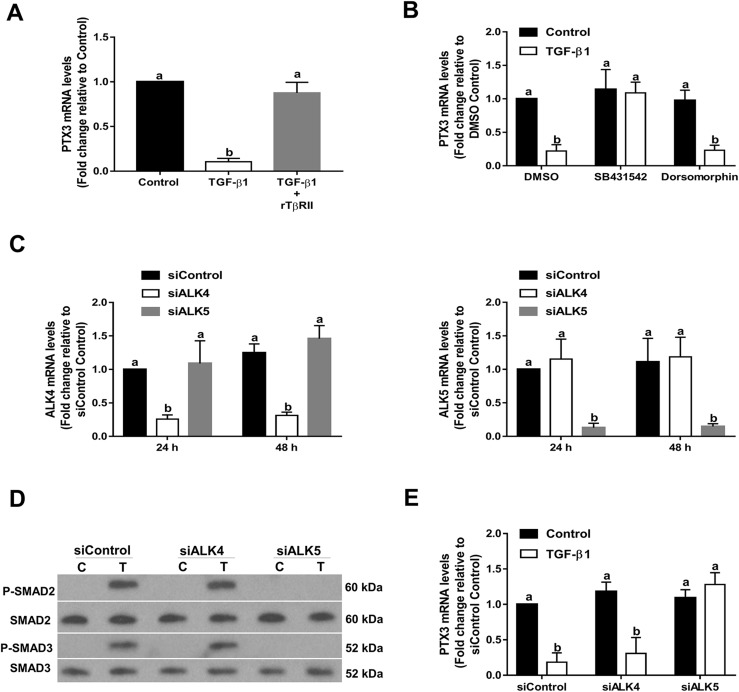
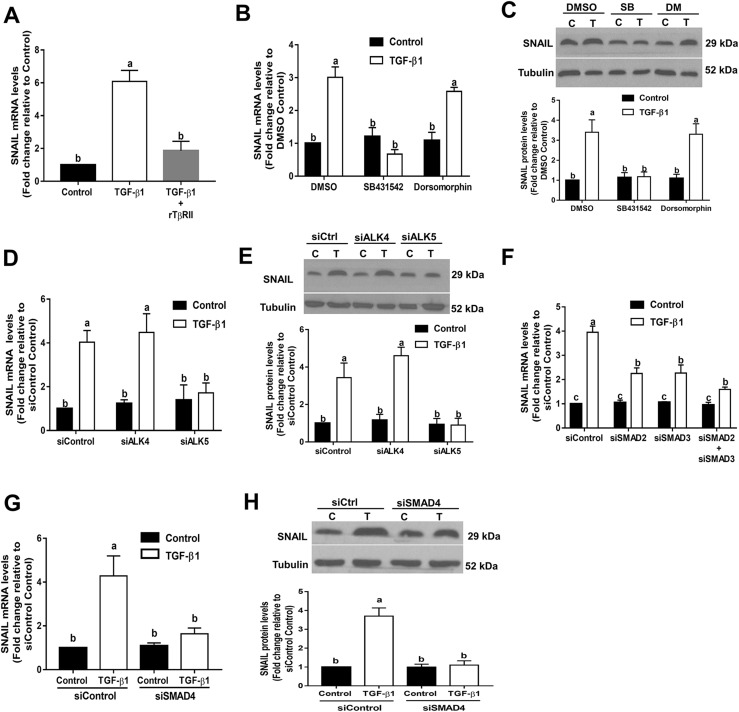
TβRII is a membrane-bound serine/threonine kinase receptor that can bind to TGF-β1 and TGF-β3 with high affinity compared with binding to TGF-β2 (33). rTβRII is a soluble TβRII that is capable of binding to TGF-β1 and TGF-β3 with sufficient affinity to act as an inhibitor at high concentrations (34). To determine whether the TβRII is involved in TGF-β1–induced downregulation of PTX3 expression, rTβRII was used as a decoy receptor to compete with endogenous TβRII. As shown in Fig. 3A, application of 5 µg/mL rTβRII, preincubated with 10 ng/mL recombinant TGF-β1 at room temperature for 1 hour, completely abolished its inhibitory effects on PTX3 expression. To determine which TβRI is required for TGF-β1–induced downregulation of PTX3 expression, SVOG cells were treated with TGF-β1 in the presence or absence of 10 μM SB431542 (a specific inhibitor of ALK4/5/7) and 10 μM dorsomorphin dihydrochloride (a specific inhibitor of ALK2/3/6) (35, 36). As shown in Fig. 3B, treatment of SVOG cells with SB431542 but not dorsomorphin dihydrochloride reversed the TGF-β1–induced downregulation of PTX3 expression. To further determine which ALKs mediate TGF-β1–induced downregulation of PTX3 expression, specific siRNAs were used to knockdown endogenous ALK4 or ALK5 in SVOG cells. As shown in Fig. 3C, transfection of SVOG cells with ALK4 or ALK5 siRNA substantially downregulated the mRNA levels of only the targeted ALK. To investigate the TGF-β1–induced downstream signaling pathway, phosphorylation levels of SMAD2 and SMAD3 were examined using western blot analysis after TGF-β1 treatment for 45 minutes. As shown in Fig. 3D, TGF-β1 treatment substantially increased the phosphorylation levels of SMAD2 and SMAD3, and the knockdown of ALK5, but not ALK4, completely abolished the TGF-β1–induced increases in phosphorylation levels of SMAD2 and SMAD3. Only knockdown of ALK5 reversed the TGF-β1–induced downregulation of PTX3 expression. In contrast, downregulation of ALK4 had no effect (Fig. 3E). These results demonstrate that TβRII and ALK5 type I receptor are the functional receptors that mediate the effect of TGF-β1 in suppressing PTX3 expression in SVOG cells.
Figure 3.
TGF-β1 downregulates the expression of PTX3 via a TβRII/ALK5-mediated signaling pathway in SVOG cells. (A) A total of 5 µg/mL rTβRII was preincubated with 10 ng/mL recombinant TGF-β1 at room temperature for 1 hour and was then added to SVOG cells for 24 hours. PTX3 mRNA levels were examined using RT-qPCR. (B) Cells were treated with 10 ng/mL TGF-β1 for 6 hours in the presence of vehicle control [dimethyl sulfoxide (DMSO)], 10 μM SB431542 or 10 μM dorsomorphin dihydrochloride. PTX3 mRNA levels were examined using RT-qPCR. (C) Cells were transfected with 25 nM siRNA [small interfering (si)Control, siALK4, or siALK5] for 24 or 48 hours, and the mRNA levels of ALK4 or ALK5 were examined using RT-qPCR. (D) Cells were transfected with siRNA (siControl, siALK4, or siALK5) for 48 hours and then treated with 10 ng/mL TGF-β1 for 45 minutes. Phosphorylation levels of SMAD2 and SMAD3 were examined using western blot analysis. (E) Cells were transfected with siRNA (siControl, siALK4, or siALK5) for 48 hours and then treated for an additional 6 hours with vehicle control or 10 ng/mL TGF-β1. PTX3 mRNA levels were examined using RT-qPCR. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05). C, control; P, phosphorylated; T, TGF-β1.
SMAD2/3-SMAD4 signaling pathway is required for TGF-β1–induced downregulation of PTX3 expression in SVOG cells
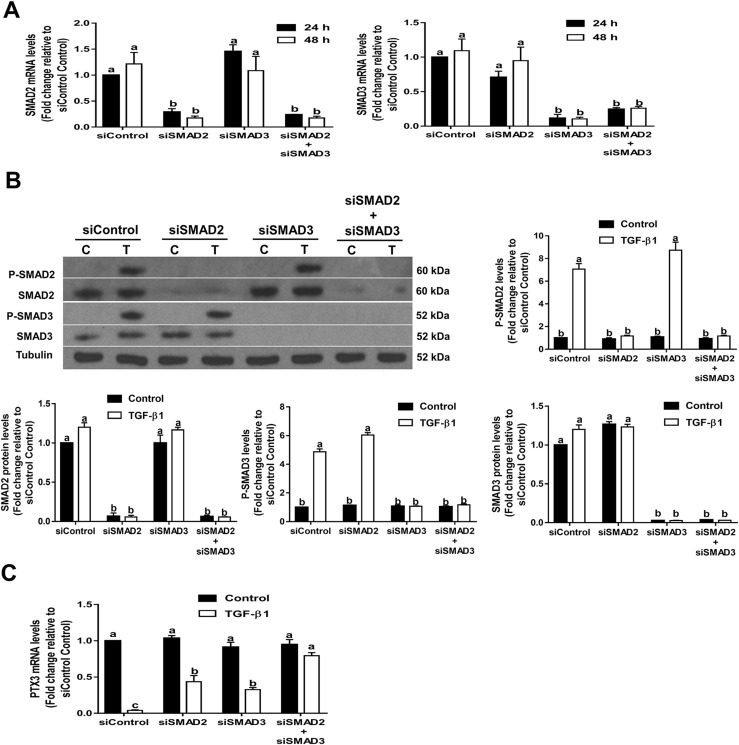
Previous studies have demonstrated that SMAD2/3 and SMAD4 are canonical downstream mediators of TGF-β1–induced cellular signaling in human GCs (32, 37). To examine the functional roles of SMAD2 and SMAD3 in TGF-β1–induced downregulation of PTX3 expression, we treated SVOG cells with 10 ng/mL TGF-β1 after siRNA-mediated knockdown of SMAD2, SMAD3, or SMAD2 and SMAD3 (concomitant knockdown). The knockdown efficiency of each SMAD (SMAD2, SMAD3, and SMAD2/3) was confirmed by RT-qPCR (Fig. 4A) and western blot analysis (Fig. 4B). Additionally, we examined the phosphorylation levels of SMAD2/3 during TGF-β1 treatment after SMAD2/3 knockdown. The results showed that the phosphorylation levels of SMAD2/3 were completely abolished after knockdown of SMAD2/3 (Fig. 4B). Knockdown of SMAD2 alone or SMAD3 alone only partially reversed the downregulation of PTX3 induced by TGF-β1 (Fig. 4C). However, concomitant knockdown of SMAD2 and SMAD3 totally reversed the suppressive effect induced by TGF-β1 (Fig. 4C).
Figure 4.
SMAD2/3-mediated signaling is required for TGF-β1–induced downregulation of PTX3 in SVOG cells. (A and B) Cells were transfected for 48 hours with 25 nM control siRNA (siControl), SMAD2 siRNA (siSMAD2), SMAD3 siRNA (siSMAD3), or both SMAD2 siRNA and SMAD3 siRNA (siSMAD2+siSMAD3). The (A) mRNA or (B) phosphorylated (P) and total protein levels of SMAD2 and SMAD3 were examined using RT-qPCR and western blot analysis, respectively. (C) Cells were transfected for 48 hours with 25 nM siControl, siSMAD2, siSMAD3, or siSMAD2+siSMAD3 and treated with 10 ng/mL TGF-β1 for an additional 6 hours. PTX3 mRNA levels were examined using RT-qPCR. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05).
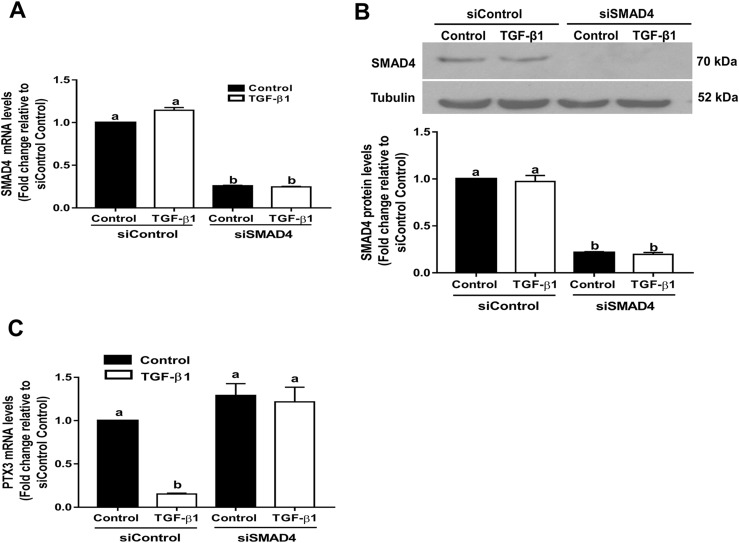
A similar approach was used to further confirm the role of SMAD signaling in TGF-β1–regulated PTX3 expression. We further treated SVOG cells with 10 ng/mL TGF-β1 after siRNA-mediated knockdown of the common SMAD, SMAD4 (knockdown efficiency was confirmed using RT-qPCR and western blot analysis; Fig. 5A and 5B). Depletion of SMAD4 reversed the suppressive effect on PTX3 expression induced by TGF-β1 (Fig. 5C).
Figure 5.
SMAD4-mediated signaling is required for TGF-β1–induced downregulation of PTX3 in SVOG cells. (A and B) Cells were transfected for 48 hours with 25 nM control siRNA (siControl) or SMAD4 siRNA (siSMAD4) and treated with 10 ng/mL TGF-β1 for an additional 6 hours. (A) mRNA and (B) protein levels of SMAD4 were examined using RT-qPCR and western blot analysis, respectively. (C) Cells were transfected for 48 hours with 25 nM siControl or siSMAD4 and were treated with 10 ng/mL TGF-β1 for an additional 6 hours. mRNA levels of PTX3 were examined using RT-qPCR. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05).
TGF-β1 upregulates expression of SNAIL in SVOG cells
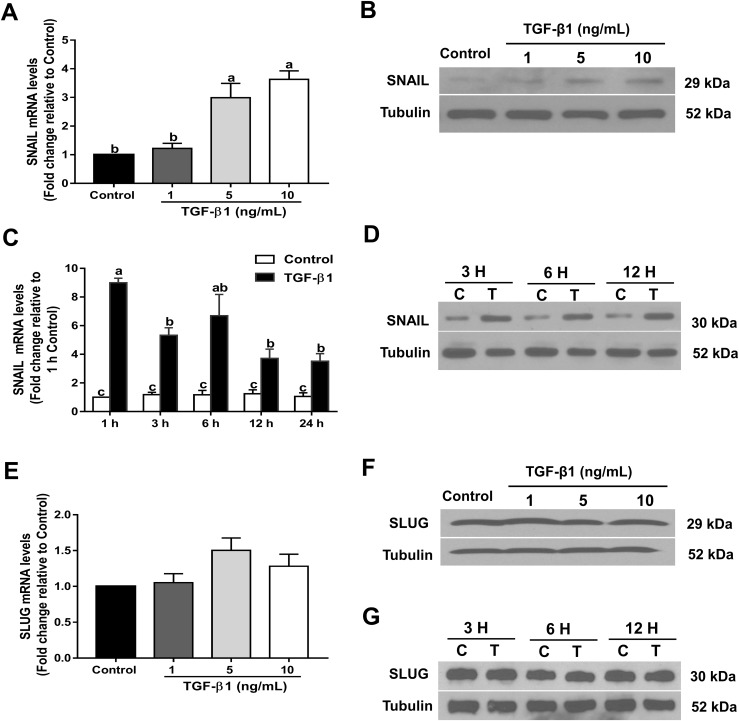
The TGF-β superfamily of signaling molecules has been implicated in the upregulation of SNAIL and SLUG (38, 39). To investigate whether TGF-β1 can upregulate SNAIL and SLUG in human GCs, we treated SVOG cells with TGF-β1, and the mRNA and protein levels of these two transcription factors were examined using RT-qPCR and western blot analysis, respectively. As shown in Fig. 6A and 6B, SNAIL mRNA and protein levels were substantially increased after treatment with increasing concentrations (1, 5, or 10 ng/mL) of TGF-β1 in SVOG cells. The time-course study showed that 10 ng/mL TGF-β1 substantially increased the levels of SNAIL mRNA (starting at 1 hour; Fig. 6C) and protein (starting at 3 hours; Fig. 6D) in SVOG cells. However, TGF-β1 did not stimulate the expression of SLUG in SVOG cells (Fig. 6E–6G).
Figure 6.
TGF-β1 upregulates SNAIL expression in SVOG cells. SVOG cells were treated for 24 hours with vehicle control or different concentrations (1, 5, or 10 ng/mL) of TGF-β1, and the (A) mRNA and (B) protein levels were examined using RT-qPCR and western blot analysis, respectively. (C) SVOG cells were treated with 10 ng/mL TGF-β1 for 1, 3, 6, 12, or 24 hours, and SNAIL mRNA levels were examined using RT-qPCR. (D) SVOG cells were treated with 10 ng/mL TGF-β1 for 3, 6, or 12 hours, and SNAIL protein levels were examined using western blot analysis. SVOG cells were treated for 24 hours with vehicle control or different concentrations (1, 5, or 10 ng/mL) of TGF-β1, and the (E) mRNA and (F) protein levels were examined using RT-qPCR and western blot analysis, respectively. (G) SVOG cells were treated with 10 ng/mL TGF-β1 for 3, 6, or 12 hours, and SLUG protein levels were examined using western blot analysis. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05).
TβRII/ALK5-mediated SMAD signaling is required for TGF-β1–induced upregulation of SNAIL expression in SVOG cells
Next, we used soluble recombinant receptors and both pharmacological and siRNA-based approaches to investigate the requirement of TGF-β receptors in the TGF-β1–induced downregulation of SNAIL. As shown in Fig. 7A, preincubation with rTβRII totally abolished the upregulation of SNAIL induced by TGF-β1 in SVOG cells. Treatment with the ALK4/5/7 inhibitor SB431542 abolished the TGF-β1–induced increase in SNAIL mRNA (Fig. 7B) and protein (Fig. 7C) levels. In contrast, the ALK2/3/6 inhibitor dorsomorphin dihydrochloride did not have such effects. Additionally, knockdown of ALK5 but not ALK4 abolished the increases in SNAIL mRNA (Fig. 7D) and protein (Fig. 7E) levels. Similarly, knockdown of either SMAD2 or SMAD3 attenuated the TGF-β1–induced increases in SNAIL mRNA (Fig. 7F). Furthermore, knockdown of SMAD4 completely abolished the TGF-β1–induced increases in SNAIL mRNA (Fig. 7G) and protein (Fig. 7H) levels. These results indicate that TβRII/ALK5-mediated SMAD signaling is required for TGF-β1–induced upregulation of SNAIL expression in SVOG cells.
Figure 7.
TβRII/ALK5-mediated SMAD signaling is required for TGF-β1–induced upregulation of SNAIL in SVOG cells. (A) A total of 5 µg/mL rTβRII was preincubated with 10 ng/mL TGF-β1 at room temperature for 1 hour and was then added to cells for 24 hours. SNAIL mRNA levels were examined using RT-qPCR. (B and C) Cells were treated with 10 ng/mL TGF-β1 for 24 hours in the presence of vehicle control [dimethyl sulfoxide (DMSO)], 10 μM SB431542 or 10 μM dorsomorphin dihydrochloride. SNAIL (B) mRNA and (C) protein levels were examined using RT-qPCR and western blot analysis, respectively. (D and E) Cells were transfected with siRNAs (siControl, siALK4, or siALK5) for 48 hours and then treated for 6 hours with vehicle control or 10 ng/mL TGF-β1. SNAIL (D) mRNA and (E) protein levels were examined using RT-qPCR and western blot analysis, respectively. (F) Cells were transfected for 48 hours with 25 nM control siRNA (siControl), SMAD2 siRNA (siSMAD2), SMAD3 siRNA (siSMAD3), or both SMAD2 siRNA and SMAD3 siRNA (siSMAD2+siSMAD3) and then treated with 10 ng/mL TGF-β1 for an additional 6 hours. SNAIL mRNA levels were examined using RT-qPCR. (G and H) Cells were transfected for 48 hours with 25 nM siControl or siSMAD4 and then treated with 10 ng/mL TGF-β1 for an additional 6 hours. SNAIL (G) mRNA and (H) protein levels were examined using RT-qPCR and western blot analysis, respectively. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05). C, control; Ctrl, Control; DM, dorsomorphin dihydrochloride; SB, SB431542; T, TGF-β1.
SNAIL mediates TGF-β1–induced downregulation of PTX3 in SVOG cells
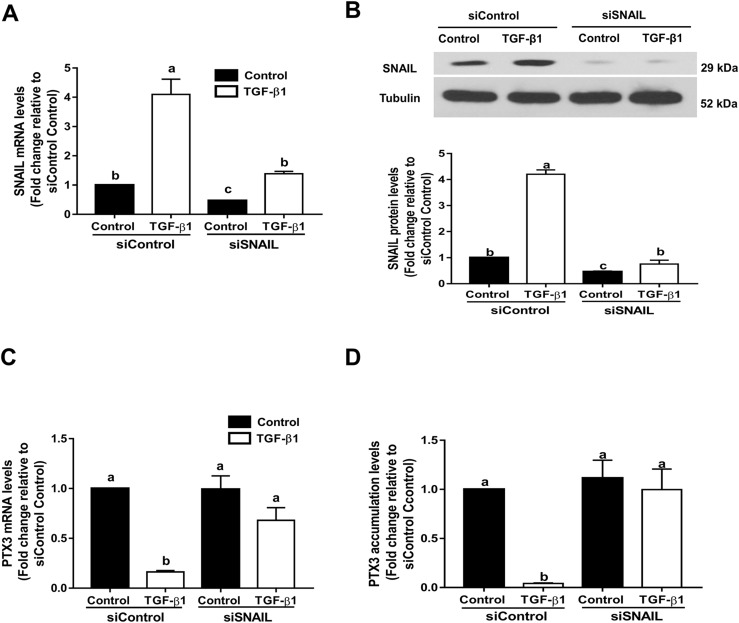
To investigate whether SNAIL mediates TGF-β1–induced downregulation of PTX3, a siRNA-based knockdown approach was used by transfecting SVOG cells with siRNA targeting SNAIL. The results were compatible with other siRNA-based studies that transfection of cells with SNAIL siRNA substantially reduced SNAIL mRNA (Fig. 8A) and protein (Fig. 8B) levels. Knockdown of SNAIL reversed the downregulation of PTX3 mRNA in response to TGF-β1 in SVOG cells (Fig. 8C). Additionally, knockdown of SNAIL reversed the decrease in PTX3 accumulation in response to TGF-β1 in SVOG cells (Fig. 8D).
Figure 8.
SNAIL mediates TGF-β1–induced downregulation of PTX3 in SVOG cells. (A and B) Cells were transfected for 48 hours with 25 nM control siRNA (siControl) or SNAIL siRNA (siSNAIL) and then treated with 10 ng/mL TGF-β1 for an additional 6 hours. SNAIL (A) mRNA and (B) protein levels were examined using RT-qPCR and western blot analysis, respectively. (C and D) Cells were transfected for 48 hours with 25 nM control siRNA (siControl) or SNAIL siRNA (siSNAIL) and then treated with 10 ng/mL TGF-β1 for an additional 6 hours. PTX3 (C) mRNA and (D) PTX3 accumulation levels were examined using RT-qPCR and an enzyme immunoassay, respectively. The results are expressed as the mean ± standard error of the mean of at least three independent experiments; lowercase letters indicate statistically significant differences (P < 0.05).
Discussion
In the present study, we have, to the best of our knowledge, demonstrated for the first time that recombinant TGF-β1 downregulates the mRNA and protein expression of PTX3 in SVOG, KGN, and primary hGL cells. This finding might provide insight into the molecular regulation of PTX3 in human GCs. Throughout follicular development, GC differentiation, oocyte maturation, cumulus expansion, and ovulation are highly dependent on an orchestrated interaction between the oocyte and its surrounding follicle cells (40). We have recently demonstrated that the intrafollicular growth factors derived from oocytes, theca, and GCs play critical roles in regulating various ovarian functions (40). Previous studies have shown that the synthesis of the cumulus–oophorus complex and the gene expression of cumulus cells in this complex are highly correlated with the quality and developmental capacity of the corresponding oocyte (41). Moreover, the degree of cumulus expansion has been used as a selection criterion to identify high-quality oocytes during in vitro fertilization treatment (42). Most importantly, the expression levels of PTX3 in GCs are correlated with oocyte quality and fertilization rates (43). Data from clinical studies have shown that TGF-β1 bioavailability is altered in women with PCOS during controlled ovarian stimulation (21). Together with our findings in GCs, the present data suggest that TGF-β1 downregulates PTX3 expression, which could lead to disrupted cumulus expansion and the subsequent ovulation dysfunction. Because TGF-β1–deficient mice die during development (44), it would be of great interest to generate ovarian tissue-specific conditional TGF-β1 mutants or overexpression lines and examine their effects on ovarian function.
In normal physiological conditions, the morphological changes involved in proliferation and dispersion of cumulus cells are essential for ovulation, transport of the cumulus–oophorus complex in the fallopian tubes and successful in vivo fertilization (4). In mice, oocyte-derived the bone morphogenetic protein (BMP)15 and growth differentiation factor (GDF)9 are key factors that upregulate several genes (Has2, Ptx3, and Ptgs2) in mouse GCs related to cumulus expansion and subsequently promote cumulus expansion (45). Our previous studies demonstrated that theca cell-derived BMP4 and BMP7 and GC-derived GDF8 downregulated the expression and protein production of PTX3 in human GCs (1, 2). Collectively, these studies indicate that oocyte-derived growth factors (mainly BMP15 and GDF9) promote cumulus expansion and maintain the cumulus cell phenotype. In contrast, theca cell-derived BMPs (BMP4 and BMP7), GC-derived GDF8 and TGF-β1 (which can be expressed in GCs and theca cells) decompose the structure of the extracellular matrix by downregulating PTX3 in neighboring mural GCs. These principle ovarian cells work together to facilitate separation of the cumulus–oophorus complex and mural GCs to subsequently achieve successful ovulation in the human ovary.
Given its potential role in the pathogenesis of ovulation dysfunction, a comprehensive understanding of the molecular mechanisms underlying cellular responses to TGF-β1 is essential for developing pharmacological strategies for clinical application. In the present study, using a soluble TβRII (rTβRII) as a competitive inhibitor, we confirmed that the TβRII is required for TGF-β1–induced cellular action in human GCs. Additionally, using pharmacological inhibitors and specific siRNA-based approaches, we demonstrated that ALK5 is the specific TβRI that primarily determines the biological responses to TGF-β1 in human GCs. Furthermore, our SMAD knockdown experiments confirmed that SMAD2/3–SMAD4-dependent signaling is required for the TGF-β1–mediated effects on PTX3 in human GCs. Consistent with our previous studies, another TGF-β superfamily member GDF8 exerts the same suppressive effect on PTX expression via an ALK5-mediated SMAD2/3-SMAD4–dependent signaling in human GCs (1). Our previous studies also demonstrated that BMP4 and BMP7 act to downregulate the expression of PTX3, an effect mediated by different subsets of type I receptors (ALK3/6 for BMP4 and ALK2/3 for BMP7) (2). Moreover, these BMPs use SMAD1/5/8-SMAD4, but TGF-β1 uses SMAD2/3-SMAD4 as the downstream signaling transducer to regulate the expression of PTX3 in the same cells. These findings raise the question of whether the combined effect or heterodimers of TGF-β1 with other TGF-β superfamily members can initiate the formation of additional receptor complexes involving ALK2/3/5/6 and induce a synergistic or unique biological response in human ovarian follicle.
In the present study, we found that SNAIL but not SLUG is required for TGF-β1–induced downregulation of PTX3 by showing that depletion of SNAIL completely reversed this suppressive effect in human GCs. Members of the SNAIL domain family of zinc finger proteins are well-established to recognize the canonical E-box sequences, CANNTG basic helix-loop-helix motifs (46). Previous studies have shown that several genes are regulated by TGF-β1–upregulated SNAIL via binding to the promoter sequences, CANNTG helix-loop-helix motifs (47, 48). At least seven CANNTG-like motifs have been found at the promoter of the human PTX3 gene (GenBank™ accession no. X97748.1), indicating that the effect of SNAIL on PTX3 could be direct. We found that TGF-β1 upregulates the expression of SNAIL, which in turn downregulates the expression of PTX3 (most likely by binding to its promoter region), indicating that SNAIL might participate in the modulation of cumulus expansion during the periovulatory phase. Furthermore, our results suggest that SNAIL and SLUG might have distinct functional roles in human GCs during follicular development.
In conclusion, we have demonstrated that recombinant TGF-β1 upregulates the expression of the transcription factor SNAIL via a TβRII/ALK5-mediated SMAD2/3-SMAD4 signaling pathway. Upregulation of SNAIL but not SLUG further downregulated the expression of PTX3, a key component of the formation of cumulus expansion. These findings inform the physiological roles of TGF-β1 and SNAIL in regulating follicular function and might provide therapeutic targets for the treatment of ovulation dysfunction.
Appendix.
Antibody Table
| Name of Antibody | Manufacturer, Catalog No. | Species Raised In; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|
| Anti-Tubulin | Santa Cruz Biotechnology, sc-23948 | Mouse; monoclonal | 1:2000 | AB_628410 |
| Anti-SMAD2 | Cell Signaling Technology, 3103 | Mouse; monoclonal | 1:1000 | AB_490816 |
| Anti-pSMAD2 | Cell Signaling Technology, 3101 | Rabbit; polyclonal | 1:1000 | AB_331673 |
| Anti-SMAD3 | Cell Signaling Technology, 9523 | Rabbit; monoclonal | 1:1000 | AB_2193182 |
| Anti-pSMAD3 | Cell Signaling Technology, 9520 | Rabbit; polyclonal | 1:1000 | AB_2193207 |
| Anti-SMAD4 | Cell Signaling Technology, 9515 | Rabbit; polyclonal | 1:1000 | AB_2193344 |
| Anti-SNAIL | Cell Signaling Technology, 3879 | Rabbit; monoclonal | 1:1000 | AB_10828214 |
| Anti-SLUG | Cell Signaling Technology, 9585 | Mouse; monoclonal | 1:1000 | AB_2239535 |
Abbreviations: p, phosphorylated; RRID, Research Resource Identifier.
Acknowledgments
Financial Support: This research was supported by Canadian Institutes of Health Research Foundation Scheme Grant 143317 (to P.C.K.L.). The present study was also supported by National Science Foundation of China Grant 31402080 and Natural Science Foundation of Jiangsu Province Grant BK20151365 (to H.L.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMP
bone morphogenetic protein
- cDNA
complementary DNA
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GC
granulosa cell
- GDF
growth differentiation factor
- hGL
human granulosa–lutein
- mRNA
messenger RNA
- PCOS
polycystic ovary syndrome
- PTX3
pentraxin 3
- RT-qPCR
reverse transcription quantitative polymerase chain reaction
- rTβRII
recombinant human transforming growth factor-β RII Fc chimera protein
- siRNA
small interfering RNA
- StAR
steroidogenic acute regulatory protein
- TGF-β
transforming growth factor-β
- TβRI
transforming growth factor-β type I receptor
- TβRII
transforming growth factor-β type II receptor
References
- 1. Chang HM, Fang L, Cheng JC, Klausen C, Sun YP, Leung PC. Growth differentiation factor 8 down-regulates pentraxin 3 in human granulosa cells. Mol Cell Endocrinol. 2015;404:82–90. [DOI] [PubMed] [Google Scholar]
- 2. Chang HM, Cheng JC, Fang L, Qiu X, Klausen C, Taylor EL, Leung PC. Recombinant BMP4 and BMP7 downregulate pentraxin 3 in human granulosa cells. J Clin Endocrinol Metab. 2015;100(3):E365–E374. [DOI] [PubMed] [Google Scholar]
- 3. Goodman AR, Cardozo T, Abagyan R, Altmeyer A, Wisniewski HG, Vilcek J. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996;7(2):191–202. [DOI] [PubMed] [Google Scholar]
- 4. Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, Mahoney DJ, Day AJ, Siracusa G, Romani L, Mantovani A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131(7):1577–1586. [DOI] [PubMed] [Google Scholar]
- 5. Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16(6):1154–1167. [DOI] [PubMed] [Google Scholar]
- 6. Huang X, Hao C, Shen X, Zhang Y, Liu X. RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients. Reprod Biol Endocrinol. 2013;11(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98(2):257–265. [DOI] [PubMed] [Google Scholar]
- 8. Chegini N, Flanders KC. Presence of transforming growth factor-beta and their selective cellular localization in human ovarian tissue of various reproductive stages. Endocrinology. 1992;130(3):1707–1715. [DOI] [PubMed] [Google Scholar]
- 9. Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod. 2014;20(4):293–308. [DOI] [PubMed] [Google Scholar]
- 10. Rosairo D, Kuyznierewicz I, Findlay J, Drummond A. Transforming growth factor-beta: its role in ovarian follicle development. Reproduction. 2008;136(6):799–809. [DOI] [PubMed] [Google Scholar]
- 11. Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor-beta: biological function and chemical structure. Science. 1986;233(4763):532–534. [DOI] [PubMed] [Google Scholar]
- 12. Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3(3):155–166. [DOI] [PubMed] [Google Scholar]
- 13. Zhou XM, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives. Tumour Biol. 2014;35(10):9523–9530. [DOI] [PubMed] [Google Scholar]
- 14. Nieto MA, Bennett MF, Sargent MG, Wilkinson DG. Cloning and developmental expression of Sna, a murine homologue of the Drosophila snail gene. Development. 1992;116(1):227–237. [DOI] [PubMed] [Google Scholar]
- 15. Savagner P, Karavanova I, Perantoni A, Thiery JP, Yamada KM. Slug mRNA is expressed by specific mesodermal derivatives during rodent organogenesis. Dev Dyn. 1998;213(2):182–187. [DOI] [PubMed] [Google Scholar]
- 16. Guo C, Meng X, Bai J, Chen C, Liu T, Liu S, Zhang C, Li WP. Expression and localization of transcription factors SNAIL and SLUG in mouse ovaries and pre-implantation embryos. Cell Tissue Res. 2014;358(2):585–595. [DOI] [PubMed] [Google Scholar]
- 17. Jabara S, Coutifaris C. In vitro fertilization in the PCOS patient: clinical considerations. Semin Reprod Med. 2003;21(3):317–324. [DOI] [PubMed] [Google Scholar]
- 18. Irani M, Seifer DB, Grazi RV, Julka N, Bhatt D, Kalgi B, Irani S, Tal O, Lambert-Messerlian G, Tal R, Vitamin D. Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2015;100(11):4307–4314. [DOI] [PubMed] [Google Scholar]
- 19. Liu M, Gao J, Zhang Y, Li P, Wang H, Ren X, Li C. Serum levels of TSP-1, NF-κB and TGF-β1 in polycystic ovarian syndrome (PCOS) patients in northern China suggest PCOS is associated with chronic inflammation. Clin Endocrinol (Oxf). 2015;83(6):913–922. [DOI] [PubMed] [Google Scholar]
- 20. Raja-Khan N, Kunselman AR, Demers LM, Ewens KG, Spielman RS, Legro RS. A variant in the fibrillin-3 gene is associated with TGF-β and inhibin B levels in women with polycystic ovary syndrome. Fertil Steril. 2010;94(7):2916–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tal R, Seifer DB, Shohat-Tal A, Grazi RV, Malter HE. Transforming growth factor-β1 and its receptor soluble endoglin are altered in polycystic ovary syndrome during controlled ovarian stimulation. Fertil Steril. 2013;100(2):538–543. [DOI] [PubMed] [Google Scholar]
- 22. Agrawal R, Sladkevicius P, Engmann L, Conway GS, Payne NN, Bekis J, Tan SL, Campbell S, Jacobs HS. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum Reprod. 1998;13(3):651–655. [DOI] [PubMed] [Google Scholar]
- 23. Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007;92(11):4191–4198. [DOI] [PubMed] [Google Scholar]
- 24. Ewens KG, Stewart DR, Ankener W, Urbanek M, McAllister JM, Chen C, Baig KM, Parker SC, Margulies EH, Legro RS, Dunaif A, Strauss JF III, Spielman RS. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocrinol Metab. 2010;95(5):2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lie BL, Leung E, Leung PC, Auersperg N. Long-term growth and steroidogenic potential of human granulosa-lutein cells immortalized with SV40 large T antigen. Mol Cell Endocrinol. 1996;120(2):169–176. [DOI] [PubMed] [Google Scholar]
- 26. Chang HM, Cheng JC, Klausen C, Leung PC. BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol Endocrinol. 2013;27(12):2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang HM, Cheng JC, Klausen C, Leung PC. Recombinant BMP4 and BMP7 increase activin A production by up-regulating inhibin βA subunit and furin expression in human granulosa-lutein cells. J Clin Endocrinol Metab. 2015;100(3):E375–E386. [DOI] [PubMed] [Google Scholar]
- 28. Chang HM, Cheng JC, Huang HF, Shi FT, Leung PC, Activin A. Activin A, B and AB decrease progesterone production by down-regulating StAR in human granulosa cells. Mol Cell Endocrinol. 2015;412:290–301. [DOI] [PubMed] [Google Scholar]
- 29. Chang HM, Pan HH, Cheng JC, Zhu YM, Leung PCK. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol Cell Endocrinol. 2016;422:9–17. [DOI] [PubMed] [Google Scholar]
- 30. Chang HM, Cheng JC, Liu Y, Klausen C, Xu C, Leung PC. Activin A-induced increase in LOX activity in human granulosa-lutein cells is mediated by CTGF. Reproduction. 2016;152(4):293–301. [DOI] [PubMed] [Google Scholar]
- 31. Chang HM, Fang L, Cheng JC, Taylor EL, Sun YP, Leung PC. Effects of growth differentiation factor 8 on steroidogenesis in human granulosa-lutein cells. Fertil Steril. 2016;105(2):520–528. [DOI] [PubMed] [Google Scholar]
- 32. Fang L, Chang HM, Cheng JC, Leung PC, Sun YP. TGF-β1 downregulates StAR expression and decreases progesterone production through Smad3 and ERK1/2 signaling pathways in human granulosa cells. J Clin Endocrinol Metab. 2014;99(11):E2234–E2243. [DOI] [PubMed] [Google Scholar]
- 33. Qian SW, Burmester JK, Tsang ML, Weatherbee JA, Hinck AP, Ohlsen DJ, Sporn MB, Roberts AB. Binding affinity of transforming growth factor-beta for its type II receptor is determined by the C-terminal region of the molecule. J Biol Chem. 1996;271(48):30656–30662. [DOI] [PubMed] [Google Scholar]
- 34. Huang T, Hinck AP. Production, isolation, and structural analysis of ligands and receptors of the TGF-β superfamily. Methods Mol Biol. 2016;1344:63–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62(1):65–74. [DOI] [PubMed] [Google Scholar]
- 37. Chen YC, Chang HM, Cheng JC, Tsai HD, Wu CH, Leung PC. Transforming growth factor-β1 up-regulates connexin43 expression in human granulosa cells. Hum Reprod. 2015;30(9):2190–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278(23):21113–21123. [DOI] [PubMed] [Google Scholar]
- 39. Choi J, Park SY, Joo CK. Transforming growth factor-beta1 represses E-cadherin production via slug expression in lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48(6):2708–2718. [DOI] [PubMed] [Google Scholar]
- 40. Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update. 2016;23(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99(4):979–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cetica PD, Dalvit GC, Beconi MT. Study of evaluation criteria used for in vitro bovine oocyte selection and maturation. Biocell. 1999;23(2):125–133. [PubMed] [Google Scholar]
- 43. McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, Amato P, Matzuk MM. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19(12):2869–2874. [DOI] [PubMed] [Google Scholar]
- 44. Kulkarni AB, Karlsson S. Transforming growth factor-beta 1 knockout mice: a mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 45. Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA. 2013;110(8):E776–E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mauhin V, Lutz Y, Dennefeld C, Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993;21(17):3951–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu J, Zhou XJ, Sun X, Xia TS, Li XX, Shi L, Zhu L, Zhou WB, Wei JF, Ding Q. RBM38 is involved in TGF-β-induced epithelial-to-mesenchymal transition by stabilising zonula occludens-1 mRNA in breast cancer. Br J Cancer. 2017;117(5):675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chiang C, Ayyanathan K. Characterization of the E-box binding affinity to snag-zinc finger proteins. Mol Biol (Mosk). 2012;46(6):907–914. [PubMed] [Google Scholar]